Play all audios:
ABSTRACT Cancer stem cells (CSC) are recently proposed to be the cancer initiating cells responsible for tumorigenesis and contribute to cancer resistance. Advances have been made in
identifying and enriching CSC in leukemia and several solid tumors, including breast, brain and lung cancers. These studies suggest that, like normal stem cells, CSCs should be rare,
quiescent, and capable of self-renewing and maintaining tumor growth and heterogeneity. Although the concept of CSC originates from that of normal stem cells, CSCs are not necessarily
aberrant counterparts of normal stem cells. In fact, they may arise from stem cells or committed progenitors of corresponding tissues, and even cells from other tissues. At the molecular
level, the alteration of stem cell self-renewal pathway(s) has been recognized as an essential step for CSC transformation. Better understanding of CSC will no doubt lead to a new era of
both basic and clinical cancer research, re-classification of human tumors and development of novel therapeutic strategies specifically targeting CSC. SIMILAR CONTENT BEING VIEWED BY OTHERS
CANCER STEM CELLS: ADVANCES IN KNOWLEDGE AND IMPLICATIONS FOR CANCER THERAPY Article Open access 05 July 2024 TARGETING CANCER STEM CELLS FOR REVERSING THERAPY RESISTANCE: MECHANISM,
SIGNALING, AND PROSPECTIVE AGENTS Article Open access 15 February 2021 CHARACTERIZATION OF STEM CELL LANDSCAPE AND ASSESSING THE STEMNESS DEGREE TO AID CLINICAL THERAPEUTICS IN HEMATOLOGIC
MALIGNANCIES Article Open access 10 October 2024 MAIN Stem cells are defined as undifferentiated cells that are capable of self-renewing and differentiating into a large number of diverse
mature progeny. Amongst the various categories of stem cells, the embryonic stem (ES) cells are totipotent and able to differentiate into many cell types under appropriate conditions _in
vitro_ and contribute to all different tissues _in vivo_ (1–3), making them a very promising foundation for stem cell-based therapeutics. Somatic stem cells from different organs, on the
other hand, are pluripotent and responsible for tissue regeneration and repair. Adult stem cells have been identified in several organs, such as the hematopoietic system, brain, skin,
mammary gland and lung, but it is not yet clear whether they are present in all other adult organs (4,5). The best-studied somatic stem cells are hematopoietic stem cells (HSC). With the aid
of cell surface markers for positive identification, fluorescence-activated cell sorting (FACS) for prospective isolation, and _in vitro_ and _in vivo_ assays for functional testing; HSCs
in mice and humans have been positively identified and successfully isolated by Weissman and colleagues (5,6). HSCs are known to be responsible for the generation of all cell types in the
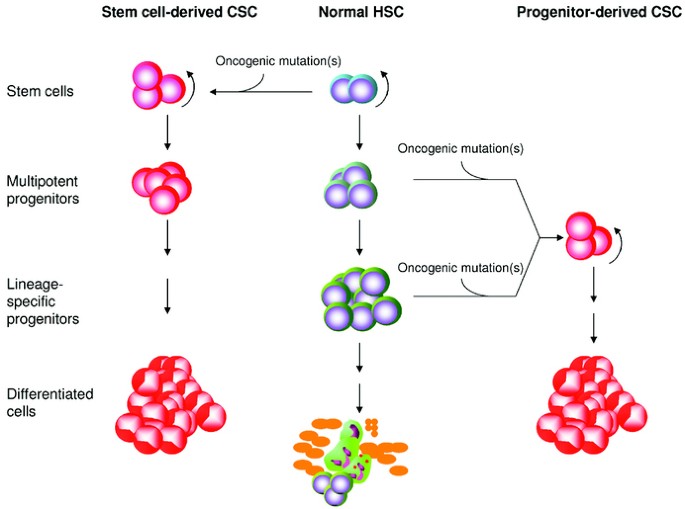
blood (Fig. 1, middle panel), although their potential for giving rise to other tissues (or plasticity) is still controversial (4,5). Dick et al. have recently revealed that, like the normal
hematopoietic system, leukemia is organized as a hierarchy in which only a rare population retains a clonogenic capacity upon transplantation (7). Similarly, a solid tumor can be likened to
an organ developed in an aberrant way, as it contains a heterogeneous mixture of cell types and abnormal tissue structures. More importantly, such an aberrant organ can be maintained and
even formed at remote sites if no therapeutic intervention is performed. It is well established that tumor engraftment, although requiring a large number of cells, results in the formation
of secondary tumors that recapitulate primary ones. The clonogenic and heterogenic nature of tumors suggests that a rare cell population in cancer, which acts like stem cells, is responsible
for tumor growth and metastasis. These rare cells are named cancer stem cells (CSC) after normal stem cells, as both have similar abilities to self-renew and to give rise to heterogeneous
differentiated cell types (8). Recent advances have begun to disclose the biologic identity and origin of CSC in several types of cancers and to elucidate the mechanisms underlying the
transformation of normal cells into CSC. This review will highlight recent progress in the field, and discuss key issues of CSC research and their clinical implications. THE IDENTITY AND
ORIGIN OF CSC To draw a true cellular and molecular picture of CSC, it is critical to identify CSC or purify the population to homogeneity. Recently, efforts have been made in isolating CSCs
from human cancer samples as well as animal models as summarized in Table 1 (7,9–17). Although most of these studies are able to show cancers initiated by certain enriched populations for
CSCs, homogeneity has not been reached. In fact, data revealing that CSCs can originate from either stem cells or progenitors (Table 1) raise the possibility that multiple CSC populations
may be formed during cancer progression and even co-exist in advanced cancers. The stem cell population is a logical candidate as a target for oncogenic transformation because of the
inherent abilities of self-renewal and multilineage differentiation (Fig. 1, left panel). Using the hematopoietic system as an example, it has been known for some time that an oncogenic
event can initiate at HSC level. It is well accepted that the t(9;22) chromosomal translocation (or the Philadelphia chromosome), which leads to the formation of the p210 BCR-ABL1
oncoprotein, is present in the HSCs of patients with chronic myelogenous leukemia (CML) (18,19). In a subset of acute lymphoblastic leukemia (ALL), this t(9;22) breakpoint was detected in
the CD34+CD38−CD19− HSCs (13). The transcripts of another leukemic fusion oncogene, _AML1-ETO_, were also detected in the CD34+Thy+CD38−Lin− HSCs of patients with acute myelogenous leukemia
(AML) in long-term remission (10). Consistent with the observations in human leukemia, only the murine _JunB__−/−_ HSC population was capable of transplanting myeloproliferative disorder
(MPD) to recipients (20). These lines of evidence underscore the stem cell origin of CSC. However, in most of the cases described above, additional mutations appear to be required for their
malignant transformation. In addition to stem cell origin, recent findings point out that CSC can also arise from committed progenitors that acquire self-renewal capacity (Fig. 1, right
panel). Such progenitors are normally derived from self-renewable HSC but have no or very limited self-renewal capacity. With progressive proliferation and differentiation, these progenitor
cells are capable of producing terminally differentiated functional cells. The oncogenic fusion genes, such as _ETV6-RUNX1_ or p190 _BCR-ABL_, could only be detected in CD34+CD38−CD19+ B
progenitor population of some ALL patients (13). From these patients, the purified CD19+ B cells, but not the CD34+CD38− CD19− HSC, exclusively reconstituted CD19+ leukemia in
immunodeficient nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice (13), providing conclusive evidence of a B progenitor origin for CSC. In the case of acute promyelocytic
leukemia (APML), the fusion gene PML-RARα, resulting from the t(15;17) translocation, was detected only in CD34−CD38+ progenitors, but not in CD34+CD38− HSC (9). The progenitor origin of CSC
in APML is supported by the transgenic mouse model of PML-RARα under the control of a human myeloid-specific promoter of MRP8, which is expressed in common myeloid progenitors (CMP) and
granulocyte/monocyte progenitors (GMP). These PML-RARα transgenic mice recapitulated human APML (21). In contrast, the transgenic mice expressing PML-RARα in more differentiated CD11b+
myelomonocytic cells failed to develop leukemia (22). Results from these studies of hematopoietic malignancies suggest that CSC can also originate from lineage-committed and stage-specific
progenitors (Fig. 1, right panel). In addition to differentiation stage-dependent origin, a study of ependymoma, a CNS (CNS) tumor, adds regional difference to the origin of CSC. Taylor _et
al._ reported that histologically identical ependymomas from the supratentorial, the posterior fossa and the spinal cord region might be initiated from altered radial glia cells (RGC,
CD133+Nestin+RC2+BLBP+ progenitors). However, these tumors maintained different chromosomal abnormalities and only recapitulated the gene expression profiles of the progenitors from their
respective anatomic sites (16). Although CSCs are generally considered to be derived from mutated stem cells or progenitors of corresponding tissues or organs, some surprisingly originate
from cells recruited from other tissues. This was shown in a mouse model of gastric cancer induced by chronic infection with _Helicobacter felis_, analogous to human _H. pylori_ infection.
In lethally irradiated mice transplanted with _LacZ_-positive bone marrow (BM) cells, these _LacZ_+ BM-derived cells could home to and repopulate the gastric mucosa and contribute to
metaplasia, dysplasia and intraepithelial cancer after _H. felis_ infection (23). _In vitro_ culture further suggested that BM-derived mesenchymal stem cells (MSC) may be a candidate origin
for CSC, as they, but not HSC, can acquire a gastric mucosa gene expression pattern when exposed to primary gastric cell cultures (23). However, it remains to be elucidated how the
BM-derived cells are transformed to CSC. MOLECULAR MECHANISMS UNDERLYING CSC FORMATION Recent studies suggest that both cell intrinsic and environmental factors can control the normal stem
cells and thus may contribute to the formation of CSCs. As for cell intrinsic effects, studies of human hematopoietic malignancies in murine models indicate the existence of a hierarchy
among oncogenic fusion proteins in their ability of endowing a self-renewal capacity to committed progenitors and blocking cell differentiation (24,25). _MOZ-TIF2_ and _MLL-ENL_ are
AML-associated oncogenes that are capable of conferring a self-renewal capacity to myeloid progenitors and blocking cell differentiation. These oncoproteins alone are sufficient to transform
committed progenitors to CSC (24,26). On the other hand, the oncoprotein p210 BCR-ABL1 in human CML, which provides HSC proliferation and survival advantage but not self-renewal activity,
is not sufficient to trigger leukemic transformation on committed myeloid progenitors (24,27). This class of oncoproteins usually requires additional alteration(s), or a 2nd hit in the
self-renewal pathways, _e.g._ Bmi-1 or Wnt/β-catenin, for complete transformation. The differences in cellular transformation potency between these oncogenic fusion proteins remain to be
further confirmed in human diseases. Since disease-specific chromosomal translocations are generally rare in epithelial tumors (28,29), multi-step mutational mechanisms are likely to be
required for CSC formation in most of them. Based upon the above observations, alteration of self-renewal pathways seems to be an important mechanism underlying CSC formation. It is known
that the signaling pathways required for normal stem cell self-renewal are also involved in cancer development, such as Hox genes, Wnt, Sonic Hedgehog, and Notch signaling pathways (30,31).
Recently, we have demonstrated that the PTEN tumor suppressor appears to negatively regulate the self-renewal of neural stem cells (NSC) (32) by modulating their G0-G1 cell cycle entry (33).
The roles for these genes/pathways in normal and cancer stem cells have drawn an increased attention from both developmental and cancer biologists. It is hypothesized that the self-renewal
and differentiation of stem cells are maintained by asymmetric division, through which a stem cell gives rise to two unequal daughter cells: one resembling the parental stem cell in the
niche and the other proceeding toward differentiation. Any change in the control of asymmetric division may result in aberrant self-renewal activity of stem cells. Support for this
hypothesis came from _Drosophila_ studies. Aberrations in the stem cell asymmetric division, caused by mutations in polarity-controlling genes, _e.g. raps_, _mira_, _numb_, or _pros_,
resulted in enhanced self-renewal activity and altered neuroblasts to form neuroblastoma-like tumors in adult hosts (34). It is suggested that the human tumor suppressor gene _LKB1_, which
is reported to regulate polarity and is lost in the Peutz-Jeghers cancer syndrome, may contribute to tumorigenesis in mammalian systems via a similar mechanism (35). Of those self-renewal
regulators, the polycomb family transcriptional repressor Bmi-1 and Wnt/β-catenin signaling pathway have recently been studied in the regulation of CSC self-renewal (25,36). Bmi-1 has been
shown to be required for the self-renewal of adult HSC and NSC (37–39). Park _et al._ demonstrated that the number of HSC was normal in the fetal liver of _Bmi-1_−/− mice but markedly
reduced in postnatal BM. Moreover, _Bmi-1_−/− fetal liver and bone marrow cells were only able to transiently reconstitute hematopoiesis (37). The studies of Bmi-1 in NSC further confirmed
that NSC self-renewal is also dependent on Bmi-1, distinct from the Bmi-1-independent proliferation of restricted progenitors (39). Intriguingly, Bmi-1 plays an essential role in the
self-renewal of CSC. Although introduction of _Hoxa9_ and _Meis1_ into _Bmi-1__−/−_ fetal liver cells resulted in AML in host mice, leukemic cells from _Bmi-1__−/−__Hoxa9–Meis1_ recipients
failed to reconstitute AML in any of secondary recipient mice (36). Besides Bmi-1, Wnt/β-catenin signaling is reported to regulate _HoxB4_ and _Notch1_, two critical regulators of HSC
self-renewal activity (40). The ectopic expression of _Axin_ or a frizzled ligand-binding domain, inhibitors of Wnt/β-catenin signaling, led to inhibition of HSC growth _in vitro_ and
reduced reconstitution _in vivo_. This self-renewal role for Wnt/β-catenin signaling appears to be conserved in self-renewing CSC. For example, committed myeloid progenitors or GMP, putative
CSC from CML patients in accelerated phase or blast crisis, showed increased nuclear β-catenin activity and self-renewal in a replating assay. Correspondingly, their replating capacity was
reduced by the lentiviral introduction of _Axin_ (25). PI3 K/AKT signaling is reported to positively regulate Wnt/β-catenin signaling through AKT-mediated phosphorylation of GSK-3β (41). In
a murine MPD model carrying a conditional deletion of _Pten_, a potent negative regulator of PI3 K/AKT signaling, we also observed elevated cytosolic and nuclear β-catenin in leukemic blasts
(W.G., J.L.L., and H.W., unpublished data). Interestingly, these two self-renewal pathways appear to play important roles in the regulation of metastasis. Brabletz _et al._ observed that a
high level of β-catenin was detected in mesenchyme-like colorectal tumor cells at the invasive fronts, implicating Wnt/β-Catenin signaling in the epithelial-to-mesenchymal transition (EMT)
or dissemination process of primary tumors (42). They proposed that low-level activation of β-catenin in the nucleus may be enough to confer self-renewal capacity, but its higher activation
is required to trigger EMT, an essential step for metastasis (43). This phenomenon appears to hold true for the Bmi-1 pathway. Expression profiling on metastatic versus primary prostate
tumors from human patients and the murine TRAMP transgenic model revealed substantial elevation of Bmi-1 and its associated molecular signature of 11 genes in metastatic cancer (44).
Furthermore, this metastasis/stem cell signature was used to predict the risk of metastatic recurrence and poor clinical outcomes using samples from 1,153 cancer patients with 11 different
types of cancer (44). Future studies are required to investigate the link between stem-ness and metastatic potential and the dual roles that Wnt/β-catenin or Bmi-1 pathway may play. As stem
cell niches control normal stem cells, it is currently unknown how extrinsic or environmental factors control CSC formation. Studies on the development of _Drosophila_ germ cells suggest
that stem cell faith is determined by the instructive signals from their microenvironment—the “stem-cell niche” (45). In _Drosophila_ germ cell niches, instructive signals are reported to be
comprised of _dpp_ and _Yb/Piwi/hh_ in ovary or Unpaired (Jak-Stat signaling) in testis (45). In mammalian systems, signaling pathways conserved in niche-mediated stem cell control are
reported to include TGFβ/BMP, NOTCH, JAK-STAT, and context-dependent WNT (46,47). Furthermore, the size of a stem cell niche determines the number of stem cells in this niche. In
_Drosophila_, the number of germline stem cells is positively correlated with the number of cap cells, a component of germline niches (48). Consistently, an increased number of murine
osteoblasts, a component of HSC niches, leads to an increase in the number of HSCs (49,50). Although the role of the stem cell or CSC-specific niches in CSC formation remains unclear, some
indirect evidence underscores their importance. For example, _dpp_ overexpression induced by heat shock in _Drosophila_ germaria resulted in stem cell-like germline tumors (51). CLINICAL
IMPLICATIONS The introduction of the CSC concept has provided exciting insights into the roots of carcinogenesis and sheds light on the future cure of cancer. The impact from current and
future studies of CSC will revolutionize clinical practice with regards to both cancer diagnosis and therapy. Two of the implicated changes will be the re-classification of human tumors and
development of novel therapeutic strategies targeting CSC. The current diagnosis and classification of human cancer is mainly based on pathologic characterization of the entire tumor.
However, we now understand that a rare CSC population(s) initiates and maintains cancer. As indicated by the above cases (13,16), although tumors are histologically identical, their CSC may
be derived from different stem cell or progenitor populations, depending on where the transformation occurs. This difference at the level of CSC will likely be very important for diagnosis
and therapy, especially for future therapies targeting CSC. Once CSC isolation approaches are established and verified, it will be critical to characterize human cancers based on both CSC
and bulk tumor and establish a new system of cancer classification. However, since the current identification of CSC is fully dependent on _in vivo_ reconstitution assays, a more practical
and quicker approach to identify CSC will be essential for diagnosis. Both molecular signatures for altered self-renewal pathways, _e.g._ Bmi-1 (39) and β-catenin (25) in CSC, and _in vitro_
replating capacity may serve as biomarkers for diagnosis based on CSC. In most cases, current therapeutic strategies are developed to target the bulk of cancer and likely do not eradicate
CSC completely, although they may have some effects on CSC, _e.g._ inhibition of CSC proliferation, and so reduce CSC number. For example, imatinib administration for CML can achieve
complete remission, but the _BCR-ABL1_ fusion gene is often detected in HSC of patients in remission, suggesting a potential risk of CML relapse. Thus, the complete eradication of CSC is
likely the key to the cure of cancer. For clinical elimination of CSC, the cellular and molecular properties described below need to be taken into account. The rarity of CSC will require
therapeutic strategies different from conventional ones. Specific recognition of CSC from the tumor mass will be the first challenge. The identification of CSC-specific antigens may help
develop specific targeting. Since the origins of CSC vary from cancer to cancer, the development of therapeutic strategies targeting different CSC population(s) will also be necessary. For
stem cell-derived CSC which usually require additional mutations to generate malignancy, the use of inductive differentiation (_i.e._ trans-retinoic acid therapy for APML) or replacement
with normal stem cells may be required, if it is hard to distinguish between normal stem cells and CSC. For progenitor-derived malignant CSC, eradication therapies targeting CSC and
progenitors can be applied as long as a normal stem cell pool is still available for reconstitution in cancer patients (13). Finally, multiple pathways/mechanisms will likely need to be
targeted together for effective elimination. CSC may have or acquire stem cell properties that are more resistant to therapies, such as survival advantage with increased anti-apoptotic
activities and drug resistance due to increased levels of drug efflux pumps such as BCRP (breast cancer resistance protein) and MDR (multiple drug resistance) complexes. Future therapeutic
strategies will need to integrate inhibition of these resistant mechanisms with CSC killing components. Moreover, combination therapies will help to prevent the generation of resistant CSC
colonies due to mutations (52). PROSPECTIVE Despite recent progress in CSC research, our knowledge of these rare populations is still limited and many questions remain to be answered.
Certain types of cancer are known to be multi-stage diseases, which generally progress into more malignant forms with the sequential accumulation of genetic and molecular alterations. For
instance, hematological malignancies, such as CML, are often found to have two distinct phases: chronic phase and blast crisis (or leukemia). Similarly, some epithelial tumors, _e.g._ colon
tumors, are thought to progress through at least five stages: pretumor patches/fields, hyperplasia, carcinoma _in situ_, invasive carcinoma and metastasis (30). One of the central questions
in the CSC research is: how to link CSC to cancer progression in these tumors? Given sequential requirements of genetic and molecular alterations and distinct pathologic abnormalities
associated with different stages of cancer progression, one may postulate that there could be multiple CSC populations, either intrinsically linked or generated independently, responsible
for different stages of cancer progression. To advance CSC research, we need to first understand the normal stem cells and critical pathways controlling stem cell properties. For this,
identification of cell surface molecules for prospective stem cell isolation and biologically relevant stem cell assays are essential. In addition, technical improvement will expedite the
studies of these rare and heterogeneous population(s). We should investigate the molecular mechanisms for the CSC formation and maintenance, especially their self-renewal regulation, which
holds the key for the development of effective therapeutic strategies against CSC. Although stem cell niches have been shown to play an instructive and pivotal role in the regulation of stem
cells, their implication in the CSC formation remains to be elucidated. Ultimately, with further improvements in our understanding of CSC, we will be able to develop better diagnostic and
therapeutic methodologies, with which to classify, treat, and cure cancer. ABBREVIATIONS * ALL: acute lymphoblastic leukemia * AML: acute myelogenous leukemia * APML: acute promyelocytic
leukemia * CML: chronic myelogenous leukemia * CSC: cancer stem cells * HSC: hematopoietic stem cells * MPD: myeloproliferative disorder * NSC: neural stem cells REFERENCES * Dewey MJ,
Martin DW, Martin GR, Mintz B 1977 Mosaic mice with teratocarcinoma-derived mutant cells deficient in hypoxanthine phosphoribosyltransferase. _Proc Natl Acad Sci U S A_ 74: 5564–5568 Article
CAS Google Scholar * Evans MJ, Kaufman MH 1981 Establishment in culture of pluripotential cells from mouse embryos. _Nature_ 292: 154–156 Article CAS Google Scholar * Martin GR 1975
Teratocarcinomas as a model system for the study of embryogenesis and neoplasia. _Cell_ 5: 229–243 Article CAS Google Scholar * Blau HM, Brazelton TR, Weimann JM 2001 The evolving concept
of a stem cell: entity or function?. _Cell_ 105: 829–841 Article CAS Google Scholar * Weissman IL 2000 Stem cells: units of development, units of regeneration, and units in evolution.
_Cell_ 100: 157–168 Article CAS Google Scholar * Weissman IL 2002 The road ended up at stem cells. _Immunol Rev_ 185: 159–174 Article CAS Google Scholar * Bonnet D, Dick JE 1997 Human
acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. _Nat Med_ 3: 730–737 Article CAS Google Scholar * Reya T, Morrison SJ, Clarke MF,
Weissman IL 2001 Stem cells, cancer, and cancer stem cells. _Nature_ 414: 105–111 Article CAS Google Scholar * Turhan AG, Lemoine FM, Debert C, Bonnet ML, Baillou C, Picard F, Macintyre
EA, Varet B 1995 Highly purified primitive hematopoietic stem cells are PML-RARA negative and generate nonclonal progenitors in acute promyelocytic leukemia. _Blood_ 85: 2154–2161 CAS
PubMed Google Scholar * Miyamoto T, Weissman IL, Akashi K 2000 AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. _Proc Natl Acad
Sci U S A_ 97: 7521–7526 Article CAS Google Scholar * Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ 1997 Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells
with long-term proliferative ability in vitro and in vivo. _Blood_ 89: 3104–3112 CAS PubMed Google Scholar * Al-Hajj M, Wicha MS, Benito-Hernandez Morrison SJ, Clarke MF 2003 Prospective
identification of tumorigenic breast cancer cells. _Proc Natl Acad Sci U S A_ 100: 3983–3988 Article CAS Google Scholar * Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez
C, Anderson K, Strombeck B, Garwicz S, Bekassy AN, Schmiegelow K, Lausen B, Hokland P, Lehmann S, Juliusson G, Johansson B, Jacobsen SE 2005 Distinct patterns of hematopoietic stem cell
involvement in acute lymphoblastic leukemia. _Nat Med_ 11: 630–637 Article CAS Google Scholar * Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE,
Herlyn M 2005 A tumorigenic subpopulation with stem cell properties in melanomas. _Cancer Res_ 65: 9328–9337 Article CAS Google Scholar * Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani
J, Hide T, Henkelman RM, Cusimano MD, Dirks PB 2004 Identification of human brain tumour initiating cells. _Nature_ 432: 396–401 Article CAS Google Scholar * Taylor MD, Poppleton H,
Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ 2005 Radial glia cells are candidate stem cells
of ependymoma. _Cancer Cell_ 8: 323–335 Article CAS Google Scholar * Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T 2005 Identification of
bronchioalveolar stem cells in normal lung and lung cancer. _Cell_ 121: 823–835 Article CAS Google Scholar * Holyoake T, Jiang X, Eaves C, Eaves A 1999 Isolation of a highly quiescent
subpopulation of primitive leukemic cells in chronic myeloid leukemia. _Blood_ 94: 2056–2064 CAS PubMed Google Scholar * Takahashi N, Miura I, Saitoh K, Miura AB 1998 Lineage involvement
of stem cells bearing the philadelphia chromosome in chronic myeloid leukemia in the chronic phase as shown by a combination of fluorescence-activated cell sorting and fluorescence in situ
hybridization. _Blood_ 92: 4758–4763 CAS PubMed Google Scholar * Passegue E, Wagner EF, Weissman IL 2004 JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic
stem cells. _Cell_ 119: 431–443 Article CAS Google Scholar * Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, Atwater S, Bishop JM 1997 A PMLRARalpha transgene initiates
murine acute promyelocytic leukemia. _Proc Natl Acad Sci U S A_ 94: 2551–2556 Article CAS Google Scholar * Early E, Moore MA, Kakizuka A, Nason-Burchenal K, Martin P, Evans RM, Dmitrovsky
E 1996 Transgenic expression of PML/RARalpha impairs myelopoiesis. _Proc Natl Acad Sci U S A_ 93: 7900–7904 Article CAS Google Scholar * Houghton J, Stoicov C, Nomura S, Rogers AB,
Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC 2004 Gastric cancer originating from bone marrow-derived cells. _Science_ 306: 1568–1571 Article CAS Google Scholar * Huntly BJ,
Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, Rowan R, Amaral S, Curley D, Williams IR, Akashi K, Gilliland DG 2004 MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem
cells to committed murine hematopoietic progenitors. _Cancer Cell_ 6: 587–596 Article CAS Google Scholar * Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li
K, Manz MG, Keating A, Sawyers CL, Weissman IL 2004 Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. _N Engl J Med_ 351: 657–667 Article CAS Google
Scholar * Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL 2003 Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid
progenitors. _Genes Dev_ 17: 3029–3035 Article CAS Google Scholar * Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL 2003 Expression of BCR/ABL and BCL-2 in myeloid
progenitors leads to myeloid leukemias. _Proc Natl Acad Sci U S A_ 100: 10002–10007 Article CAS Google Scholar * Mitelman F 2000 Recurrent chromosome aberrations in cancer. _Mutat Res_
462: 247–253 Article CAS Google Scholar * Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta
KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. _Science_ 310: 644–648 Article CAS Google Scholar * Miller SJ, Lavker
RM, Sun TT 2005 Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. _Biochim Biophys Acta_ 1756: 25–52 CAS PubMed Google
Scholar * Woodward WA, Chen MS, Behbod F, Rosen JM 2005 On mammary stem cells. _J Cell Sci_ 118: 3585–3594 Article CAS Google Scholar * Groszer M, Erickson R, Scripture-Adams DD, Lesche
R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H 2001 Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. _Science_ 294: 2186–2189
Article CAS Google Scholar * Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind D, Liu X, Kornblum HI, Wu H 2005 PTEN Negatively Regulates Neural Stem
Cell Self-Renewal by Modulating G0-G1 Cell Cycle Entry. _Proc Natl Acad Sci U S A_ Dec 22; [Epub ahead of print] * Caussinus E, Gonzalez C 2005 Induction of tumor growth by altered
stem-cell asymmetric division in Drosophila melanogaster. _Nat Genet_ 37: 1125–1129 Article CAS Google Scholar * Clevers H 2005 Stem cells, asymmetric division and cancer. _Nat Genet_ 37:
1027–1028 Article CAS Google Scholar * Lessard J, Sauvageau G 2003 Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. _Nature_ 423: 255–260 Article CAS
Google Scholar * Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF 2003 Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells.
_Nature_ 423: 302–305 Article CAS Google Scholar * Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R 2005 Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse
growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. _Genes Dev_ 19: 1432–1437 Article CAS Google Scholar * Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke
MF, Morrison SJ 2003 Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. _Nature_ 425: 962–967 Article CAS Google Scholar * Reya T, Duncan AW,
Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL 2003 A role for Wnt signalling in self-renewal of haematopoietic stem cells. _Nature_ 423: 409–414 Article CAS
Google Scholar * Pap M, Cooper GM 1998 Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. _J Biol Chem_ 273: 19929–19932 Article CAS Google
Scholar * Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T 2001 Variable beta-catenin expression in colorectal cancers indicates tumor progression
driven by the tumor environment. _Proc Natl Acad Sci U S A_ 98: 10356–10361 Article CAS Google Scholar * Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T 2005 Opinion: migrating
cancer stem cells - an integrated concept of malignant tumour progression. _Nat Rev Cancer_ 5: 744–749 Article CAS Google Scholar * Glinsky GV, Berezovska O, Glinskii AB 2005 Microarray
analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. _J Clin Invest_ 115: 1503–1521 Article CAS Google Scholar * Lin H
2002 The stem-cell niche theory: lessons from flies. _Nat Rev Genet_ 3: 931–940 Article CAS Google Scholar * Fuchs E, Tumbar T, Guasch G 2004 Socializing with the neighbors: stem cells
and their niche. _Cell_ 116: 769–778 Article CAS Google Scholar * Spradling A, Drummond-Barbosa D, Kai T 2001 Stem cells find their niche. _Nature_ 414: 98–104 Article CAS Google
Scholar * Xie T, Spradling AC 2000 A niche maintaining germ line stem cells in the Drosophila ovary. _Science_ 290: 328–330 Article CAS Google Scholar * Calvi LM, Adams GB, Weibrecht KW,
Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT 2003 Osteoblastic cells regulate the haematopoietic stem cell niche.
_Nature_ 425: 841–846 Article CAS Google Scholar * Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L 2003
Identification of the haematopoietic stem cell niche and control of the niche size. _Nature_ 425: 836–841 Article CAS Google Scholar * Xie T, Spradling AC 1998 decapentaplegic is
essential for the maintenance and division of germline stem cells in the Drosophila ovary. _Cell_ 94: 251–260 Article CAS Google Scholar * Shah NP, Tran C, Lee FY, Chen P, Norris D,
Sawyers CL 2004 Overriding imatinib resistance with a novel ABL kinase inhibitor. _Science_ 305: 399–401 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Drs.
Bangyan Stiles and Shunyou Wang for comments and suggestions, and Bahram Valamehr for proofreading. AUTHOR INFORMATION Author notes * Joseph L Lasky: J.L.L. is a fellow of NCI Scholar for
Oncology and Molecular Imaging Training Program. AUTHORS AND AFFILIATIONS * Department of Molecular and Medical Pharmacology, University of California, Los Angeles, 90095, CA Wei Guo, Joseph
L Lasky & Hong Wu * Department of Pediatrics, University of California, Los Angeles, 90095, CA Joseph L Lasky Authors * Wei Guo View author publications You can also search for this
author inPubMed Google Scholar * Joseph L Lasky View author publications You can also search for this author inPubMed Google Scholar * Hong Wu View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Wei Guo. ADDITIONAL INFORMATION This work is supported by grants from DOD PC031130 and NCI UO1 CA84128-06 and
RO1 CA107166 (H.W.). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Guo, W., Lasky, J. & Wu, H. Cancer Stem Cells. _Pediatr Res_ 59 (Suppl 4), 59–64
(2006). https://doi.org/10.1203/01.pdr.0000203592.04530.06 Download citation * Received: 02 December 2005 * Accepted: 28 December 2005 * Issue Date: 01 April 2006 * DOI:
https://doi.org/10.1203/01.pdr.0000203592.04530.06 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative