Play all audios:
ABSTRACT Crizotinib is a clinically approved tyrosine kinase inhibitor for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring EML4-ALK
fusion. Crizotinib was originally developed as an inhibitor of MET (HGF receptor), which is involved in the metastatic cascade. However, little is known about whether crizotinib inhibits
tumor metastasis in NSCLC cells. In this study, we found that crizotinib suppressed TGFβ signaling by blocking Smad phosphorylation in an ALK/MET/RON/ROS1-independent manner in NSCLC cells.
Molecular docking and in vitro enzyme activity assays showed that crizotinib directly inhibited the kinase activity of TGFβ receptor I through a competitive inhibition mode. Cell tracking,
scratch wound, and transwell migration assays showed that crizotinib simultaneously inhibited TGFβ- and HGF-mediated NSCLC cell migration and invasion. In addition, in vivo bioluminescence
imaging analysis showed that crizotinib suppressed the metastatic capacity of NSCLC cells. Our results demonstrate that crizotinib attenuates cancer metastasis by inhibiting TGFβ signaling
in NSCLC cells. Therefore, our findings will help to advance our understanding of the anticancer action of crizotinib and provide insight into future clinical investigations. SIMILAR CONTENT
BEING VIEWED BY OTHERS TARGETING STAT3 SIGNALING OVERCOMES GEFITINIB RESISTANCE IN NON-SMALL CELL LUNG CANCER Article Open access 31 May 2021 ANLOTINIB ENHANCES THE EFFICACY OF KRAS-G12C
INHIBITORS THROUGH C-MYC/ORC2 AXIS INHIBITION IN NON-SMALL CELL LUNG CANCER Article Open access 02 May 2025 DEOXYBOUVARDIN TARGETS EGFR, MET, AND AKT SIGNALING TO SUPPRESS NON-SMALL CELL
LUNG CANCER CELLS Article Open access 06 September 2024 INTRODUCTION Tumor metastasis is the end result of a series of cell biological events that enable cancer cells to disseminate from
primary tumors and survive in the new tumor microenvironment in distant tissues1,2. Since most metastatic cancers are resistant to conventional therapeutic agents, tumor metastasis is the
major cause of mortality in patients with cancer, including non-small cell lung cancer (NSCLC), which therefore remains a major clinical challenge3,4. Therefore, targeting metastasis as a
strategy for the prevention or inhibition of initial or recurrent metastasis and for the treatment of established metastatic tumors holds clinical promise for patients with or at risk of
metastatic cancer5. During the invasion-metastasis cascade, tumor cells undergo distinct changes in signaling pathways, such as transforming growth factor β (TGFβ) and
c-mesenchymal-epithelial transition factor (MET), which lead to epithelial plasticity, cell migration and invasion, and metastatic colonization6,7. In particular, aberrant TGFβ signaling
promotes tumor metastasis by mediating the multiple steps of metastasis, such as epithelial-mesenchymal transition (EMT), cell invasion, the interaction of circulating tumor cells with
platelets, and transendothelial migration, and is associated with poor therapeutic outcome and prognosis in patients with NSCLC8,9,10,11,12. Therefore, TGFβ signaling has attracted much
attention as a therapeutic approach to cancer metastasis. Crizotinib was originally developed as a MET tyrosine kinase inhibitor and was later found to inhibit anaplastic lymphoma kinase
(ALK), v-ros UR2 sarcoma virus oncogene homolog 1 (ROS1), and recepteur d’origine nantais (RON) kinases13,14,15,16. Crizotinib demonstrated potent antitumor activity in patients with NSCLC
harboring echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion or ROS1 rearrangement, which led to FDA approval for its clinical use13,14,15,17. However, emerging evidence
suggests that the anticancer effect of crizotinib is largely attributable to uncharacterized off-target mechanisms18. MET, the hepatocyte growth factor (HGF) receptor, is involved in the
development of metastasis in various types of human cancer19,20, including NSCLC21,22,23. Given that crizotinib inhibits MET, these findings suggested that crizotinib can suppress tumor
metastasis. In addition, recent evidence has shown that crizotinib may be beneficial for patients with metastatic EML4-ALK-positive NSCLC24,25. However, little is known about whether
crizotinib attenuates tumor metastasis in NSCLC cells. Furthermore, the effect of crizotinib on TGFβ signaling has not been investigated in NSCLC cells. Based on previous findings and our
data-driven inference, in this study, we hypothesized that crizotinib exerts antimetastatic activity by inhibiting TGFβ signaling. We provided evidence that crizotinib suppresses tumor
metastasis by directly inhibiting TGFβ receptor I (TβRI) kinase activity in NSCLC cells. Our results will enhance our knowledge about the molecular mechanisms underlying the anticancer
actions of crizotinib, providing insight into the clinical usefulness of crizotinib in patients with advanced NSCLC. MATERIALS AND METHODS CELL CULTURE AND REAGENTS NCI-H3122 cells were
kindly provided by Professor Pasi A. Janne (Dana Faber Cancer Institute, Boston, MA, USA). A549, Calu-1, Calu-3, H1975, H2228, PC-9, and SNU2535 cells were supplied by the American Type
Culture Collection (ATCC, Manassas, VA, USA) or the Korean Cell Line Bank (KCLB, Seoul, Korea). All cell lines were confirmed to be mycoplasma free. All cell culture reagents were obtained
from Gibco (Grand Island, NY, USA) or HyClone (Logan, UT, USA). Before treatment with TGFβ1 (R&D Systems, Minneapolis, MN, USA), cells were maintained in RPMI 1640 medium containing 0.2%
fetal bovine serum (FBS) for 2 h. Crizotinib was purchased from Tocris Bioscience (Ellisville, MO, USA). Alectinib, ceritinib, PHA-665752, and savolitinib were obtained from Selleckchem
(Houston, TX, USA). SB431542 was purchased from EMD Millipore (Darmstadt, Germany). All other reagents not specified were supplied by Sigma-Aldrich (St. Louis, MO, USA). DNA MICROARRAY
EXPERIMENTS DNA microarray experiments were performed using total RNA from A549 cells following treatment with 10 μM crizotinib for 24 h in the presence or absence of 1 ng/ml TGFβ. The
microarray data are available through the Gene Expression Omnibus (GEO) database under the accession number GSE189047. BIOINFORMATIC ANALYSIS We used GSE89127 (public RNA-seq datasets of
NCI-H3122 cells treated with crizotinib), GSE31210 (public microarray datasets of patients with NSCLC), and GSE189047. For GSE89127 data analysis, sequence read alignment was conducted using
Rsubread (version 1.28.1), and normalization was performed using edgeR (version 3.22.3) and Limma R (version 3.36.2) packages to determine gene expression levels in log2 transcripts per
million (TPM). Sequence reads were aligned to the human reference genome GRCh38, and the aligned sequences were mapped to NCBI gene IDs by using NCBI gene annotation data. For GSE31210 and
GSE189047 data analysis, SCAN normalization was used to normalize gene expression levels, and microarray probe sets were mapped to 17,052 NCBI Entrez gene IDs using a custom mapping file
(version 22.0.0) from the BrainArray resource. Then, we performed hierarchical clustering with Euclidean distance and principal component analysis (PCA) to verify that both datasets had
distinctive gene expression profiles. We used the Linear Models for Microarray Data (Limma) Bioconductor R package to identify differentially expressed genes (DEGs). The _t_ values in the
Limma results were used to supply the ranked list of genes into GSEAPreranked to examine gene signature enrichment in the hallmark or canonical pathways of the Molecular Signature Database
(v7.2). The significant gene signatures were determined by the threshold false discovery rate (FDR) _q_ value of 0.1. LUCIFERASE ASSAY Cells were transfected with pGL2-3TP-luciferase and
pCMV-β-galactosidase gene constructs using FuGENE 6 reagent (Promega, Indianapolis, IN, USA) as previously described in ref. 26. After 24 h of transfection, the cells were incubated with
TGFβ, crizotinib, or both for 24 h and then assayed for reporter gene activity using a commercial kit (Promega, Madison, WI, USA). The luciferase activity was normalized to β-galactosidase
activity as previously described26. WESTERN BLOT ANALYSIS The crude extracts were prepared by incubation with RIPA buffer containing protease and phosphatase inhibitor cocktails (EMD
Millipore, La Jolla, CA, USA). The samples were resolved using 6 or 10% SDS-PAGE and probed with the indicated antibodies. Antibodies against AKT, phospho-AKT, ALK, pALK, E-cadherin, MET,
pMET, Smad3, pSmad3, S6K, p S6K, and TβRI were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against Myc, RON, vimentin, and fibronectin were obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). An anti-β-tubulin antibody was supplied by Sigma-Aldrich. The signals were determined using an Amersham ECL western blotting detection reagent (GE
Healthcare, NJ, USA). The data were representative of at least three independent experiments. Full-scan images of western blots are shown in Supplementary Fig. 15. TRANSFECTION WITH SMALL
INTERFERING RNAS (SIRNAS) The siRNAs against ALK (siALK)-1 (5′-CCUGUAUACCGGAUAAUGAUU-3′), siALK-2 (5′-CCGCUUUGCCGAUAGAAUAUU-3′), siMET-1 (5′-AGAAUGUCAUUCUACAUGAGCUU-3′), siMET-2
(5′-CAUAUUCACAUUCAUCUCGGAUU-3′), siTβRI-1 (5′-CCAUCGAGUGCCAAAUGAAUU-3′), siTβRI-2 (5′-GCAUCUCACUCAUGUUGAUGGUCUAUU-3′), siRON-2 (5′-GGGCGACAGAAAUGAGAGUUU-3′), and siGFP
(5′-GCAAGCUGACCCUGAAGUUCAUUU-3′) as negative control were obtained from Genolution (Seoul, Korea). siRON-1 (5′-GGGCGACAGAAAUGAGAGUUU-3′) was purchased from Santa Cruz Biotechnology. Cells
were transfected with each siRNA (50 nM) using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA) for 48 h and then treated with or without TGFβ for the indicated times prior to
western blot analysis or luciferase assay. IN VITRO TΒRI KINASE ACTIVITY ASSAY The TGFβRI Kinase Enzyme System (Promega, Madison, WI, USA) was used in this study. TβRI kinase activity was
measured using an in vitro ADP-Glo kinase assay according to the manufacturer’s protocols. TβRI (20 ng/μL), TβRI substrate peptide (200 ng/μL), ATP (25–800 μM), and crizotinib (the indicated
concentrations) in kinase assay buffer (Promega) were incubated for 1 h at room temperature. Luminescence was measured using a plate-reading luminometer (Tecan, Männedorf, Switzerland).
CELL TRACKING ASSAY Cells were plated in 35 mm collagen-coated glass-bottom dishes and grown to 60% confluence. Cells were starved with RPMI 1640 containing 0.2% FBS and 1% antibiotics for 2
h and then treated with the indicated reagents, inhibitors, or both. Migrated cells were observed at intervals of 2 min for 12 h under a BioStation time-lapse imaging system (Nikon, Tokyo,
Japan), and fifteen different fields were counted. Data analyses were performed with the manual tracking plugin in ImageJ software, and plots were obtained from the chemotaxis and migration
tool program. SCRATCH-WOUND ASSAY A scratch-wound assay was used to assess cell migration, as previously described in ref. 27. Briefly, cells (1.5 × 105 cells/well) were grown in six-well
plates until they were fully confluent. A scratch was made on the cell monolayer using a sterile 10-μL pipette tip, and then cells were treated with the indicated reagents, inhibitors, or
both in RPMI 1640 medium containing 0.2% FBS for 24 h. Migrated cells in scratched space were counted using a phase-contrast microscope (Nikon Eclipse TS100, Nikon Instruments, Inc.,
Melville, NY, USA). The wound closure area was calculated using ImageJ software. TRANSWELL MIGRATION ASSAY Transwell filters (8-μm pore size, 24-well) were coated with 0.1 mg/mL collagen for
1 h. Cells (1.5 × 105 cells/well) were placed in the upper transwell in a serum-free medium (100 μL). Then, TGFβ (5 ng/mL), HGF (50 ng/mL), or both of them, as chemoattractants in
serum-free medium (800 μL), were placed in the lower chamber. After incubation for 24 h, migrated cells in the lower chamber were fixed with 4% formaldehyde in phosphate-buffered saline,
stained with 0.2% crystal violet, and counted under a phase-contrast microscope. IN VIVO BIOLUMINESCENCE IMAGING Calu-1-Luc cells were prepared by stable transfection with IVISbrite Red
F-luc-GFP Lentiviral Particles (PerkinElmer, Waltham, MA, USA). BALB/c-nude mice (male, 8 weeks old, Charles River, Yokohama, Japan) were injected intravenously with 1 × 106 Calu-1-Luc cells
via the tail-vein as previously described in ref. 28. Vehicle (water, 200 µL) or crizotinib (with 10 or 25 mg/kg, 200 μL) was administered by oral gavage daily for 2 weeks. For in vivo
bioluminescence imaging (BLI), mice were injected intraperitoneally with D-Luciferin (150 mg/kg, 200 μL, Gold Biotechnology, St. Louis, MO) and imaged 10 min later using the IVIS spectrum
system (PerkinElmer, Hopkinton, MA USA). Ex vivo BLI was performed on the organs isolated from mice after the last in vivo BLI. BLI intensity was measured using region of interest (ROI)
analysis. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Asan Institute for Life Sciences at
the Asan Medical Center (approval no. 2020‑13‑122). IMMUNOFLUORESCENCE STAINING ANALYSIS Immunofluorescence staining of metastatic tumor tissues was performed using a Leica Bond Rx™
Automated Stainer (Nussloch, Germany), and the images were analyzed using a Vectra Polaris Automated Quantitative Pathology Imaging System and inForm Image Analysis software (Akoya
Biosciences, CA, USA) as previously described in ref. 29. Tissue slides were labeled with anti-pSmad3 antibody (Cell Signaling Technology) and goat anti-rabbit IgG HRP polymer (Abcam,
Cambridge, UK) and probed with Tyramide Signal Amplification (Akoya Biosciences) and DAPI (Thermo Fisher Scientific, Dreieich, Germany). TUMOR XENOGRAFT EXPERIMENTS BALB/c-nude mice (male, 5
weeks old) were subcutaneously inoculated with Calu-1 cells (1 × 107 cells per mouse) in the right flank of each mouse. When the tumor reached approximately 70–90 mm3, the mice were
randomly divided into three groups (five in each group) and orally administered crizotinib (0, 25, or 100 mg/kg) every other day for 22 days. The tumor size was assessed every 2 days. The
mice were sacrificed after 22 days, and tumors were excised to measure volume and weight. These animal experiments were performed in accordance with protocols approved by the Institutional
Animal Care and Use Committee of the Asan Institute for Life Sciences at the Asan Medical Center (approval no. 2021-13-165). STATISTICAL ANALYSIS A comparison of mean values among
experimental groups was performed using one-way ANOVA followed by a post hoc test. _p_ < 0.05 was considered statistically significant. RESULTS CRIZOTINIB SPECIFICALLY INHIBITS TGFΒ
SIGNALING BY BLOCKING SMAD3 PHOSPHORYLATION To infer clinically significant latent relationships between drug-cell signatures and disease signatures, we investigated the gene expression
signatures of the GSE89127 (EML4-ALK-positive NCI-H3122 NSCLC cells treated with crizotinib) and GSE31210 (patients with EML4-ALK-positive NSCLC) datasets (Supplementary Fig. 1a–d). From
1697 DEGs that showed anti-similarity (or inverse correlation) between these two datasets (Supplementary Fig. 1e, f), we identified EMT as a clinically significant hallmark signature; the
EMT signature was enhanced in patients with NSCLC bearing EML4-ALK, whereas it was decreased by crizotinib (Table 1 and Supplementary Fig. 2). Based on the fact that TGFβ is a major mediator
of EMT30,31, we hypothesized that crizotinib inhibits TGFβ signaling in EML4-ALK-positive NSCLC cells. To confirm this data-driven hypothesis, we first performed reporter gene assays in
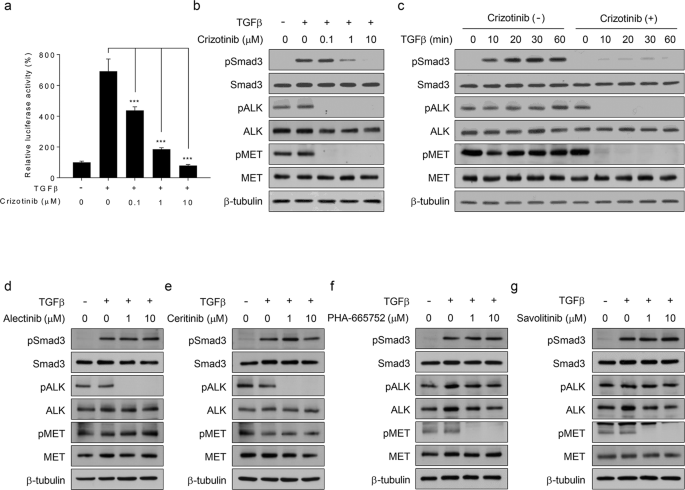
EML4-ALK-positive NCI-H3122 cells. Crizotinib inhibited TGFβ-mediated luciferase activity in a dose-dependent manner (Fig. 1a and Supplementary Fig. 3). Western blot analysis showed that
crizotinib abolished the phosphorylation of Smad3 in TGFβ-treated NCI-H3122 cells without affecting its total expression level (Fig. 1b). The time course analysis also showed that crizotinib
blocked TGFβ-induced Smad3 phosphorylation (Fig. 1c). These results indicate that crizotinib inhibits TGFβ signaling by blocking Smad3 phosphorylation. To determine whether the results from
Fig. 1a–c are specific to crizotinib, we repeated the abovementioned experiments with other ALK-specific inhibitors (alectinib and ceritinib) or MET-specific inhibitors (PHA-665752 and
savolitinib) in NCI-H3122 cells. Western blot analysis showed that none of the inhibitors used abolished TGFβ-induced Smad3 phosphorylation (Fig. 1d–g). Under these assay conditions, we
confirmed that the ALK- or MET-specific inhibitors suppressed the phosphorylation of only their own targets (Fig. 1d–g), whereas crizotinib simultaneously inhibited both ALK and MET activity
(Fig. 1b, c). Altogether, our results demonstrate that crizotinib specifically suppresses TGFβ signaling by blocking Smad3 phosphorylation. However, it is unclear whether ALK or MET
mediates the inhibitory effect of crizotinib on TGFβ signaling. CRIZOTINIB INHIBITS TGFΒ SIGNALING IN AN ALK/MET/RON/ROS1-INDEPENDENT MANNER We first questioned whether crizotinib inhibits
TGFβ signaling by an on-target mechanism (e.g., through an ALK-, MET-, RON-, or ROS1-dependent mechanism). When siRNAs against ALK, MET, or RON were employed in the experiments, these siRNAs
did not interfere with TGFβ-mediated luciferase activity in NCI-H3122 cells (Supplementary Fig. 4a–c). In contrast, siTβRI, as a positive control, noticeably inhibited TGFβ signaling
(Supplementary Fig. 4d). Western blot analysis also showed that siALK, siMET, or siRON did not affect TGFβ-induced Smad3 phosphorylation in NCI-H3122 cells compared to siTβRI (Fig. 2a–d and
Supplementary Fig. 4e–h). Since our RT-PCR analysis found that ROS1 is not detectable in NCI-H3122 cells as previously described in refs. 32,33, we excluded the involvement of ROS1 in
crizotinib-mediated inhibition of TGFβ signaling. Therefore, our results demonstrate that crizotinib attenuates TGFβ signaling through an ALK/MET/RON/ROS1-independent mechanism. Then, we
further examined the effect of crizotinib on TGFβ signaling in EML4-ALK-negative A549 and Calu-1 cells. Similar to the results obtained from NCI-H3122 cells (Fig. 1a–c), crizotinib inhibited
TGFβ-mediated luciferase activity and Smad3 phosphorylation in a dose-dependent manner (Supplementary Figs. 5,6 and Fig. 2e, h, i). Crizotinib also inhibited TGFβ-induced Smad3
phosphorylation in EML4-ALK-positive (H2228 and SNU2535) and EML4-ALK-negative (H1975, Calu-3, and PC-9) NSCLC cells (Supplementary Fig. 7). The time course analysis also showed that
crizotinib almost completely blocked TGFβ-induced Smad3 phosphorylation (Fig. 2f). Since TGFβ is known to induce EMT, which facilitates the migration and invasion of cancer cells34,35, we
analyzed the expression levels of EMT marker proteins in A549 cells. TGFβ decreased the expression levels of E-cadherin and increased those of vimentin and fibronectin, and these effects of
TGFβ were abolished by crizotinib (Fig. 2g, j). In addition, other ALK- or MET-specific inhibitors did not affect TGFβ-induced Smad3 phosphorylation (Fig. 2k–n) in A549 cells. Therefore,
these results confirm that crizotinib inhibits TGFβ signaling through an off-target mechanism. CRIZOTINIB DIRECTLY INHIBITS TΒRI KINASE ACTIVITY THROUGH COMPETITIVE INHIBITION To determine
the mechanism by which crizotinib inhibits TGFβ-induced Smad3 phosphorylation, we first assessed whether crizotinib stimulates the dephosphorylation of Smad3. After pretreatment with TGFβ
for 30 min, the cells were washed and then treated with the TβRI kinase inhibitor SB431542 to prevent rephosphorylation of the dephosphorylated Smad3. Under this assay condition, crizotinib
did not affect the phosphorylation levels of Smad3 in NCI-H3122 (Fig. 3a) and A549 cells (Fig. 3b). We also ascertained that crizotinib did not affect the expression level of TβRI (Fig. 3a,
b). These results indicate that crizotinib inhibits the phosphorylation of Smad3 rather than stimulating its dephosphorylation or degradation of TβRI. Based on these results (Fig. 3a, b), we
investigated whether crizotinib directly acts on the kinase activity of TβRI. Molecular docking and amino acid sequence analyses provided clues to the inhibitory mechanism of crizotinib on
TβRI; our model showed that crizotinib directly binds to the kinase domain of TβRI (Supplementary Figs. 8a–c, 9; see their legends for details). If our computational model is correct,
crizotinib should directly inhibit the kinase activity of TβRI. To examine this assumption, we performed an in vitro ADP-Glo kinase activity assay using active recombinant TβRI and its
substrate peptide. Crizotinib inhibited the in vitro kinase activity of TβRI, similar to the TβRI inhibitor SB431542, in a concentration-dependent manner (the IC50 values of crizotinib and
SB431542 were 276.9 and 96.88 nM, respectively) (Fig. 3c). Therefore, these results demonstrate that crizotinib suppresses TGFβ signaling by directly inhibiting the kinase activity of TβRI.
Since molecular docking analysis also showed that crizotinib acts as an ATP-competitive inhibitor for TβRI (Supplementary Figs. 8, 9), we measured TβRI activity at various ATP concentrations
in the presence of increasing concentrations of crizotinib. Lineweaver–Burk plot analysis showed that Vmax was not changed, whereas Km was increased (Fig. 3d), revealing that crizotinib
behaves as an ATP-competitive inhibitor for TβRI. Altogether, our results demonstrate that crizotinib directly inhibits the kinase activity of TβRI in a competitive inhibition mode. We also
found that other ALK- or MET-specific inhibitors did not affect the in vitro kinase activity of TβRI (Fig. 3e), confirming the specificity of crizotinib action on TβRI inhibition. If
crizotinib directly inhibits TβRI activity, it should suppress noncanonical TGFβ signaling pathways, such as AKT, S6K, and Myc. Western blot analysis showed that crizotinib inhibited
TGFβ-mediated AKT and S6K activation and Myc induction in NCI-H3122, A549, and Calu-1 cells (Fig. 3f). Altogether, our results demonstrate that crizotinib directly inhibits TβRI activity in
a competitive inhibitory manner. CRIZOTINIB SUPPRESSES TGFΒ-INDUCED CELL MIGRATION AND INVASION TGFβ is known to regulate the migratory behaviors and invasion-metastasis cascade of cancer
cells30,34. In addition, our own microarray experiments revealed that crizotinib significantly affected the expression of EMT signature genes in A549 cells (Supplementary Fig. 10 and Fig.
4a). To address the pathophysiological significance of our findings, we first examined whether crizotinib inhibits the migration of A549 cells treated with TGFβ, crizotinib, or both for 24
h. A scratch-wound assay showed that crizotinib decreased TGFβ-induced cell migration (Fig. 4b and Supplementary Fig. 11a). In addition, a transwell migration assay showed that crizotinib
inhibited TGFβ-mediated invasion of A549 cells (Fig. 4c and Supplementary Fig. 11b). Similarly, crizotinib suppressed TGFβ-induced cell migration (Fig. 4d and Supplementary Fig. 11c) and
invasion (Fig. 4e and Supplementary Fig. 11d) of Calu-1 cells. These results demonstrate that crizotinib potently inhibits TGFβ-induced migration and invasion of cancer cells. The TGFβ and
MET signaling pathways participate in the development of cancer metastasis6,7. The present study found that crizotinib concomitantly inhibits the TGFβ and MET signaling pathways (Fig. 1b,
c). Based on these results, we investigated whether crizotinib suppresses cell migration and invasion by simultaneously inhibiting TGFβ and MET signaling in A549 cells. Cell tracking
analysis showed that TGFβ, HGF (a ligand for MET), or both increased the velocity and accumulated distance of cell migration, which was reverted to basal levels by crizotinib (Fig. 5a and
Supplementary Fig. 12a, b). In addition, scratch-wound and transwell migration assays showed that TGFβ, HGF, or both markedly increased cancer cell migration and invasion, and these effects
were abolished by crizotinib (Fig. 5b, c and Supplementary Fig. 13a, b). These results demonstrate that crizotinib attenuates cell migration and invasion by concurrent inhibition of TGFβ and
MET signaling. To assess the potential benefit of simultaneous inhibition of two independent metastasis-related signaling pathways, we examined the effect of savolitinib, SB431542, and
crizotinib on the migration and invasion of A549 cells in the presence of both TGFβ and HGF. As presented in Fig. 5d–f (also Supplementary Figs. 12c, d, 13c, d), combined treatment with
savolitinib and SB431542 potently inhibited cell migration and invasion, which is comparable to crizotinib. In contrast, both savolitinib and SB431542 partially suppressed these cell
behaviors. These results show that crizotinib acts as a useful multitarget antimetastatic agent for the treatment of advanced NSCLC. CRIZOTINIB EXERTS ANTIMETASTATIC ACTIVITY IN VIVO We then
investigated whether crizotinib suppresses tumor metastasis in vivo using highly metastatic Calu-1 NSCLC cells, which readily colonize the lungs following intravenous injection28. In vivo
bioluminescence imaging analysis using luciferase-expressing Calu-1 (Calu-1-Luc) cells showed that luciferase activity was observed mainly in lung tissues compared to nonlung tissues (liver,
spleen, kidney, and heart) in the tail-vein injection model of metastasis (Fig. 6a, b). Crizotinib markedly reduced luminescence signals from a lung metastasis of Calu-1-Luc cells (Fig. 6a,
c, and Supplementary Fig. 14a). Ex vivo luminescence signals were observed mainly in isolated lung organs and were diminished by crizotinib (Fig. 6b, d and Supplementary Fig. 14b). In
addition, immunofluorescence staining analysis showed that crizotinib decreased the number of phospho-Smad3-positive cells in metastatic cancer tissues (Fig. 6e, f). Tumor xenograft
experiments showed that crizotinib at a dose of 25 mg/kg did not affect tumor growth compared to the 100 mg/kg dose (Fig. 6g–i), suggesting that the antimetastatic activity of crizotinib is
independent of its antiproliferative activity. These results indicate that crizotinib suppresses metastatic colonization of circulating tumor cells without affecting tumor growth. These
results demonstrate that crizotinib exerts antimetastatic activity in vivo, suggesting that crizotinib has a potential benefit for the prevention or inhibition of initial or recurrent
metastasis and for the treatment of established metastatic NSCLC. DISCUSSION Crizotinib is a clinically approved agent for the treatment of patients with NSCLC harboring EML4-ALK or ROS1
rearrangement13,14,15,17. However, the mechanism of the anticancer action of crizotinib is still largely unknown. In this study, we identified a novel molecular mechanism by which crizotinib
suppresses the metastatic capacity of NSCLC cells. Our main findings are as follows: (1) crizotinib blocks TGFβ-induced Smad activation in NSCLC cells via an ALK/MET/RON/ROS1-independent
mechanism; (2) crizotinib directly inhibits TβRI kinase activity in a competitive inhibitory manner; (3) crizotinib suppresses TGFβ- and HGF-induced cell migration and invasion in NSCLC
cells; and (4) crizotinib exerts antimetastatic activity in NSCLC cells in vivo without affecting cell growth. Based on previous findings that MET is involved in the development of tumor
metastasis19,20,21,23 and because crizotinib was originally developed as a MET inhibitor16, the possibility was raised that crizotinib can suppress tumor metastasis. However, crizotinib
exerts antimetastatic activity in NSCLC cells. Our bioinformatic analysis provided a clue to the novel off-target mechanism of action of crizotinib (Table 1 and Supplementary Fig. 2). Based
on our hypothesis- and data-driven inference and subsequent experiments, we found that crizotinib potently suppressed cell migration and invasion by simultaneous inhibition of at least two
independent prometastatic signaling pathways involving the HGF-MET and TGFβ-TβRI pathways (Fig. 5), which contributes to its antimetastatic activity (Fig. 6). Therefore, these results deepen
our understanding of the mechanism of the anticancer action of crizotinib and provide insight into the clinical usefulness of crizotinib in the treatment of metastatic cancer. We found that
TGFβ induces Smad3 phosphorylation without affecting ALK or MET phosphorylation (Fig. 1b, c), indicating that TGFβ signaling does not regulate the activity of ALK or MET signaling.
Conversely, inhibition of ALK or MET using siRNAs or specific inhibitors did not affect TGFβ-induced Smad3 phosphorylation (Fig. 2k, l and Supplementary Fig. 4e, f), indicating that ALK or
MET signaling does not lead to deregulated TGFβ signaling. Therefore, our results demonstrate that these oncogenic signaling pathways are independent of each other and independently
contribute to tumor metastasis, suggesting that simultaneous inhibition of multiple oncogenic pathways is crucial for the prevention or inhibition of metastasis. Our findings suggest that
crizotinib-resistant metastatic NSCLC cells can develop cellular resilience mechanisms to resist molecular or cellular perturbation by inhibition of TGFβ signaling as well as ALK and MET,
providing new insight into the mechanism of cancer drug resistance. In this regard, it is possible that the tumor cells acquiring resistance to crizotinib-induced TGFβ inhibition may rapidly
result in highly metastatic cancer. Therefore, careful clinical observation is required to assess the potential association between the crizotinib dose and metastatic progression. However,
a previous study showed that aberrant TGFβ signaling plays a crucial role in the acquisition of resistance to crizotinib in ALK-rearranged NSCLC cells in response to chronic, repeated
exposure to crizotinib36, suggesting the presence of a latent association between TGFβ and ALK signaling with respect to a complex mechanism of cancer drug resistance. We showed that
crizotinib inhibited TGFβ-induced EMT, cell migration, and invasion and attenuated metastatic colonization in NSCLC cells. Previous clinical studies have shown that crizotinib is beneficial
for patients with established metastatic NSCLC22. Therefore, our results suggest that crizotinib may be useful for the prevention or inhibition of initial or recurrent metastasis and the
treatment of established metastatic tumors. Since TGFβ plays a crucial role in immune evasion, angiogenesis, and metastatic niche establishment2,6, crizotinib can suppress tumor metastasis
by targeting the tumor microenvironment (i.e., noncell autonomous mechanism), suggesting that crizotinib exerts multilayered, multitargeted antimetastatic properties. This phenomenon may
explain the effectiveness of crizotinib in inhibiting cancer metastasis in vivo, although it exerted antimetastatic activity in our in vitro model at relatively high concentrations. In
addition, our results suggest that crizotinib can be exploited as a valuable chemical probe for dissecting metastatic signaling networks in NSCLC, indicating that crizotinib can be used as a
lead compound for structure-based optimization studies to maximize antimetastatic therapeutic efficacy. The present study demonstrates that crizotinib attenuates cancer metastasis by
ALK/MET/RON/ROS1-independent inhibition of TGFβ signaling in NSCLC cells. Our results will contribute to enhancing the understanding of the molecular mechanism underlying the anticancer
actions of crizotinib and providing further insight into its clinical usefulness. REFERENCES * Chitty, J. L. et al. Recent advances in understanding the complexities of metastasis.
_F1000Res._ 7, F1000 Faculty Rev–F1000 Faculty1169 (2018). Article Google Scholar * Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis.
_Cell_ 168, 670–691 (2017). Article CAS Google Scholar * Nurwidya, F., Takahashi, F., Murakami, A. & Takahashi, K. Epithelial mesenchymal transition in drug resistance and metastasis
of lung cancer. _Cancer Res. Treat._ 44, 151–156 (2012). Article Google Scholar * Rotow, J. & Bivona, T. G. Understanding and targeting resistance mechanisms in NSCLC. _Nat. Rev.
Cancer_ 17, 637–658 (2017). Article CAS Google Scholar * Steeg, P. S. Targeting metastasis. _Nat. Rev. Cancer_ 16, 201–218 (2016). Article CAS Google Scholar * Colak, S. & Ten
Dijke, P. Targeting TGF-beta signaling in cancer. _Trends Cancer_ 3, 56–71 (2017). Article CAS Google Scholar * Gherardi, E., Birchmeier, W., Birchmeier, C. & Vande Woude, G.
Targeting MET in cancer: rationale and progress. _Nat. Rev. Cancer_ 12, 89–103 (2012). Article CAS Google Scholar * Dongre, A. & Weinberg, R. A. New insights into the mechanisms of
epithelial-mesenchymal transition and implications for cancer. _Nat. Rev. Mol. Cell Biol._ 20, 69–84 (2019). Article CAS Google Scholar * Eser, P. O. & Janne, P. A. TGFbeta pathway
inhibition in the treatment of non-small cell lung cancer. _Pharmacol. Ther._ 184, 112–130 (2018). Article CAS Google Scholar * Neuzillet, C. et al. Targeting the TGFbeta pathway for
cancer therapy. _Pharmacol. Ther._ 147, 22–31 (2015). Article CAS Google Scholar * Perlikos, F., Harrington, K. J. & Syrigos, K. N. Key molecular mechanisms in lung cancer invasion
and metastasis: a comprehensive review. _Crit. Rev. Oncol. Hematol._ 87, 1–11 (2013). Article Google Scholar * Vazquez, P. F. et al. TGF-beta specifically enhances the metastatic
attributes of murine lung adenocarcinoma: implications for human non-small cell lung cancer. _Clin. Exp. Metastasis_ 30, 993–1007 (2013). Article CAS Google Scholar * Kwak, E. L. et al.
Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. _N. Engl. J. Med._ 363, 1693–1703 (2010). Article CAS Google Scholar * Ou, S. H. et al. Activity of crizotinib
(PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. _J.
Thorac. Oncol._ 6, 942–946 (2011). Article Google Scholar * Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. _N. Engl. J. Med._ 371, 2167–2177
(2014). Article Google Scholar * Zou, H. Y. et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative
and antiangiogenic mechanisms. _Cancer Res._ 67, 4408–4417 (2007). Article CAS Google Scholar * Shaw, A. T. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. _N. Engl. J.
Med._ 371, 1963–1971 (2014). Article Google Scholar * Lin, J. J., Riely, G. J. & Shaw, A. T. Targeting ALK: precision medicine takes on drug resistance. _Cancer Discov._ 7, 137–155
(2017). Article CAS Google Scholar * Sierra, J. R. & Tsao, M. S. c-MET as a potential therapeutic target and biomarker in cancer. _Ther. Adv. Med. Oncol._ 3, S21–S35 (2011). Article
CAS Google Scholar * Spina, A. et al. HGF/c-MET axis in tumor microenvironment and metastasis formation. _Biomedicines_ 3, 71–88 (2015). Article CAS Google Scholar * Benedettini, E. et
al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. _Am. J. Pathol._ 177, 415–423 (2010).
Article CAS Google Scholar * Finocchiaro, G., Toschi, L., Gianoncelli, L., Baretti, M. & Santoro, A. Prognostic and predictive value of MET deregulation in non-small cell lung cancer.
_Ann. Transl. Med._ 3, 83 (2015). PubMed PubMed Central Google Scholar * Salgia, R. MET in lung cancer: biomarker selection based on scientific rationale. _Mol. Cancer Ther._ 16, 555–565
(2017). Article CAS Google Scholar * Costa, D. B. et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. _J.
Clin. Oncol._ 33, 1881–1888 (2015). Article CAS Google Scholar * Zhang, I., Zaorsky, N. G., Palmer, J. D., Mehra, R. & Lu, B. Targeting brain metastases in ALK-rearranged
non-small-cell lung cancer. _Lancet Oncol._ 16, e510–e521 (2015). Article CAS Google Scholar * Park, E. J. et al. Schisandrin B suppresses TGFbeta1 signaling by inhibiting Smad2/3 and
MAPK pathways. _Biochem. Pharmacol._ 83, 378–384 (2012). Article CAS Google Scholar * Chun, J. N. et al. Schisandrin B suppresses TGFbeta1-induced stress fiber formation by inhibiting
myosin light chain phosphorylation. _J. Ethnopharmacol._ 152, 364–371 (2014). Article CAS Google Scholar * Larsen, J. E. et al. ZEB1 drives epithelial-to-mesenchymal transition in lung
cancer. _J. Clin. Invest._ 126, 3219–3235 (2016). Article Google Scholar * Hong, S. W. et al. Immune profile by multiplexed immunohistochemistry associated with recurrence after
chemoradiation in rectal cancer. _J. Gastroenterol. Hepatol._ 37, 542–550 (2022). Article CAS Google Scholar * Xu, J., Lamouille, S. & Derynck, R. TGF-beta-induced epithelial to
mesenchymal transition. _Cell Res._ 19, 156–172 (2009). Article CAS Google Scholar * Hao, Y., Baker, D. & Ten Dijke, P. TGF-beta-mediated epithelial-mesenchymal transition and cancer
metastasis. _Int. J. Mol. Sci._ 20, 2767 (2019). Article CAS Google Scholar * Rimkunas, V. M. et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung
cancer: identification of a FIG-ROS1 fusion. _Clin. Cancer Res._ 18, 4449–4457 (2012). Article CAS Google Scholar * Yasuda, H., de Figueiredo-Pontes, L. L., Kobayashi, S. & Costa, D.
B. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. _J. Thorac. Oncol._ 7, 1086–1090 (2012).
Article CAS Google Scholar * Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. _Nat. Rev. Mol. Cell Biol._ 15, 178–196 (2014). Article
CAS Google Scholar * Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. _J. Clin. Invest._ 119, 1429–1437 (2009). Article CAS Google Scholar * Kim, H.
R. et al. Epithelial-mesenchymal transition leads to crizotinib resistance in H2228 lung cancer cells with EML4-ALK translocation. _Mol. Oncol._ 7, 1093–1102 (2013). Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS We thank Professor San-Duk Yang for the helpful discussion about RNA-seq data analysis. This study was supported by a grant of the Korea Health
Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110057), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT)
(2017M3A9D8062960 and 2018R1A4A1023822), the Education and Research Encouragement Fund of Seoul National University Hospital, and the Asan Institute for Life Sciences (2019IP0585-1). AUTHOR
INFORMATION Author notes * These authors contributed equally: Soonbum Park, Eun A Cho, Jung Nyeo Chun. AUTHORS AND AFFILIATIONS * Department of Physiology and Biomedical Sciences, Seoul
National University College of Medicine, Seoul, Korea Soonbum Park, Jung Nyeo Chun, Da Young Lee, So Hee Lee, Insuk So & Ju-Hong Jeon * ASAN Institute for Life Sciences, ASAN Medical
Center, Seoul, Korea Eun A Cho, Mi Yeon Kim, Sang Mun Bae, Su In Jo & Sang-Yeob Kim * Department of Medical Science, Asan Medical Center, University of Ulsan College of Medicine, Seoul,
South Korea Eun A Cho & Mi Yeon Kim * Institute of Human-Environment Interface Biology, Seoul National University, Seoul, Korea Jung Nyeo Chun, Insuk So & Ju-Hong Jeon * Department
of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT, USA Sanghoon Lee * College of Pharmacy, Chung-Ang University, Seoul, Korea Hyun Ho Park * Cancer Research
Institute, Seoul National University College of Medicine, Seoul, Korea Tae Min Kim * Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea Tae Min Kim Authors *
Soonbum Park View author publications You can also search for this author inPubMed Google Scholar * Eun A Cho View author publications You can also search for this author inPubMed Google
Scholar * Jung Nyeo Chun View author publications You can also search for this author inPubMed Google Scholar * Da Young Lee View author publications You can also search for this author
inPubMed Google Scholar * Sanghoon Lee View author publications You can also search for this author inPubMed Google Scholar * Mi Yeon Kim View author publications You can also search for
this author inPubMed Google Scholar * Sang Mun Bae View author publications You can also search for this author inPubMed Google Scholar * Su In Jo View author publications You can also
search for this author inPubMed Google Scholar * So Hee Lee View author publications You can also search for this author inPubMed Google Scholar * Hyun Ho Park View author publications You
can also search for this author inPubMed Google Scholar * Tae Min Kim View author publications You can also search for this author inPubMed Google Scholar * Insuk So View author publications
You can also search for this author inPubMed Google Scholar * Sang-Yeob Kim View author publications You can also search for this author inPubMed Google Scholar * Ju-Hong Jeon View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.P., E.A.C., J.N.C., S.-Y.K., and J.-H.J. designed the study. S.P., E.A.C., J.N.C., D.Y.L., S.L.,
M.Y.K., S.M.B., and S.H.L. conducted the experiments. J.N.C., T.M.K., H.H.P., and I.S. performed data analysis and interpretation. S.P., E.A.C., J.N.C., S.-Y.K., and J.-H.J. wrote the
manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Sang-Yeob Kim or Ju-Hong Jeon. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Park, S., Cho, E.A., Chun, J.N. _et al._ Crizotinib attenuates cancer metastasis by inhibiting TGFβ signaling in non-small cell lung cancer
cells. _Exp Mol Med_ 54, 1225–1235 (2022). https://doi.org/10.1038/s12276-022-00835-8 Download citation * Received: 24 January 2022 * Revised: 09 June 2022 * Accepted: 16 June 2022 *
Published: 23 August 2022 * Issue Date: August 2022 * DOI: https://doi.org/10.1038/s12276-022-00835-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative