Play all audios:
ABSTRACT OBJECTIVE Characterize C-reactive protein (CRP) within 72 postnatal hours in early-onset sepsis (EOS). STUDY DESIGN Secondary analysis of a prospective surveillance study of
neonates with EOS 2015–2017. We examined CRP use by center and neonatal characteristics, and CRP levels by time, neonatal characteristics, clinical signs, and pathogen. RESULTS CRP was
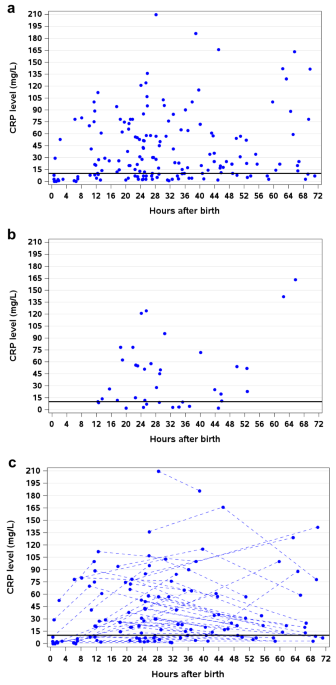
obtained for 96/235 neonates with EOS, which varied by center (p < 0.001). 71/95 had CRP > 10 mg/L (1 missing). Neonatal characteristics with and without CRP did not differ. There was
no relationship between CRP level and timing (_p_ = 0.41) or neonate characteristics. Median CRP was higher with ≥5 vs <5 clinical signs (56, 23 mg/L; _p_ = 0.002), and was not different
in Gram-positive vs Gram-negative sepsis (43, 51 mg/L; _p_ = 0.37) or preterm neonates who died vs survived (38, 28 mg/L; _p_ = 0.37). CONCLUSIONS Among neonates with EOS, CRP use varied by
center. CRP levels did not differ by time, neonate characteristics, pathogen, or death. CLINICAL TRIALS REGISTRATION ClinicalTrials.gov ID Early-Onset Sepsis an NICHD/CDC Surveillance Study
(EOSII): NCT02410486. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DETERMINANTS OF CRP MEASUREMENTS AND CRP DYNAMICS DURING EARLY NEONATAL SEPSIS WORK UP Article Open access 23 May 2025
C-REACTIVE PROTEIN TO PLATELET RATIO AS AN EARLY BIOMARKER IN DIFFERENTIATING NEONATAL LATE-ONSET SEPSIS IN NEONATES WITH PNEUMONIA Article Open access 28 March 2025 ASSOCIATION BETWEEN
PROGNOSTIC FACTORS AND THE CLINICAL DETERIORATION OF PRETERM NEONATES WITH NECROTIZING ENTEROCOLITIS Article Open access 17 August 2022 DATA AVAILABILITY Data reported in this paper may be
requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home. REFERENCES * Stoll BJ, Puopolo KM, Hansen NI, Sánchez
PJ, Bell EF, Carlo WA, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174:e200593. Article
PubMed PubMed Central Google Scholar * Kuzniewicz MW, Mukhopadhyay S, Li S, Walsh EM, Puopolo KM. Time to positivity of neonatal blood cultures for early-onset sepsis. Pediatr Infect Dis
J. 2020;39:634–40. Article PubMed Google Scholar * Ramasethu J, Kawakita T. Antibiotic stewardship in perinatal and neonatal care. Semin Fetal Neonatal Med. 2017;22:278–83. Article
PubMed Google Scholar * Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, Newman TB, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA
Pediatr. 2017;171:365–71. Article PubMed Google Scholar * Puopolo KM, Mukhopadhyay S, Hansen NI, Cotten CM, Stoll BJ, Sanchez PJ, et al. Identification of extremely premature infants at
low risk for early-onset sepsis. Pediatrics. 2017;140:e20170925. * Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37:421–38.
Article PubMed Google Scholar * Sturgeon JP, Zanetti B, Lindo D. C-Reactive Protein (CRP) levels in neonatal meningitis in England: an analysis of national variations in CRP cut-offs for
lumbar puncture. BMC Pediatr. 2018;18:380. Article CAS PubMed PubMed Central Google Scholar * Mukherjee A, Davidson L, Anguvaa L, Duffy DA, Kennea N. NICE neonatal early onset sepsis
guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed. 2015;100:F248–9. Article PubMed Google Scholar * Cantey JB, Prusakov
P. A proposed framework for the clinical management of neonatal “culture-negative” sepsis. J Pediatr. 2022;244:203–11. Article PubMed Google Scholar * Gude SS, Peddi NC, Vuppalapati S,
Venu Gopal S, Marasandra Ramesh H, Gude SS. Biomarkers of neonatal sepsis: from being mere numbers to becoming guiding diagnostics. Cureus. 2022;14:e23215. PubMed PubMed Central Google
Scholar * Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102:25–36.
Article CAS PubMed Google Scholar * Tiozzo C, Mukhopadhyay S. Noninfectious influencers of early-onset sepsis biomarkers. Pediatr Res. 2022;91:425–31. Article PubMed Google Scholar *
Committee on Infectious Diseases AAoP, Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;
2021. * Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. Article CAS PubMed Google Scholar * von
Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting
observational studies. J Clin Epidemiol. 2008;61:344–9. Article Google Scholar * Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely
preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. Article PubMed Google Scholar * Murthy K, Dykes FD, Padula MA, Pallotto EK, Reber KM, Durand DJ, et
al. The Children’s Hospitals Neonatal Database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34:582–6. Article CAS PubMed Google Scholar * Goodman
DC, Little GA, Harrison WN, Moen A, Mowitz ME, Ganduglia Cazaban C, et al. The Dartmouth Atlas of Neonatal Intensive Care. Lebanon, NH: The Dartmouth Institute of Health Policy &
Clinical Practice, Geisel School of Medicine at Dartmouth, 2019. * Puopolo KM, Benitz WE, Zaoutis TE. Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven
early-onset bacterial sepsis. Pediatrics. 2018;142:e20182896. * Puopolo KM, Benitz WE, Zaoutis TE. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven
early-onset bacterial sepsis. Pediatrics. 2018;142:e20182894. * Weitkamp Jr-H, Aschner JL. Diagnostic use of C-Reactive Protein (CRP) in assessment of neonatal sepsis. NeoReviews.
2005;6:e508–15. Article Google Scholar * Coggins SA, Wynn JL, Hill ML, Slaughter JC, Ozdas-Weitkamp A, Jalloh O, et al. Use of a computerized C-reactive protein (CRP) based sepsis
evaluation in very low birth weight (VLBW) infants: a five-year experience. PLoS One. 2013;8:e78602. Article CAS PubMed PubMed Central Google Scholar * Songer CN, Calip GS, Srinivasan
N, Barbosa VM, Pham JT. Factors influencing antibiotic duration in culture-negative neonatal early-onset sepsis. Pharmacotherapy. 2021;41:148–61. Article CAS PubMed Google Scholar *
Singh N, Gray JE. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J Perinatol. 2021;41:2488–94. Article
CAS PubMed Google Scholar * Yochpaz S, Friedman N, Zirkin S, Blumovich A, Mandel D, Marom R. C-reactive protein in early-onset neonatal sepsis - a cutoff point for CRP value as a
predictor of early-onset neonatal sepsis in term and late preterm infants early after birth? J Matern Fetal Neonatal Med. 2022;35:4552–7. Article CAS PubMed Google Scholar * Eschborn S,
Weitkamp JH. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol. 2019;39:893–903. Article PubMed Google Scholar *
Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998;102:E41. Article CAS PubMed Google Scholar *
Pourcyrous M, Bada HS, Korones SB, Baselski V, Wong SP. Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics. 1993;92:431–5. Article CAS
PubMed Google Scholar * Dhudasia MB, Benitz WE, Flannery DD, Christ L, Rub D, Remaschi G, et al. Diagnostic performance and patient outcomes with C-reactive protein use in early-onset
sepsis evaluations. J Pediatr. 2023;256:98–104.e106. Article CAS PubMed Google Scholar * Guerti K, Devos H, Ieven MM, Mahieu LM. Time to positivity of neonatal blood cultures: fast and
furious? J Med Microbiol. 2011;60:446–53. Article PubMed Google Scholar * Garcia-Prats JA, Cooper TR, Schneider VF, Stager CE, Hansen TN. Rapid detection of microorganisms in blood
cultures of newborn infants utilizing an automated blood culture system. Pediatrics. 2000;105:523–7. Article CAS PubMed Google Scholar * Dunne WM Jr., Case LK, Isgriggs L, Lublin DM.
In-house validation of the BACTEC 9240 blood culture system for detection of bacterial contamination in platelet concentrates. Transfusion. 2005;45:1138–42. Article PubMed Google Scholar
* Nanua S, Weber C, Isgriggs L, Dunne WM Jr. Performance evaluation of the VersaTREK blood culture system for quality control testing of platelet units. J Clin Microbiol. 2009;47:817–8.
Article PubMed PubMed Central Google Scholar * Sabui T, Tudehope DI, Tilse M. Clinical significance of quantitative blood cultures in newborn infants. J Paediatr Child Health.
1999;35:578–81. Article CAS PubMed Google Scholar * Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J
Pediatr. 1996;129:275–8. Article CAS PubMed Google Scholar * Chiesa C, Natale F, Pascone R, Osborn JF, Pacifico L, Bonci E, et al. C reactive protein and procalcitonin: reference
intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412:1053–9. Article CAS PubMed Google Scholar * Perrone S, Lotti F, Longini M, Rossetti A,
Bindi I, Bazzini F, et al. C reactive protein in healthy term newborns during the first 48 h of life. Arch Dis Child Fetal Neonatal Ed. 2018;103:F163–6. Article PubMed Google Scholar *
Macallister K, Smith-Collins A, Gillet H, Hamilton L, Davis J. Serial C-reactive protein measurements in newborn infants without evidence of early-onset infection. Neonatology.
2019;116:85–91. Article CAS PubMed Google Scholar * Rallis D, Balomenou F, Kappatou K, Karantanou K, Tzoufi M, Giapros V. C-reactive protein in infants with no evidence of early-onset
sepsis. J Matern Fetal Neonatal Med. 2022;35:5659–64. * Mjelle AB, Guthe HJT, Reigstad H, Bjørke-Monsen AL, Markestad T. Serum concentrations of C-reactive protein in healthy term-born
Norwegian infants 48–72 h after birth. Acta Paediatr. 2019;108:849–54. Article CAS PubMed Google Scholar * Hofer N, Müller W, Resch B. Non-infectious conditions and gestational age
influence C-reactive protein values in newborns during the first 3 days of life. Clin Chem Lab Med. 2011;49:297–302. Article CAS PubMed Google Scholar * Ishibashi M, Takemura Y, Ishida
H, Watanabe K, Kawai T. C-reactive protein kinetics in newborns: application of a high-sensitivity analytic method in its determination. Clin Chem. 2002;48:1103–6. Article CAS PubMed
Google Scholar * Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics.
2014;133:30–6. Article PubMed PubMed Central Google Scholar * Berardi A, Fornaciari S, Rossi C, Patianna V, Bacchi Reggiani ML, Ferrari F, et al. Safety of physical examination alone for
managing well-appearing neonates ≥ 35 weeks’ gestation at risk for early-onset sepsis. J Matern Fetal Neonatal Med. 2015;28:1123–7. Article PubMed Google Scholar * Joshi NS, Gupta A,
Allan JM, Cohen RS, Aby JL, Kim JL, et al. Management of chorioamnionitis-exposed infants in the newborn nursery using a clinical examination-based approach. Hosp Pediatr. 2019;9:227–33.
Article PubMed Google Scholar * Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature
infants. JAMA Netw Open. 2018;1:e180164. Article PubMed PubMed Central Google Scholar * Franz AR, Bauer K, Schalk A, Garland SM, Bowman ED, Rex K, et al. Measurement of interleukin 8 in
combination with C-reactive protein reduced unnecessary antibiotic therapy in newborn infants: a multicenter, randomized, controlled trial. Pediatrics. 2004;114:1–8. Article PubMed Google
Scholar * Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. Article CAS PubMed Google Scholar * Mihara M, Hashizume
M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143–59. Article CAS PubMed Google Scholar
* Lai MY, Tsai MH, Lee CW, Chiang MC, Lien R, Fu RH, et al. Characteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (≦10 mg/L). BMC
Infect Dis. 2015;15:320. Article PubMed PubMed Central Google Scholar Download references FUNDING This study was supported by the Eunice Kennedy Shriver National Institute of Child
Health and Human Development cooperative agreements, which provided infrastructure and study support to the NRN (grants UG1 HD27904, UG1 HD21364, UG1 HD27853, UG1 HD40492, UG1 HD27851, UG1
HD27856, UG1 HD68278, UG1 HD36790, UG1 HD27880, UG1 HD34216, UG1 HD68270, UG1 HD53109, UG1 HD53089, UG1 HD68244, UG1 HD68263, UG1 HD40689, UG1 HD21385, and UG1 HD87229 from the NICHD), the
National Center for Advancing Translational Sciences, which provided infrastructure support to the NRN (grants UL1 TR1425, UL1 TR1117, UL1 TR454, UL1 TR1108, UL1 TR1085, UL1 TR442, UL1
TR1449, and UL1 TR42 from NCATS), and the Centers for Disease Control and Prevention, which provided study support to the NRN (Interagency Agreement #14FED1412884 from the CDC). AUTHOR
INFORMATION Author notes * A full list of members and their affiliations appears in the Supplementary Information. AUTHORS AND AFFILIATIONS * Division of Newborn Medicine, Tufts University
School of Medicine, Boston, MA, USA Ryan Kilpatrick * Department of Pediatrics, Duke University, Durham, NC, USA Rachel Greenberg & C. Michael Cotten * Social, Statistical and
Environmental Sciences Unit, RTI International, Research Triangle Park, NC, USA Nellie I. Hansen * Department of Pediatrics, Wayne State University, Detroit, MI, USA Seetha Shankaran *
Division of Neonatology, University of Alabama at Birmingham, Birmingham, AL, USA Waldemar A. Carlo * Department of Pediatrics, McGovern Medical School at The University of Texas Health
Science Center at Houston, Houston, TX, USA Barbara J. Stoll Authors * Ryan Kilpatrick View author publications You can also search for this author inPubMed Google Scholar * Rachel Greenberg
View author publications You can also search for this author inPubMed Google Scholar * Nellie I. Hansen View author publications You can also search for this author inPubMed Google Scholar
* Seetha Shankaran View author publications You can also search for this author inPubMed Google Scholar * Waldemar A. Carlo View author publications You can also search for this author
inPubMed Google Scholar * C. Michael Cotten View author publications You can also search for this author inPubMed Google Scholar * Barbara J. Stoll View author publications You can also
search for this author inPubMed Google Scholar CONSORTIA THE EUNICE KENNEDY SHRIVER NATIONAL INSTITUTE OF CHILD HEALTH AND HUMAN DEVELOPMENT NEONATAL RESEARCH NETWORK CONTRIBUTIONS RK
conceptualized and designed the study, drafted the manuscript, and interpreted the data analysis. RG contributed to the conception and design of the study, data interpretation and critical
revision of the manuscript for important intellectual content. NIH contributed to the design of the study, completed the data analysis, contributed to data interpretation, and reviewed and
revised the manuscript. SS contributed to the conception and design of the study, data interpretation, and critical revision of the manuscript for important intellectual content. WAC
contributed to the conception and design of the study, data interpretation, and critical revision of the manuscript for important intellectual content. MC contributed to the conception and
design of the study, data interpretation, and critical revision of the manuscript for important intellectual content. BJS contributed to the conception and design of the study, data
interpretation, and critical revision of the manuscript for important intellectual content. CORRESPONDING AUTHOR Correspondence to Ryan Kilpatrick. ETHICS DECLARATIONS COMPETING INTERESTS
Ryan Kilpatrick, Nellie I. Hansen, Seetha Shankaran, Waldemar A. Carlo, C. Michael Cotten, and Barbara J. Stoll have nothing to disclose. Rachel Greenberg has received support from industry
for research services (https://dcri.org/about-us/conflict-of-interest/). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds
exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely
governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kilpatrick, R., Greenberg, R., Hansen, N.I. _et al._ Use
and utility of C-reactive protein (CRP) in neonatal early-onset sepsis: a secondary analysis of a prospective surveillance study. _J Perinatol_ 45, 139–145 (2025).
https://doi.org/10.1038/s41372-024-02064-5 Download citation * Received: 12 February 2024 * Revised: 19 June 2024 * Accepted: 25 June 2024 * Published: 05 August 2024 * Issue Date: January
2025 * DOI: https://doi.org/10.1038/s41372-024-02064-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative