Play all audios:
ABSTRACT The intestinal epithelium is the first line of defense and acts as an interface between the vast microbial world within the gastrointestinal tract and the body’s internal milieu.
The intestinal epithelium not only facilitates nutrient absorption but also plays a key role in defending against pathogens and regulating the immune system. Central to maintaining a healthy
epithelium are intestinal stem cells (ISCs), which are essential for replenishing the intestinal epithelium throughout an individual’s lifespan. Recent research has unveiled the intricate
interplay between ISCs and their niche, which includes various cell types, extracellular components, and signaling molecules. In this review, we delve into the most recent advances in ISC
research, with a focus on the roles of ISCs in maintaining mucosal homeostasis and how ISC functionality is influenced by the niche environment. In this review, we explored the regulatory
mechanisms that govern ISC behavior, emphasizing the dynamic adaptability of the intestinal epithelium in the face of various challenges. Understanding the intricate regulation of ISCs and
the impact of aging and environmental factors is crucial for advancing our knowledge and developing translational approaches. Future studies should investigate the interactive effects of
different risk factors on intestinal function and develop strategies for improving the regenerative capacity of the gut. SIMILAR CONTENT BEING VIEWED BY OTHERS INTESTINAL PLASTICITY AND
METABOLISM AS REGULATORS OF ORGANISMAL ENERGY HOMEOSTASIS Article 17 November 2022 DIETARY AND METABOLIC EFFECTS ON INTESTINAL STEM CELLS IN HEALTH AND DISEASE Article 02 October 2024
IFNΓ-STAT1 AXIS DRIVES AGING-ASSOCIATED LOSS OF INTESTINAL TISSUE HOMEOSTASIS AND REGENERATION Article Open access 30 September 2023 INTRODUCTION The gastrointestinal tract is an
extraordinary biological system that serves as a complex interface between the vast microbial world within the digestive system and the internal milieu of the body1. At the forefront of this
intricate ecosystem lies the intestinal epithelium, which is a single layer of specialized cells that lines the inner surface of the gut2,3. This remarkable tissue is responsible for vital
functions such as nutrient absorption, the maintenance of a robust barrier against pathogens, and the regulation of immune responses within the digestive system. Intestinal stem cells (ISCs)
are central to the continuous regeneration of this essential tissue3,4,5. ISCs play a pivotal role in maintaining the structural integrity and functional capacity of the intestinal
epithelium. These remarkable cells reside within small pockets, known as crypts, and are entrusted with the extraordinary responsibility of ensuring the continual renewal and replenishment
of the intestinal lining throughout an individual’s lifetime6. ISCs exhibit remarkable plasticity and are capable of self-renewal and differentiation into diverse cell types that are
required for normal intestinal function7. Through a precisely orchestrated process, ISCs proliferate and generate into specialized cell lineages, including absorptive enterocytes,
mucus-secreting goblet cells, hormone-producing enteroendocrine cells, antimicrobial peptide-releasing Paneth cells, etc.8. This careful balance of self-renewal and differentiation is
orchestrated by intricate signaling pathways, such as the Wnt pathway, which is crucial for coordinating the seamless regeneration of the intestinal epithelium. In this review, we focus
primarily on recent advances in understanding the regulatory function of ISCs in homeostasis and how it is influenced by various risk factors for disease, particularly tumorigenesis and
inflammation. REGULATION OF CANONICAL ISC FUNCTIONS Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) is a receptor for R-spondin that promotes Wnt signaling by stabilizing
β-catenin9. The intricate balance between the self-renewal and differentiation of Lgr5hi ISCs plays a vital role in maintaining the continuous replenishment of the intestinal epithelium,
which allows for the preservation of its structural and functional integrity. Originally identified as a marker of actively cycling cells located at the base of crypts8, Lgr5 has become a
widely utilized stem cell marker for tracking and isolating actively cycling stem cells in the intestine and other organs3. The regulation of ISCs and their behavior are influenced by many
factors, both intrinsic and extrinsic. Disruptions in the regulation of Lgr5hi ISCs can lead to perturbations in mucosal homeostasis, potentially resulting in gastrointestinal diseases,
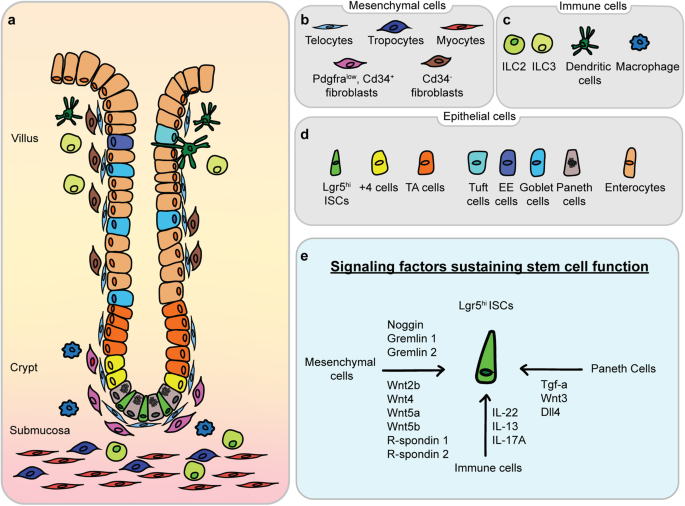
including inflammation, inflammatory bowel disease, and tumorigenesis. The complex interplay between cellular composition and paracrine signaling molecules, which shape the intestinal
epithelium and its supportive niche, is depicted in Fig. 1. SIGNALING PATHWAYS THAT REGULATE THE FUNCTION OF ISCS Multiple developmental signaling pathways, including the Wnt, Notch, BMP,
and Hedgehog pathways, play pivotal roles in governing the fate and function of ISCs10,11,12,13. The Wnt pathway, in particular, is a major contributor to the maintenance and function of
ISCs. Transcriptional regulators such as Lgr5 and Ascl2 are critical for sustaining the ISC pool and controlling the proliferative potential of ISCs14. Recent research has highlighted the
importance of TRIM27 in maintaining gut homeostasis. TRIM27 competes with Axin for binding to the conserved Armadillo (ARM) repeat domain of β-catenin, thus disrupting the Axin-β-catenin
interaction. This interference leads to the stabilization of β-catenin and its subsequent translocation from the cytoplasm to the nucleus, thereby activating the Wnt/β-catenin signaling
pathway. Such activation is fundamental for the self-renewal of Lgr5hi ISCs and the production of epithelial lineages and is crucial for maintaining the integrity of the intestinal
epithelial barrier and gut homeostasis15. Vitamin D receptor (Vdr) expression is also important for maintaining the function of ISCs. The Vdr gene was identified as one of the 30 core
components of the stem cell gene signature of Lgr5hi ISCs. The robust expression of Vdr in Lgr5hi cells is immediately downregulated in daughter cells, coinciding with their loss of function
as stem cells16. The deletion of Vdr in Lgr5hi ISCs inhibited lineage tracing, thereby confirming the necessity of Vdr expression for normal ISC function17. In addition, activation or
upregulation of Vdr expression attenuates radiation-induced intestinal damage in mice and promotes the repair of epithelial damage in intestinal stem cells; thus, these results further
establish the key role of Vdr signaling in the function of Lgr5hi ISCs18. INTESTINAL STEM CELL NICHE Within the complex microenvironment of the gastrointestinal tract lies a vital
determinant of mucosal homeostasis — the stem cell niche. This specialized microenvironment delivers essential signals and cues critical for the maintenance, regulation, and functionality of
ISCs19. A fundamental component of the ISC niche is the Paneth cell, which interacts with Lgr5hi ISCs at the base of crypts. Moreover, Paneth cells secrete growth factors, antimicrobial
peptides, and Wnt ligands, thereby contributing to mucosal immune defense and sustaining stem cell function. The role of these cells as niche cells encompasses the release of important
growth factors, including Egf, Tgf-a, Wnt3, and the Notch ligand Dll4, each of which activates critical signals that are necessary for stem cell maintenance and function20. Mesenchymal cells
also form a significant part of the niche, with distinct subsets including pericryptal myofibroblasts, fibroblasts, pericytes, endothelial cells, immune cells, neural cells, and smooth
muscle cells12,21,22. Single-cell RNA sequencing (scRNAseq) has revealed a diverse population of intestinal mesenchymal cells characterized by the expression of marker genes, such as Gli1,
Pdgfra, and Foxl123,24,25. Twist2 stromal cells were recently identified as a niche subpopulation for canonical ISCs that maintain homeostasis through the secretion of Wnt ligands26. Further
studies utilizing scRNAseq revealed new stromal cell types, including MAP3K2-regulated stromal cells and RSPO3+GREM1+ fibroblasts, which were sources of RSPO1 and RSPO327,28. The regulation
of ISC potential is also influenced by signals from lymphatic cells. In addition to the newly identified protein REELIN, Wnt2 and R-spondin3, which are both Wnt signaling factors, directly
regulate the regenerative capacity of ISCs as crypt lymphatic signals29. Immune cells predominantly reside in the lamina propria just beneath crypts30. Together with other stromal cells,
such as fibroblasts and endothelial cells, immune cells constitute the ISC microenvironment and orchestrate the complicated processes of ISC self-renewal and differentiation31. Immune cells
regulate ISCs through the production of cytokines and other stimulatory factors, thereby influencing the integrity of the intestinal barrier, which plays an important role in the
pathogenesis of intestinal and systemic diseases32. For example, IL-22 secreted by type 3 innate lymphoid cells (ILC3s) can promote ISC-mediated epithelial regeneration after intestinal
damage and drive ISC self-renewal33. In addition, ILC2s can promote ISC self-renewal via the IL-13 pathway34. Cytokines secreted by immune cells also guide the differentiation of Lgr5hi
ISCs. A recent study showed that IL-17A regulates the expression of Atoh1, a key component in lineage specification in the Notch pathway in Lgr5hi ISCs35, thus inducing differentiation
toward secretory cell types36. EXTRINSIC FACTORS THAT DISRUPT MUCOSAL HOMEOSTASIS Homeostasis is a continuous process of tissue/organ modification and adaptation37. In the intestine, this
process alters mucosal structure, function, and regenerative capabilities through reprogramming of ISCs, progenitor cells, and their lineages. Perturbations in the adaptive response of the
intestinal epithelium cause maladaptation to extrinsic environmental factors, further aggravating mucosal homeostasis, as depicted in Fig. 2. AGING Aging is a natural and inevitable process
that is characterized by progressive physiological and functional decline across multiple organ systems, and the gastrointestinal tract is no exception. This decline is critical for
understanding the increase in vulnerability to gastrointestinal disorders such as inflammation and tumorigenesis. A key age-related alteration is the reduced regenerative capacity of the
intestinal epithelium38. ISCs, which are critical for continual replenishment of the epithelium, exhibit a marked reduction in their proliferative potential and self-renewal ability with
age39,40. Investigations of aging mice have shown that they have lower survival rates and their intestinal crypts undergo significantly less cell division than normal crypts41. Gene
expression profiling revealed aging-associated changes in mRNAs associated with the cell cycle, oxidative stress and apoptosis39. Using scRNAseq technology, a recent study demonstrated that
a functional decline in ISCs leads to a delay in progenitor cell maturation, potentially leading to the overall impairment of regenerative capacity42. This results in a diminished capacity
to replace damaged or dying cells, ultimately compromising the regenerative response to injury or stress within the mucosal lining and reducing the overall efficiency of maintaining mucosal
function and integrity. Additionally, the altered behavior and functionality of ISC niches, including changes in the secretory profile of Paneth cells and the composition of the
extracellular matrix, contribute to the perturbation of mucosal homeostasis during aging. The functional decline in ISCs was caused by a decrease in Wnt signaling due to the production of
Notum, an extracellular Wnt inhibitor, in aged Paneth cells43. The mechanism involved the high activity of mammalian target of rapamycin complex 1 (mTORC1) in aged Paneth cells, which
inhibited the activity of peroxisome proliferator activated receptor α (PPAR-α), and decreased PPAR-α activity, which increased Notum expression43. Another study employed bulk genome-wide
chromatin accessibility and transcriptome analysis combined with scRNAseq and revealed that promoters of polycomb target genes become differentially accessible in aged ISCs, resulting in
increased expression of genes involved in enteroendocrine cell specification and biased differentiation44. In addition, the ability to repair cellular organization in the intestinal crypt is
also diminished in aging mice45. This study revealed the unique ‘soccer ball-like’ mosaic pattern formed by Lgr5hi ISCs surrounding Paneth cells, which is rapidly restored upon disruption
by the rearrangement of cellular organization. This process accompanies the active clearance of cellular debris, depending on orchestrated movement within the stem cell niche. Importantly,
the aged ISC niche exhibited less repair due to diminished motility, resulting in delays in post-damage recovery45. Aging has a multifactorial impact on the immune system in the intestine.
The proper immune functions of the tissue are impaired, but the number of immune cells is also elevated, leading to low-level chronic inflammation. This process has been termed inflammaging,
a hallmark of aging that significantly impacts mucosal homeostasis. Intestinal levels of proinflammatory cytokines, in particular TNFα, IL-1β, IFNγ and IL-6, tend to increase with age5,46.
The sustained inflammatory state disrupts the delicate balance of immune responses within the gastrointestinal tract, thereby altering the gut microbiome composition, affecting nutrient
absorption, and impairing the barrier function of the intestinal epithelium. Ultimately, the accumulation of age-associated changes contributes to an altered mucosal microenvironment, which
disrupts the functional harmony of the intestinal epithelium and predisposes older individuals to gastrointestinal ailments47. Studies have also shown that the amount of CD8+ T cells in
intestinal lamina propria mononuclear cells increases in elderly individuals, with a striking increase in CD45R- memory T cells with age48. The profile of highly proliferative and activated
T cells in the aged gut reflects the skewing of lymphocytes from a naïve state to a memory and/or effector state during lifetime antigen exposure49. Despite extensive investigations in this
area, further work is necessary to determine the impact of aging on intestinal immunity, and additional studies need to investigate how alterations in immune cell populations and the
phenomenon of inflammaging influence the function of ISCs and progenitor cells. DIET Dietary patterns and nutritional components are among the most influential environmental factors that
affect mucosal homeostasis. A diet rich in processed foods, high in sugars and unhealthy fats, and low in fiber has been linked to inflammation, altered gut microbiota composition, and
compromised barrier function50. Conversely, fiber-rich diets with ample antioxidants and essential nutrients support a healthy gastrointestinal tract by promoting microbial diversity,
enhancing immune function, and facilitating proper digestion and absorption51. One challenge is pinpointing specific changes to individual nutrients, as they often substantially interact to
influence cellular reprogramming in the intestine and are contingent on the presence and levels of other nutrients. This complexity is not surprising, given that even potent oncogenes and
tumor suppressor genes can exhibit varying or null effects across different tissues, depending on their distinct transcriptional programs. Despite these complexities, focused research has
yielded significant insights. A recent study demonstrated that a high-fat diet comprising 60% fat enhances the self-renewal capacity of Lgr5hi ISCs through PPARα/δ-dependent activation of
downstream pathways, including β-catenin and fatty acid oxidation52. Extended exposure to the same high-fat diet suppresses the expression of major histocompatibility complex (MHC) class II
molecules in ISCs53. However, the adaptive response to diet varies depending on the diet composition and relative nutrient levels. In another study, a Western-style diet in which a number of
nutrients were adjusted was linked to increased risk54, which induced impairment of Lgr5hi ISCs via epigenetic downregulation of the expression of _Ppargc1a_, a master regulator of
mitochondrial biogenesis. In this study, alternate stem cells that were Bmi1+ Ascl2hi were recruited to compensate for the functional impairment of Lgr5hi ISCs, which subsequently led to the
remodeling of the lineages55. Nutrient exposure also has a tremendous impact on the development of abnormal phenotypes in Paneth cells. Mice fed a Western-style diet adjusted to reflect
nutrient levels typically found in humans with a higher risk of tumorigenesis exhibited ectopic expression of Paneth cell markers throughout the intestinal mucosa56. A high-fat diet with a
fat content of 40% was used to induce Paneth cell defects through two key pathways within the intestinal epithelium—the farnesoid X receptor (FXR) pathway and the type I interferon (IFN)
pathway. Both pathways are needed to induce alterations, as inhibition of either FXR or type I IFN signaling prevents high-fat diet-induced Paneth cell defects57. Dietary exposure also
affects the intestinal immune system. Multiple studies using dietary conditions that reflect an elevated risk for tumorigenesis have shown low-grade chronic inflammation. Extended exposure
to a Western-style diet increased MHC II complex expression in mature enterocytes and increased the number of tissue-resident immune cells55. This condition has been documented in various
etiologies of human inflammatory bowel disease and is also protumorigenic58,59. Feeding a high-fat high-sugar (HFHS) diet dramatically increased the proportion of regulatory T (Treg) cells,
as well as that of CD4+ intraepithelial lymphoid-T cells (IEL-T cells), CD4+ lamina propria lymphoid-T cells, and conventional type 1 dendritic cells (cDC1s), which are critical for Treg
induction. Moreover, memory-like CD8αα + IEL-T cells and memory-like CD8αβ + IEL-T cells accumulate in the intestines of mice fed a HFHS diet; these cells express high levels of XCL1 and are
likely involved in the recruitment of cDC1s via the XCL1–XCR1 signaling axis in response to the diet60. Obesity has also been linked to a nonspecific, low-grade inflammatory state in the
intestine accompanied by the activation of proinflammatory signaling pathways, such as the NF-κB pathway, and increased expression of cytokines, including IL-1β, TNFα, and IL-12p4061,62. A
high-fat diet also shifts the immune cell balance in the small intestinal lamina propria by elevating the level of IFNγ-producing Th1 cells and CD8 + T cells while reducing that of
immunosuppressive Treg cells63. Additionally, feeding a high-fat diet also reduced the abundance of IgA+ B cells in the gut of mice, especially in the small intestine and lamina propria64.
MICROBIOME The gut microbiome, which is a complex consortium of microorganisms within the gastrointestinal tract, plays a pivotal role in shaping mucosal homeostasis. Importantly, the
intestinal microbiome has a bidirectional impact on gut homeostasis and pathogenesis. In aging individuals, the important role of the gut microbiota is highlighted by the notion that in
centenarians, “longevity adaptation” is characterized by enrichment in health-associated gut microbes65,66. The majority of adults over 65 years of age exhibit reduced microbiota diversity
compared to younger adults, along with greater interindividual variation in microbiota composition67. This may be reflected in the observation that mice from the same colony and subjected to
the same environmental conditions (i.e., housing, diet, temperature, humidity, etc.) exhibited an age-dependent drift in their microbiota profile68,69, likely indicating that normal
mechanisms that help maintain the balance of complex ecosystems deteriorate with age. Moreover, in a murine model, intrinsic, environment-independent aging itself drove alterations in the
gut microbiome composition70. The senescence-related remodeling of microorganisms mediates an increase in chronic inflammation, which is strongly correlated with microbial metabolites and
induced immune responses68,71. Disruptions of the gut microbiota composition, often triggered by antibiotic use, microbial infections, or excessive hygiene, can lead to dysbiosis,
inflammation, and compromised mucosal barrier integrity. A recent study showed that defects in Paneth cells induced dysbiosis, resulting in the activation of tuft cells and further
triggering type 2 immune responses72. Furthermore, lactate from lactic acid-producing bacteria plays a pivotal role in promoting ISC proliferation and epithelial development73. Nevertheless,
further research is required to comprehensively understand the mechanisms by which microbial metabolites influence the function of ISCs, reprogramming of the epithelial mucosa, and the
onset of pathogenesis. CONCLUSION Epithelial regeneration serves as the linchpin for preserving intestinal barrier function and facilitating efficient nutrient absorption. A decrease in
regenerative capacity is a defining hallmark of aging in the intestine; thus, there has been a push for extensive research into how regeneration is stimulated and regulated. To advance our
understanding and develop translational approaches for addressing age-related intestinal decline, the complexity of the gut ecosystem and the intricacies of stem cell regulation need to be
addressed. While significant progress has been made, there are notable gaps, especially regarding the comprehensive assessment of multiple risk factors in the context of the complex
interplay of aging, diet, the microbiome, and other environmental variables. While this research is challenging, it is essential for developing new approaches to promote gastrointestinal
function and health across the lifespan. REFERENCES * Neish, A. S. The gut microflora and intestinal epithelial cells: a continuing dialogue. _Microbes Infect._ 4, 309–317 (2002). Article
PubMed Google Scholar * Bellmann, S. et al. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. _Wiley
Interdiscip. Rev. Nanomed. Nanobiotechnol._ 7, 609–622 (2015). Article CAS PubMed PubMed Central Google Scholar * van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and
differentiation in the intestinal epithelium. _Annu. Rev. Physiol._ 71, 241–260 (2009). Article PubMed Google Scholar * de Santa Barbara, P., van den Brink, G. R. & Roberts, D. J.
Development and differentiation of the intestinal epithelium. _Cell Mol. Life Sci._ 60, 1322–1332 (2003). Article PubMed PubMed Central Google Scholar * Branca, J. J. V., Gulisano, M.
& Nicoletti, C. Intestinal epithelial barrier functions in ageing. _Ageing Res. Rev._ 54, 100938 (2019). Article CAS PubMed Google Scholar * Barker, N. Adult intestinal stem cells:
critical drivers of epithelial homeostasis and regeneration. _Nat. Rev. Mol. Cell Biol._ 15, 19–33 (2014). Article CAS PubMed Google Scholar * Cheng, H. & Leblond, C. P. Origin,
differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. _Am. J. Anat._ 141,
537–561 (1974). Article CAS PubMed Google Scholar * Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. _Nature_ 449, 1003–1007 (2007).
Article CAS PubMed Google Scholar * Kim, K. A. et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. _Science_ 309, 1256–1259 (2005). Article CAS PubMed Google
Scholar * Yeung, T. M., Chia, L. A., Kosinski, C. M. & Kuo, C. J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. _Cell Mol. Life Sci._ 68, 2513–2523
(2011). Article CAS PubMed PubMed Central Google Scholar * Fre, S. et al. Notch signals control the fate of immature progenitor cells in the intestine. _Nature_ 435, 964–968 (2005).
Article CAS PubMed Google Scholar * Powell, D. W., Pinchuk, I. V., Saada, J. I., Chen, X. & Mifflin, R. C. Mesenchymal cells of the intestinal lamina propria. _Annu. Rev. Physiol._
73, 213–237 (2011). Article CAS PubMed PubMed Central Google Scholar * Santos, A. J. M., Lo, Y. H., Mah, A. T. & Kuo, C. J. The intestinal stem cell niche: homeostasis and
adaptations. _Trends Cell Biol._ 28, 1062–1078 (2018). Article CAS PubMed PubMed Central Google Scholar * van der Flier, L. G. et al. Transcription factor achaete scute-like 2 controls
intestinal stem cell fate. _Cell_ 136, 903–912 (2009). Article PubMed Google Scholar * Wang, J. et al. TRIM27 maintains gut homeostasis by promoting intestinal stem cell self-renewal.
_Cell Mol. Immunol._ https://doi.org/10.1038/s41423-022-00963-1 (2023). * Munoz, J. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers.
_EMBO J._ 31, 3079–3091 (2012). Article CAS PubMed PubMed Central Google Scholar * Peregrina, K. et al. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions.
_Carcinogenesis_ 36, 25–31 (2015). Article CAS PubMed Google Scholar * Lin, Y. et al. Protective effects of activated vitamin D receptor on radiation-induced intestinal injury. _J Cell
Mol Med_ https://doi.org/10.1111/jcmm.17645 (2022). * Gola, A. & Fuchs, E. Environmental control of lineage plasticity and stem cell memory. _Curr. Opin. Cell Biol._ 69, 88–95 (2021).
Article CAS PubMed PubMed Central Google Scholar * Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. _Nature_ 469, 415–418 (2011). Article CAS
PubMed Google Scholar * Roulis, M. & Flavell, R. A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. _Differentiation_ 92, 116–131 (2016).
Article CAS PubMed Google Scholar * McCarthy, N. et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. _Cell Stem Cell_ 26, 391–402.e395
(2020). Article CAS PubMed PubMed Central Google Scholar * Paerregaard, S. I. et al. The small and large intestine contain related mesenchymal subsets that derive from embryonic Gli1(+)
precursors. _Nat. Commun._ 14, 2307 (2023). Article CAS PubMed PubMed Central Google Scholar * Aoki, R. et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell
niche. _Cell Mol. Gastroenterol. Hepatol._ 2, 175–188 (2016). Article PubMed Google Scholar * Shoshkes-Carmel, M. et al. Subepithelial telocytes are an important source of Wnts that
supports intestinal crypts. _Nature_ 557, 242–246 (2018). Article CAS PubMed PubMed Central Google Scholar * Xiang, J. et al. A stromal lineage maintains crypt structure and villus
homeostasis in the intestinal stem cell niche. _BMC Biol._ 21, 169 (2023). Article CAS PubMed PubMed Central Google Scholar * Wu, N. et al. MAP3K2-regulated intestinal stromal cells
define a distinct stem cell niche. _Nature_ https://doi.org/10.1038/s41586-021-03283-y (2021). * Goto, N. et al. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and
injury. _Cell Stem Cell_ 29, 1246–1261.e1246 (2022). Article CAS PubMed PubMed Central Google Scholar * Niec, R. E. et al. Lymphatics act as a signaling hub to regulate intestinal stem
cell activity. _Cell Stem Cell_ 29, 1067–1082.e1018 (2022). Article CAS PubMed PubMed Central Google Scholar * Viola, M. F. & Boeckxstaens, G. Niche-specific functional
heterogeneity of intestinal resident macrophages. _Gut_ https://doi.org/10.1136/gutjnl-2020-323121 (2020). * Kayisoglu, O. et al. Location-specific cell identity rather than exposure to GI
microbiota defines many innate immune signalling cascades in the gut epithelium. _Gut_ https://doi.org/10.1136/gutjnl-2019-319919 (2020). * Naik, S., Larsen, S. B., Cowley, C. J. &
Fuchs, E. Two to Tango: dialog between immunity and stem cells in health and disease. _Cell_ 175, 908–920 (2018). Article CAS PubMed PubMed Central Google Scholar * Lindemans, C. A. et
al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. _Nature_ 528, 560–564 (2015). Article CAS PubMed PubMed Central Google Scholar * Zhu, P. et al. IL-13
secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. _Nat. Immunol._ 20, 183–194 (2019). Article CAS PubMed Google Scholar * Gerbe, F. et
al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. _J. Cell Biol._ 192, 767–780 (2011). Article CAS PubMed PubMed
Central Google Scholar * Lin, X. et al. IL-17RA-signaling in Lgr5(+) intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment.
_Immunity_ 55, 237–253.e238 (2022). Article CAS PubMed PubMed Central Google Scholar * Davies, K. J. Adaptive homeostasis. _Mol. Asp. Med_. 49, 1–7 (2016). Article Google Scholar *
Jasper, H. Intestinal stem cell aging: origins and interventions. _Annu. Rev. Physiol._ 82, 203–226 (2020). Article CAS PubMed Google Scholar * Moorefield, E. C. et al. Aging effects on
intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. _Aging (Albany NY)_ 9, 1898–1915 (2017). Article CAS PubMed Google Scholar * Brunet,
A., Goodell, M. A. & Rando, T. A. Ageing and rejuvenation of tissue stem cells and their niches. _Nat. Rev. Mol. Cell Biol._ 24, 45–62 (2023). Article CAS PubMed Google Scholar *
Nalapareddy, K. et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. _Cell Rep._ 18, 2608–2621 (2017). Article CAS PubMed PubMed Central Google Scholar * Choi, J.
et al. Intestinal stem cell aging at single-cell resolution: Transcriptional perturbations alter cell developmental trajectory reversed by gerotherapeutics. _Aging Cell_ e13802.
https://doi.org/10.1111/acel.13802 (2023). * Pentinmikko, N. et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. _Nature_ 571, 398–402 (2019).
Article CAS PubMed PubMed Central Google Scholar * Tauc, H. M. et al. Age-related changes in polycomb gene regulation disrupt lineage fidelity in intestinal stem cells. _Elife_ 10.
https://doi.org/10.7554/eLife.62250 (2021). * Choi, J. et al. Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. _Sci. Rep._
8, 10989 (2018). Article PubMed PubMed Central Google Scholar * Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and
macrophage dysfunction. _Cell Host Microbe_ 21, 455–466.e454 (2017). Article CAS PubMed PubMed Central Google Scholar * Li, X. et al. Inflammation and aging: signaling pathways and
intervention therapies. _Signal Transduct. Target Ther._ 8, 239 (2023). Article PubMed PubMed Central Google Scholar * Booth, J. S. et al. Age-dependency of terminal ileum tissue
resident memory T cell responsiveness profiles to S. Typhi following oral Ty21a immunization in humans. _Immun. Ageing_ 18, 19 (2021). Article CAS PubMed PubMed Central Google Scholar *
Sirvinskas, D. et al. Single-cell atlas of the aging mouse colon. _iScience_ 25, 104202 (2022). Article CAS PubMed PubMed Central Google Scholar * Tu, W. B., Christofk, H. R. &
Plath, K. Nutrient regulation of development and cell fate decisions. _Development_ 150. https://doi.org/10.1242/dev.199961 (2023). * Wu, Q., Gao, Z. J., Yu, X. & Wang, P. Dietary
regulation in health and disease. _Signal Transduct. Target Ther._ 7, 252 (2022). Article PubMed PubMed Central Google Scholar * Mana, M. D. et al. High-fat diet-activated fatty acid
oxidation mediates intestinal stemness and tumorigenicity. _Cell Rep._ 35, 109212 (2021). Article CAS PubMed PubMed Central Google Scholar * Beyaz, S. et al. Dietary suppression of MHC
class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. _Cell Stem Cell_ https://doi.org/10.1016/j.stem.2021.08.007 (2021). * Newmark, H. L. et al.
Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. _Carcinogenesis_ 30, 88–92
(2009). Article CAS PubMed Google Scholar * Choi, J. et al. Dynamic intestinal stem cell plasticity and lineage remodeling by a nutritional environment relevant to human risk for
tumorigenesis. _Mol. Cancer Res._ https://doi.org/10.1158/1541-7786.MCR-22-1000 (2023). * Wang, D. et al. Paneth cell marker expression in intestinal villi and colon crypts characterizes
dietary induced risk for mouse sporadic intestinal cancer. _Proc. Natl Acad. Sci. USA_ 108, 10272–10277 (2011). Article CAS PubMed PubMed Central Google Scholar * Liu, T. C. et al.
Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. _Cell Host Microbe_ 29, 988–1001.e1006 (2021). Article CAS
PubMed PubMed Central Google Scholar * Parikh, K. et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. _Nature_ 567, 49–55 (2019). Article CAS PubMed
Google Scholar * Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. _Cell_ 178, 714–730.e722 (2019). Article CAS PubMed PubMed Central
Google Scholar * Wang, Y. C. et al. Intestinal cell type-specific communication networks underlie homeostasis and response to Western diet. _J. Exp. Med._ 220
https://doi.org/10.1084/jem.20221437 (2023). * Ding, S. et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin
resistance in mouse. _PLoS ONE_ 5, e12191 (2010). Article PubMed PubMed Central Google Scholar * Li, H. et al. Intestinal, adipose, and liver inflammation in diet-induced obese mice.
_Metabolism_ 57, 1704–1710 (2008). Article CAS PubMed Google Scholar * Hong, C. P. et al. Gut-specific delivery of T-Helper 17 Cells Reduces Obesity and Insulin Resistance in Mice.
_Gastroenterology_ 152, 1998–2010 (2017). Article CAS PubMed Google Scholar * Sakamoto, Y., Niwa, M., Muramatsu, K. & Shimo, S. High-fat diet and age-dependent effects of IgA-bearing
cell populations in the small intestinal lamina propria in mice. _Int. J. Mol. Sci._ 22. https://doi.org/10.3390/ijms22031165 (2021). * Biagi, E. et al. The gut microbiota of centenarians:
signatures of longevity in the gut microbiota profile. _Mech. Ageing Dev._ 165, 180–184 (2017). Article PubMed Google Scholar * Deng, F., Li, Y. & Zhao, J. The gut microbiome of
healthy long-living people. _Aging (Albany NY)_ 11, 289–290 (2019). Article PubMed Google Scholar * Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in
the elderly. _Nature_ 488, 178–184 (2012). Article CAS PubMed Google Scholar * Langille, M. G. et al. Microbial shifts in the aging mouse gut. _Microbiome_ 2, 50 (2014). Article PubMed
PubMed Central Google Scholar * Zhang, L. et al. Improving intestinal inflammaging to delay aging? A new perspective. _Mech. Ageing Dev._ 214, 111841 (2023). Article CAS PubMed Google
Scholar * Miyoshi, J. et al. Minimizing confounders and increasing data quality in murine models for studies of the gut microbiome. _PeerJ_ 6, e5166 (2018). Article PubMed PubMed Central
Google Scholar * Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. _Science_ 308, 1635–1638 (2005). Article PubMed PubMed Central Google Scholar * Coutry, N. et
al. Cross talk between Paneth and tuft cells drives dysbiosis and inflammation in the gut mucosa. _Proc. Natl Acad. Sci. USA_ 120, e2219431120 (2023). Article CAS PubMed PubMed Central
Google Scholar * Lee, Y. S. et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. _Cell Host Microbe_ 24, 833–846.e836 (2018). Article CAS
PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part by P30 AG038072 and 5T32AG023475-21 from the NIA and R01CA214625, R01CA229216, R01CA222358, and
P30-013330 from the NCI. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY, USA Jiahn Choi & Leonard H. Augenlicht
* Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, USA Leonard H. Augenlicht Authors * Jiahn Choi View author publications You can also search for this author inPubMed
Google Scholar * Leonard H. Augenlicht View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.C. and L.H.A. drafted and edited the manuscript.
CORRESPONDING AUTHOR Correspondence to Jiahn Choi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Choi, J., Augenlicht, L.H. Intestinal stem cells: guardians of homeostasis in
health and aging amid environmental challenges. _Exp Mol Med_ 56, 495–500 (2024). https://doi.org/10.1038/s12276-024-01179-1 Download citation * Received: 14 November 2023 * Accepted: 11
December 2023 * Published: 01 March 2024 * Issue Date: March 2024 * DOI: https://doi.org/10.1038/s12276-024-01179-1 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative