Play all audios:
ABSTRACT An ethnoracial minority density (EMD) effect in studies of psychotic spectrum disorders has been observed, whereby the risk of psychosis in ethnoracial minority group individuals is
inversely related to the proportion of minorities in their area of residence. The authors investigated the relationships among area-level EMD during childhood, cortical thickness (CT), and
social engagement (SE) in clinical high risk for psychosis (CHR-P) youth. Data were collected as part of the North American Prodrome Longitudinal Study. Participants included 244 ethnoracial
minoritized (predominantly Hispanic, Asian and Black) CHR-P youth and ethnoracial minoritized healthy controls. Among youth at CHR-P (_n_ = 164), lower levels of EMD during childhood were
associated with reduced CT in the right fusiform gyrus (adjusted β = 0.54; 95% CI 0.17 to 0.91) and right insula (adjusted β = 0.40; 95% CI 0.05 to 0.74). The associations between EMD and CT
were significantly moderated by SE: among youth with lower SE (SE at or below the median, _n_ = 122), lower levels of EMD were significantly associated with reduced right fusiform gyrus CT
(adjusted β = 0.72; 95% CI 0.29 to 1.14) and reduced right insula CT (adjusted β = 0.57; 95% CI 0.18 to 0.97). However, among those with greater SE (_n_ = 42), the associations between EMD
and right insula and fusiform gyrus CT were not significant. We found evidence that lower levels of ethnic density during childhood were associated with reduced cortical thickness in
regional brain regions, but this association may be buffered by greater levels of social engagement. You have full access to this article via your institution. Download PDF SIMILAR CONTENT
BEING VIEWED BY OTHERS POPULATION-LEVEL NORMATIVE MODELS REVEAL RACE- AND SOCIOECONOMIC-RELATED VARIABILITY IN CORTICAL THICKNESS OF THREAT NEUROCIRCUITRY Article Open access 19 June 2024
NEIGHBORHOOD SOCIAL FRAGMENTATION IN RELATION TO IMPAIRED MISMATCH NEGATIVITY AMONG YOUTH AT CLINICAL HIGH RISK FOR PSYCHOSIS AND HEALTHY COMPARISONS Article 02 April 2025 SOCIOECONOMIC
FACTORS, BRAIN-DERIVED NEUROTROPHIC FACTOR VAL66MET POLYMORPHISM, AND CORTICAL STRUCTURE IN CHILDREN AND ADOLESCENTS Article Open access 29 May 2025 INTRODUCTION The relationship between
adverse experiences and brain morphology has been documented in numerous studies in the past decades [1,2,3]. This relationship has been demonstrated in numerous experimental studies with
animals [4,5,6,7] and, more recently, similar findings have been reported in studies of humans [5, 8, 9]. Exposure to stress appears to have a cumulative effect on gray matter morphology in
humans, such that increased stress is linked with reduced thickness and volume in cortical regions. This has recently been demonstrated in youth at clinical high risk for psychosis (CHR-P),
as well as healthy controls [10]. In fact, there is growing evidence that different types of stressors (e.g., threat versus deprivation) during childhood may impact distinct regions of the
brain [11, 12]. While the relationship between early adversity and psychopathology is well established [13], the underlying biological mechanisms (e.g., changes in brain structure) that link
early adversity, protective factors, and cortical thickness among ethnoracial minority youth at CHR-P have yet to be examined. It has long been known that ethnoracial minority individuals
in the U.S. and Europe experience greater stressful life events compared to white people [14, 15]. Greater life event stress may partially explain the higher rates of certain mental
illnesses, including psychosis, among ethnoracial minorities, especially those who reside in predominantly white communities [16, 17]. It has been shown that ethnoracial minorities who live
in neighborhoods with greater proportions of other ethnoracial minorities, referred to as ethnoracial minority density (EMD), have a reduced risk of developing psychopathology, including
psychotic disorders [18, 19]. These findings may be explained by lower exposure to perceived discrimination and other stressors as well as greater engagement in social networks [17,
20,21,22]. In our previous study, we found that among ethnoracial minority youth at CHR-P, higher levels of EMD during childhood predicted lower perceived discrimination at baseline, which
in turn predicted remission of psychosis-risk symptoms within two-year follow-up [23]. It is possible that the inverse relationship between childhood EMD and psychosis-risk may be partially
explained by discrimination related to ethnoracial minority status and other adverse social experiences, which in turn impacts select cortical regions [24, 25]. The Stimulation Discrepancy
Deprivation (SDD) model of psychosis categorizes different types of structural exposures along with their proposed influences on different neural systems [26]. The SDD model proposes that
EMD falls under “social discrepancy exposure” such that chronic exposure to lower levels of EMD and greater discrepancy from peers in culture and appearance may increase feelings of being
‘different’ for ethnoracial minority children, as they develop and become aware of social cues and social hierarchy in respect to their minority status [27]. Their enhanced ethnoracial
minority status in context of living in areas with fewer people who also share a minority status may predispose them to greater perceived discrimination, lack of social cohesion, lower sense
of belonging and support, and less robust social networks [27]. The SDD model posits these social stressors and circumstances may impact certain “social brain” regions from the
oxytocinergic system, which has been linked to social attachment, empathy, and social threat [26, 28, 29]. Studies have found intranasal delivery of oxytocin to modulate the evaluation of
socially relevant faces through the fusiform gyrus [30, 31]. Administration of intranasal oxytocin has also been shown to decrease activity in the anterior insula in response to social
versus nonsocial positive stimuli compared with placebo treatment [32]. Other neural regions that have been shown to be impacted by oxytocin in The SDD model include the orbitofrontal cortex
[33], anterior cingulate cortex [34], and posterior cingulate cortex [26, 35]. These brain regions also process social norm compliance [36], conflict monitoring [37], and social connection
[26, 38]. Interestingly, loss of gray matter in these regions has also been shown to be associated with conversion to psychosis, although results have been mixed across studies
[39,40,41,42,43,44,45,46,47,48]. In sum, exposure to lower levels of childhood EMD may predispose ethnoracial minority youth to experience greater social stress [25, 49], which may in turn
lead to gray matter loss of social brain regions [4]. In addition, recent evidence suggests that engaging in desirable social activities may buffer the deleterious effects of early social
adversity on gray matter volume [50]. Therefore, it is possible that social engagement also moderates the association between lower levels of EMD and cortical thickness among ethnoracial
minority youth. To our knowledge, no study has explored the relationships among childhood EMD, cortical thickness, and social engagement. The present study examines these relations in
samples of ethnoracial minoritized CHR-P and healthy participants, with EMD indexed as the proportion of residents who did not identify as white non-Hispanics in the area. We predict the
following: (1) Lower levels of childhood EMD will specifically be associated with reduced cortical thicknesses of the “social brain” regions as suggested from The SDD Model, which include
the fusiform gyrus, insula, rostral anterior cingulate, lateral orbitofrontal, medial orbitofrontal, and posterior cingulate at baseline. We also predict that there will be greater effects
on the right as compared to the left hemisphere based on prior evidence from animal models and human studies suggesting that early-life environmental stressors appear to have a greater
impact on brain morphometry in the right hemisphere [51,52,53,54]. (2) Social engagement will moderate the relationships between EMD and selected brain regions. Specifically, it is
hypothesized that social engagement will reduce the adverse impacts of lower levels of EMD on cortical thickness among ethnoracial minorities. In addition to testing the above hypotheses, we
will test the potential moderating effects of demographic and clinical characteristics including age, sex, and CHR-P status. METHODS AND MATERIALS PARTICIPANTS Baseline data were collected
as part of the North American Prodrome Longitudinal Study-Phase 2 (NAPLS2), a multi-site consortium that recruited help-seeking youth at CHR-P and healthy controls. CHR-P individuals met the
Criteria of Prodromal Syndromes, which is based on the Structured Interview for Psychosis Risk Syndromes (SIPS) [55]. The aims and recruitment methods have been described in prior
literature [56, 57]. The participants in the present sample include those who identified with a non-White ethnoracial or Hispanic group, had complete data on childhood residence
(cities/towns) in the USA suitable for geocoding, and whose structural magnetic resonance imaging (MRI) data passed quality assurance metrics. Table 1 lists characteristics of the sample and
a comparison of these characteristics between participants included and those excluded due to missing data is shown in Table S1. The Institutional Review Boards of each study site reviewed
and approved the study protocol and consent forms, and procedures complied with the ethical standards of relevant committees. INSTRUMENTS Demographic and clinical variables were obtained
from self-report and interview-based measures during baseline assessments and included age, sex, race and ethnicity, family history of mental illnesses, the towns where participants spent
the longest time during childhood, and social engagement. Family history of mental illnesses included bipolar disorder, depression, psychosis, and schizophrenia among first- or second-degree
relatives [58, 59]. AREA-LEVEL VARIABLES Towns where individuals lived for the longest time during childhood were linked to the primary county 5-digit Federal Information Processing
Standards (FIPS) codes [60]. Then, 1990 county-level characteristics were linked to these FIPS codes for those born between 1985 and 1994, whereas census data from 2000 were used for
individuals born between 1995 and 2000 [61]. Censuses from these two time periods were chosen to capture the area (county) characteristics during childhood for each participant. _Area-level
EMD_ was defined as percentage of all residents who did not self-identify their race/ethnicity as White non-Hispanic as done in prior literature [19, 62]. Other definitions of ethnic or
group density (including the proportion of others belonging to the same ethnoracial group) were not used in this study due to missing area-level characteristics regarding specific
ethnoracial minority groups, including those who identify as Interracial, for example. _Area-level Socioeconomic Deprivation_ was defined as the average z-score of the percentage of
residents living below the poverty level, percentage of residents who did not complete high school and/or obtain GED, percentage of residents in the civilian labor force who were unemployed,
and percentage of owner-occupied housing units (reversed) as done in prior literature [63, 64]. SOCIAL ENGAGEMENT Social engagement was calculated using the total count of five items from
the Desirable Social Activities subscale of Life Event Stress (LES) scale as used in prior studies [50, 65, 66] and this score ranges from zero to five. The LES scale was assessed at
baseline regarding events that have occurred in the participant’s life. These items include: (1) Involvement in church or synagogue, club, neighborhood, or other organization; (2) Took a
vacation; (3) Took up a new hobby, sport, craft, or recreational activity; (4) Acquired a pet; and (5) Made new friends. IMAGING DATA ACQUISITION AND PROCESSING Cortical thickness of all
brain regions was obtained at baseline for this study. Five sites used Siemens scanners, which used 12-channel head coils, and three sites used GE scanners, which used 8-channel head coils.
All scanners had a magnetic field strength of 3-Tesla. Sequence parameters were optimized for each scanner manufacturer, software version, and coil configuration according to the ADNI
protocol [67]. To extract regional parcels for cortical thicknesses, automated surface-based cortical reconstruction, cortical parcellation, and subcortical segmentation were carried out
using the Freesurfer software suite version 5.3 [68, 69]. Regional parcels for cortical thicknesses were extracted using a gyral and sulcal pattern-based Desikan atlas with 34 parcels in
each hemisphere [70]. DATA ANALYSIS A priori 12 cortical ROIs were derived from prior literature on The SDD Model [26] and included the fusiform gyrus, insula, lateral orbitofrontal cortex,
medial orbitofrontal cortex, posterior cingulate cortex, rostral anterior cingulate cortex of the right and left hemispheres. For Aim 1, we first calculated the Pearson’s correlations
between EMD and cortical thicknesses of these 12 ROIs, adjusting for multiple comparisons using the False Discovery Rate (FDR). In this study, the outcomes were cortical brain ROIs, and we
did not include subcortical structures that may have been relevant (e.g., amygdala). However, we did assess for possible structural effects outside the pre-hypothesized anatomical regions,
and they were significant if they survived multiple comparisons correction across the whole brain. Then, each significant association between EMD and ROI was tested using a generalized
linear mixed (GLM) model with the inclusion of covariates. Fixed factors included age, sex, CHR-P status, family history of mental illnesses, and area-level deprivation. Random factors
included unique counties and sites. Variance inflation factors of less than or equal to five was used to rule out multicollinearity in these models. We tested whether the association between
EMD and each ROI may differ based upon age, sex, and CHR-P status. Each significant association between EMD and ROI in the adjusted multi-level model was further tested with their
respective interaction terms. For each significant interaction term, we analyzed the relationships between EMD and cortical thicknesses by subgroups. For Aim 2, we assessed social engagement
as a potential moderator. Each significant association between EMD and ROI in the adjusted multi-level model was further tested with the interaction term, EMD-by-social engagement. For each
significant interaction term, we conducted a secondary analysis to further analyze the significant interactions observed. We stratified the sample into subgroups of participants based upon
the median value of the total social engagement score and assessed the association between EMD and ROI. R version 4.2.1 was used for statistical analyses. RESULTS SAMPLE CHARACTERISTICS This
study (_N_ = 244; mean (SD) age, 18.2 (3.4) years) included 139 males (57.0%). There were 164 (67.2%) youth at CHR-P. Other participant sociodemographic information is presented in Table 1.
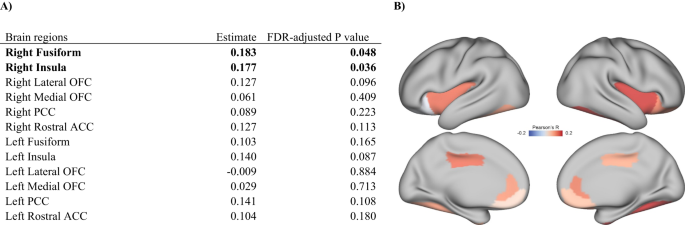
Childhood EMD was positively correlated with cortical thicknesses of the right fusiform gyrus (Pearson’s r = 0.183; FDR-adjusted _p_ < 0.05) and right insula (Pearson’s r = 0.177;
FDR-adjusted _p_ < 0.05) (Fig. 1). Childhood EMD was not significantly associated with cortical thicknesses of other brain regions (Table S2). GENERALIZED LINEAR MIXED MODELS PREDICTING
CORTICAL THICKNESSES OF FUSIFORM GYRUS AND INSULA In the main analysis, lower levels of childhood EMD were associated with reduced cortical thickness of the right fusiform gyrus (adjusted β
= 0.31; 95% CI: 0.02 to 0.59; _p_ = 0.041) and the right insula (adjusted β = 0.30; 95% CI = 0.06 to 0.53; _p_ = 0.019). To test whether these associations would differ by age, sex, and
CHR-P status, six additional GLM models were constructed assessing each interaction term with childhood EMD for the right fusiform gyrus and right insula (Table S3). Only the interaction
term, CHR-P status-by-childhood EMD was significantly associated with right fusiform gyrus cortical thickness (adjusted β = 0.25; 95% CI = 0.01 to 0.50; _p_ = 0.040), suggesting that there
may be differences in the association between childhood EMD and cortical thickness between healthy youth and those at CHR-P. Stratifying this association by healthy youth and those at CHR-P
demonstrated that the associations between childhood EMD and right fusiform gyrus (adjusted β = 0.54; 95% CI = 0.17 to 0.91; _p_ = 0.006) and right insula (adjusted β = 0.40; 95% CI = 0.05
to 0.74; _p_ = 0.028) were only significant among youth at CHR-P, but not among healthy youth (Table 2). MODERATING EFFECT OF SOCIAL ENGAGEMENT Among ethnoracial minority youth at CHR-P, the
interaction term EMD-by-social engagement was significantly associated with cortical thickness of the right fusiform gyrus (adjusted β = −0.15; 95% CI = −0.26 to −0.04; _p_ = 0.010) and
right insula (adjusted β = −0.14; 95% CI = −0.26 to −0.03; _p_ = 0.018). To further analyze the significant interactions observed, we stratified the sample into two subgroups of
participants, those whose total social engagement score was at or below (_n_ = 122) and above (_n_ = 42) four, the median social engagement score. Among those with lower social engagement,
lower levels of EMD were significantly associated with reduced cortical thickness of the right fusiform gyrus (adjusted β = 0.72; 95% CI = 0.29 to 1.14; _p_ = 0.002) and right insula
(adjusted β = 0.57; 95% CI = 0.18 to 0.97; _p_ = 0.007). Conversely, among those with higher social engagement, EMD was not significantly associated with cortical thickness of the right
fusiform gyrus (adjusted β = 0.03; 95% CI = −0.61 to 0.67; _p_ = 0.921) or right insula (adjusted β = 0.29; 95% CI = −0.85 to 0.29; _p_ = 0.338) (Table 3). Best-fit lines of the associations
between EMD and cortical thicknesses of the right fusiform gyrus and right insula for each subgroup is shown in Fig. 2. DISCUSSION The current study builds upon prior studies on ethnic
density effect in psychosis and finds neurobiological correlates of exposure to EMD during childhood. In addition, we showed that the associations between childhood EMD and right insula and
fusiform gyrus cortical thicknesses were significant among minority youth at CHR-P and not among those who are healthy, although it is possible that we could not detect significant
associations between EMD and cortical thicknesses due to the relatively small sample size of healthy participants. Among youth at CHR-P, we found that social engagement moderated the
association between childhood EMD and right insula and right fusiform gyrus cortical thicknesses. Those who are more socially engaged already have consistently high cortical thickness
regardless of levels of EMD. However, for those who are less socially engaged, levels of EMD are correlated with cortical thickness and this subgroup may benefit from social support and
greater levels of EMD during childhood. This highlights the importance of not only social engagement, but also of having those protective connectedness cultural factors during childhood in
the event there is less social engagement in a person’s life trajectory. It is possible that ethnoracial minority youths growing up in communities with greater number of people who share
similar minority status may feel less of a “minority” in their local communities [71] and therefore, they may experience less perceived discrimination [72]. Conversely, for minority youths
who grew up in communities with fewer people who share similar minority status, their minority status may be accentuated, which may impact their ability to “fit in” to social groups [71].
Perhaps, this chronic experience of being an “outsider” or othered may abnormally activate the fusiform gyrus [72] and insula [73, 74], as abnormal activation has been shown to be associated
with cortical thinning over time [75]. In accordance with our prediction, we found that EMD exposure during childhood was associated with cortical thickness in regions of the right
hemisphere. Prior literature suggests that greater plasticity during younger age would lead to early recruitment of the right hemisphere [54, 76, 77]. Perhaps, early exposure to the social
environment may result in greater changes to the gray matter of certain social brain regions in the right hemisphere. The fusiform gyrus is particularly sensitive to human faces [31, 78,
79]. Studies have shown that this area of the brain is activated during social categorization tasks. In these experiments, participants are asked to distinguish between different social
groups, such as males and females, or people of different races [80,81,82,83,84,85]. Other studies have found that the fusiform gyrus is more active when participants have to update their
own or others' social status [86, 87]. The frequent activation of the fusiform gyrus during explicit social categorization may indicate its role in modulating social perception from the
top down. It is possible that the initial role of the fusiform gyrus in discriminating between social categories and identifying significant social cues based on pre-existing expectations
and attention to social context may be relevant to the processing of socially relevant environmental exposures. The right insula has been shown to play a role in distinguishing between
in-group and out-group membership, which may arise from its role in connecting the mirror neuron system and the limbic system [88,89,90,91]. This brain region has also been specifically
shown to demonstrate strong outgroup bias. Numerous studies have found that the insula exhibits greater activation in response to various social categories and tasks. These include racial
and political outgroups [92,93,94,95,96], unfamiliar faces [97], dominant faces [98], and the ability to discriminate against minimal outgroups [86]. It is possible that hyperactivation of
the fusiform gyrus and insula from heightened social threat of being an ethnoracial minority may lead to dendritic shrinkage and progressive cortical gray matter reduction of these brain
regions over time [99], which have been shown to be associated with conversion to psychosis [43, 100, 101]. However, greater social engagement may moderate this effect by helping to minimize
the perception of being in an out-group. Through engaging with other people in the community (i.e., making new friends, being part of a community organization or church), people of
different backgrounds can come together, which might attenuate some of the in-group/out-group differences based on other characteristics and partially mitigate the psychological and
neurobiological impact of ethnoracial minority status [24, 102]. An alternative interpretation of the significant moderation of social engagement on the association between EMD and cortical
thickness is that social engagement may be differentiating those who have a more severe illness. Since one of the early premorbid changes and indicators of psychotic disorders is impaired
social functioning, it may be possible that the association between lower EMD and reduced cortical thickness may be more evident among the group of CHR-P who are more ill. Future research
should explore whether social engagement and its interaction with EMD predicts conversion to psychosis. This study has several limitations. First, this study has a modest sample size and has
a cohort design. Prospective longitudinal studies with large sample size are warranted to explore the causal directionality of EMD, cortical thickness, and social engagement as well as
sensitive developmental exposure time periods in which EMD and social engagement might have their greatest impact on cortical thickness. Larger studies that include more ethnoracial
minorities may also allow for the investigation of ethnoracial differences in the relationship between EMD and cortical thickness as well as investigating the relationship between area-level
own group ethnic density and cortical thickness. It is important to note that in our study, EMD refers to the proportion of ethnoracial minorities and not the proportion of those who belong
to the same ethnoracial group. Nevertheless, it is possible that social support from one’s own as well as other ethnoracial minority groups may impact cortical thickness. Second, this study
identified a priori cortical brain regions based on the SDD model. However, it is possible that other subcortical brain regions (i.e. hippocampus and amygdala) may be impacted by social
stress via the HPA axis [50]. Future research should examine the potential impact of EMD on other subcortical brain areas. Third, this study did not identify which part of the insula or
fusiform gyrus may have been impacted by EMD. Future studies should investigate the relationship of EMD and specific sub-regions (e.g., anterior and posterior regions). Fourth, the
assessment of childhood exposures at the county level was limited by availability of data provided. It is possible exposure to ethnic density may be more important at home versus at school,
for example [103]. The non-specificities of both exposure and outcome measures may explain the modest effect sizes of the correlation between EMD and cortical thicknesses. For example,
ethnic density was measured at the county level (as opposed to the more granular neighborhood or school levels) and cortical thickness of insula includes both the anterior and posterior
insula. Follow-up studies assessing more specific area-level measurements of EMD and sub-regions of the brain are warranted. Lastly, the measurement of social engagement used in this study
was limited, and it is still unclear which components of participant social engagement and what period (e.g., childhood or adolescence) might be most important in buffering the deleterious
effects of adverse social environment on cortical thickness. CONCLUSIONS This study provides novel evidence for the associations between childhood EMD and right fusiform gyrus and right
insula cortical thickness, with more prominent effects among minority youth at CHR-P. Furthermore, social engagement moderates this relationship as those with greater social engagement
already seem to exhibit greater cortical thickness regardless of levels of ethnic density. These findings suggest there are aspects of the social environment that may have protective effects
on neurobiological markers of conversion to psychosis. Further research is needed to better understand mechanisms linking environment, brain, and behavior. REFERENCES * Belleau EL, Treadway
MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53. Article PubMed Google
Scholar * Sanacora G, Yan Z, Popoli M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:86–103. Article CAS
PubMed Google Scholar * Lenart-Bugla M, Szcześniak D, Bugla B, et al. The association between allostatic load and brain: a systematic review. Psychoneuroendocrinology. 2022;145:105917.
Article PubMed Google Scholar * Alleva E, Santucci D. Psychosocial vs. “physical” stress situations in rodents and humans. Physiol Behav. 2001;73:313–20. Article CAS PubMed Google
Scholar * Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. Article CAS PubMed Google Scholar *
Hodges T, McCormick C. Stress and social development in adolescence in a rodent model, in Routledge International Handbook of Social Neuroendocrinology. London, Routledge: Taylor &
Francis Group; 2018. p. 479–503. Google Scholar * Patel D, Kas MJ, Chattarji S, et al. Rodent models of social stress and neuronal plasticity: relevance to depressive-like disorders.
Behavioural Brain Research. 2019;369:111900. Article PubMed Google Scholar * Ulmer Yaniv A, Salomon R, Waidergoren S, et al. Synchronous caregiving from birth to adulthood tunes humans’
social brain. Proc Natl Acad Sci USA. 2021;118:e2012900118. Article PubMed PubMed Central Google Scholar * Shaw GA, Dupree JL, Neigh GN: Adolescent maturation of the prefrontal cortex:
Role of stress and sex in shaping adult risk for compromise [Internet]. Genes, Brain and Behavior 2020; 19 [cited 2023 Apr 5] Available from: https://doi.org/10.1111/gbb.12626. * Aberizk K,
Collins MA, Addington J, et al. Life event stress and reduced cortical thickness in youth at clinical high risk for psychosis and healthy control subjects. Biol Psychiatry Cogn Neurosci
Neuroimaging. 2022;7:171–9. PubMed Google Scholar * LoPilato AM, Goines K, Addington J, et al. Impact of childhood adversity on corticolimbic volumes in youth at clinical high-risk for
psychosis. Schizophr Res. 2019;213:48–55. Article PubMed Google Scholar * Machlin L, Egger HL, Stein CR, et al. Distinct associations of deprivation and threat with alterations in brain
structure in early childhood. J Am Acad Child Adolesc Psychiatry. 2023;S0890-8567(23):00061–8. Google Scholar * Copeland WE, Keeler G, Angold A, et al. Traumatic events and posttraumatic
stress in childhood. Arch Gen Psychiatry. 2007;64:577–84. Article PubMed Google Scholar * Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by
race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol. 2007;40:313–32. Article PubMed Google Scholar * Muscatell KA, Alvarez GM, Bonar AS, et al.
Brain–body pathways linking racism and health. American Psychologist. 2022;77:1049–60. Article PubMed Google Scholar * Faris REL, Dunham HW. Mental disorders in urban areas: an ecological
study of schizophrenia and other psychoses. Oxford, England: Univ. Chicago Press; 1939. Google Scholar * Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other
psychoses in England, 1950-2009: a systematic review and meta-analyses. PLoS One. 2012;7:e31660. Article CAS PubMed PubMed Central Google Scholar * Bécares L, Dewey ME, Das-Munshi J.
Ethnic density effects for adult mental health: systematic review and meta-analysis of international studies. Psychol Med. 2018;48:2054–72. Article PubMed Google Scholar * Baker SJ,
Jackson M, Jongsma H, et al. The ethnic density effect in psychosis: a systematic review and multilevel meta-analysis. Br J Psychiatry. 2021;219:632–43. Article PubMed PubMed Central
Google Scholar * Veling W. Ethnic minority position and risk for psychotic disorders. Curr Opin Psychiatry. 2013;26:166–71. Article PubMed Google Scholar * Crush E, Arseneault L, Jaffee
SR, et al. Protective factors for psychotic symptoms among poly-victimized children. Schizophrenia Bulletin. 2018;44:691–700. Article PubMed Google Scholar * Anglin DM, Ereshefsky S,
Klaunig MJ, et al. From womb to neighborhood: a racial analysis of social determinants of psychosis in the United States. AJP. 2021;178:599–10. Article Google Scholar * Anglin DM, Espinosa
A, Addington J, et al. Childhood Area-Level Ethnic Density in the U.S. and Psychosis Risk among Ethnoracial Minoritized Individuals in the NAPLS2 Cohort. JAMA Psychiatry. 2023; In press. *
Bécares L, Nazroo J, Stafford M. The buffering effects of ethnic density on experienced racism and health. Health Place. 2009;15:670–8. Article PubMed Google Scholar * Das-Munshi J,
Becares L, Dewey ME, et al. Understanding the effect of ethnic density on mental health: multi-level investigation of survey data from England. BMJ. 2010;341:c5367. Article PubMed PubMed
Central Google Scholar * Vargas T, Conley RE, Mittal VA. Chronic stress, structural exposures and neurobiological mechanisms: A stimulation, discrepancy and deprivation model of psychosis.
Int Rev Neurobiol. 2020;152:41–69. Article CAS PubMed Google Scholar * Kearns A, Whitley E. Perceived neighborhood ethnic diversity and social outcomes: context-dependent effects within
a postindustrial city undergoing regeneration. J Urban Aff. 2018;40:186–208. Article PubMed Google Scholar * Heinrichs M, Baumgartner T, Kirschbaum C, et al. Social support and oxytocin
interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98. Article CAS PubMed Google Scholar * Norman GJ, Hawkley LC, Cole SW, et
al. Social neuroscience: The social brain, oxytocin, and health. Social Neurosci. 2012;7:18–29. Article Google Scholar * Petrovic P, Kalisch R, Singer T, et al. Oxytocin attenuates
affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–15. Article CAS PubMed PubMed Central Google Scholar * Petrovic P, Kalisch R, Pessiglione M,
et al. Learning affective values for faces is expressed in amygdala and fusiform gyrus. Soc Cogn Affect Neurosci. 2008;3:109–18. Article PubMed PubMed Central Google Scholar * Yao S,
Zhao W, Geng Y, et al. Oxytocin facilitates approach behavior to positive social stimuli via decreasing anterior insula activity. Int J Neuropsychopharmacol. 2018;21:918–25. Article PubMed
PubMed Central Google Scholar * Chen Y, Becker B, Zhang Y, et al. Oxytocin increases the pleasantness of affective touch and orbitofrontal cortex activity independent of valence. Eur
Neuropsychopharmacol. 2020;39:99–10. Article PubMed Google Scholar * Li X-H, Matsuura T, Xue M, et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional
anxiety by inhibiting presynaptic long-term potentiation. Cell Reports. 2021;36:109411. Article CAS PubMed Google Scholar * Riem MME, Van IJzendoorn MH, Tops M, et al. Oxytocin effects
on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol. 2013;23:1288–95. Article CAS PubMed Google Scholar * Spitzer M, Fischbacher
U, Herrnberger B, et al. The neural signature of social norm compliance. Neuron. 2007;56:185–96. Article CAS PubMed Google Scholar * Adolphs R. The social brain: neural basis of social
knowledge. Annu Rev Psychol. 2009;60:693–16. Article PubMed PubMed Central Google Scholar * Eisenberger NI. The neural bases of social pain: evidence for shared representations with
physical pain. Psychosomatic Med. 2012;74:126–35. Article Google Scholar * Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a
cross-sectional and longitudinal MRI comparison. The Lancet. 2003;361:281–8. Article Google Scholar * Borgwardt S, Mcguire P, Aston J, et al. Reductions in frontal, temporal and parietal
volume associated with the onset of psychosis. Schizophrenia Res. 2008;106:108–14. Article Google Scholar * Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior
cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–65. Article PubMed Google Scholar * Sun D, Phillips L, Velakoulis D, et
al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia Res. 2009;108:85–92. Article Google Scholar * Takahashi T, Wood SJ, Yung AR,
et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366. Article PubMed Google Scholar * Jung WH, Kim JS,
Jang JH, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophrenia Bull. 2011;37:839–49. Article Google Scholar * Ziermans TB, Schothorst PF,
Schnack HG, et al. Progressive structural brain changes during development of psychosis. Schizophrenia Bull. 2012;38:519–30. Article Google Scholar * Cannon TD, Chung Y, He G, Sun D,
Jacobson A, van Erp TGM, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol
Psychiatry. 2015;77:147–57. Article PubMed Google Scholar * Collins MA, Ji JL, Chung Y, et al.: Accelerated cortical thinning precedes and predicts conversion to psychosis: The NAPLS3
longitudinal study of youth at clinical high-risk [Internet]. Mol Psychiatry 2022; [cited 2023 Mar 10] Available from: https://www.nature.com/articles/s41380-022-01870-7. * Del ReEC, Stone
WS, Bouix S, et al. Baseline cortical thickness reductions in clinical high risk for psychosis: brain regions associated with conversion to psychosis versus non-conversion as assessed at
one-year follow-up in the Shanghai-at-risk-for-psychosis (SHARP) study. Schizophr Bull. 2021;47:562–74. Article Google Scholar * Shell AM, Peek MK, Eschbach K. Neighborhood Hispanic
composition and depressive symptoms among Mexican-descent residents of Texas City, Texas. Soc Sci Med. 2013;99:56–63. Article PubMed PubMed Central Google Scholar * Ku BS, Aberizk K,
Addington J, et al. The association between neighborhood poverty and hippocampal volume among individuals at clinical high-risk for psychosis: the moderating role of social engagement.
Schizophr Bull. 2022;48:1032–42. Article PubMed PubMed Central Google Scholar * Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. Article CAS PubMed
Google Scholar * Taylor RL, Cooper SR, Jackson JJ, et al. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open.
2020;3:e2023774. Article PubMed PubMed Central Google Scholar * Ku BS, Addington J, Bearden CE, et al. The associations between area-level residential instability and gray matter volumes
from the North American Prodrome Longitudinal Study (NAPLS) consortium. Schizophr Res. 2022;241:1–9. Article PubMed PubMed Central Google Scholar * Esteves M, Ganz E, Sousa N, et al.
Asymmetrical brain plasticity: physiology and pathology. Neuroscience. 2021;454:3–14. Article CAS PubMed Google Scholar * McGlashan T, Walsh B, Woods S: The Psychosis-Risk Syndrome:
Handbook for Diagnosis and Follow-Up [Internet]. Oxford University Press, 2010. Available from https://books.google.com/books?id=FXM\_utb3D8EC. * Addington J, Cadenhead KS, Cornblatt BA, et
al. North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophrenia Research. 2012;142:77–82. Article PubMed Google Scholar * Addington J, Piskulic D, Liu L,
et al. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophrenia Research. 2017;190:90–95. Article PubMed Google Scholar * NIMH Genetics Initiative. Family Interview
for Genetic Studies. Rockville: National Institute of Mental Health; 1992. Google Scholar * Georgopoulos G, Stowkowy J, Liu L, et al. The role of a family history of psychosis for youth at
clinical high risk of psychosis. Early Intervent Psychiatry. 2019;13:251–6. Article Google Scholar * US Cities Database [Internet]. Simple Maps Available from: Simplemaps.Com. * Selected
housing characteristics, 1995–2000 American Community Survey 5-year estimates. US Census Bureau, 2002. * Bécares L, Shaw R, Nazroo J, et al. Ethnic density effects on physical morbidity,
mortality, and health behaviors: a systematic review of the literature. Am J Public Health. 2012;102:e33–66. Article PubMed PubMed Central Google Scholar * Nagendra A, Halverson TF,
Pinkham AE, et al. Neighborhood socioeconomic status and racial disparities in schizophrenia: An exploration of domains of functioning. Schizophrenia Res. 2020;224:95–101. Article Google
Scholar * Karcher NR, Schiffman J, Barch DM. Environmental risk factors and psychotic-like experiences in children aged 9–10. J Am Acad Child Adolesc Psychiatry. 2021;60:490–500. Article
PubMed Google Scholar * Falcón LM, Todorova I, Tucker K. Social support, life events, and psychological distress among the Puerto Rican population in the Boston area of the United States.
Aging Mental Health. 2009;13:863–73. Article PubMed PubMed Central Google Scholar * Rodríguez-Galán MB, Falcón LM. Patterns of Social Activity Engagement Among Older Hispanics and Their
Relationship to Sociodemographic and Health Variables. Activit Adapt Aging. 2010;34:251–75. Google Scholar * MRI Acquisition [Internet]. ADNI 2021; Available from:
http://adni.loni.usc.edu/methods/mri-tool/mri-analysis/. * Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. NeuroImage. 1999;9:179–94. Article CAS PubMed Google Scholar *
Fischl B. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. Article PubMed Google Scholar * Desikan RS, Ségonne F, Fischl B, et al. An automated
labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. Article PubMed Google Scholar * Finney N, Jivraj
S. Ethnic group population change and neighbourhood belonging. Urban Stud. 2013;50:3323–41. Article Google Scholar * Bécares L, Cormack D, Harris R. Ethnic density and area deprivation:
neighbourhood effects on Māori health and racial discrimination in Aotearoa/New Zealand. Soc Sci Med. 2013;88:76–82. Article PubMed PubMed Central Google Scholar * Bach P, Frischknecht
U, Bungert M, et al. Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. Eur Neuropsychopharmacol. 2019;29:291–305. Article CAS PubMed
Google Scholar * Mwilambwe-Tshilobo L, Spreng RN. Social exclusion reliably engages the default network: a meta-analysis of Cyberball. Neuroimage. 2021;227:117666. Article PubMed Google
Scholar * Putcha D, Brickhouse M, O’Keefe K, et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J
Neurosci. 2011;31:17680–8. Article CAS PubMed PubMed Central Google Scholar * Hull R, Vaid J. Bilingual language lateralization: A meta-analytic tale of two hemispheres*.
Neuropsychologia. 2007;45:1987–2008. Article PubMed Google Scholar * Keenan JP, Thangaraj V, Halpern AR, et al. Absolute pitch and planum temporale. Neuroimage. 2001;14:1402–8. Article
CAS PubMed Google Scholar * Platek SM, Wathne K, Tierney NG, et al. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Research. 2008;1232:173–84. Article
CAS PubMed Google Scholar * Jung S, Kim J-H, Kang N-O, et al. Fusiform gyrus volume reduction associated with impaired facial expressed emotion recognition and emotional intensity
recognition in patients with schizophrenia spectrum psychosis. Psychiatry Res Neuroimaging. 2021;307:111226. Article PubMed Google Scholar * O’Doherty J, Winston J, Critchley H, et al.
Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–55. Article PubMed Google Scholar * Singer T, Seymour B, O’Doherty J, et
al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. Article CAS PubMed Google Scholar * Van Bavel JJ, Packer DJ, Cunningham WA.
The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychol Sci. 2008;19:1131–9. Article PubMed Google Scholar * Van Bavel JJ, Packer DJ,
Cunningham WA. Modulation of the fusiform face area following minimal exposure to motivationally relevant faces: evidence of in-group enhancement (not out-group disregard). J Cognit
Neurosci. 2011;23:3343–54. Article Google Scholar * Marsh LE, de C Hamilton AF. Dissociation of mirroring and mentalising systems in autism. NeuroImage. 2011;56:1511–9. Article PubMed
Google Scholar * Farrow TFD, Jones SC, Kaylor-Hughes CJ, et al. Higher or lower? The functional anatomy of perceived allocentric social hierarchies. NeuroImage. 2011;57:1552–60. Article
PubMed Google Scholar * Rilling JK, Goldsmith DR, Glenn AL, et al. The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46:1256–66. Article
PubMed Google Scholar * Zink CF, Tong Y, Chen Q, et al. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–83. Article CAS PubMed PubMed Central
Google Scholar * Augustine J. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–44. Article CAS PubMed Google Scholar *
Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–51. Article CAS PubMed Google Scholar * Bud Craig AD. How do you
feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. Article Google Scholar * Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting
interoceptive awareness. Nat Neurosci. 2004;7:189–95. Article CAS PubMed Google Scholar * Phelps EA, O’Connor KJ, Cunningham WA, et al. Performance on indirect measures of race
evaluation predicts amygdala activation. J Cognit Neurosci. 2000;12:729–38. Article CAS Google Scholar * Richeson JA, Baird AA, Gordon HL, et al. An fMRI investigation of the impact of
interracial contact on executive function. Nat Neurosci. 2003;6:1323–8. Article CAS PubMed Google Scholar * Richeson JA, Todd AR, Trawalter S, et al. Eye-gaze direction modulates
race-related amygdala activity. Group Processes Intergroup Relat. 2008;11:233–46. Article Google Scholar * Kaplan JT, Aziz-Zadeh L, Uddin LQ, et al. The self across the senses: an fMRI
study of self-face and self-voice recognition. Soc Cognit Affect Neurosci. 2008;3:218–23. Article Google Scholar * Falk EB, Spunt RP, Lieberman MD. Ascribing beliefs to ingroup and
outgroup political candidates: neural correlates of perspective-taking, issue importance and days until the election. Philos Trans R Soc Lond B Biol Sci. 2012;367:731–43. Article PubMed
PubMed Central Google Scholar * Ida Gobbini M, Leibenluft E, Santiago N, et al. Social and emotional attachment in the neural representation of faces. NeuroImage. 2004;22:1628–35. Article
PubMed Google Scholar * Chiao JY, Adams RB, Tse PU, et al. Knowing Who’s Boss: fMRI and ERP Investigations of Social Dominance Perception. Group Processes Intergroup Relat.
2008;11:201–14. Article Google Scholar * McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29.
Article CAS PubMed PubMed Central Google Scholar * Smieskova R, Fusar-Poli P, Aston J, et al. Insular volume abnormalities associated with different transition probabilities to
psychosis. Psychol Med. 2012;42:1613–25. Article CAS PubMed Google Scholar * ENIGMA Clinical High Risk for Psychosis Working Group, Jalbrzikowski M, Hayes RA, et al. Association of
Structural Magnetic Resonance Imaging Measures With Psychosis Onset in Individuals at Clinical High Risk for Developing Psychosis: An ENIGMA Working Group Mega-analysis. JAMA Psychiatry.
2021;78:753. Article Google Scholar * Bhugra D, Becker MA. Migration, cultural bereavement and cultural identity. World Psychiatry. 2005;4:18–24. PubMed PubMed Central Google Scholar *
Ku BS, Addington J, Bearden CE, et al. Associations Between Childhood Area-Level Social Fragmentation Maladaptation to School and Social Functioning Among Healthy Youth and Those at Clinical
High Risk for Psychosis. Schizophrenia Bulletin. 2023; In press. Download references FUNDING This work was supported in part by the National Institute of Mental Health (NIMH) grants U01
MH081902 to TDC, P50 MH066286 to CEB, U01 MH081857 to BAC, U01 MH82022 to SWW, U01 MH066134 to JA, U01 MH081944 to KSC, R01 U01 MH066069 to DOP, R01 MH076989 to DHM, U01 MH081988 to EFW, and
K23 MH129684 to BSK. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA, USA Benson S. Ku *
Department of Psychology, Yale University, New Haven, CT, USA Meghan Collins & Tyrone D. Cannon * Department of Psychology, The City College of New York, City University of New York, New
York, NY, USA Deidre M. Anglin & Kristin S. Cadenhead * The Graduate Center, City University of New York, New York, NY, USA Deidre M. Anglin * Department of Psychiatry, University of
California, San Diego, CA, USA Anthony M. Diomino & Ming T. Tsuang * Department of Psychiatry, University of Calgary, Calgary, AB, Canada Jean Addington & Scott W. Woods *
Departments of Psychiatry and Biobehavioral Sciences and Psychology, Semel Institute for Neuroscience and Human Behavior, UCLA, Los Angeles, CA, USA Carrie E. Bearden * Department of
Psychiatry, Yale University, New Haven, CT, USA Tyrone D. Cannon * Division of Psychiatry Research, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, NY, USA Barbara A. Cornblatt *
Department of Psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA Barbara A. Cornblatt * Department of Health Policy and Management, Rollins
School of Public Health, Emory University, Atlanta, GA, USA Benjamin G. Druss * Harvard Medical School, Departments of Psychiatry at Massachusetts Mental Health Center Public Psychiatry
Division, Beth Israel Deaconess Medical Center, and Massachusetts General Hospital, Boston, MA, USA Matcheri Keshavan & William S. Stone * Department of Psychiatry, University of
California, and San Francisco Veterans Affairs Medical Center, San Francisco, CA, USA Daniel H. Mathalon * Department of Psychiatry, University of North Carolina, Chapel Hill, NC, USA Diana
O. Perkins * Department of Psychology, Emory University, Atlanta, GA, USA Elaine F. Walker Authors * Benson S. Ku View author publications You can also search for this author inPubMed Google
Scholar * Meghan Collins View author publications You can also search for this author inPubMed Google Scholar * Deidre M. Anglin View author publications You can also search for this author
inPubMed Google Scholar * Anthony M. Diomino View author publications You can also search for this author inPubMed Google Scholar * Jean Addington View author publications You can also
search for this author inPubMed Google Scholar * Carrie E. Bearden View author publications You can also search for this author inPubMed Google Scholar * Kristin S. Cadenhead View author
publications You can also search for this author inPubMed Google Scholar * Tyrone D. Cannon View author publications You can also search for this author inPubMed Google Scholar * Barbara A.
Cornblatt View author publications You can also search for this author inPubMed Google Scholar * Benjamin G. Druss View author publications You can also search for this author inPubMed
Google Scholar * Matcheri Keshavan View author publications You can also search for this author inPubMed Google Scholar * Daniel H. Mathalon View author publications You can also search for
this author inPubMed Google Scholar * Diana O. Perkins View author publications You can also search for this author inPubMed Google Scholar * William S. Stone View author publications You
can also search for this author inPubMed Google Scholar * Ming T. Tsuang View author publications You can also search for this author inPubMed Google Scholar * Scott W. Woods View author
publications You can also search for this author inPubMed Google Scholar * Elaine F. Walker View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
The authors confirm contribution to the paper as follows: study conception and design: BK, JA, CB, KC, TC, BC, BD, MK, DM, DP, WS, MT, SW, EW; data collection: BK, JA, CB, KC, TC, BC, MK,
DM, DP, WS, MT, SW, EW; analysis and interpretation of results: BK, MC, DA, AD; draft manuscript preparation: BK, MC, DA, AD, BD, EW; or revise critically for important intellectual content:
JA, CB, KC, TC, BC, MK, DM, DP, WS, MT, SW. All authors reviewed the results, approved the final version of the manuscript, and agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CORRESPONDING AUTHOR Correspondence to Benson S. Ku. ETHICS
DECLARATIONS COMPETING INTERESTS TDC has served as a consultant for Boehringer-Ingelheim Pharmaceuticals and Lundbeck A/S. DHM has served as a consultant for Aptinyx, Boehringer-Ingelheim
Pharmaceuticals, Cadent Therapeutics, and Greenwich Biosciences. DOP has served as a consultant for Sunovion and Alkermes, has received research support from Boehringer-Ingelheim, and has
received royalties from American Psychiatric Association Publishing. SWW has received investigator-initiated research support from Pfizer and sponsor-initiated research support from Auspex
and Teva; he has served as a consultant for Biomedisyn (unpaid), Boehringer-Ingelheim, and Merck and as an unpaid consultant to DSM-5; he has been granted a patent for a method of treating
prodromal schizophrenia with glycine; and he has received royalties from Oxford University Press. The other authors report no financial relationships with commercial interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL
MATERIAL RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or
other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ku, B.S., Collins, M., Anglin, D.M. _et al._ Associations between childhood ethnoracial minority density, cortical thickness, and social
engagement among minority youth at clinical high-risk for psychosis. _Neuropsychopharmacol._ 48, 1707–1715 (2023). https://doi.org/10.1038/s41386-023-01649-6 Download citation * Received: 28
April 2023 * Revised: 16 June 2023 * Accepted: 29 June 2023 * Published: 12 July 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41386-023-01649-6 SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by
the Springer Nature SharedIt content-sharing initiative