Play all audios:
ABSTRACT Hepatic sinusoids play a key role in maintaining high activities of liver cells in the hepatic acinus. However, the construction of hepatic sinusoids has always been a challenge for
liver chips, especially for large-scale liver microsystems. Herein, we report an approach for the construction of hepatic sinusoids. In this approach, hepatic sinusoids are formed by
demolding a self-developed microneedle array from a photocurable cell-loaded matrix in a large-scale liver-acinus-chip microsystem with a designed dual blood supply. Primary sinusoids formed
by demolded microneedles and spontaneously self-organized secondary sinusoids can be clearly observed. Benefiting from significantly enhanced interstitial flows by formed hepatic sinusoids,
cell viability is witnessed to be considerably high, liver microstructure formation occurs, and hepatocyte metabolism is enhanced. In addition, this study preliminarily demonstrates the
effects of the resulting oxygen and glucose gradients on hepatocyte functions and the application of the chip in drug testing. This work paves the way for the biofabrication of fully
functionalized large-scale liver bioreactors. SIMILAR CONTENT BEING VIEWED BY OTHERS 3D BIOPRINTING OF HEPATOCYTES: CORE–SHELL STRUCTURED CO-CULTURES WITH FIBROBLASTS FOR ENHANCED
FUNCTIONALITY Article Open access 04 March 2021 3D BIOPRINTING OF BICELLULAR LIVER LOBULE-MIMETIC STRUCTURES VIA MICROEXTRUSION OF CELLULOSE NANOCRYSTAL-INCORPORATED SHEAR-THINNING BIOINK
Article Open access 26 November 2020 A VERSATILE MICROFLUIDIC TOOL FOR THE 3D CULTURE OF HEPARG CELLS SEEDED AT VARIOUS STAGES OF DIFFERENTIATION Article Open access 07 July 2021
INTRODUCTION The liver is one of the most important organs in the human body and plays a key role in the metabolism of many substances, such as blood glucose and drugs1,2. To understand the
complex liver structure and associated function at the microscale, researchers define two main divisions for liver microstructures, the hepatic lobule3,4,5 and hepatic acinus6,7,8. Compared
with the hepatic lobule, a hexagonal division that relies mostly on the characteristics of liver microstructure, the hepatic acinus, a fusiform or olive-shaped structure centered on the
portal area and approximately one-third of the volume of the hepatic lobule, is defined by both structural and functional properties9. In addition, the hepatic acinus is also more concise in
structure10. In the hepatic acinus, blood enters the hepatic sinusoid via the portal vein (PV) and hepatic artery (HA) and exits via the central vein (CV)6. This unique dual blood supply
with tri-vascular microcirculation spatially generates concentration gradients of both oxygen and nutrients (e.g., glucose) in the hepatic acinus11 and then significantly forms structure and
function differences in various regions of the hepatic acinus, e.g., differences in metabolism12, liver gene expression13 and sinusoid morphology14. These differences may be magnified under
varying physiological and pathological conditions15 or in the presence of drugs16. To discover the mechanism of liver function, investigate the hepatotoxicity of drugs, and mimic
liver-related diseases, animal models are routinely used17, but the application of animal models is limited due to interspecies differences in structure and function18. 2D and 3D cell
cultures are alternatives to minimize the difference between human and nonhuman cells19,20,21. However, 2D culture is very different from in vivo 3D cell growth environments22,23, and
traditionally proposed 3D culture only occasionally forms a few very simple structures24 and rarely generates self-organized perfusable vascular structures25,26, let alone a complex dual
blood supply and tri-vascular system. Very recently, organ-on-a-chip techniques have undergone rapid development27 and enable the biomimicry of human tissue culture on a microfluidic
platform28,29,30. In addition, the organ-on-a-chip technique can build microstructures and microenvironments more similar to physiological ones than traditionally developed 3D culture
methods24. As one of the main members of the organ-on-a-chip, although liver chips have always been focused extensively31, they are very different from in vivo livers, and they still present
many challenges, especially in the construction of peripheral vascular systems32 and hepatic sinusoids2,33,34. In terms of the construction of hepatic sinusoids, initially reported chips
achieved only 2D dynamic endotheliocyte culture; that is, endothelial cells are simply laid on the surface of the culture zone, and the culture medium flows through the cell
surface35,36,37,38. Later, researchers directly constructed customized scaffold structures as sinusoids using approaches such as photolithography39,40, laser-based cavitation41,
dielectrophoresis42, and magnetic field induction43. However, these approaches have limitations either in spatial resolution (usually >100 µm) or in structural complexity (it is difficult
to form a complex 3D structure)44. Then, pioneering researchers tried to form sinusoids by the self-assembly of endotheliocytes with the help of growth-factor gradients45,46,47 or culture
medium flows48,49. Physiologically similar sinusoids are the basis of large-scale liver tissue culture, as oxygen and nutrients can be transported to all cells2. However, the assembly
process is slow, the likelihood of the formation of perfusable sinusoids is low, and the growth and migration of endotheliocytes are usually unordered, leading to poor reproducibility41. In
terms of the construction of the tri-vascular (HA–PV–CV) system for feeding cultured cells, many of the early liver chips neglected the tri-vascular structure and even the dynamic flow50,51,
which has been proven to promote cell function and long-term culture51,52. Later, liver chips with a single-flow pathway as a vascular substitute emerged, supplying the cultured cells with
oxygen and nutrients and removing waste35,51,53,54,55. However, it is difficult for the single-vascular structure to form physiologically similar oxygen and nutrient gradients in the cell
culture area, which are considered one of the main factors resulting in the differentiated functional regions of hepatic acinus56. Furthermore, it is also difficult to reach large-scale
tissues because of the transportation limitation of oxygen and nutrients. Currently, a dual-vascular structure, originally used for the organ-on-a-chip57,58,59, has been used for liver
chips60,61. In this structure, the cell culture area is located between two fluid pathways, forming an artery-tissue-vein microcirculation system. This system can form microvascular networks
and concentration gradients39,60. However, it still fails to achieve a double blood supply, as observed in the liver, much less physiologically similar oxygen and nutrient gradients48,49.
There are two general design approaches to pursue the tri-vascular structure based on the two divisions for liver microstructures. Statistically, chips with the hepatic lobule design are in
the majority, although the hepatic acinus design is simple, especially for the tri-vascular structure. In our previous work, we proposed two designs of the dual blood supply tri-vascular
structure for a single hepatic lobule chip and a multiple hepatic lobules chip48,49. For the single hepatic lobule chip, the structure is simple, and it only consists of 3 layers of
polydimethylsiloxane (PDMS). This chip forms sinusoid-like structures49. However, the perfusable sinusoid is barely observed. The multiple hepatic lobule chip consists of 5 layers of
polymethyl methacrylate (PMMA) and one layer of PDMS with continuously arranged hexagon areas. With the micropillar arrays specifically designed as PV, HA, and CV, this chip forms perfusable
sinusoids via flow-guided self-assembly and replicates the convergence of venues from PV and arterioles from HA to the connection between sinusoids and CV48. However, the structure of the
multiple hepatic lobule chip is very complicated, and the degree of formation of perfusable sinusoids is unsatisfactory. In summary, although the dual blood supply tri-vascular structure has
already been developed, the formation of perfusable sinusoids for large-scale liver-tissue culture is still lacking60,61,62,63. In this study, therefore, we present a microneedle-assisted
hepatic sinusoid construction for a large-scale liver-acinus-chip microsystem with a dual blood supply. The research process is as follows: first, we designed a dual blood supply liver chip
based on the hepatic acinus structure; then, we fabricated a microneedle array using 3D printing and promoted the formation of perfusable hepatic sinusoids by demolding a self-developed
microneedle array from a photocurable cell-loaded matrix in the liver acinus chip; afterward, we investigated the effects of microneedle array-induced hepatic sinusoids on fluid flows inside
the culture area, liver metabolism, cellular activity, and tissue formation; finally, we preliminarily demonstrated the effects of the resulting oxygen and glucose gradients on hepatocyte
functions and the application of the chip in drug testing. This work provides insights into the construction of more biomimetic liver chips with sinusoids. RESULTS FABRICATION OF THE
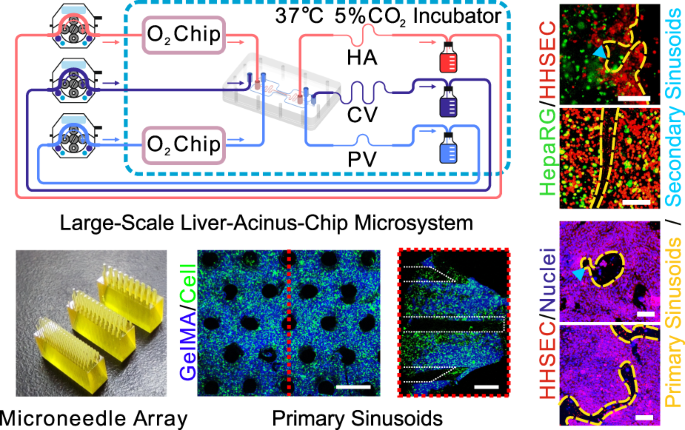
MICRONEEDLE-ASSISTED HEPATIC ACINUS CHIP (MHAC) The design of the cell culture area of the mHAC is inspired by the structure of the human hepatic acinus. That is, a single culture area is
designed as a triangular prism, consisting of 1/6 of a hepatic lobule or 1/2 of a hepatic acinus (Fig. 1a). In the mHAC, the CV pathway is located on the lower edge of the triangular prism,
while the PV pathway is located on the other two edges of the triangular prism, and the HA pathway is between the PV pathways. In the culture area, the hepatic sinusoids are considered to
extend through the whole culture area, perpendicular to the top surface of the triangular prism (Fig. 1a). In the cultured chip, the culture media from PV and HA partially infiltrate into
the culture area, and the culture medium flows through the cell-cultured area into the CV, as reported physiologically11. The chip was designed as shown in Fig. 1b. It was composed of 3
layers of PMMA: Layer 1 was for CV (green), Layer 2 was for the culture area (light blue; isosceles trapezoid), and Layer 3 was for PV (blue) and HA (red). In addition, the layers were
separated/sandwiched with a silicone membrane (SM) and a porous membrane (only for the culture area). The detailed design parameters (Fig. S1) and pictures of the chip (Fig. S2) are shown in
the Supplementary Information. In addition, a microneedle array was designed to assist in the formation of the primary hepatic sinusoids (Fig. 1c and Fig. S3). Then, the chip was assembled
according to the procedure in Fig. 1d. Finally, the assembled chip (Fig. 1e) was connected to a culture system (Fig. 1f and Fig. S4). In experiments, the perfusable chip microsystem could
run stably for more than 14 days. CHARACTERIZATION OF SINUSOIDS FORMED BY MICRONEEDLES In this study, 3D printed microneedles with radii of 100, 150, and 200 μm were designed to inspire the
generation of primary sinusoids (Fig. 2a; the volume fraction of microposts is designed to be close to the porosity of sinusoids). The confocal laser scanning microscopy (CLSM) results
showed that after demolding, sinusoid pathways were formed well in both cell-free and cell-laden gelatin methacryloyl (GelMA) extracellular matrix (ECM) (Fig. 2b). Although small breakages
may occur due to demolding damage, the majority of pathways are intact. In addition, the radius of the pathways is slightly larger than the radius of the microneedles, possibly resulting
from the dehydration of GelMA during scanning with CLSM. Generally, the pathways for primary sinusoids were formed as expected (Fig. 2c). MICRONEEDLE-INSPIRED LIVER CHIP INCREASES FLOWS
INSIDE THE ECM To demonstrate the advantages of sinusoids formed using microneedles in terms of flows inside ECM, the effects of formed sinusoids on flows were theoretically and
experimentally investigated. The simulation shows that the flow directions of fluids are mainly from PV and HA to CV via the formed sinusoids, with the convergence of parts of fluids from
sinusoids to CV in the culture area (Fig. 3a). The flow rate across the culture area (_Q__CO_ − _Q__CI_ or _Q__HI_ − _Q__HO_ + _Q__PI_ − _Q__PO_) increases with the radius of the formed
sinusoids, resulting from the decreasing flow resistance (Fig. 3b and Fig. S5). For formed sinusoids of radius 150 μm, the flow rate, which is close to the value experimentally reported in
the literature, simulates the interstitial flow across hepatocytes more physiologically64. To experimentally confirm the flow directions of fluids in PV, HA, and CV, a blue dye was added in
PV, HA, and CV separately (Fig. 3c). When the dye was injected from one of the vascular pathways (e.g., HA), the concentrations of the dye in the rest of the vascular pathways (e.g., PV and
CV) were relatively negligible (Fig. 3d and Fig. S6). Thus, mixing of HA and PV beyond the culture area barely occurred, and the flow directions of fluids in the culture area were definitely
from PV and HA to CV. In vivo, there are differences between HA and PV in terms of supplied oxygen and nutrients. These results also imply that differences in oxygen and nutrients can be
maintained if applied. MICRONEEDLE-INSPIRED LIVER CHIP HEIGHTENS THE METABOLISM OF HEPATOCYTES To demonstrate the advantages of microneedle-assisted constructed sinusoids in terms of the
metabolic function of hepatocytes, the concentrations of specific biomarkers were analyzed (Fig. 4a). For the uninduced group, the concentrations of the studied biomarkers decreased rapidly
after a small rise at the beginning of culture (Fig. S7; the daily metabolism also indirectly confirms the stability of the microsystem). However, for the induced groups, the concentrations
of the studied biomarkers maintained an upward trend, then reached a maximum at the end of the middle stage, and finally decreased slightly (although the concentrations dropped slightly, the
values were still high). Collectively, compared with the microneedle-unused group (i.e., _r_ = 0 μm), the concentrations of ALB (Fig. 4b), BUN (Fig. 4c), and TBA (Fig. 4d) in the
microneedle-assisted groups were significantly higher, whether in the early stage (1–4 days), in the mid-stage (5–9 days), or in the late stage (10–14 days). In addition, the group with 150
μm microneedles is significantly better than the other groups with microneedles, probably profiting from the more physiologically similar flows provided by sinusoids formed with 150 μm
microneedles. We further analyzed the activities of 2 cytochrome P450 enzymes, CYP3A4 (Fig. 4e) and CYP1A2 (Fig. 4f), on Day 7. The results show that the activities of 2 enzymes in the
microneedle-assisted groups are also significantly higher than in the microneedle-unused group. MICRONEEDLE-ASSISTED LIVER CHIP INCREASES THE VIABILITY OF CELLS AND PROMOTES THE DEVELOPMENT
OF MICROSTRUCTURES To demonstrate the effects of induced sinusoids on liver tissue formation, the viability of cells and the formed microstructures were examined. The induced group presented
higher cell viability than the uninduced group (Fig. 5a, b and Fig. S8). Among the induced groups, the viability of the group with a radius of 150 μm microneedles was the highest. In the
induced group with 150 μm radius microneedles, the sinusoids formed with microneedles were clearly visible and could be observed more at different positions in the tissue (Fig. 5c shows the
checked position; Fig. 5d–g); more interestingly, secondary sinusoid formation occurred, resulting from flow-driven angiogenesis across the primary sinusoids (the formed secondary hepatic
sinusoids can further deliver nutrients to the cells far away from the primary hepatic sinusoids). In addition, staining for the enzyme CYP and the bile canaliculus showed the cord-like
endothelial structure, the plate-like hepatocyte cluster, and occasionally the bile canaliculus (Fig. S8), which also confirmed the previously tested metabolic results (Fig. 4).
RECONSTRUCTION OF CONCENTRATION GRADIENTS AND CHIP APPLICATION To demonstrate the physiological similarity of the chip, the effects of the reconstructed oxygen and glucose gradients on cell
functions were preliminarily presented (Fig. 6a and Fig. S9). Previous studies have shown that oxygen gradients promote the emergence of hepatocyte diversity and enhance hepatocyte
metabolism48,56,65. Here, the reconstructed oxygen gradients (simulated in Fig. S10A) also significantly increased the metabolism of hepatocytes as measured by ALB, BUN, TAB, CYP1A2, and
CYP3A4 (Fig. 6b). We also checked the influence of glucose gradients (simulated in Fig. S10B) on cell metabolism. One of the key functions of hepatocytes is their response to glucose
gradients and their subsequent launch of differentiated metabolism66,67. The experimental results of this study show that the more physiologically similar the glucose gradients are, the
higher the metabolic activity of hepatocytes (Fig. 6c). That is, the formed sinusoids in the induced groups significantly improved the physiological similarity of oxygen and glucose
gradients in mHAC and thus increased the metabolic capacity of hepatocytes. When hepatocytes are exposed to more physiologically similar conditions, they are expected to show a more
physiologically similar response to the tested drugs68. To demonstrate the application of the mHAC in drug testing, the drug acetaminophen (APAP) was used (APAP is a widely used analgetic
and antipyretic drug, and the excessive use of APAP can cause hepatotoxicity48,69). The results of this study showed that the hepatocytes in the induced groups were more sensitive to drugs
than those in the uninduced groups, especially in terms of CYP enzymes (Fig. 6d). DISCUSSION Structure is the basis of function70,71,72. Existing few studies show that the formation of
hepatic sinusoids (i.e., liver microvascular networks) is not only of great significance for the formation of the liver and its tissue function35 and the expansion of liver-chip
applications68 but also provides a prerequisite for the high activity of large-scale liver tissue cultured in vitro73. However, the formation of hepatic sinusoids has always been a challenge
for liver chips. This is because the size of hepatic sinusoids is very small (only a few microns74). This accuracy issue exists for most organ-on-a-chip techniques, even 3D bioprinting75.
Induced self-assembly by flow, as well as chemical factors, is promising48,68, but the few methods proposed by previously reported work have not reproduced the in vivo structural features
well34. In this paper, a method is proposed for the construction of hepatic sinusoids. That is, a 3D-printed microneedle array is used as a mold; larger primary hepatic sinusoids are formed
by demolding technology, and then smaller secondary hepatic sinusoids are self-assembled with the help of microflows. This work clearly shows that primary hepatic sinusoids are formed,
followed gradually by secondary hepatic sinusoids. In addition, the fluid flow supplied by the microsystem could exert a positive effect on the viability and function of hepatocytes in
long-term culture, as reported previously48. Due to the limitations of the printing technique, the microneedles used in this paper are still large (on the order of 100 microns), but the
proposed method has the potential to further optimize the formation of the primary hepatic sinusoids by controlling the size, density, and array of the microneedles. Here, the liver chip is
designed based on the physiological hepatic acinus structure, which solves the difficulty of designing a dual blood supply liver chip with a tri-vascular structure. Benefiting from the
realization of dual blood supply (PV and HA supply blood separately) and the assistance of the oxygen chip, the liver-chip microsystem can reproduce oxygen zonation well48, thus providing a
basis for further improvement of liver function. The liver chip can also be used to simulate the dynamic blood glucose distribution1 or drug distribution76 in a hepatic lobule. In this
study, as an example, only one triangular prism was designed on the mHAC; however, more triangular prisms can be carved on the mHAC to obtain a larger-scale liver acinus chip (Fig. S11). In
addition, due to the limitation of the chip size, it is very difficult to directly observe the gradient distribution on the chip, so current research on the influence of the gradient is
focused on metabolic analysis. In future work, an in-depth study will be performed to fully demonstrate these applications using the liver chip we designed. In addition, the liver
acinus-based design with PV, HA, and CV could also provide a reference for the construction of a more biomimetic liver77. CONCLUSIONS In summary, we proposed a new method for the
construction of hepatic sinusoids in a liver-acinus-on-a-chip microsystem using a self-developed microneedle array. We confirmed the formation of hepatic sinusoids and explored the effects
of microneedle sizes. Additionally, we preliminarily demonstrated the effects of the resulting oxygen and glucose gradients on hepatocyte functions and the application of the microsystem in
drug testing. In the future, liver organoids will be seeded in the cell culture area to further enhance the structure and functional formation of hepatic sinusoids. MATERIALS AND METHODS
CELL CULTURE Undifferentiated HepaRG cells were obtained from Beina Chuanglian Biotechnology Co., Ltd. (Beijing, China). To obtain differentiated HepaRG cells, undifferentiated HepaRG cells
were cultured in William’s E medium (Thermo Fisher Scientific, MA, USA) supplemented with 10% fetal bovine serum (Life Science Products & Services, Australia), 1% penicillin–streptomycin
(Sangon Biotech Co., Ltd., Shanghai, China), 2 mM glutamine (Thermo Fisher Scientific, MA, USA), 5 µg/mL insulin (Wanbang Pharmaceutical Co., Ltd., Jiangsu, China) and 50 µM hydrocortisone
hemisuccinate (Aladdin Industrial Corporation, Shanghai, China) in a cell incubator (Thermo Fisher Scientific, Waltham, MA, USA) with 5% CO2 at 37 °C for 2 weeks and then transferred to the
above culture medium containing 2% dimethyl sulfoxide (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for 2 weeks78. HHSECs were obtained from Tongpai Biotechnology Co., Ltd.
(Shanghai, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Life Technologies, USA) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin and 2 mM
glutamine in an incubator at 37 °C with 5% CO2. HEPATIC ACINUS CHIP FABRICATION Here, the hepatic acinus chip was made of three PMMA (Qingsheng Information Technology Co., Ltd., Hefei,
China) plates and two SMs (Jin Yi Hong Plastic Materials Co., Hefei, China). The chip was designed with SolidWorks software (Fig. S1) and fabricated using a CNC drill/tap center (DT-1, Haas
Automation, Inc., CA, USA) at the Experiment Center for Life Sciences at the University of Science and Technology of China (USTC), Anhui, China (Fig. S2). MICRONEEDLE ARRAY AND AUXILIARY
MOLD FABRICATION The microneedle array and auxiliary mold for demolding were designed with SolidWorks and fabricated using a high-precision 3D printer (S130, 2 μm precision, BMF Material
Technology Inc.) at the Engineering and Materials Experiment Center in USTC, Anhui, China (Fig. S3). Here, three microneedle arrays were designed and fabricated, and the ratio of microneedle
space (s) to microneedle diameter (2_r_) was 5:2 (Fig. 1C). Due to its excellent photocuring properties and biocompatibility, GelMA was adopted as the ECM79,80. After the microneedle array
was removed from the GelMA hydrogel, the cylindrical channel arrays modeling hepatic sinusoids formed in the hydrogel. In this study, the porosity (_ε_) of the liver tissue is defined as the
volume ratio of the hepatic sinusoids to the liver tissue in the following equation: $${\rm{\varepsilon }}=\frac{{V}_{{sinusoid}}}{{V}_{{tissue}}}=\frac{\pi
{r}^{2}}{\frac{\sqrt{3}}{2}{s}^{2}}$$ the value of _ε_ can be calculated as 0.145, which is in the range (0.143 ± 0.028) of the porosity of human liver tissue reported in the literature81.
The microneedle space of the through-holes in the auxiliary mold is the same as that of the microneedle array, and the radius of the through-holes in the auxiliary mold is 200 μm larger than
that of the microneedle array. CELL LOADING AND CHIP ASSEMBLY Before chip assembly, the GelMA precursor solution was prepared according to the supplier’s instructions. Briefly, GelMA solid
was dissolved in phosphate-buffered saline (PBS) containing 0.25% photosensitizer at 60 °C for 30 min to obtain the GelMA precursor solution (w/v: 10%). The solution was sterilized using a
filter (pore size: 0.22 μm) before use. For chip assembly, Layer 2 of the mHAC, microneedle array, and auxiliary mold were assembled in the order shown in Fig. 1d. The differentiated HepaRG
cells and HHSECs were mixed with GelMA precursor solution (the cell densities were approximately 2 × 107 cells/mL and 4 × 106 cells/mL, respectively), and the cell-laden GelMA solution was
injected into the culture area in layer 2 and irradiated with 405 nm ultraviolet (UV) light for 30 s (short-term UV illumination has very little effect on cell viability82,83,84). Afterward,
the auxiliary mold and microneedle array was carefully demolded in sequence (it should be noted that due to mold assembly error, blocking of primary sinusoids sometimes occurred after
demolding, especially at the bottom; thus, a slightly longer microneedle array is proposed; moreover, the flow shear force may also alleviate the blocking). Then, Layers 1, 2, and 3 of the
mHAC, SMs, and porous membranes (10 mm × 5 mm × 0.1 mm) were assembled in that order (Fig. 1e). Finally, the cell suspension of HHSECs (the cell density was approximately 4 × 106 cells/mL)
was injected into the chip from PV and HA inlets, and the chip was cultured in an incubator at 37 °C with 5% CO2 overnight to allow cell attachment (Fig. 1f). OXYGEN CONCENTRATION REGULATING
CHIP FABRICATION The oxygen concentration regulating chip (ORC) contains two PMMA plates and one SM layer. The detailed fabrication parameters are given in Fig. S12. Compared to the oxygen
chip we previously reported48, the stability of the ORC reported here was enhanced, especially under high flow rates, by increasing the width of the chip. ASSEMBLY AND OPERATION OF THE
LIVER-CHIP MICROSYSTEM The mHAC, ORC, glass flasks, silicone tubes, and PTFE tubes were connected as shown in Fig. 1f (the picture of the culture system is in Fig. S4). It should be noted
that the lengths of the silicone tubes were the same, while the lengths of the PTFE tubes for PV, HA, and CV (wavy lines in Fig. 1f) were 10 cm, 5 cm, and 2 cm, respectively, to promote the
generation of interstitial flow. Here, the tape was used to prevent fluid leakage at the connections. The liver chip, the glass flasks, and the oxygen concentration regulating chips were
placed in the cell incubator, while the peristaltic pumps were placed outside the incubator. The flow rates of the PV, HA, and CV channels were set to 2, 1, and 1 mL/min, respectively, and
operated continuously for 7–14 days. MICRONEEDLE ARRAYS AND SINUSOID CHARACTERIZATION In this study, SEM (EVO18, Zeiss, Germany) was used to characterize the morphology of the microneedle
array. To enable observation of the nonconductive microneedle array under SEM, the microneedle array was gilded by a vacuum evaporation instrument. Then, the gilded microneedle array was
observed and photographed by SEM. We measured the bottom circle area of the cylindrical microneedles and calculated the radius using ImageJ software. CLSM (DMi8, Leica, Germany) was used to
characterize the GelMA hydrogel, and the three-dimensional structure was reconstructed. To observe the 3D structure of ECM, GelMA labeled with blue fluorescence was used (the operation
procedure in the section “_cell loading and chip assembly”_). The drilling GelMA hydrogel was removed from the chip with a scalpel and immediately photographed using CLSM at different
resolutions. For the measurement of the area and radius of the bottom circle of the cylindrical channel, ImageJ software was used. FLOWS INSIDE ECM ANALYSIS In this study, we analyzed the
flow and direction of interstitial flow. The uninduced cell-free tri-vascular system and the induced systems (induced by different microneedle arrays with _r_ = 100 μm, 150 μm, and 200 μm)
were constructed as described in the section “_assembly and operation of the liver-chip microsystem”_. We used COMSOL to simulate the flow in the tissue, and more detailed information can be
found in Fig. S5. Based on the simulation results, the flow rates of the PV, HA, and CV inlets were set to 2, 1, and 1 mL/min, respectively, the outlet pressure difference was 8 Pa, and the
system was operated for 1 h. After the system was stable, the liquid from the CV outlet within 5 minutes was collected. In this study, interstitial flow is defined as the flow from HA and
PV to CV, which was calculated using the following equations: $${Q}_{{CO}}-{Q}_{{CI}}={Q}_{{PO}}-{Q}_{{PI}}+{Q}_{{HO}}-{Q}_{{HI}}={Q}_{t}$$ where \({Q}_{{CO}}\), \({Q}_{{PO}}\) and
\({Q}_{{HO}}\) are the outlet flow rates of CV, PV, and HA, respectively. \({Q}_{{CI}}\), \({Q}_{{PI}}\), and \({Q}_{{HI}}\) are the inlet flow rates of CV, PV, and HA, respectively, and
\({Q}_{t}\) is the interstitial flow. To confirm the flow direction on the chip, deionized water with blue dye was used. We first measured the absorbance of water with blue dye (0.12 mg/mL)
in the wavelength range of 400–800 nm and found that the dye had an absorption peak at 622 nm. As the dye concentration ranged from 0 to 0.3 mg/mL, there was a linear correlation between the
dye concentration and the dye absorbance at 622 nm, as shown in Fig. S13. Thus, deionized water with the dye added at a concentration of 0.3 mg/mL was pumped into the mHAC through the PV,
HA, or CV inlet, and the absorbance of the water with dye from all the inlets and outlets of the mHAC at 622 nm was recorded using a microplate reader (Multiskan GO; Thermo Fisher
Scientific, MA, USA). HEPATOCYTE METABOLISM ANALYSIS To analyze liver function, the concentrations of TBA, ALB, and BUN metabolized by the HepaRG cells were tested, as well as the
concentrations of CYP1A2 and CYP3A4 enzymes. To estimate the TBA, ALB, and BUN concentrations, medium samples collected from the medium bottle connected to the CV outlet of the chip were
analyzed with an enzyme-labeled reader using a human ELISA kit (Mlbio, Shanghai, China) according to the literature59,68,85. For CYP1A2 and CYP3A4, the device was disassembled on the 7th
day, and the cultured tissue was placed in a 48-well plate. The CYP1A2 and CYP3A4 activities were measured using P450-Glo™ CYP1A2 and CYP3A4 assays (Promega, Madison, WI, USA) according to
the manufacturer’s instructions, and the luminescence was read by a microplate reader (iD5, Molecular Devices, USA)26,53,86,87. ANALYSIS OF CELL VIABILITY Cell viability was determined by
cell staining. The device was disassembled on Day 7, and the cultured tissue was placed in a 48-well plate for cell staining. Calcein-AM (Aladdin Industrial Corporation, Shanghai, China),
propidium iodide (PI, Sangon Biotech Co., Ltd., Shanghai, China), and Hoechst 33342 (Sangon Biotech Co., Ltd., Shanghai, China) were used to identify living cells, dead cells, and cell
nuclei, respectively (Fig. S14A). The cells were stained according to the manufacturer’s instructions and observed using a fluorescence microscope (IX73; Olympus, Tokyo, Japan)49. ImageJ
software was used to analyze the viability of cells (i.e., the ratio of the number of living cells to the number of cell nuclei). IMMUNOSTAINING AND IMAGING After 7 days of culture, the
device was disassembled, and the cultured tissue was fixed in 4% paraformaldehyde for 30 min at room temperature and then permeabilized with 0.2% Triton X-100 (Sigma) in PBS solution for 15
min at room temperature. After blocking with 2% bovine serum albumin (BSA, Sigma) in PBS for 1 h at room temperature, samples were incubated overnight at 4 °C with primary antibodies
(anti-CD31, anti-ZO-1, anti-CYP1A2, and anti-CYP3A4; 1:100 in PBS; Proteintech Co., Ltd., Hubei, China) and then incubated for 4 h at 4 °C with the corresponding secondary antibodies (Alexa
Fluor 488 or 594; 1:250 in PBS; ABclonal Biotech Co., Ltd., Hubei, China). Cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI; 1:100 in PBS; Proteintech Co., Ltd., Hubei,
China) for 30 min at room temperature. The samples were washed 3 times with PBS after each step (Fig. S14B and Fig. S14C). Images of the samples were taken using CLSM. BILE CANALICULI
STAINING The successful formation of bile canaliculi was verified using 5(6)-carboxy-2’,7’-dichlorofluorescein diacetate (CDFDA, Sigma‒Aldrich, MO, USA)88. After staining with CDFDA (1:300
in PBS dilution) for 30 min at 37 °C, the cells were washed 3 times with PBS and then observed using a fluorescence microscope (Fig. S14D). PRESTAINING Before cell loading and device
assembly, cells were stained with DiI (red, 1:100 in PBS) or DiO (green, 1:100 in PBS) (Jiangsu Keygen Biotechnology Co., Ltd., Jiangsu, China) dye at 37 °C for 30 min, followed by washing 3
times with PBS (Fig. S14E). OXYGEN GRADIENT CULTURE To create an oxygen gradient in the chip, the ORC was connected to the liver chip to generate an oxygen gradient in the tissue area.
According to the literature, the dissolved oxygen concentrations for HA and PV were maintained at 3.5 and 1.5 mg/L, respectively48. The device was run for 7 days in an incubator at 37 °C
with 5% CO2 (Fig. S9A). GLUCOSE GRADIENT CULTURE To develop a glucose gradient in the chip, the PV, HA, and CV bottles were loaded with high-glucose DMEM (4.5 g/L, Gibco Life Technologies,
USA), low glucose DMEM (1.0 g/L, Gibco Life Technologies, USA) and low-glucose DMEM (1.0 g/L), respectively. The cell medium in each bottle was supplemented with 10% fetal bovine serum, 1%
penicillin–streptomycin, and 2 mM glutamine. The device was run for 7 days in an incubator at 37 °C with 5% CO2 (Fig. S9B). DRUG TEST Drug testing of the liver chip was demonstrated (Fig.
S9C). The chip was cultured for 6 days. For the microneedle-induced group (_r_ = 150 μm) and the uninduced group, medium mixed with APAP (1 mM in DMEM, as reported in the literature48) was
pumped into the chip through the PV. For the control group, medium mixed with APAP (1 mM in DMEM) was added to the petri dish. After treatment with the drug for 24 h, the CYP activity and
viability of cells in the three groups were tested. STATISTICAL ANALYSIS Data of continuous variables are presented as the means ± standard deviations of triplicate experimental results. _p_
Values were analyzed using a two-tailed Student’s _t_-test or one-way ANOVA. _p_ Values < 0.05 were considered significant. The significance levels are indicated by * for _p_ < 0.05,
** for _p_ < 0.01, *** for _p_ < 0.001, and N.S. for no significance. REFERENCES * Kemas, A. M., Youhanna, S., Zandi Shafagh, R. & Lauschke, V. M. Insulin-dependent glucose
consumption dynamics in 3D primary human liver cultures measured by a sensitive and specific glucose sensor with nanoliter input volume. _FASEB J._ 35, 21305 (2021). Article Google Scholar
* Moradi, E., Jalili-Firoozinezhad, S. & Solati-Hashjin, M. Microfluidic organ-on-a-chip models of human liver tissue. _Acta Biomater._ 116, 67–83 (2020). Article Google Scholar *
Paris, J. & Henderson, N. C. Liver zonation, revisited. _Hepatology_ 76, 1219–1230 (2022). Article Google Scholar * Hong, G. et al. Production of multiple cell-laden microtissue
spheroids with a biomimetic hepatic-lobule-like structure. _Adv. Mater._ 33, 2102624 (2021). Article Google Scholar * Kang, D. et al. Bioprinting of multiscaled hepatic lobules within a
highly vascularized construct. _Small_ 16, 1905505 (2020). Article Google Scholar * Rappaport, A., Borowy, Z., Lougheed, W. & Lotto, W. Subdivision of hexagonal liver lobules into a
structural and functional unit. Role in hepatic physiology and pathology. _Anat. Rec._ 119, 11–33 (1954). Article Google Scholar * Zhang, S., Chen, W. & Zhu, C. in _Artificial Liver_
(ed Lanjuan Li) Ch. 1, 21–47 (Springer, Singapore, 2021). * Sasikumar, S., Chameettachal, S., Kingshott, P., Cromer, B. & Pati, F. 3D hepatic mimics—the need for a multicentric approach.
_Biomed. Mater._ 15, 052002 (2020). Article Google Scholar * Teutsch, H. F. The modular microarchitecture of human liver. _Hepatology_ 42, 317–325 (2005). Article Google Scholar *
Hammad, S. et al. Protocols for staining of bile canalicular and sinusoidal networks of human, mouse and pig livers, three-dimensional reconstruction and quantification of tissue
microarchitecture by image processing and analysis. _Arch. Toxicol._ 88, 1161–1183 (2014). Article Google Scholar * Manco, R. & Itzkovitz, S. Liver zonation. _J. Hepatol._ 74, 466–468
(2021). Article Google Scholar * Ben-Moshe, S. & Itzkovitz, S. Spatial heterogeneity in the mammalian liver. _Nat. Rev. Gastrol. Hepat._ 16, 395–410 (2019). Article Google Scholar *
Halpern, K. B. et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. _Nature_ 542, 352–356 (2017). Article Google Scholar * Nagy, P.,
Thorgeirsson, S. S. & Grisham, J. W. in _The Liver: Biology and Pathobiolog_y (ed Harvey J. Alter, Irwin M. Arias, James L. Boyer, David E. Cohen, David A. Shafritz, Snorri S.
Thorgeirsson, Allan W., Wolkoff) Ch. 1, 1–13 (John Wiley & Sons Ltd., 2020). * Wang, B., Zhao, L., Fish, M., Logan, C. Y. & Nusse, R. Self-renewing diploid Axin2(+) cells fuel
homeostatic renewal of the liver. _Nature_ 524, 180–185 (2015). Article Google Scholar * Wei, Y. L. et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. _Science_ 371,
906 (2021). Article Google Scholar * Nevzorova, Y. A., Boyer-Diaz, Z., Cubero, F. J. & Gracia-Sancho, J. Animal models for liver disease—a practical approach for translational
research. _J. Hepatol._ 73, 423–440 (2020). Article Google Scholar * Delire, B., Starkel, P. & Leclercq, I. Animal models for fibrotic liver diseases: what we have, what we need, and
what is under development. _J. Clin. Transl. Hepatol._ 3, 53–66 (2015). Article Google Scholar * Park, D. Y. et al. One-stop microfiber spinning and fabrication of a fibrous
cell-encapsulated scaffold on a single microfluidic platform. _Biofabrication_ 6, 024108 (2014). Article Google Scholar * Ma, C. et al. On-chip construction of liver lobule-like
microtissue and its application for adverse drug reaction assay. _Anal. Chem._ 88, 1719–1727 (2016). Article Google Scholar * Pamies, D. et al. Good cell culture practice for stem cells
and stem-cell-derived models. _ALTEX_ 34, 95–132 (2017). Google Scholar * Carter, K. et al. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human
mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. _Acta Biomater._ 99, 247–257 (2019). Article Google Scholar * Flaim, C. J., Chien, S. & Bhatia, S. N. An
extracellular matrix microarray for probing cellular differentiation. _Nat. Methods_ 2, 119–125 (2005). Article Google Scholar * No, D. Y., Lee, K. H., Lee, J. & Lee, S. H. 3D liver
models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. _Lab Chip_ 15, 3822–3837 (2015). Article Google Scholar * Deng, J. et al. A cell lines
derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. _Biomicrofluidics_ 13, 024101 (2019). Article Google Scholar * Lee, J. B. et al.
Implantable vascularized liver chip for cross-validation of disease treatment with animal model. _Adv. Funct. Mater._ 29, 1900075 (2019). Article Google Scholar * Zhang, Z. Y., Liu, Z.,
Deng, H. H. & Chen, Q. Effects of acupuncture on vascular dementia (VD) animal models: a systematic review and meta-analysis. _BMC Complement. Altern. M._ 18, 302 (2018). Article Google
Scholar * Ma, H., Xu, H. & Qin, J. Biomimetic tumor microenvironment on a microfluidic platform. _Biomicrofluidics_ 7, 011501 (2013). Article Google Scholar * Xie, R. et al.
Composable microfluidic spinning platforms for facile production of biomimetic perfusable hydrogel microtubes. _Nat. Protoc._ 16, 937–964 (2021). Article Google Scholar * Huh, D.,
Torisawa, Y. S., Hamilton, G. A., Kim, H. J. & Ingber, D. E. Microengineered physiological biomimicry: organs-on-chips. _Lab Chip_ 12, 2156–2164 (2012). Article Google Scholar *
Polidoro, M. A., Ferrari, E., Marzorati, S., Lleo, A. & Rasponi, M. Experimental liver models: from cell culture techniques to microfluidic organs-on-chip. _Liver Int._ 41, 1744–1761
(2021). Article Google Scholar * Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. _Science_ 276, 60–66 (1997). Article Google Scholar * Meng, X. et al. Rebuilding the
vascular network: in vivo and in vitro approaches. _Front. Cell Dev. Biol._ 9, 639299 (2021). Article Google Scholar * Ehrlich, A., Duche, D., Ouedraogo, G. & Nahmias, Y. Challenges
and opportunities in the design of liver-on-chip microdevices. _Annu. Rev. Biomed. Eng._ 21, 219–239 (2019). Article Google Scholar * Du, Y. et al. Mimicking liver sinusoidal structures
and functions using a 3D-configured microfluidic chip. _Lab Chip_ 17, 782–794 (2017). Article Google Scholar * Moya, A. et al. Online oxygen monitoring using integrated inkjet-printed
sensors in a liver-on-a-chip system. _Lab Chip_ 18, 2023–2035 (2018). Article Google Scholar * Jeon, J. W., Lee, S. H., Kim, D. & Sung, J. H. In vitro hepatic steatosis model based on
gut-liver-on-a-chip. _Biotechnol. Progr._ 37, 3121 (2021). Article Google Scholar * Slaughter, V. L. et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical
therapeutic efficacy evaluation. _Sci. Rep._ 11, 13159 (2021). Article Google Scholar * Banaeiyan, A. A. et al. Design and fabrication of a scalable liver-lobule-on-a-chip
microphysiological platform. _Biofabrication_ 9, 015014 (2017). Article Google Scholar * Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. _Nat. Biotechnol._ 32, 760–772
(2014). Article Google Scholar * Enrico, A. et al. 3D microvascularized tissue models by laser-based cavitation molding of collagen. _Adv. Mater._ 34, 2109823 (2022). Article Google
Scholar * Ahadian, S. et al. Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. _Adv. Healthc. Mater._ 7, 1700506 (2018). Article Google
Scholar * Kim, E., Takeuchi, M. & Fukuda, T. in _Field-Driven Micro and Nanorobots for Biology and Medicine_ (eds Yu Sun, Xian Wang, & Jiangfan Yu) Ch. 12, 285–304 (Springer
International Publishing, 2022). * Placone, J. K. & Engler, A. J. Recent advances in extrusion-based 3D printing for biomedical applications. _Adv. Healthc. Mater._ 7, 1701161 (2018).
Article Google Scholar * Shin, Y. et al. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. _Lab Chip_ 11, 2175–2181 (2011). Article Google Scholar
* Rouwkema, J. & Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. _Trends Biotechnol._ 34, 733–745 (2016). Article Google
Scholar * Spiller, K. L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds.
_Biomaterials_ 37, 194–207 (2015). Article Google Scholar * Ya, S. et al. On-chip construction of liver lobules with self-assembled perfusable hepatic sinusoid networks. _ACS Appl. Mater.
Inter._ 13, 32640–32652 (2021). Article Google Scholar * Du, K. et al. Modeling nonalcoholic fatty liver disease on a liver lobule chip with dual blood supply. _Acta Biomater._ 134,
228–239 (2021). Article Google Scholar * Zhang, P., Li, X., Chen, J. Y. & Abate, A. Controlled fabrication of functional liver spheroids with microfluidic flow cytometric printing.
_Biofabrication_ 14, 045011 (2022). Article Google Scholar * Lee, S. A. et al. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions
and flow effects. _Lab Chip_ 13, 3529–3537 (2013). Article Google Scholar * Dash, A. et al. Hemodynamic flow improves rat hepatocyte morphology, function, and metabolic activity in vitro.
_Am. J. Physiol._ 304, 1053–1063 (2013). Article Google Scholar * Ortega-Prieto, A. et al. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus
infection. _Nat. Commun._ 9, 682 (2018). Article Google Scholar * Prodanov, L. et al. Long‐term maintenance of a microfluidic 3D human liver sinusoid. _Biotechnol. Bioeng._ 113, 241–246
(2016). Article Google Scholar * Liu, J. et al. Large-scale high-density culture of hepatocytes in a liver microsystem with mimicked sinusoid blood flow. _J. Tissue Eng. Regen. M._ 12,
2266–2276 (2018). Article Google Scholar * Kietzmann, T. Metabolic zonation of the liver: the oxygen gradient revisited. _Redox Biol._ 11, 622–630 (2017). Article Google Scholar * Mu,
X., Zheng, W. F., Xiao, L., Zhang, W. & Jiang, X. Y. Engineering a 3D vascular network in hydrogel for mimicking a nephron. _Lab Chip_ 13, 1612–1618 (2013). Article Google Scholar *
Adriani, G., Ma, D., Pavesi, A., Kamm, R. D. & Goh, E. L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier.
_Lab Chip_ 17, 448–459 (2017). Article Google Scholar * Wang, X. et al. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. _Lab
Chip_ 16, 282–290 (2016). Article Google Scholar * Shih, M. C., Tseng, S. H., Weng, Y. S., Chu, I. M. & Liu, C. H. A microfluidic device mimicking acinar concentration gradients across
the liver acinus. _Biomed. Microdevices_ 15, 767–780 (2013). Article Google Scholar * Ding, W. et al. Simulation of blood and oxygen distributions in a hepatic lobule with sinusoids
obstructed by cancer cells. _J. Theor. Biol._ 446, 229–237 (2018). Article MathSciNet Google Scholar * Lee-Montiel, F. T. et al. Control of oxygen tension recapitulates zone-specific
functions in human liver microphysiology systems. _Exp. Biol. Med._ 242, 1617–1632 (2017). Article Google Scholar * Bhushan, A. et al. Towards a three-dimensional microfluidic liver
platform for predicting drug efficacy and toxicity in humans. _Stem Cell Res. Ther._ 4, S16 (2013). Article Google Scholar * Ho, H. & Zhang, E. Virtual lobule models are the key for
multiscale biomechanical and pharmacological modeling for the liver. _Front. Physiol._ 11, 1061 (2020). Article Google Scholar * Sato, A., Kadokura, K., Uchida, H. & Tsukada, K. An in
vitro hepatic zonation model with a continuous oxygen gradient in a microdevice. _Biochem. Biophys. Res. Commun._ 453, 767–771 (2014). Article Google Scholar * Hijmans, B. S., Grefhorst,
A., Oosterveer, M. H. & Groen, A. K. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. _Biochimie_ 96, 121–129 (2014). Article Google
Scholar * Berndt, N. & Holzhutter, H. G. Dynamic metabolic zonation of the hepatic glucose metabolism is accomplished by sinusoidal plasma gradients of nutrients and hormones. _Front.
Physiol._ 9, 1786 (2018). Article Google Scholar * Delalat, B. et al. Microengineered bioartificial liver chip for drug toxicity screening. _Adv. Funct. Mater._ 28, 1801825 (2018). Article
Google Scholar * Athersuch, T. J. et al. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: a perspective. _Toxicol. Res._ 7, 347–357 (2018). Article
Google Scholar * Ho, C. T. et al. Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. _Lab Chip_ 13, 3578–3587 (2013). Article Google Scholar * Horowitz, L.
F., Rodriguez, A. D., Ray, T. & Folch, A. Microfluidics for interrogating live intact tissues. _Microsyst. Nanoeng._ 6, 69 (2020). Article Google Scholar * Albillos, A., de Gottardi,
A. & Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. _J. Hepatol._ 72, 558–577 (2020). Article Google Scholar * Hassan, S. et al.
Liver-on-a-chip models of fatty liver disease. _Hepatology_ 71, 733–740 (2020). Article Google Scholar * Pulitano, C. et al. Postreperfusion microcirculatory derangements after liver
transplantation: relationship to hemodynamics, serum mediators, and outcome. _Liver Transpl._ 23, 527–536 (2017). Article Google Scholar * Yang, H. et al. Three-dimensional bioprinted
hepatorganoids prolong survival of mice with liver failure. _Gut_ 70, 567–574 (2021). Article Google Scholar * Kubota, N., Kubota, T. & Kadowaki, T. Midlobular zone 2 hepatocytes: a
gatekeeper of liver homeostasis. _Cell Metab._ 33, 855–856 (2021). Article Google Scholar * Gough, A. et al. Human biomimetic liver microphysiology systems in drug development and
precision medicine. _Nat. Rev. Gastrol. Hepat._ 18, 252–268 (2021). Article Google Scholar * Malinen, M. M. et al. Differentiation of liver progenitor cell line to functional organotypic
cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. _Biomaterials_ 35, 5110–5121 (2014). Article Google Scholar * Yue, K. et al. Synthesis, properties, and biomedical
applications of gelatin methacryloyl (GelMA) hydrogels. _Biomaterials_ 73, 254–271 (2015). Article Google Scholar * Liu, H. et al. Advances in hydrogels in organoids and organs-on-a-chip.
_Adv. Mater._ 31, 1902042 (2019). Article Google Scholar * Debbaut, C. et al. Perfusion characteristics of the human hepatic microcirculation based on three-dimensional reconstructions and
computational fluid dynamic analysis. _J. Biomech. Eng._ 134, 011003 (2012). Article Google Scholar * Pepelanova, I., Kruppa, K., Scheper, T. & Lavrentieva, A. Gelatin-Methacryloyl
(GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. _Bioengineering_ 5, 55 (2018). Article Google Scholar *
Bertassoni, L. E. et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. _Lab Chip_ 14, 2202–2211 (2014). Article Google Scholar * Mohamed,
M. G. A. et al. An integrated microfluidic flow-focusing platform for on-chip fabrication and filtration of cell-laden microgels. _Lab Chip_ 19, 1621–1632 (2019). Article Google Scholar *
Zheng, X. et al. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. _Nat. Commun._ 6, 5834 (2015). Article Google Scholar * Weng, Y. S., Chang, S. F., Shih, M.
C., Tseng, S. H. & Lai, C. H. Scaffold‐free liver‐on‐a‐chip with multiscale organotypic cultures. _Adv. Mater._ 29, 1701545 (2017). Article Google Scholar * Leite, S. B. et al. Novel
human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. _Biomaterials_ 78, 1–10 (2016). Article Google Scholar * Ehrlich, A. et al. Microphysiological flux
balance platform unravels the dynamics of drug induced steatosis. _Lab Chip_ 18, 2510–2522 (2018). Article Google Scholar * Jungermann, K. & Kietzmann, T. Oxygen: modulator of
metabolic zonation and disease of the liver. _Hepatology_ 31, 255–260 (2000). Article Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge the funding support from the
National Natural Science Foundation of China (82072018, 81571768), the Anhui Provincial Natural Science Foundation (2208085QH256), the Strategic Priority Research Program (C) of the CAS
(XDC07040200), the China Postdoctoral Science Foundation (2022M713055), the National Key Research and Development Program of China (2018AAA0100300), and the Fundamental Research Funds for
Central Universities (WK2480000006, WK9100000001, WK9110000125). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Electronic Engineering and Information Science, University of
Science and Technology of China, Hefei, Anhui, 230027, China Shibo Li, Chengpan Li & Zhengdi Shi * Department of Oncology, the First Affiliated Hospital of USTC, Division of Life
Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230001, China Shibo Li & Weiping Ding * Center for Biomedical Imaging, University of Science and
Technology of China, Hefei, Anhui, 230027, China Muhammad Imran Khan & Bensheng Qiu * School of Biology, Food and Environment, Hefei University, Hefei, Anhui, 230601, China Jing Liu *
Department of Mechanical Engineering, University of Washington, Seattle, WA, 98195, USA Dayong Gao Authors * Shibo Li View author publications You can also search for this author inPubMed
Google Scholar * Chengpan Li View author publications You can also search for this author inPubMed Google Scholar * Muhammad Imran Khan View author publications You can also search for this
author inPubMed Google Scholar * Jing Liu View author publications You can also search for this author inPubMed Google Scholar * Zhengdi Shi View author publications You can also search for
this author inPubMed Google Scholar * Dayong Gao View author publications You can also search for this author inPubMed Google Scholar * Bensheng Qiu View author publications You can also
search for this author inPubMed Google Scholar * Weiping Ding View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.L. performed the
experiments, analyzed the data, and wrote the paper. C.L. assisted with data collection and analysis and edited the paper. M.I.K. provided suggestions for editing the paper. J.L. assisted
with the experimental design. Z.S. assisted with data collection. B.Q. and D.G. supervised the study. W.D. conceived the study, designed the experiments, and edited the paper. All authors
discussed the results and approved the submission. CORRESPONDING AUTHOR Correspondence to Weiping Ding. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, S., Li, C., Khan, M.I. _et al._ Microneedle array facilitates hepatic sinusoid construction in a large-scale liver-acinus-chip
microsystem. _Microsyst Nanoeng_ 9, 75 (2023). https://doi.org/10.1038/s41378-023-00544-w Download citation * Received: 05 October 2022 * Revised: 03 February 2023 * Accepted: 24 February
2023 * Published: 07 June 2023 * DOI: https://doi.org/10.1038/s41378-023-00544-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative