Play all audios:
ABSTRACT BACKGROUND The role of fatty acids (FA) in the pathogenesis of insulin resistance and hyperlipidemia is a subject of intensive research. Several recent works have suggested
_cis_-vaccenic acid (cVA) in plasma lipid compartments, especially in plasma phospholipids (PL) or erythrocyte membranes, could be associated with markers of insulin sensitivity and
cardiovascular health. Nevertheless, not all the results of research work testify to these beneficial effects of cVA. Therefore, we decided to investigate the relations of proportion of cVA
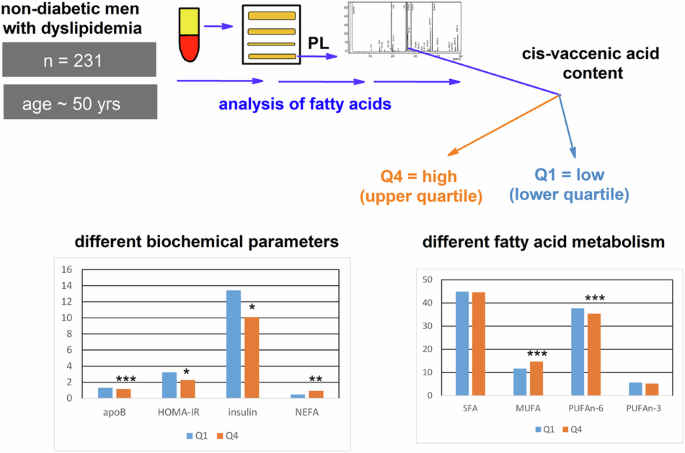
in plasma PL to markers of insulin resistance in hyperlipidemic men. SUBJECTS In 231 men (median age 50) with newly diagnosed hyperlipidemia, we analyzed basic clinical parameters together
with FA composition of plasma PL and stratified them according to the content of cVA into upper quartile (Q4) and lower quartile (Q1) groups. We examined also small control group of 50
healthy men. RESULTS The individuals in Q4 differed from Q1 by lower plasma insulin (_p_ < 0.05), HOMA-IR values (_p_ < 0.01), and apolipoprotein B concentrations (_p_ < 0.001), but
by the higher total level of nonesterified FA (_p_ < 0.01). Both groups had similar age, anthropometrical, and other lipid parameters. In plasma PL, the Q4 group had lower content of the
sum of n-6 polyunsaturated FA, due to decrease of γ-linolenic and dihomo-γ-linolenic acids, whereas the content of monounsaturated FA (mainly oleic and palmitoleic) was in Q4 higher.
CONCLUSIONS Our results support hypothesis that plasma PL cVA could be associated with insulin sensitivity in men with hyperlipidemia. SIMILAR CONTENT BEING VIEWED BY OTHERS OBESITY AND
ACYLCARNITINE DERIVATES INTERPLAY WITH CORONARY ARTERY DISEASE Article Open access 05 May 2025 LIPID AND SATURATED FATTY ACIDS INTAKE AND CARDIOVASCULAR RISK FACTORS OF OBESE CHILDREN AND
ADOLESCENTS Article 05 December 2020 ATHEROGENIC INDEX OF PLASMA AS PREDICTORS FOR METABOLIC SYNDROME, HYPERTENSION AND DIABETES MELLITUS IN TAIWAN CITIZENS: A 9-YEAR LONGITUDINAL STUDY
Article Open access 10 May 2021 INTRODUCTION Fatty acids (FA), as essential components of the lipid structure, play a number of important structural and functional roles in living organisms.
They affect the properties of cell membranes and cell signaling, are ligands of nuclear factors, and are precursors of the plethora of biologically active molecules [1]. The composition of
FAs in human body compartments depends on nutritional intake, endogenous synthesis, the functioning of enzymes desaturases and elongases (see Figs. 1 and 2), and different degradation
mechanisms [2]. Moreover, racial and genetic factors play a role, as well [3, 4]. Dysregulation of FA is involved in the etiopathogenesis of insulin resistance. Inflammation and oxidative
stress are important factors affecting insulin signaling, as well [5]. Insulin resistance (IR) was recognized to be associated with metabolic syndrome [6], type 2 diabetes mellitus (T2DM)
development [7], and different pathological conditions, such as dyslipidemia, but also some cancers or neuropsychiatric diseases [8,9,10]. The relationships of individual classes of FA to
insulin resistance, as well as to inflammation and oxidative stress, are different. Generally, a higher content of saturated fatty acids in the diet (especially palmitic acid) and a lower
content of PUFA (polyunsaturated fatty acids) n-3, as well as monounsaturated FA (principally oleic acid) are mostly associated with insulin resistance [11], while the role of the n-6 PUFA
class in this regard is less clear. In a recently published analysis of 20 studies, high plasma linoleic acid (LA, 18:2n-6) levels were associated with a significant decrease in the relative
risk of T2DM [12], whereas the proportion of dihomo-γ-inolenic (20:3n-6) acid in serum cholesteryl esters positively correlated with the incidence of type 2 diabetes mellitus [13]. The
different effects of individual SFA on cardiovascular risk in humans are a well-known phenomenon [14], and it seems that SFA has variable associations with insulin sensitivity, too. These
findings show us that the relationship of individual fatty acids to insulin resistance is apparently more complex. For the reasons stated, the specific metabolic effects of individual FAs in
insulin resistance pathogenesis became a subject of intensive research. For example, when investigating the effects of palmitoleic acid (16:1n-7, POA), it was demonstrated that POA
synthetized in adipose tissue favorably influences lipid and glucose homeostasis in other tissues, such as liver or muscle, and attenuates liver steatosis induced by high-fat diet or
diabetes [15, 16]. Nevertheless, the findings on the effects of circulating palmitoleate on cardiometabolic health in humans are still controversial, as the content of POA in plasma lipid
classes was an independent marker of abdominal obesity in men [17] or high plasma glucose concentration [18]. Moreover, POA content in erythrocyte membranes has been shown to be associated
with inflammation and a higher risk of metabolic syndrome [19]. These discrepant results can be assigned to many factors, including metabolic conversion of POA. The elongation of POA
produces _cis_-vaccenic acid (18:1n-7, cVA), and interestingly, POA, increases the transcription of the genes encoding elongase enzymes, especially ELOVL5 [20], in endothelial cells in a
dose-dependent manner [21]. The results of some studies have shown beneficial effects on human health associated with cVA. Erythrocyte membrane cVA was inversely related to the risk of heart
failure with antecedent CHD [22], and in one prospective study, lower cVA in PL predicted consistently lower insulin sensitivity and β-cell function over 6 years [23]. In a large
prospective cohort study in the US, comprising 3004 participants free of diabetes (men and women) aged >65 years, it was proved that circulating plasma phospholipid cVA was inversely
associated with the risk of diabetes mellitus development [24]. These findings indicate that the potential beneficial effects of cVA on cardiometabolic risk in humans should be further
studied, also taking into account sex and depot differences between men and women [25]. Hormonal differences are responsible for different metabolism of MUFA and PUFA due to upregulation of
desaturases and elongases resulting in higher PUFA n-3 and different deposition and mobilization of fatty acids from visceral and gluteofemoral adipose tissues [26, 27]. The aim of this
study was to investigate relations of proportion of cVA in plasma phospholipids to markers of insulin resistance in non-diabetic men with high cardiovascular risk. MATERIAL AND METHODS STUDY
DESIGN AND PARTICIPANTS The study population comprised 231 men consecutively examined as outpatients at the 4th Department of Internal Medicine between the years 2020–2022. As a control
group, we examined 50 apparently healthy men (volunteers and university staff). The study protocol was approved by the Joint Ethical Committee of the General University Hospital and the
first Medical Faculty at Charles University, Prague. Written informed consent was obtained from all participants prior to inclusion. The study was performed in accordance with the
Declaration of Helsinki. These patients were referred to Lipid Clinic to consider the use of hypolipidemic treatment. Subjects studied had an LDL-C level >3.0 mmol/L (7% of probands)
and/or triacylglycerolemia >1.70 mmol/L (68.5% of probands). Moreover, we observed low concentrations of high-density lipoprotein cholesterol (HDL-C) (<1.00 mmol/L) in 24% of them. The
following exclusion criteria were applied to the study: ongoing lipid-lowering, antidiabetic or antioxidant medication, excessive alcohol consumption (>30 g/day), hormonal replacement
therapy, supplementation with polyunsaturated FA (PUFA n-3 and n-6 families), manifestation of cardiovascular disease and/or cerebrovascular disease, diabetes mellitus, liver disease (except
for nonalcoholic fatty liver disease), kidney disease (creatinine >130 μmol/L), hypothyroidism, and recent infections and malignancies. Six weeks before the investigation, they were
recommended to adhere to the AHA Step One diet and to maintain stable dietary patterns. ANTHROPOMETRIC AND BLOOD PRESSURE MEASUREMENTS Basic clinical and anthropometric data, including the
assessment of body fat, were analyzed using standard methods as described previously [28]. Moreover, the bioelectrical impedance method was used for the body fat mass measurements with
Tanita SC-240 (Tanita Corporation, Tokyo, Japan). Blood pressure measurements were performed according to standardized procedures after resting for 10 min, using automated blood pressure
devices (Omron M3 Comfort, OMRON Healthcare Europe) by trained study personnel, and the average of two measurements was presented as a result. LABORATORY MEASUREMENTS Plasma concentrations
of biochemical parameters were measured using routine methods, whereas concentrations of nonesterified FA (NEFA) were determined using an enzymatic colorimetric method (Randox Laboratories,
UK). The homeostasis model assessment method (HOMA-IR) was used as the index of insulin resistance [29]. The glomerular filtration rate (eGFR) was estimated from serum creatinine according
to the 2021 CKD-EPI equation. Fatty acids were analyzed in plasma phospholipids as methyl esters by GC-FID [30] using LN-FAME-HT (60 m × 0.25 mm ID, 0.20 μm df) column (Chromservis s.r.o.,
Prague, Czech Republic). This setting allowed proper separation of isomers of monounsaturated FA (see Supplementary Fig. 1 for sample chromatogram). The retention times for FA methyl esters
were verified using commercially available standards (NuChek Prep Inc., Elysian, MN, USA, Cat. No. GLC-566C and GLC-674). The FID detector performance was checked on the basis of the
linearity of the response; the correction factors were calculated using the abovementioned standard mixtures with defined composition. Method variability, presented as a relative standard
deviation, ranged from 1.07% for PA (16:0) to 8.60% for 16:1n-9 [31]. Desaturase and elongase activities were estimated using FA product/precursor ratios [32]. DIETARY HABITS Average intake
of energy, proteins, fats, saturated FA, carbohydrates, and fiber were analyzed by a 3-day dietary questionnaire. The nutritional data were analyzed by Nutrimaster SE software, version 1.0
[33]. STATISTICAL METHODS The overall group of men was for further comparisons stratified into quartiles according to the content of _cis_-vaccenic acid in plasma phospholipids; the
investigators were blinded according to the quartile assignment. The statistical analysis was performed using the R statistical software Version 4.1.3 [34], or with the STATISTICA® CZ
software for Windows (ver. 12). Studies on insulin resistance in subjects with high or low cVA concentrations have not been published to allow sample size calculations; nevertheless, the a
posteriori sample size calculations gave power 1-beta 0.76 (HOMA-IR) and 0.66 (insulin) for the current setting. Categorical data were summarized using absolute and relative frequencies.
Continuous data were expressed as the median and interquartile range (IQR, 25th–75th percentile). The normality of the distribution was tested using the Shapiro–Wilks _W_ test. Comparisons
between the groups were determined using the independent _t_-test and the Wilcoxon test, respectively. _P_-values for both continuous and categorical data were adjusted for multiple
comparisons using the Benjamini–Hochberg correction. Multivariate linear regression with backward stepwise analysis was used to distinguish the significance of intercorrelated relevant
predictors. RESULTS CLINICAL AND BIOCHEMICAL CHARACTERISTICS OF THE STUDIED GROUP Basic demographic, anthropometric, and metabolic data of the group of 231 men with hyperlipidemia are shown
in Table 1. The median age was 50 years, and the patients were mostly overweight/obese. Table 2 shows demographic, anthropometric, and metabolic data according to _cis_-vaccenic acid
concentrations in plasma phospholipids. Quartile 4 was compared with Quartile 1 (Q1 and Q4 groups, median [25th–75th percentiles]). Between the groups Q4 and Q1, there were no differences
between the daily energy intake and the content of proteins, fats, and carbohydrates, as well. Similarly, we did not observe significant differences in the intakes of dietary FA—saturated
FA, monounsaturated FA. and PUFA (as the sum of both PUFA n-3 and n-6) (data not presented). In comparison with the Q1 group, the Q4 group was characterized by significantly lower values of
insulin, apolipoprotein, and HOMA-IR index, while by significantly higher concentrations of plasma NEFA. Persons in the Q4 group had significantly lower concentrations of conjugated dienes
in LDL particles (CD-LDL). Differences in all other variables were not significant (age, BMI, waist circumference, waist-to-hip ratio, fat mass, systolic and diastolic blood pressure),
percentage of smokers, as well. There were no significant differences in concentrations of total plasma cholesterol, triacylglycerols, HDL-cholesterol, LDL-cholesterol, non-HDL-cholesterol,
apolipoprotein A1, fasting glycemia, uricemia, or hs-CRP. When comparing the control and patient groups, no significant differences were found in age, BMI, proportion of hypertensives,
smokers, systolic, and diastolic BP. As expected, significant differences were found in plasma lipid levels (patients with hyperlipidemia differed in significantly higher levels of plasma
total cholesterol, triacylglycerols, LDL-C, non-HDL-C, apo B), uric acid, glucose, waist circumference, and index values WHCR. Moreover, controls had a higher proportion of cVA and the sum
of MUFA (see Supplementary Tables 3 and 4). FATTY ACID PROFILES Fatty acid profiles (mol %) in plasma PL of the Q4 and Q1 groups are shown in Table 3. Q4 group (high _cis_-vaccenic group)
had a higher molar percentage of the sum of MUFA caused by a high content of oleic acid, 18:1n-9, palmitoleic acid, _cis_-vaccenic and minor FA of this family (_cis_-hexadecenoic and gondoic
acids). The lower content of PUFAn-6 in the Q4 group was more pronounced in minor FA (γ-linolenic and dihomo-γ-linolenic acids); we did not observe significant differences in the content of
linoleic and arachidonic acids. The remaining differences in FA concentrations were insignificant after the Benjamini–Hochberg correction. Surrogate markers of estimated activities of FA
desaturases and elongases, computed as the (product/substrate) ratio of respective FA in plasma PL of both Q4 and Q1 groups, are shown in Table 4. In the Q4 group we found raised estimated
activity of delta-9-desaturase (D9D) for stearic acid (D9D18) and for palmitic acid (D9D16). Furthermore, we found increased estimated activities for ELOVL5 (calculated as 18:1n-7/16:1n-7
ratio), and ELOVL 2/5 (ratio 22:5n-3/20:5n-3) and 22:4n-6/20:4n-6, respectively. Concurrently, the estimated activity of D6D (ratio 18:3n-6/18:2n-6) was decreased in the Q4 group.
CORRELATIONS BETWEEN FATTY ACID PROFILES AND SELECTED VARIABLES Relevant correlations (Spearman´s coefficients, _p_ values) of investigated variables of the total group of 231 men with
dyslipidemia are presented in Supplementary Table 1. _Cis_-vaccenic acid negatively correlated with insulin (_r_ = −0.183, _p_ < 0.01), HOMA-IR (_r_ = −0.196, _p_ < 0.01), apo-B (_r_ =
−0.203, _p_ < 0.01). We observed significant correlations with insulin and HOMA-IR for several FA (Supplementary Table 1). Because of high intercorrelations between all monounsaturated
fatty acids (see Supplementary Table 1), we performed backward stepwise regression analysis for all analyzed MUFA to identify significant determination of parameters of insulin resistance
with these MUFA. According to backward stepwise analysis, only association with 18:1n-7c remains significant for insulin (_p_ = 0.015) and HOMA-IR (_p_ = 0.006) (see Supplementary Table 2
for detailed results). DISCUSSION In this study, we have found significant differences in the parameters of glucose and lipid homeostasis between the patients belonging to the upper (group
Q4) vs. lower (Q1) quartile of the _cis_-vaccenic acid content in plasma phospholipids (PL). Fatty acid profiles of the Q4 and Q1 groups significantly differed, as well. To our knowledge, it
is the first study investigating associations of high vs. low proportions of cis-vaccenic acid in plasma phospholipids (PL) with markers of insulin resistance in men with hyperlipidemia.
The main finding of the study was that individuals in group Q4 differed from those in Q1 by the more favorable profile of insulin resistance markers. They had significantly lower plasma
insulin, HOMA-IR values, and apolipoprotein B concentrations. Moreover, both quartiles differed significantly in the composition of FA in plasma PL. The individuals in the Q4 group had lower
proportions of FA 14:0, 18:3n-6, 20:3n-6, and the sum of PUFA n-6. On the other hand, the Q4 group had, besides cVA, significantly elevated FA 16:1n-9, 16:1n-7, 18:1n-9, 20:1n-9, 18:3n-3,
sum of MUFA and total plasma NEFA. We did not prove different dietary habits between Q1 and Q4 subjects (data not shown). It is known that dietary assessment methods have many limitations.
The accuracy for individual dietary components reaches a maximum of 70–80% [35]. While insulin and HOMA-IR values serve as surrogate markers of insulin resistance, elevated apolipoprotein B,
concurrently with elevated serum triacylglycerols, increased small LDL particles, and reduced level of HDL characterize atherogenic dyslipidemia that is typical for insulin resistant
states. Interestingly, the whole group of 231 men with hyperlipidemia had a significantly smaller proportion of cVA and oleic acid in their plasma PL, in comparison with a small control
group of 50 normolipidemic men (see Supplementary Tables S2, S3). Our findings of associations of plasma phospholipid cis-vaccenic acid (18:1n-7) with cardiometabolic risk factors are in
line with the results of several studies. In participants of a prospective Cardiovascular Health Study, free of diabetes, concentrations of 18:1n-7 in plasma PL were inversely associated
with incident T2DM [24]. From another perspective, in a 6-year study in the Canadian population at risk for diabetes, lower _cis_-vaccenic acid (cVA) predicted lower insulin sensitivity and
β-cell function [23]. Ethnic factors may also play a role in the functioning of cVA. In Multi-Ethnic Study of Atherosclerosis (MESA) participants, higher levels of plasma cVA were inversely
associated with insulin resistance scores across four races/ethnicities (Caucasian, Black, American Chinese, Hispanic), and related to 17%, 39%, and 32% lower risks of incident T2DM in
Black, Chinese American, and Hispanic participants, respectively. In Caucasians, the significant association of cVA with incident T2DM was not found [36]. In other studies, different results
were found, too. In the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam study, cVA by quintile of erythrocyte membrane fatty acid proportions did not correlate
with the risk of T2DM [37]. The Q4 group differed from the Q1 group by a higher level of individual MUFAs (16:1n-9, 18:1n-9, 20:1n-9) and palmitoleic acid (16:1n-7, POA), which is a
precursor of cVA. The levels of α-linolenic acid (18:3n-3, LnA) and total plasma NEFA were higher in the Q4 group, as well. MUFA, mostly oleic acid, is recognized as beneficial in
influencing insulin sensitivity and preventing DM2. However, not all FA in this class have the same effects. Oleic acid (OA) content has been linked with a number of mechanisms, such as
reduced expression of pro-inflammatory genes, upregulation of enzymes responsible for fatty acid oxidation, beneficial effects on endothelial dysfunction, and others [38]. Negative
correlations with T2DM were found for OA in both plasma CE [39] and plasma PL [13]. On the other hand, some studies also produced opposite results - in one prospective study, oleic and
palmitoleic (POA) acids were independently associated with incident T2DM [40]. Q1 differed from Q4 by significantly higher concentrations of γ-linolenic acid (GLA) and dihomo-γ-linolenic
acid (DGLA) while lower proportions of sum PUFA n-6 (at the expense of linoleic acid, LA) and α-linolenic acid (ALA, 18:3n-3). In our previous work, higher proportions of POA, GLA, and DGLA,
while lower content of LA in plasma phosphatidylcholines were able to identify in the patients with metabolic syndrome those with higher IR or an oxidative stress marker [33]. Also, DGLA
was associated with abdominal fat expressed as waist circumference or waist/hip ratio [41]. In this work, we observed higher levels of NEFA in the Q4 vs. Q1 group. It was generally accepted
that elevated plasma NEFA is associated with insulin resistance and T2DM in humans [42]. NEFA was elevated in diabetic subjects with overweight/obesity in comparison with healthy subjects
[43] and was prospectively associated with an increased risk of both impaired glucose tolerance and T2DM [44]. Nevertheless, individual NEFA may have different effects on the progress of
insulin resistance and the development of type 2 diabetes mellitus [45]. Saturated NEFA causes insulin resistance, while monounsaturated FA increases insulin sensitivity in both diabetic
[46] and healthy persons [45]. Still, some issues are to be resolved dealing with different NEFA contribution to insulin resistance and other pathologic states: FA profiles in plasma lipid
compartments differ significantly between men and women and among different ethnic groups, as well [47], and reference range for main plasma FA in healthy subjects are missing [48]. The
reasons for the higher NEFA concentration in the Q4 group are not clear. Some findings suggest that increased content of cis vaccenic acid, a product of ELOVL5 activity, could be related to
a decrease in ectopic fat deposition. Supplementation of obese rats with trans-vaccenic acid (replacing oleic acid) led to increased insulin sensitivity, reduced hepatic steatosis, and
decreased nonalcoholic fatty liver disease activity scores. The action of cVA on PPARγ (peroxisome proliferator activator receptor γ) in adipose tissue may counteract excessive ectopic lipid
accumulation [49]. In our study, in set of men with hyperlipidemia group of patients belonging to the fourth quartile of serum PL cVA proportions had higher values of estimated ELOVL2/5
activity, calculated both as a 22:5n-3/20:5 n-3 and 22:4n-6/20:4n-6 ratios and activity of ELOVL5 calculated as a ratio 18:1n-7/16:1n-7. In experimental work, increasing hepatic ELOVL5
activity improved glycemia, insulinemia, HOMA-IR, and glucose tolerance to normal values in obese mice [50]. In mice with a knockout of the Elovl5 gene, the lack of endogenously formed
long-chain PUFA leads to the derepression of SREBP-1c, the activation of lipogenesis, and hepatic steatosis [51]. ELOVL5 activity was also associated with increased catabolism of
triacylglycerols, suppressed expression of enzymes involved in gluconeogenesis [52], and induced the expression of fibroblast growth factor 21 (FGF21), which increases hepatic insulin
sensitivity, decreases lipogenesis, potentiates fatty acid β-oxidation, reduces hepatic ER (endoplasmic reticulum) stress, and diminishes VLDL (very low-density lipoproteins) delivery to the
liver [53] and can improve ectopic lipid deposition in liver and muscle [54]. The patients in the Q4 group had significantly higher estimated activities of delta-9-desaturase (D9D) both for
16:0 (D9D16) and 18:0 (D9D18), whereas lower estimated activity of D6D (calculated as 18:3n-6/18:2n-6 ratio). Elevated activity of D9D is a marker of de novo lipogenesis, and in this study,
both D9D16 and D9D18 correlated significantly with the total plasma NEFA levels and with all principal n-9 FA (16:1n-7, 18:1n-9, 20:1n-9, and cVA. Interestingly, the estimated activity
(D9D18) index correlated negatively with markers of insulin resistance, whereas the D9D16 index correlated positively (see Supplementary Table 1). This probably could be related to the
possible positive association of POA with insulin resistance in this set of patients. Proportion of cVA correlated negatively with insulin, HOMA-IR, and apolipoprotein B, showing that in our
set of hyperlipidemic patients with obesity/overweight, cVA in plasma PL may function to improve insulin sensitivity. In contrast, POA correlated rather differently both with anthropometric
parameters and markers of glucose and lipid homeostasis. Its proportion in plasma PL correlated positively with BMI, WHCR, fat mass, and triacylglycerols. This finding corresponds with some
results of previous human studies, indicating unfavorable effects of POA on glucose homeostasis [19], though not all studies did prove such results [24]. Patients of Q4, in comparison with
the Q1 group, had significantly lower values of an estimated D6D (18:3n6/18:2n6) (_p_ < 0.001). This finding is in line with the results of other studies, where elevated estimated
activity of D6D was described in patients with metabolic syndrome and T2DM [31], impaired fasting glycemia [30], and other pathologic conditions such as cardiovascular disease [55], tumors
[9], or depression [10] Inverse correlations have been found between POA and cVA with estimated D6D activity (while POA correlated positively, cVA correlated negatively, both _p_ <
0.001). We have analyzed in our group of hyperlipidemic men markers of oxidative stress and inflammation (conjugated dienes in LDL and hs-CRP), as these conditions are associated with
insulin resistance. Probands in the Q4 group had significantly lower concentrations of CD-LDL in comparison with the Q1 group. Concentrations of CD-LDL are considered a marker of systemic
oxidative stress, partly reflecting the levels of minimally modified LDL, in which only the lipid component is oxidatively modified [56]. This finding is in line with the results of our
earlier studies dealing with hypertriglyceridemia, severity of metabolic syndrome, or pancreatic cancer, as well [9, 26, 57]. The significance of lipid peroxidation in the pathogenesis of
insulin resistance has been proven in experimental and clinical studies [58]. On the other hand, we were not able to find differences in hs-CRP concentrations. To our knowledge, this is the
first work describing significant associations of serum PL cVA with parameters of insulin sensitivity in male patients with hyperlipidemia. The study was conducted in a relatively large set
of men with overweight or obesity, who were not treated with lipid-lowering drugs, supplements of n-3 or n-6 PUFA, or antioxidants. Limitations include a cross-sectional type of study, so
causality could not be proved. Moreover, the profile of individual plasma NEFA was not investigated, and the content of individual MUFA and PUFA was not assessed in dietary questionnaires,
as well. Also, the data about the physical activity were not available. Our results support the hypothesis that plasma PL cis-vaccenic acid could be associated with insulin sensitivity in
men with hyperlipidemia and high cardiovascular risk. Moreover, the results indicate that individual FA in the same class can have different pathophysiological effects. The results should be
further studied and applied to other populations, e.g. healthy subjects, women, or type 2 diabetics. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study
are available from the corresponding author upon reasonable request. REFERENCES * de Carvalho CCCR, Caramujo MJ. The various roles of fatty acids. Molecules. 2018;23:2583. Article PubMed
PubMed Central Google Scholar * Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice.
Prog Lipid Res. 2010;49:186–99. Article CAS PubMed Google Scholar * Zeman M, Vecka M, Jáchymová M, Jirák R, Tvrzická E, Staňková B, et al. Fatty acid CoA ligase-4 gene polymorphism
influences fatty acid metabolism in metabolic syndrome, but not in depression. Tohoku J Exp Med. 2009;217:287–93. Article CAS PubMed Google Scholar * Smith CE, Ordovás JM. Fatty acid
interactions with genetic polymorphisms for cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2010;13:139–44. Article CAS PubMed PubMed Central Google Scholar * Yaribeygi H,
Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152–61. Article CAS PubMed Google Scholar * Warensjö E,
Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. Article PubMed Google Scholar *
Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. Article CAS PubMed Google Scholar * Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle
insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 2010;30:273–90. Article CAS
PubMed PubMed Central Google Scholar * Macášek J, Vecka M, Žák A, Urbánek M, Krechler T, Petruželka L, et al. Plasma fatty acid composition in patients with pancreatic cancer:
correlations to clinical parameters. Nutr Cancer. 2012;64:946–55. Article PubMed Google Scholar * Vařeka T, Vecka M, Jirák R, Tvrzická E, Macášek J, Žák A, et al. Plasma fatty acid
profile in depressive disorder resembles insulin resistance state. Neuro Endocrinol Lett. 2012;33:83–86. PubMed Google Scholar * Sears B, Perry M. The role of fatty acids in insulin
resistance. Lipids Health Dis. 2015;14:121 https://doi.org/10.1186/s12944-015-0123-1 Article CAS PubMed PubMed Central Google Scholar * Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson
Korat AV, de Goede J, et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies.
Lancet Diabetes Endocrinol. 2017;5:965–74. Article CAS PubMed PubMed Central Google Scholar * Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and
incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–8. Article CAS PubMed Google Scholar * Perna M, Hewlings S.
Saturated fatty acid chain length and risk of cardiovascular disease: a systematic review. Nutrients. 2022;15:30. Article PubMed PubMed Central Google Scholar * Cao H, Gerhold K, Mayers
JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–44. Article CAS PubMed PubMed
Central Google Scholar * de Souza CO, Teixeira AAS, Biondo LA, Lima Junior EA, Batatinha HAP, Rosa Neto JC. Palmitoleic acid improves metabolic functions in fatty liver by PPARα-dependent
AMPK activation. J Cell Physiol. 2017;232:2168–77. Article PubMed Google Scholar * Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, et al. Plasma palmitoleic acid, a
product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–40. Article CAS PubMed Google
Scholar * Lindgärde F, Vessby B, Ahrén B. Serum cholesteryl fatty acid composition and plasma glucose concentrations in Amerindian women. Am J Clin Nutr. 2006;84:1009–13. Article PubMed
Google Scholar * Zong G, Ye X, Sun L, Li H, Yu Z, Hu FB, et al. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged
and older Chinese. Am J Clin Nutr. 2012;96:970–6. Article CAS PubMed PubMed Central Google Scholar * Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, et al. Regulation
of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47:2028–41. Article CAS PubMed Google Scholar * de Souza CO, Vannice GK, Rosa Neto
JC, Calder PC. Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol Nutr Food Res. 2018;62:1700504. Article
Google Scholar * Djoussé L, Matsumoto C, Hanson NQ, Weir NL, Tsai MY, Gaziano JM. Plasma cis-vaccenic acid and risk of heart failure with antecedent coronary heart disease in male
physicians. Clin Nutr. 2014;33:478–82. Article PubMed Google Scholar * Johnston LW, Harris SB, Retnakaran R, Zinman B, Giacca A, Liu Z, et al. Longitudinal associations of phospholipid
and cholesteryl ester fatty acids with disorders underlying diabetes. J Clin Endocrinol Metab. 2016;101:2536–44. Article CAS PubMed Google Scholar * Ma W, Wu JHY, Wang Q, Lemaitre RN,
Mukamal KJ, Djousse I, et al. Prospective association of fatty acids in de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study1–5. Am J Clin Nutr.
2015;101:153–63. Article CAS PubMed Google Scholar * Ramos P, Bush NC, Jensen MD. Sex and depot differences in palmitoleic acid content of human blood and fat. Lipids. 2020;55:63–72.
Article CAS PubMed PubMed Central Google Scholar * Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc.
2008;67:19–27. Article CAS PubMed Google Scholar * Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne).
2015;5:241. Article PubMed Google Scholar * Žák A, Tvrzická E, Vecka M, Jáchymová M, Duffková L, Staňková B, et al. Severity of metabolic syndrome unfavorably influences oxidative stress
and fatty acid metabolism in men. Tohoku J Exp Med. 2007;212:359–71. Article PubMed Google Scholar * Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis
model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. Article CAS PubMed Google Scholar
* Vecka M, Tvrzická E, Staňková B, Žák A. Effect of column and software on gas chromatographic determination of fatty acids. J Chromatogr B Anal Technol Biomed Life Sci. 2002;770:91–9.
Article CAS Google Scholar * Tvrzická E, Vecka M, Staňková B, Žák A. Analysis of fatty acids in plasma lipoproteins by gas chromatography-flame ionisation detection. Quantitative aspects.
Anal Chim Acta. 2002;465:337–50. Article Google Scholar * Macášek J, Zeman M, Žák A, Staňková B, Vecka M. Altered indices of fatty acid elongases ELOVL6, ELOVL5, and ELOVL2 activities in
patients with impaired fasting glycemia. Metab Syndr Relat Disord. 2021;19:386–92. Article PubMed Google Scholar * Žák A, Burda M, Vecka M, Zeman M, Tvrzická E, Staňková B. Fatty acid
composition indicates two types of metabolic syndrome independent of clinical and laboratory parameters. Physiol Res. 2014;63:S375–85. Article PubMed Google Scholar * R Core Team. R: a
language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2024 * Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM,
Boushey CJ, Ferruzzi MG, editors. Nutrition in the prevention and treatment of disease. USA: Elsevier Inc.; 2013. pp. 5–46. * Weir NL, Steffen BT, Guan W, Johnson LM, Djousse L, Mukamal KJ,
et al. Circulating omega-7 fatty acids are differentially related to metabolic dysfunction and incident type II diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Metab.
2020;46:319–25. Article CAS PubMed Google Scholar * Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase
activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr.
2011;93:127–42. Article PubMed Google Scholar * Rehman K, Haider K, Jabeen K, Akash MSH. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and
endothelial functioning against insulin resistance and diabetes. Rev Endocr Metab Disord. 2020;21:631–43. Article CAS PubMed Google Scholar * Vessby B, Aro A, Skarfors E, Berglund L,
Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43:1353–7. Article CAS PubMed Google Scholar *
Qureshi W, Santaren ID, Hanley AJ, Watkins SM, Lorenzo C, Wagenknecht LE. Risk of diabetes associated with fatty acids in the de novo lipogenesis pathway is independent of insulin
sensitivity and response: the Insulin Resistance Atherosclerosis Study (IRAS). BMJ Open Diabetes Res Care. 2019;7:e000691. Article PubMed PubMed Central Google Scholar * Zeman M, Vecka
M, Burda M, Tvrzická E, Staňková B, Macášek J, et al. Fatty acid composition of plasma phosphatidylcholine determines body fat parameters in subjects with metabolic syndrome-related traits.
Metab Syndr Relat Disord. 2017;15:371–8. Article CAS PubMed Google Scholar * Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10.
Article CAS PubMed Google Scholar * Ni Y, Zhao L, Yu H, Ma X, Bao Y, Rajani C, et al. Circulating unsaturated fatty acids delineate the metabolic status of obese individuals.
EBioMedicine. 2015;2:1513–22. Article PubMed PubMed Central Google Scholar * Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels
are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab. 2012;97:3302–9. Article CAS PubMed Google Scholar
* Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC.KANWU Study et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men
and women: the KANWU study. Diabetologia. 2001;44:312–9. Article CAS PubMed Google Scholar * Parillo M, Rivelles AA, Ciardullo AV, Capaldo B, Giacco A, Genovese S, et al. A high
monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism. 1992;41:1373–8. Article CAS PubMed Google Scholar
* Abdelmagid SA, Clarke SE, Nielsen DE, Badawi A, El-Sohemy A, Mutch DM, et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE.
2015;10:e0116195. Article PubMed PubMed Central Google Scholar * Chandra K, Jain V, Jain SK. Plasma non-esterified fatty acids (NEFA) in type 2 diabetes mellitus: evidence on
pathophysiology. J Diabetes Clin Res. 2021;3:46–50. Google Scholar * Jacome-Sosa MM, Borthwick F, Mangat R, Uwiera R, Reaney MJ, Shen J, et al. Diets enriched in trans-11 vaccenic acid
alleviate ectopic lipid accumulation in a rat model of NAFLD and metabolic syndrome. J Nutr Biochem. 2014;25:692–701. Article CAS PubMed Google Scholar * Tripathy S, Torres-Gonzalez M,
Jump DB. Elevated hepatic fatty acid elongase-5 activity corrects dietary fat induced hyperglycemia in obese C57BL/6J mice. J Lipid Res. 2010;51:2642–54. Article CAS PubMed PubMed Central
Google Scholar * Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res. 2009;50:412–23. Article CAS PubMed PubMed
Central Google Scholar * Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33:249–69. Article CAS PubMed PubMed Central
Google Scholar * Falamarzi K, Malekpour M, Tafti MF, Azarpira N, Behboodi M, Zarei M. The role of FGF21 and its analogs on liver associated diseases. Front Med (Lausanne). 2022;9:967375.
Article PubMed Google Scholar * Jia Y, Yu H, Liang J, Zhang Q, Sun J, Yang H, et al. Increased FGF-21 improves ectopic lipid deposition in the liver and skeletal muscle. Nutrients.
2024;16:1254. Article CAS PubMed PubMed Central Google Scholar * Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as
redictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88:203–9. Article PubMed Google Scholar * Ahotupa M, Ruutu M, Mäntylä E.
Simple methods of quantifying oxidation products and antioxidant potential of low density lipoproteins. Clin Biochem. 1996;29:139–44. Article CAS PubMed Google Scholar * Zeman M, Žák A,
Vecka M, Tvrzická E, Romaniv S, Konárková M. Treatment of hypertriglyceridaemia with fenofibrate, fatty acid composition of plasma and LDL, and their relations to parameters of
lipoperoxidation of LDL. Ann N Y Acad Sci. 2002;967:336–41. Article CAS PubMed Google Scholar * Murdolo G, Piroddi M, Luchetti F, Tortoioli C, Canonico B, Zerbinati C, et al. Oxidative
stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–94. Article CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS This research was funded by the Ministry of Health of the Czech Republic, grants number MH CZ DRO-VFN64165 and NU23-01-00288, and the Ministry
of Education, Youth and Sports of the Czech Republic, grant the Charles University Research program, Cooperatio—Gastroenterology, The statistical help of Barbora Pejchalová is greatly
acknowledged. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Fourth Department of Internal Medicine, First Faculty of Medicine, Charles University and General University Hospital in Prague, U
Nemocnice 2, 128 08, Prague, Czech Republic Jaroslav Macášek, Barbora Staňková, Aleš Žák, Markéta Růžičková, Radan Brůha, Simona Kutová, Marek Vecka & Miroslav Zeman * Institute of
Clinical Chemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University and General University Hospital in Prague, Na Bojišti 3, 121 08, Prague, Czech Republic Barbora
Staňková & Marek Vecka Authors * Jaroslav Macášek View author publications You can also search for this author inPubMed Google Scholar * Barbora Staňková View author publications You can
also search for this author inPubMed Google Scholar * Aleš Žák View author publications You can also search for this author inPubMed Google Scholar * Markéta Růžičková View author
publications You can also search for this author inPubMed Google Scholar * Radan Brůha View author publications You can also search for this author inPubMed Google Scholar * Simona Kutová
View author publications You can also search for this author inPubMed Google Scholar * Marek Vecka View author publications You can also search for this author inPubMed Google Scholar *
Miroslav Zeman View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization, JM, MZ, RB, and MV; methodology, MV and BS;
writing—original draft preparation, MZ, JM, MV, and AZ; writing—review and editing, MV, M.Z, and JM; super-vision, MZ, RB, and JM; project administration, MV, MR, and SK; funding
acquisition, AZ and RB. All authors have read and agreed to the published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Marek Vecka. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES 1-5 AND SUPPLEMENTARY FIGURE 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Macášek, J., Staňková, B., Žák, A. _et al._ Associations of plasma
phospholipid cis-vaccenic acid with insulin resistance markers in non-diabetic men with hyperlipidemia. _Nutr. Diabetes_ 14, 73 (2024). https://doi.org/10.1038/s41387-024-00332-z Download
citation * Received: 23 January 2024 * Revised: 22 August 2024 * Accepted: 30 August 2024 * Published: 11 September 2024 * DOI: https://doi.org/10.1038/s41387-024-00332-z SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative