Play all audios:
ABSTRACT Chronic inflammation in type 2 diabetes (T2D), characterized by constitutively activated immune cells and elevated pro-inflammatory mediators along with hyperglycaemia and increased
free fatty acids and branched chain amino acid levels, significantly alters the immuno-metabolic axis. Over the years, dietary intervention has been explored as an effective strategy for
managing T2D. Evidence from experimental and clinical studies indicates that various diets, including Mediterranean, Nordic, Palaeolithic and ketogenic diets, increase insulin sensitivity,
decrease gluconeogenesis, and adiposity, and exert anti-inflammatory effects, thus preserving immuno-metabolic homeostasis in individuals with T2D. Indian dietary sources are categorized as
_Sattvic_, _Rajasic_, and _Tamasic_, depending on their impact on health and behaviour. The Yogic diet, commonly recommended during yoga practice, is predominantly _Sattvic_, emphasizing
plant-based whole foods while limiting processed and high-glycaemic-index items. Yogic diet is also recommended for _Mitahara_, emphasizing mindful eating, which is attributed to calorie
restriction. Adopting a Yogic diet, featuring low-fat vegetarian principles, strongly reduces inflammatory mediator levels. This diet not only ameliorates insulin resistance and maintains a
healthy body weight but also regulates immunomodulation, enhances gut microbiome diversity and provides essential phytonutrients, collectively preventing inflammation. Although, preliminary
studies show aforementioned beneficial role of Yogic diet in improving diabetes associated metabolic and inflammatory changes, precise cellular and molecular mechanisms are not yet
understood. Hence, further studies are warranted to decipher the mechanisms. This review summarizes the multiple roles of Yogic diet and related dietary components in mitigating inflammation
and enhancing glycaemic control in T2D. SIMILAR CONTENT BEING VIEWED BY OTHERS THE ROLE OF DIETARY INFLAMMATORY INDEX IN METABOLIC DISEASES: THE ASSOCIATIONS, MECHANISMS, AND TREATMENTS
Article 21 October 2024 A LEGUME-ENRICHED DIET IMPROVES METABOLIC HEALTH IN PREDIABETES MEDIATED THROUGH GUT MICROBIOME: A RANDOMIZED CONTROLLED TRIAL Article Open access 22 January 2025
ALMOND SNACKING MODULATES GUT MICROBIOME AND METABOLOME IN ASSOCIATION WITH IMPROVED CARDIOMETABOLIC AND INFLAMMATORY MARKERS Article Open access 20 March 2025 INTRODUCTION Type 2 diabetes
(T2D) is associated with chronic and low-grade inflammation characterized by elevated levels of inflammatory mediators and constitutively activated immune cells. In response to various
extrinsic and intrinsic factors, such as a sedentary lifestyle, obesity, hyperglycaemia, oxidative and ER stress and genetic and epigenetic reprogramming, the activation of inflammatory
signalling pathways, such as the Jun N-terminal kinase (JNK) and nuclear factor-κ B (NF-κB) pathways, induces the constitutive production of inflammatory mediators. Studies have shown that
inflammatory cytokines such as interleukin (IL)-1β, tumour necrosis factor-_α_ (TNF-_α_), interleukin-6 (IL-6), MCP-1 and many others regulate metabolic processes under both physiological
and pathological conditions. Chronic activation of the innate immune system associated with hyperglycaemia leads to persistent low-grade inflammation, which contributes to a range of
complications, such as impaired β cell function, insulin resistance and impaired glucose homeostasis, leading to the onset and progression of diabetes and its complications [1,2,3,4].
Diabetes and obesity are metabolic disorders that manifest as a consequence of multiple factors, including the consumption of high-calorie foods, insufficient physical activity and a genetic
predisposition. Excess consumption of processed grains, added sugars, fried foods, and processed and red meat elevates the risk of T2D-related diseases. Hence, the conventional approach to
managing T2D involves medication, lifestyle modifications, and dietary interventions. Various components in our diet induce both pro-inflammatory and anti-inflammatory signals, influencing
immune responses within the gut. Different dietary nutrients directly interact with components of both innate and adaptive immunity or exert their effects by influencing the gut microbiota
and its metabolites, thereby contributing to immune responses. In mouse models, a high-fat diet (HFD) caused an increase in the ratio of Firmicutes to Bacteroidetes, triggering the release
of pro-inflammatory cytokines such as IL-6, IFN-γ, IL-1β and TNF-α resulting in signs of endotoxemia. A comparison study between whole and refined grain revealed that whole grain led to a
reduction in energy intake, body weight, and low-grade systemic inflammatory mediators such as CRP and IL-6. Importantly, these noticeable changes were independent of the composition of the
gut microbiome, underscoring the direct immune-modulating effects of whole grains [5]. Taken together, mounting evidence indicates that dietary factors regulate inflammatory pathways; hence,
a tailored diet may help reduce inflammation in individuals with T2D and maintain metabolic homeostasis. Over the years, multiple dietary patterns, including the Mediterranean diet, Chinese
diet and Palaeolithic diet have been examined to discern their influence on inflammatory processes in T2D. The essence of the Mediterranean diet philosophy involves prioritizing the
consumption of legumes, vegetables, fruits, nuts, wholegrain foods, and fish. The results of several studies revealed that the Mediterranean diet significantly improves HbA1c levels, with
greater adherence linked to a notable 23% reduction in the risk of developing T2D [6,7,8]. Compared to a diet designed for diabetes management, the Palaeolithic diet, also known as the
caveman diet, significantly decreased the average HbA1c, triglyceride, diastolic blood pressure, weight, body mass index, and waist circumference [9]. The Yogic/_Sattvic_ diet is an integral
part of traditional yoga practices and has emerged as a potential complementary strategy in T2D management. This diet is often recommended during regular yoga, where the dietary pattern
emphasizes the consumption of plant-based, whole foods while reducing the consumption of processed and high-glycaemic-index foods [10]. Despite the promising potential of the yogic diet as
an adjunctive strategy in the holistic management and reduction of inflammation in T2D patients, further research is essential to elucidate the underlying precise mechanisms of action and
long-term effects. This review aims to deepen our comprehension of the fundamental principles of the yogic diet and assess its effectiveness in managing glycaemic levels and reducing
inflammation in individuals with T2D. MULTIPLE PATHWAYS IN T2D INDUCE CHRONIC LOW-GRADE INFLAMMATION IN DIFFERENT TISSUES Experimental and clinical studies have demonstrated the activation
of tissue-specific inflammatory pathways and the induction of metabolic alterations affecting insulin sensitivity, gluconeogenesis, adiposity and the immune system. Adipose tissue
inflammation plays an important role in regulating insulin sensitivity and glucose tolerance. During low-grade chronic adipose tissue inflammation, macrophages and other immune cell
populations infiltrate the adipose tissue. An increase in the production of pro-inflammatory chemokines and cytokines such as C-C motif chemokine ligand 2, TNF-α, IL-1β and IL-6, as well as
a decrease in the expression of the key insulin-sensitizing adipokine, adiponectin, is associated with the infiltration of pro-inflammatory cells into adipose tissue. Compared to
subcutaneous fat, visceral fat has been shown to have a stronger association with insulin resistance [11]. The insulin receptor substrate-1 (IRS-1)/PI3K/Akt signalling axis is disrupted,
contributing to reduced glucose transport and leading to hyperglycaemia [12]. Along with the increased secretion of proinflammatory cytokines (TNF-α and IL-6), adipose tissue macrophage
infiltration and activation contribute to insulin resistance and impaired insulin signalling. The Toll-like receptor (TLR) pathway and the NOD-like receptor family pyrin domain containing 3
(NLRP3) inflammasome also play roles in connecting inflammation to metabolic dysfunction [2]. In rodent models of obesity-induced diabetes, macrophage infiltration is increased in pancreatic
islets with a more pro-inflammatory phenotype [13]. Excessive lipid accumulation in non-adipose organs such as the liver and skeletal muscle leads to lipotoxicity and tissue damage.
Ceramides and the intracellular lipid metabolite diacylglycerol (DAG) both activate the protein phosphatase 2 A (PP2A) and protein kinase C (PKC) pathways, affecting insulin signalling and
reducing glucose absorption. Additionally, fatty metabolites may cause the unfolded protein response (UPR), increase ER stress and subsequently induce insulin resistance [14]. Emerging
evidence indicates that T2D and obesity alter the gut microbiota composition, characterized by a reduction in beneficial bacteria and an increase in potentially pathogenic species [15].
Dysbiosis in the gut microbiota promotes inflammation, insulin resistance and metabolic dysfunction through multiple pathways, including the production of short-chain fatty acids (SCFAs) and
endotoxins such as lipopolysaccharides (LPS). SCFAs influence insulin sensitivity and gluconeogenesis, while increased LPS levels contribute to systemic inflammation and insulin resistance
[16]. Hyperglycaemia activates the NF-κB and JNK pathways, leading to pro-inflammatory milleu, impaired insulin signalling and insulin resistance in several insulin-responsive tissues, such
as hepatocytes, skeletal muscles, adipocytes and endothelial cells [17, 18]. Compared to that in nonobese individuals, the expression of genes associated with inflammation in obese
individuals was markedly elevated in abdominal subcutaneous adipocytes. The accumulation of lipids in adipocytes causes nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
activation, which exacerbates ROS production. Inflammation in T2D is primarily triggered by cytokines and adipokines released by adipocytes, including IL-6, TNF-α, resistin, visfatin,
angiotensin, adiponectin, leptin, monocyte chemoattractant protein-1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1). Some of these proteins are also released by immune cells that are
recruited to adipose tissue and participate in a feed forward loop for inflammation [19]. The major adipokines originating from adipose tissue during T2D are adiponectin and leptin. Among
these, leptin shows significant pro-inflammatory activity [20]. PI3K signalling is required for leptin effects in the hypothalamus, and a dysfunctional pathway contributes to leptin
resistance during diet-induced obesity. Leptin also triggers immune responses and induces other inflammatory mediators [21]. Increased amounts of IL-6 increase free-flowing fatty (FFA)
synthesis and help inhibit insulin pathway signalling, which reduces the responsiveness of insulin in the liver and muscles. IL-6 regulates lipid metabolism, including suppressing
triglyceride deposition and lipoprotein lipase (LPL) activity [22]. TNF-α attenuates the insulin signalling pathway by inhibiting insulin receptor tyrosine kinase activity in adipocytes and
decreasing tyrosine phosphorylation and IRS-1 activation, which in turn reduces the ability of cells to respond to insulin. TNF-α has also been shown to decrease the expression of genes
involved in insulin signalling [23]. It has been demonstrated that TNF-α stimulates the synthesis of chemokine (C-X-C motif) ligand 5 (CXCL5), a potent macrophage chemoattractant. Animals
treated with anti-CXCL5 or those with C-X-C motif chemokine receptor 2 (CXCR2) knockouts exhibited reduced insulin resistance [24]. Resistin is a polypeptide that is crucial for numerous
biological processes, including lipid metabolism, inflammation, and insulin resistance, as studied in rodent models. Recombinant human resistin causes insulin resistance via both the AMPK
(AMP-activated protein kinase)-dependent and AMPK-independent suppressor of cytokine signalling-3 (SOCS-3) signalling pathways [25]. Data indicate that adiponectin regulates microRNAs to
reduce intracellular proinflammatory pathways such as TLR-4 signalling, which contributes to some of the anti-inflammatory effects it has on adipose tissue [26]. IL-10 blocks the synthesis
of pro-inflammatory cytokines, including TNF-α and IL-6, and exerts a key anti-inflammatory effect. Reduced amounts of IL-10, which is produced by lymphocytes and macrophages, are associated
with metabolic syndrome and inflammatory responses in individuals with T2D via the reduction of tyrosine kinase activity of insulin receptors [27]. Nucleotide-binding oligomerization
domain-like receptor 3 (NLRP3) is an inflammasome that functions in the production of IL-1β and IL-18, resulting in insulin resistance. In obese subjects with T2D, caloric restriction and
exercise-mediated weight loss are linked to decreased NLRP3 expression in adipose tissue, as well as reduced inflammation and increased insulin sensitivity [28]. Adipocytes generate MCP-1
(CCL2), a chemoattractant for monocytes, dendritic cells, and memory T cells [29]. Weisberg et al., in 2006 demonstrated that knocking out C-C chemokine receptor type 2 (CCR2), a crucial
MCP-1 receptor, shows decreased adipose tissue macrophage recruitment and inflammatory gene expression and further protected against insulin resistance in HFD models [30]. Glucose is the
source of energy for immune cells, including neutrophils; hence, hyperglycaemia has an adverse impact on neutrophil function [31]. Previous studies have demonstrated that both diabetic and
high glucose-pretreated neutrophils respond less strongly to stimuli such as LPS and form weaker and nonfunctional NETs [32]. In individuals with T2D, fasting and postprandial glucose levels
are significantly correlated with neutrophil elastase and cell-free DNA [33]. It has been suggested that inflammatory pathways and excessive activation of T cells are also associated with
the pathogenesis of T2D. The control mechanism regulating the generation of several sets of effector cytokines is closely associated with the differentiation of effector T cells [34]. T2D
patients exhibit improperly differentiated T lymphocytes [35]. Although the proportion of B cells did not differ, the expression of CD38 (cluster of differentiation 38) in B cells was
greater in normal individuals than in obese subjects with T2D [36]. Additionally, it was demonstrated that a HFD caused an increase in B-cell recruitment in adipose tissue [37]. Compared
with those in nondiabetic donors, intra-islet increases in macrophages, along with polarization markers (CD11c, CD163, and NOS2) and proinflammatory cytokines (TNF-α, IL-6, and IL-1), have
been demonstrated in T2D patients. IL-1β and IL-10 were expressed mostly by resident macrophages after clodronate-mediated depletion, whereas IL-6, TNF-α, and transforming growth factor β1
(TGFB1) were found to primarily originate from nonmacrophage sources in human islets [13]. TNF-α, IL-1β, and interferon-γ (INF-γ) have also been shown to contribute to β-cell malfunction and
enhance susceptibility to β-cell toxicity. These cytokines increase the formation of the free radical nitric oxide (NO), which significantly slows cellular metabolism by impairing
mitochondrial activity [38]. High FFA plasma levels are associated with glucose intolerance, disrupted muscle insulin signalling, increased hepatic gluconeogenesis, and reduced insulin
response to glucose [39]. Additionally, the liver releases several acute-phase proteins in response to proinflammatory cytokines, including C-reactive protein (CRP), serum amyloid-A (SAA),
alpha-1-acid glycoprotein (AGP), PAI-1 and haptoglobin [40]. The skeletal muscle is also considered a target of insulin resistance induced by inflammation [41]. Vascular cells also actively
participate in inflammatory processes. The normal endothelium does not allow circulating leukocytes to adhere to it. However, under T2D conditions, the endothelium expresses cell adhesion
molecules that adhere to leukocytes [42]. Our earlier studies showed that IL-6 induces proteasomal degradation of DNMT1 and induces DNA methylation-dependent gene expression related to
insulin signalling and angiogenesis [43]. Taken together, the above studies demonstrate that the T2D microenvironment induces tissue-specific inflammation and that inflammatory mediators
disrupt insulin signalling and consequently alter metabolic homeostasis. DIETARY COMPONENTS MODULATE INFLAMMATORY AND METABOLIC HOMEOSTASIS The influence of the immune system on nutrient
distribution is apparent in various conditions, including food intake, obesity and exercise. Dietary intake plays a pivotal role in triggering cytokine activation and subsequent
inflammation. Studies in animal models focusing on diet-induced obesity provide substantiating evidence that an organism’s diet profoundly affects the immune system. Studies have shown that
obesity induces baseline elevation of proinflammatory cytokines such as IL-6 and TNF-α in the brain, with heightened levels in the cortex and hippocampus [44, 45]. The postprandial period is
characterized by an intricate state involving endocrine, metabolic, and inflammatory processes. Studies have demonstrated that an elevated intake of dietary fat results in increased
circulating levels of bacterial endotoxins such as LPS and proinflammatory cytokines in healthy individuals [46, 47]. Postprandial elevation of serum LPS indicates that metabolic endotoxemia
induced by dietary fat may underlie the frequently observed postprandial inflammatory response. Elevated inflammatory cytokines, including TNF-α, IL-6, IFN-α, IL-1β, IFN-γ, IL-10, IL-12,
and MIP-1β, have been observed after consumption of a high-fat meal in both individuals with type 1 diabetes (T1D) and healthy individuals. Notably, in the postprandial state, concentrations
of triglycerides were found to be correlated with interleukin-10 (IL-10) and interleukin-12 (IL-12) [48, 49]. Ingestion of a high-fat, high-carbohydrate or combined diet induces
postprandial inflammatory responses in healthy subjects, marked by increased plasma lipopolysaccharides, ROS production, IL-6, TNF-α, Nf-κB and elevated leukocyte counts. Various gut
hormones, including glucagon-like peptide-1 (GLP-1), bile acids, leptin, FGF19 and ghrelin, play roles in the postprandial period and may exert anti-inflammatory effects [47, 50].
Furthermore, the levels of hormones related to the hypothalamic‒pituitary‒adrenal (HPA) axis, such as adrenocorticotropic hormone and cortisol, increase postprandially, which inhibits the
production of several cytokines [47]. Given that the gut microbiome and dietary elements, such as saturated fatty acids, initiate inflammatory pathways via TLR4 and Nf-κB, numerous studies
have focused on examining the impact of nutrition and dietary patterns on chronic low-grade inflammation. The consumption of vegetables and fruits was associated with a decrease in baseline
IL-6 levels, while the consumption of whole grains was linked to reduced TNF-α levels. Among various Mediterranean diets, the Cretan diet has been suggested to be particularly beneficial,
potentially owing to its high intake of fresh vegetables, fruits, legumes and cereals. Adhering to the Mediterranean diet revealed a reduction in inflammatory markers, including CRP, IL-6,
and ICAM-1, emphasizing the potential positive effects on inflammation and endothelial function [51]. In a cohort of healthy individuals, studies revealed a pronounced correlation between
elevated weekly grain intake, particularly exceeding the 75th percentile, and heightened serum levels of circulating TNF-α, MCP-1 and IFN-γ. Similarly, an intricate association was
identified between red meat consumption and a statistically significant increase in IL-8 and CRP and a reiterated increase in IL-8. Furthermore, subjects exhibiting a proclivity for
increased fruit consumption demonstrated elevated concentrations of interferon gamma-induced protein-10 (IP-10), IL-8, and IFN-γ. Notably, increased levels of IL-8 were detected in
individuals who tended to consume more sweets. Conversely, a significant reduction in CRP levels was observed in individuals with augmented intake of eggs, greens, or shelled fruits. Within
this subgroup, a statistically significant decrease in IL-6 and IL-1β was also observed, emphasizing the nuanced impact of specific dietary preferences on modulating inflammatory markers
among healthy individuals [52]. These data suggest that dietary patterns, especially those emphasizing plant-based and Mediterranean approaches, play a crucial role in modulating
inflammation, with specific food choices demonstrating intricate associations with pro- and anti-inflammatory markers in both healthy individuals and those with T2D. MULTIPLE TYPES OF
DIETARY PATTERNS IN THE MANAGEMENT OF T2D-ASSOCIATED METABOLIC DYSREGULATION The manifestation and heterogeneity of the prevalence of T2D are influenced by a combination of factors,
including diverse ancestry, varied dietary patterns, and heterogeneous edible oil consumption across different agroclimatic conditions. Notably, studies underscore the substantial impact of
dietary choices on T2D predisposition. A balanced diet containing vegetables, fruits, lean meat, fish, and whole grain cereals rich in fibre, vitamins, and minerals lowers the risk of
chronic diseases such as cancer, diabetes, or cardiovascular disease [53, 54]. Micha et al. [55] demonstrated that dietary variables contribute significantly to fatalities from heart
disease, stroke, and T2D. A meta-analysis in European populations highlighted the association between increased consumption of red meat, processed meat, french fries, and refined grains and
an elevated risk of developing T2D [56]. Dietary habits alter intricate molecular pathways in T2D that affect glucose metabolism, insulin sensitivity and inflammation. Hence, understanding
these mechanisms is essential for developing targeted dietary interventions to enhance glycaemic regulation and overall metabolic health in individuals with T2D. Multiple studies have shown
that poor adherence to diet and exercise regimens is a significant barrier to the use of nonpharmacological therapies for diabetes [57]. Excessive protein intake, particularly from fatty
meat, results in elevated gluconeogenesis and increased blood glucose levels in individuals with T2D [58]. The consumption of high levels of dietary fats, including saturated and trans fats,
has the potential to activate inflammatory pathways, induce TLRs and subsequently induce insulin resistance. A greater intake of processed grains, added sugars, fried foods, and processed
or red meats may increase the risk of developing T2D. In children, the consumption of sugar-sweetened beverages has been identified as one of the contributing factors to the risk of
childhood obesity [59], potentially leading to future diabetes-related complications. Chronic sugar consumption induces hyperinsulinemia and disrupts insulin receptor signalling, resulting
in reduced cellular responsiveness to insulin. Excessive fructose consumption stimulates liver lipogenesis, contributing to non-alcoholic fatty liver disease (NAFLD), a common comorbidity of
T2D. Contrary to expectations, zero-calorie drinks or diet soda appear ineffective and do not reduce the likelihood of developing T2D [60, 61]. A high intake of white rice increased the
risk of developing T2D in Japanese women [62]. Epidemiological studies have revealed a significant association between diet and the incidence of T2D, with urbanization playing a crucial
role. Nonetheless, there is a gap in information regarding the substantial contribution of fat intake to obesity, which potentially acts as a precursor to the development of T2D, as opposed
to the intake of other macronutrients. Opting to avoid fast foods, fatty meats, sugar-sweetened beverages, and untimely food consumption while incorporating a diet rich in beneficial foods
such as fruits, vegetables, and healthy dairy products reduces the likelihood of developing T2D [63]. A study by Yang et al., 2024, demonstrated a negative correlation between the onset of
T2D and adherence to a prudent diet, characterized by high intake of whole grains, fruits, fish, and vegetables. Conversely, diets that restrict wheat, dairy, and eggs, as well as meat-based
and full-cream dairy diets, exhibited positive associations with the onset of T2D [64]. Experiments on high fat diet induced obese mice by Ding et al., 2018 indicate that the administration
of resveratrol (RES), a polyphenol predominantly present in grapes and mulberries, enhances CCR2 expression in white adipose tissue, mitigates inflammation, and diminishes macrophage
infiltration, thereby improving insulin signaling markers in both subcutaneous and visceral adipose tissue. This strategy aids in preserving glucose metabolic equilibrium in obese mice
caused by a high-fat diet [65]. In parallel with contemporary scientific findings, a plant-based diet rich in fruits, vegetables, whole grains, and legumes has demonstrated efficacy in
improving lipid profiles, insulin sensitivity and glycaemic management in individuals with T2D [66]. In the context of T2D, the management of weight and glycaemic indices is effectively
addressed through the practice of structured mindful eating [67]. Essential strategies for regulating blood glucose levels include maintaining portion control and distributing meals evenly
throughout the day. The adoption of smaller, more frequent meals can mitigate sharp fluctuations in glucose levels, contributing to a more stable glycaemic profile [68]. A dietary focus on
foods with a lower glycaemic index, coupled with an increased emphasis on complex carbohydrates such as whole grains and high-fibre foods, results in a decreased rate of glucose absorption.
This dietary approach mitigates post meal spikes and augments glycaemic control [69]. Furthermore, the incorporation of unsaturated fats, particularly omega-3 fatty acids derived from
sources such as nuts and avocados, exhibits anti-inflammatory properties and enhances insulin sensitivity in individuals with T2D [70]. The inclusion of soluble fibres in the diet
contributes to the deceleration of glucose absorption, which is crucial for postprandial glucose management and overall glucose homeostasis [71, 72]. Notably, dietary fibres modulate the gut
microbiota, positively influencing metabolic health and reducing inflammation. Short-chain fatty acids (SCFAs), which are produced in the gut by specific microorganisms, possess
anti-inflammatory properties and may augment insulin sensitivity [73]. Epidemiological studies on T2D underscore that a greater consumption of fruits, vegetables, whole grains, and low-fat
dairy products may diminish the risk of developing diabetes [74]. Studies have demonstrated a positive association between the consumption of vegetables, fruits, legumes, and dairy products
and improved insulin activity, considering the glycaemic index and insulin activity alongside dietary habits [75]. Hence, cultivating a harmonious and healthy dietary regimen, coupled with
consistent engagement in physical and mental exercise, may pave the way for a lifestyle devoid of disease. In the context of T2D, caloric restriction alleviates oxidative stress and
inflammation, as does autophagy. Autophagy restores pancreatic β-cell function and enhances glucose uptake by target tissues, including the liver and skeletal muscle [76]. Moreover, calorie
restriction induces autophagy in liver cells, promoting hepatic insulin sensitivity and mitigating fatty liver disease, a prevalent consequence of T2D [77]. Notably, a deficiency in
autophagy proteins exacerbates the expression of proinflammatory markers, indicating the intricate link between caloric restriction, autophagy, and the management of T2D [78]. CLASSIFICATION
OF INDIAN DIETARY PATTERNS According to the _Triguna_ hypothesis, physical makeup and mental attitudes are significantly regulated by regular dietary patterns [79]. The food we eat help us
decides among consciousness, inertia, and agitation. Ancient yogic science divided food into three primary _categories Sattvic_ foods, _Rajasic_ foods, and _Tamasic_ foods—based on how these
food components affect the _trigunas_ of the mind-body complex [80]. Yogic diet mainly encompasses a _sattvic_ diet that includes a predominantly vegetarian diet, eaten with mindfulness and
gratitude [81]. The food components classified under the _sattvic_ category are fresh, nutrient-rich, naturally tasty, and light in the stomach, which are mostly consumed by those who
practice yoga and those who aim for physio-psychological benefits. Eating such a diet increases life expectancy, inner and exterior strength, happiness, wisdom, health and satisfaction.
_Sattvic_ foods encompass water, cereals, legumes, grains, fruits, most vegetables, nuts, and unrefined dairy products, such as unpasteurized and homogenized fresh milk, ghee, butter,
paneer, cream, yogurt, and raw honey. Among these components, fresh milk from a contented cow and fruits fallen directly from trees are considered the purest manifestations of _sattvic_
food, being unadulterated and perceived as a gracious gift from nature. Consuming a meal long after it has been prepared is not deemed _sattvic_, as the food is believed to have diminished
in its inherent aura or energy [82, 83]. Food falling under the _rajasic_ category is characterized by attributes such as spiciness, sourness, excessive sweetness or dryness. Consequently,
individuals who partake in such foods are often characterized by intense passion and lead an active lifestyle. An appetite resembling that of a king aligns with the characteristics of a
_rajasic_ diet. However, the consumption of these foods is associated with the development of diseases, suffering, melancholy and restlessness attributed to the activation of _vata_ and
_pitta_ in the body [84]. _Rajasic_ foods include curd, nonvegetarian components; vegetables, including onion and garlic; and spices, such as pepper and chilies, along with lentils and
pulses [85]. Food categorized as _tamasic_ consists of food that has been stored overnight, leftovers, or has become stale. _Tamasic_ foods include nonvegetarians, fermented foods, bread,
cakes, alcohol, meat, and underripe and overripe vegetables and fruits [86]. It also encompasses overcooked, unclean, tasteless and rotten food, which tends to undergo microbial action,
resulting in a loss of nutritional value. The consequences of consuming such a diet include feelings of laziness, lethargy, heaviness, irritability and doubt. Additionally, a _tamasic_ diet
contributes to accelerated aging and heightened drowsiness [87]. The ingestion of meat contributes to elevated levels of saturated fats and cholesterol present in a carnivorous diet,
fostering the generation of bile acids. This process, followed by the conversion of these bile acids into deoxycholic acid and lithocholic acid by the gut microbiota, can lead to a reduction
in microbial diversity and the occurrence of gut dysbiosis. These transformed acids can be toxic to beneficial gut bacteria [88]. Embracing a lifestyle rooted in yoga may enhance digestion,
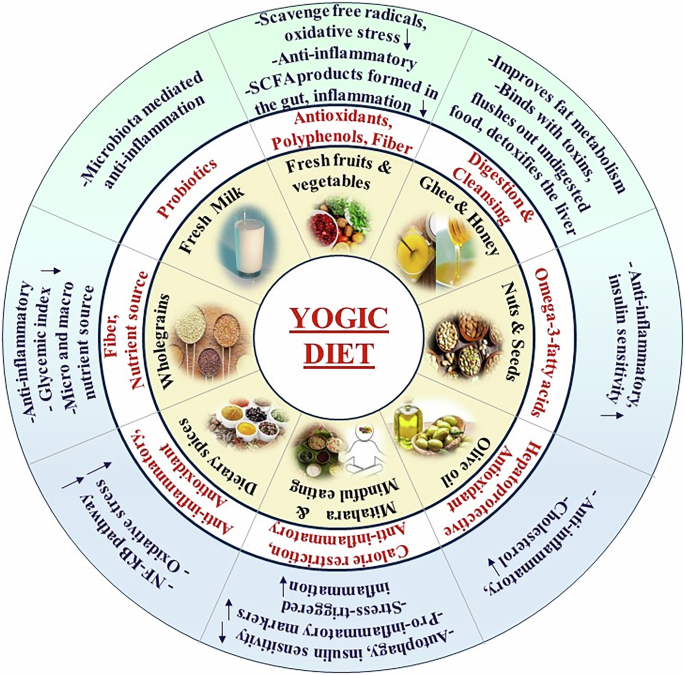
reduce inflammation, and contribute to weight loss, among various other health benefits [89]. COMPONENTS OF YOGIC DIET A yogic or _sattvic_ diet is generally fresh and light. Such dietary
practices provide mental peace and clarity along with having a balanced, easily digestible diet that focuses on a healthy immune system. The yogic diet encompasses a low-fat vegetarian diet
that ameliorates insulin resistance, helps maintain a healthy body weight, regulates immunomodulation, improves gut microbiome diversity, improves the gut microbiota, provides phytonutrients
and prevents inflammation [66, 90, 91]. Adopting a yogic lifestyle, including yogic practices and a corresponding diet, has been shown to alleviate stress, anxiety and depression while also
curbing food cravings and promoting a balanced body mass index. According to Ayurvedic concepts, adherence to a balanced _Sattvic_ diet is purported to enhance vitality, strength, and vigor
[54]. In the yogic diet, _Mitahara_ encompasses the concepts of calorie restriction, mindful eating and consuming food that are only congenial to health and wellbeing. Calorie restriction
in individuals with T2D contributes to weight loss, positively influencing insulin sensitivity and glycaemic control [92]. _The_ practice of _Mitahara_ leads to a reduction in visceral fat,
a key contributor to inflammation, thereby decreasing proinflammatory cytokines and adipokines [93]. Traditional yoga scriptures, such as _Hatha Pradipika and Gheranda Samhitha_, provide
invaluable guidance on dietary choices for Yoga Sadhakas (practitioners) to optimize their benefits [94]. The prescribed diet for yogic practitioners includes fresh whole lcereals, butter,
fruits, and vegetables, in alignment with the principle of _Mitahara_, emphasizing easily digestible and mind-pleasing food choices. A yogic diet recommends nutrient-dense foods, and mindful
eating aligns seamlessly with the overarching goals of T2D management. As a result, a burgeoning body of evidence suggests the potential benefits of integrating yoga and dietary
modifications in the comprehensive approach to T2D management, underscoring the need for more extensive research to elucidate their multifaceted roles. Fresh fruits and vegetables are an
integral part of the yogic diet and are rich sources of antioxidants that contain fibre and function in improving digestion [95]. Whole grains, rice and easily digestible pulses are
incorporated because these foods contain micro- and macronutrients and are carriers of vitamin B. Two essential components that are recommended for daily consumption are honey and ghee.
These factors help in easy digestion and overall cleansing [96]. The source of probiotics is fresh milk, which is an inevitable part of the yogic diet and contributes extensively to good
health [97]. A yogic diet is generally considered pleasant and sweet in taste. However, moderate levels of sugar, which is pleasant to the mind and aids in digestion, must be consumed [82].
Nuts are strong sources of healthy omega-3 fatty acids [66, 95]. India is known for its spices, and anti-inflammatory dietary spices, such as cinnamon, turmeric, ginger, coriander, cardamom
and saffron, are derived from plants used in Ayurveda. These compounds are extensively included in the yogic diet and have been shown to suppress inflammatory cytokines and NF-κB signalling
pathways, which contribute to the pathogenesis of diseases such as cardiovascular diseases and T2D [98] (Fig. 1). Spicy food, including chilly, onion and garlic, increases _tamas_ and
_rajas_ in the body and reduces _agni_ [10]. Dietary components, including curd, garlic, nonvegetarian food, and oils such as mustard and sesame, are considered to be stimulatory and fierce
in passion due to their salty and pungent nature. Since yoga involves internal arousal of the nervous system, a yogic diet avoids foods that cause external stimulation [85, 94, 95]. Meat is
avoided because it is highly fat and unecological and contains adrenaline, which can cause fear. Refined grains and pulses are not included in the Yogic diet due to loss of mineral content
during refining and may cause constipation. In addition, it is essential to eat a yogic diet according to the agro-climatic region and at proper times. This is because the cycle of the body
is closely connected to the natural habitat [10]. YOGIC DIET AMELIORATES INFLAMMATION IN T2D Studies have shown that dietary modifications play a crucial role in mitigating inflammation
associated with T2D [99, 100]. Some preliminary studies suggest that a yogic diet may play a beneficial role in improving diabetes-related metabolic and inflammatory changes. However, the
precise cellular and molecular mechanisms remain unclear due to the diverse dietary patterns followed across various agroclimatic regions of the Indian subcontinent, which contribute to the
vast heterogeneity and complexity in defining a sattvik or yogic diet. A study on diabetic postmenopausal women examined inflammatory mediators after a prescribed diet change and exercise
regimen. The authors demonstrated that exercise alone did not cause much change, and there was a difference in the various inflammatory markers in response to a low-fat diet. Plasma CRP
levels and leptin levels were decreased upon intervention with dietary modifications along with exercise, whereas few changes were found in adiponectin and TNF-α [101]. A yogic diet
positively affects inflammatory markers and stress markers within 10 days of the regimen. The levels of these markers, including IL-6 and TNF-α, were significantly reduced [102]. A study in
72 subjects with obesity suggested that diet-based interventions had beneficial effects on aging and inflammatory processes. The major outcomes were the relative fold changes in the
expression of genes related to oxidative stress, NF-κB, IL-6, TNF-α, and human telomerase reverse transcriptase (TERT) in peripheral blood mononuclear cells (PBMCs) [103]. According to Das
et al. (2023), a yogic diet encourages the consumption of anti-inflammatory foods such as fruits, vegetables, whole grains, nuts and seeds [91]. These dietary components are full of
bioactive substances such as vitamins, minerals, polyphenols, and antioxidants that have been found to control inflammatory pathways. Antioxidants scavenge free radicals and lessen oxidative
stress, which is a significant factor in T2D-related inflammation [104]. Polyphenols inhibit inflammatory enzymes, such as cyclooxygenase (COX) and lipoxygenase (LOX), thus suppressing the
production of pro-inflammatory mediators [105]. Plant-based diets are also associated with reduced cardiovascular risk factors in individuals suffering from T2D [66]. Ghrita, an ayurvedic
formulation which constitutes ghee from cow milk, is considered to be the superior of fats and good for oleation. Ghee is rich in conjugated linoleic acid and proven to be antidiabetic.
However, with ghee rich in linoleic acid, demonstrated a reduction in prostaglandins, leucotrines and interleukins associated with inflammation. In subjects with T2D or metabolic syndrome,
consumption of milk and dairy products did not show pro-inflammatory (neutral) or showed an anti-inflammatory effect [106,107,108]. Numerous clinical studies have demonstrated that
substituting honey for sucrose and dextrose results in lowered glucose levels, diminished postprandial glycaemia in healthy individuals, and a reduced postprandial glycaemic response
[109,110,111,112]. The effect of honey on cell cultures have shown to reduce inflammatory mediators such as TNF- α, IL-1 β, IL-6 and also inhibit TLR4/NFκB expression [113]. Several studies
have demonstrated the influence of natural honey consumption among healthy human subjects and has been reported to reduce post-prandial glycaemic response and glucose level in the blood
[110, 111, 114, 115]. The study demonstrated that hesperidin, a flavanone glycoside present in citrus fruits, plays a role in glucose homeostasis. Consumption of hesperidin supplements may
enhance glycaemic control through improved antioxidant effects. Hesperidin significantly influences glucose transporters (GLUTs) by down-regulating hepatic GLUT2 expression and up-regulating
GLUT4 expression in adipocytes. Additionally, it has been reported that hesperidin enhances the expression of adipocyte PPAR genes and glucokinase [116]. Dairy products and dairy proteins
have demonstrated efficacy in reducing inflammatory markers, including CRP, IL-6, TNF-α, and MCP-1, in individuals with obesity and overweight conditions [108, 117]. Whole grains have
contributed to stable HbA1c levels, and lower concentrations of C-peptide and leptin in healthy subjects [118]. Furthermore, legume seeds, especially peanut butter, have demonstrated
potential in decreasing inflammatory biomarkers, including total cholesterol, LDL, and apolipoprotein B (apo B), in women with T2D [119]. Furthermore, the prudent diet shown an inverse
correlation with the majority of inflammatory markers, while the full-cream dairy diet showed a positive correlation with these markers. Significantly, the majority of inflammatory markers,
especially the INFLA-score, exhibited a strong correlation with the onset of T2D. The INFLA-score mediated 13% of the link between the sensible diet and the incidence of T2D, and 34% of the
association between the full-cream dairy diet and the beginning of T2D [64]. Linoleic acid (LA) (18:2n-6), an essential fatty acid that derived primarily from plant oils and legumes,
constituted 85–90% of dietary n-6 PUFAs. According to a meta-analysis investigation of the dose-dependent relationship between LA and the risk of T2D demonstrated that greater intake of LA
reduces risk T2D [120]. Flaxseed, an excellent source of omega fatty acids, has been shown to decrease serum glucose and insulin levels in overweight/obese individuals with pre-diabetes
after 12 weeks of consumption [116, 121]. Studies indicates that the consumption of vegetables is connected with a 9% reduction in the incidence of T2D with an increased intake of up to 300
g/day, consumption of fruits up to 200–300 grams per day lowered T2D risk by 10 and dairy consumption diminished the risk of T2D by 6% with increased intake up to 400–600 g/day [122].
Plant-based proteins, such as those from legumes, nuts, and seeds have been shown to reduce inflammation, likely due to their anti-inflammatory properties and relatively high fibre content
[123]. Dietary spices have played a major role in the Indian diet since the inception of Ayurveda and are known for their anti-inflammatory properties. It has been demonstrated that
cinnamaldehyde and cinnamon treatment leads to downregulation of the NF-κB pathway and reduces the levels of proinflammatory cytokines in diseases such as T2D and atherosclerosis in ApoE−/−
mice [124, 125]. The focus of yogic diets on nutrient-dense, plant-based foods, along with the prevention of overeating, can aid in weight management, an important aspect of T2D management.
Given that adipokines such as adiponectin and leptin are secreted by adipose tissue in obesity, which leads to inflammation, weight loss following a yogic diet and lifestyle changes lessens
inflammation in adipose tissue and increases insulin sensitivity [11]. A comparative approach revealed that a low-carbohydrate diet was more beneficial than a low-fat diet (LFD) for a better
inflammatory state, as indicated by the levels of interleukins and tumor necrosis factors in the serum [126]. The emphasis of the yogic diet on fibre-rich foods supports gut health and
promotes a diverse and balanced gut microbiota composition. A healthy gut microbiota is associated with reduced inflammation and improved metabolic health. Certain gut microbes metabolize
dietary fibre into SCFAs, such as butyrate, acetate, and propionate, which possess anti-inflammatory properties [127]. SCFAs inhibit NF-κB activation, suppress proinflammatory gene
expression, and promote the generation of regulatory T cells, which help maintain immune balance [128]. Mindful eating practices, an integral part of the yogic diet, reduce stress and
promote relaxation [129]. Inflammation in T2D patients is worsened by chronic stress, which further leads to an increase in pro-inflammatory mediators [130]. Mindful eating habits, such as
being present and grateful for each meal, can lower stress hormone levels and help alleviate inflammation. The yogic diet stresses whole, unprocessed foods and the avoidance of refined
sugars and unhealthy fats that help improve insulin sensitivity. Upon promoting better glycaemic control and reducing hyperinsulinemia, the yogic diet may directly and indirectly contribute
to the reduction in inflammatory pathways associated with insulin resistance (Fig. 2). CONCLUSION The intricate interplay among chronic inflammation, lifestyle factors and T2D underscores
the importance of holistic management. From the detrimental effects of poor dietary choices to the positive impacts of mindful eating, plant-based diets, and traditional practices such as
Ayurveda and yoga, it is clear that nutrition plays a critical role in T2D development, management, and prevention. Conventional strategies involve medication, lifestyle modifications, and
dietary interventions, and hence, the practice of the yogic diet as a complementary strategy offers a promising avenue. Studies highlight the beneficial impact of dietary patterns such as
_sattvic_ and Mediterranean diets on reducing inflammation, emphasizing the nuanced role of individual food groups in influencing inflammatory markers. Dietary modifications, particularly
those promoting whole foods and avoiding inflammatory triggers, offer a potential strategy for alleviating inflammation in patients with T2D. Further research is essential to fully
comprehend the cellular and molecular mechanisms and long-term impact of the yogic diet on T2D management. REFERENCES * de Baat A, Trinh B, Ellingsgaard H, Donath MY. Physiological role of
cytokines in the regulation of mammalian metabolism. Trends Immunol. 2023;44:613–27. Article PubMed Google Scholar * Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease.
Nat Rev Immunol. 2011;11:98–107. Article CAS PubMed Google Scholar * Kalin MF, Goncalves M, John-Kalarickal J, Fonseca V. Pathogenesis of type 2 diabetes mellitus. In: Principles of
Diabetes Mellitus: Third Edition. Springer International Publishing; 2017. p. 267–77. * Sapra A, Bhandari P. Diabetes. In Treasure Island (FL); 2023. * Bilal M, Ashraf S, Zhao X. Dietary
Component-Induced Inflammation and Its Amelioration by Prebiotics, Probiotics, and Synbiotics. Front Nutr. 2022;9:931458. Article PubMed PubMed Central Google Scholar * Carter P, Achana
F, Troughton J, Gray LJ, Khunti K, Davies MJA. Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysis. J Hum Nutr
Diet. 2014;27:280–97. Article CAS PubMed Google Scholar * Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes
mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014;63:903–11. Article CAS PubMed Google Scholar * Josefs T, Barrett TJ, Brown EJ, Quezada A,
Wu X, Voisin M, et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight. 2020;5. * Klonoff DC. The
beneficial effects of a Paleolithic diet on type 2 diabetes and other risk factors for cardiovascular disease. Journal diabetes Sci Technol. 2009;3:1229–32. United States. Article Google
Scholar * Vagh MG. Influence and intimation of yogic diet over the mind. International J Yogic, Hum Mov Sports Sci. 2019;878:878–81. Google Scholar * Burhans MS, Hagman DK, Kuzma JN,
Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2019;9:1–58. Article Google Scholar * Boucher J,
Kleinridders A, Kahn CR. Insulin Receptor Signaling in Normal. Cold Spring Harb Perspect Biol. 2014;6:a009191 2014. Article PubMed PubMed Central Google Scholar * He W, Yuan T, Maedler
K. Macrophage-associated pro-inflammatory state in human islets from obese individuals. Nutr Diabetes. 2019;9:0–3. Article Google Scholar * Kojta I, Chacińska M, Błachnio-Zabielska A.
Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020;12:1305. Article CAS PubMed PubMed Central Google Scholar * Harsch IA, Konturek PC. The
Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabetes Mellitus: New Insights into ‘Old’ Diseases. Med Sci (Basel). 2018;6:1–28. Google Scholar * Scheithauer TPM, Rampanelli E,
Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol. 2020;11:1–29. Article
Google Scholar * Yerneni KKV, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor κB in cultured fibroblasts and endothelial cells.
Diabetes. 1999;48:855–64. Article CAS PubMed Google Scholar * Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal TK, Maeda K, et al. A central role for JNK in obesity and insulin
resistance Jiro Hirosumi, Gu¨rol Tuncman, Lufen Chang. Nature. 2002;420:333–6. Article CAS PubMed Google Scholar * Lin Y, Berg AH, Iyengar P, Lam TKT, Giacca A, Combs TP, et al. The
hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. Journal Biol Chem. 2005;280:4617–26. Article CAS Google Scholar * Nikolajczyk BS,
Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. Article CAS PubMed PubMed Central
Google Scholar * Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pr. 2013;99:85–92.
Article CAS Google Scholar * Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance - A mini-review. Gerontology. 2009;55:379–86. Article CAS PubMed Google Scholar *
Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-α. Journal Biol Chem. 1991;266:21839–45. Article CAS Google
Scholar * Chavey C, Lazennec G, Lagarrigue S, Clapé C, Iankova I, Teyssier J, et al. CXC Ligand 5 Is an Adipose-Tissue Derived Factor that Links Obesity to Insulin Resistance. Cell Metab.
2009;9:339–49. Article CAS PubMed PubMed Central Google Scholar * Luo Z, Zhang Y, Li F, He J, Ding H, Yan L, et al. Resistin induces insulin resistance by both AMPK-dependent and
AMPK-independent mechanisms in HepG2 cells. Endocrine. 2009;36:60–9. Article CAS PubMed Google Scholar * Ge Q, Gérard J, Noël L, Scroyen I, Brichard SM. MicroRNAs regulated by
adiponectin as novel targets for controlling adipose tissue inflammation. Endocrinology. 2012;153:5285–96. Article CAS PubMed Google Scholar * van Exel E, Gussekloo J, de Craen AJ,
Frölich M, Bootsma-Van Der Wiel A, Westendorp RG, et al. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-plus study.
Diabetes. 2002;51:1088–92. Article PubMed Google Scholar * Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced
inflammation and insulin resistance. Nat Med. 2011;17:179–89. Article CAS PubMed PubMed Central Google Scholar * Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity
and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–70. Article CAS PubMed PubMed Central Google Scholar * Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et
al. CCR2 modulates inflammatory and metabo. Journal Clin Investig. 2006;116:115–24. Article CAS Google Scholar * Dowey R, Iqbal A, Heller SR, Sabroe I, Prince LR. A Bittersweet Response
to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity. Front Immunol. 2021;12:1–21. Article Google Scholar * Joshi MB, Lad A, Bharath Prasad AS,
Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–6.
Article CAS PubMed Google Scholar * Joshi MB, Baipadithaya G, Balakrishnan A, Hegde M, Vohra M, Ahamed R, et al. Elevated homocysteine levels in type 2 diabetes induce constitutive
neutrophil extracellular traps. Sci Rep. 2016;6:36362. Article CAS PubMed PubMed Central Google Scholar * Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–18. Article PubMed Google Scholar * Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of Adaptive and Innate Immunity in
Type 2 Diabetes Mellitus. J Diabetes Res. 2018;2018. * Van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, et al. Increased systemic and adipose tissue inflammation
differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63:492–501. Article PubMed Google Scholar * DeFuria J, Belkina AC, Jagannathan-Bogdan
M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile.
Proc Natl Acad Sci USA. 2013;110:5133–8. Article CAS PubMed PubMed Central Google Scholar * Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and
beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32:24–34. Article CAS PubMed Google Scholar *
Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: Results from the san antonio metabolism study. Diabetes.
2017;66:815–22. Article PubMed Google Scholar * Al-Shukaili A, Al-Ghafri S, Al-Marhoobi S, Al-Abri S, Al-Lawati J, Al-Maskari M. Analysis of inflammatory mediators in type 2 diabetes
patients. Int J Endocrinol. 2013;2013:8–10. Article Google Scholar * Shoelson SE, Herrero L, Naaz A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology. 2007;132:2169–80.
Article CAS PubMed Google Scholar * Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. Article CAS PubMed Google Scholar
* Balakrishnan A, Guruprasad KP, Satyamoorthy K, Joshi MB. Interleukin-6 determines protein stabilization of DNA methyltransferases and alters DNA promoter methylation of genes associated
with insulin signaling and angiogenesis. Laboratory Investig. 2018;98:1143–58. Article CAS Google Scholar * Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al.
Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. Article CAS PubMed Google Scholar * Baumgarner KM, Setti
S, Diaz C, Littlefield A, Jones A, Kohman RA. Diet-induced obesity attenuates cytokine production following an immune challenge. Behavioural brain Res. 2014;267:33–41. Article CAS Google
Scholar * Laugerette F, Vors C, Géloën A, Chauvin MA, Soulage C, Lambert-Porcheron S, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade
inflammation. J Nutr Biochem. 2011;22:53–9. Article CAS PubMed Google Scholar * Meessen ECE, Warmbrunn M V, Nieuwdorp M, Soeters MR. Human Postprandial Nutrient Metabolism and Low-Grade
Inflammation: A Narrative Review. Nutrients. 2019 11. * Laugerette F, Vors C, Peretti N, Michalski MC. Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation.
Biochimie. 2011;93:39–45. Article CAS PubMed Google Scholar * Fogarty CL, Nieminen JK, Peräneva L, Lassenius MI, Ahola AJ, Taskinen MR, et al. High-fat meals induce systemic cytokine
release without evidence of endotoxemia-mediated cytokine production from circulating monocytes or myeloid dendritic cells. Acta Diabetol. 2015;52:315–22. Article CAS PubMed Google
Scholar * Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through
distinct signaling pathways. Surgery. 2008;143:334–42. Article PubMed Google Scholar * Knight A, Bryan J, Wilson C, Hodgson J, Murphy K. A randomised controlled intervention trial
evaluating the efficacy of a Mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: the MedLey study. BMC Geriatr. 2015;15:55. Article
PubMed PubMed Central Google Scholar * D’Esposito V, Di Tolla MF, Lecce M, Cavalli F, Libutti M, Misso S, et al. Lifestyle and Dietary Habits Affect Plasma Levels of Specific Cytokines in
Healthy Subjects. Front Nutr. 2022;9:913176. Article PubMed PubMed Central Google Scholar * Scarborough P, Nnoaham KE, Clarke D, Capewell S, Rayner M. Modelling the impact of a healthy
diet on cardiovascular disease and cancer mortality. J Epidemiol Community Health. 2012;66:420–6. (1978). Article PubMed Google Scholar * Chordia N. Concept yogic diet yoga Lit.
2018;3:2255–7. Google Scholar * Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2
diabetes in the United States. JAMA - J Am Med Assoc. 2017;317:912–24. Article Google Scholar * Ibsen DB, Steur M, Imamura F, Overvad K, Schulze MB, Bendinelli B, et al. Replacement of Red
and Processed Meat With Other Food Sources of Protein and the Risk of Type 2 Diabetes in European Populations: The EPIC-InterAct Study. Diabetes Care. 2020;43:2660–7. Article CAS PubMed
PubMed Central Google Scholar * Raveendran AV, Deshpandae A, Joshi SR. Therapeutic Role of Yoga in Type 2 Diabetes. Endocrinology Metab. 2018;33:307–17. Article Google Scholar *
Fromentin C, Tomé D, Nau F, Flet L, Luengo C, Azzout-Marniche D, et al. Dietary proteins contribute little to glucose production, even under optimal gluconeogenic conditions in healthy
humans. Diabetes. 2013;62:1435–42. Article CAS PubMed PubMed Central Google Scholar * Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and
childhood obesity: A prospective, observational analysis. Lancet. 2001;357:505–8. Article CAS PubMed Google Scholar * Malik V, Hu F. Fructose and Cardiometabolic Health: What the
Evidence from Sugar-Sweetened Beverages Tells Us. J Am Coll Cardiol. 2015;66:1615–24. Article CAS PubMed PubMed Central Google Scholar * Sakurai M, Nakamura K, Miura K, Takamura T,
Yoshita K, Nagasawa SY, et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr. 2014;53:251–8.
Article CAS PubMed Google Scholar * Nanri A, Mizoue T, Noda M, Takahashi Y, Matsushita Y, Poudel-Tandukar K, et al. Fish intake and type 2 diabetes in Japanese men and women: The Japan
Public Health Center-based prospective study. American J Clin Nutr. 2011;94:884–91. Article CAS Google Scholar * Seidell JC. Dietary fat and obesity: An epidemiologic perspective.
American J Clin Nutr. 1998;67:546–50. Article Google Scholar * Yang G, Du X, Wang J, Jiang X, Shi S, Shen J, et al. Unveiling the Roles of Immune Function and Inflammation in the
Associations Between Dietary Patterns and Incident Type 2 Diabetes. Journal Am Nutr Assoc. 2025;44:59–67. CAS Google Scholar * Ding S, Jiang J, Wang Z, Zhang G, Yin J, Wang X, et al.
Resveratrol reduces the inflammatory response in adipose tissue and improves adipose insulin signaling in high-fat diet-fed mice. PeerJ. 2018;6:e5173. Article PubMed PubMed Central Google
Scholar * McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. Journal Geriatr Cardiol. 2017;14:342–54. CAS Google Scholar * Miller CK, Kristeller
JL, Headings A, Nagaraja H, Miser WF. Comparative Effectiveness of a Mindful Eating Intervention to a Diabetes Self-Management Intervention among Adults with Type 2 Diabetes: A Pilot Study.
J Acad Nutr Diet. 2012;112:1835–42. Article PubMed PubMed Central Google Scholar * Mekary RA, Giovannucci E, Cahill L, Willett WC, Van Dam RM, Hu FB. Eating patterns and type 2 diabetes
risk in older women: Breakfast consumption and eating frequency. American J Clin Nutr. 2013;98:436–43. Article CAS Google Scholar * Koloverou E, Panagiotakos DB. Macronutrient composition
and management of non-insulin-dependent diabetes mellitus (NIDDM): A new paradigm for individualized nutritional therapy in diabetes patients. Review Diabet Stud. 2016;13:6–16. Article
Google Scholar * Rivellese AA, De Natale C, Lilli S. Type of Dietary Fat and Insulin Resistance. Ann N. Y Acad Sci. 2002;967:329–35. Article CAS PubMed Google Scholar * Lunn J, Buttriss
JL. Carbohydrates and dietary fibre. Nutr Bull. 2007;32:21–64. Article Google Scholar * Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food
Sci Nutr. 2017;57:1950–62. CAS PubMed Google Scholar * Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from
current human evidence to future possibilities. Diabetologia. 2017;60:943–51. Article CAS PubMed PubMed Central Google Scholar * Odegaard AO, Koh WP, Butler LM, Duval S, Gross MD, Yu
MC, et al. Dietary patterns and incident type 2 diabetes in Chinese men and women the Singapore Chinese Health Study. Diabetes Care. 2011;34:880–5. Article PubMed PubMed Central Google
Scholar * Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, et al. The Relationship between Dietary Habits, Blood Glucose and Insulin Levels among People
without Cardiovascular Disease and Type 2 Diabetes; The ATTICA Study. Review Diabet Stud. 2005;2:208–208. Article Google Scholar * Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, Karmakar
P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018;17:709–20. PubMed PubMed Central Google Scholar * Yang L, Li P, Fu S, Calay ES,
Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–78. Article CAS PubMed PubMed Central Google Scholar *
Choi AJS, Ryter SW. Autophagy in inflammatory diseases. Int J Cell Biol. 2011;2011. * Shilpa S, Venkatesha Murthy CG. Development and standardization of Mysore Triguna scale. Sage Open.
2012;2:1–10. Google Scholar * Shankar NR, Nk M, Venugopal V, Sreedhar P, Sukanya R, Hr N. Review article Concept of Holistic Diet – Blending of Yogic Diet and Balanced Diet - A Review.
2017;:59–71. * Puta, M., & Sedlmeier, P. The concept of tri-guna: A working model. In S. Schmidt & H. Walach (Eds.), Meditation — Neuroscientific approaches and philosophical
implications. 2014 (pp. 317-64). * Maurya S, Dhimdhime RS, Khandekar SB, Shinde MM, Khan TK. Concept of yogic diet for healthy modern life. World J Pharm Res. 2021;10:954–67. Google Scholar
* Kanoujia S. Yofa and Yogic Diet: Tool for Holistic Empowerment of Women. Think India. 2019;23:208–13. Google Scholar * Dhanya S, Ramesh NV, Mishra A. Traditional methods of food habits
and dietary preparations in Ayurveda — The Indian system of medicine. Journal Ethn Foods. 2019;6:1–9. Google Scholar * Junnarkar G. Principles of Diet for a Yogic Lifestyle. In: The
Principles and Practice of Yoga in Cardiovascular Medicine. 2022. p. 65–7. * Bansal A, Srivastava S. Concept of Yogic Diet and Mental Health: A Literature Review on Scientific and Scriptural
aspects. 2022;6:8364–75. * Rathore J. Indian Food Patterns and Triguna Theory of Personality. Int. j Res Humanit & Soc. Sci. 2020;8:51–3. Google Scholar * Riccio P, Rossano R.
Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: A matter of barriers and a proposal on the origin of organ specificity. International J
Multidiscip Educ Res. 2019;11:2714. CAS Google Scholar * Stephens I. Medical Yoga Therapy. Children. 2017;4:12. Article PubMed PubMed Central Google Scholar * Manchanda SC. Yoga - A
promising technique to control cardiovascular disease. Indian Heart J. 2014;66:487–9. Article CAS PubMed PubMed Central Google Scholar * Das M, Pundir M, Nayak P, Patra S, Thajuddin N.
Yogic diet on gut microbial diversity in asthma. Yoga Mimamsa. 2023;55:58. Article Google Scholar * Napoleão A, Fernandes L, Miranda C, Marum AP. Effects of Calorie Restriction on Health
Span and Insulin Resistance: Classic Calorie Restriction Diet vs. Ketosis-Inducing Diet. Nutrients. 2021;13:1302. Article PubMed PubMed Central Google Scholar * Khanna D, Khanna S,
Khanna P, Kahar P, Patel BM. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus. 2022;14:e22711. PubMed PubMed Central Google Scholar * Desai BP. Place of nutrition in
yoga. Anc Sci Life. 1990;9:147–53. CAS PubMed PubMed Central Google Scholar * Ramos-Jiménez A, Wall-Medrano A, I CHR, Hernández-Torres RP. Yoga, bioenergetics and eating behaviors: A
conceptual review. Int J Yoga. 2015;8:89–95. Article PubMed PubMed Central Google Scholar * Reddy GG. Yogic Diet for Well-Being. International J Multidiscip Educ Res. 2022;11:65–7.
Google Scholar * Kishore R, Zaidi A, Dixit A, Srivastav AK. Yogic concept of diet and their scientific aspect. European Chem Bull. 2023;12:5064–7. Google Scholar * Kocchar KP, Sunil, Ghosh
T, Arora J. Yogic Diet and its Anti-inflammatory Effect in Relation to CVD. In: The Principles and Practice of Yoga in Cardiovascular Medicine. 2022. p. 395–403. * Santos L. The impact of
nutrition and lifestyle modification on health. Eur J Intern Med. 2022;97:18–25. Article PubMed Google Scholar * Guo Y, Huang Z, Sang D, Gao Q, Li Q. The Role of Nutrition in the
Prevention and Intervention of Type 2 Diabetes. Front Bioeng Biotechnol. 2020; 15:8:575442 * Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, et al. Effects of
diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–75. Article CAS PubMed Google Scholar *
Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: Preliminary results. Journal Alternative
Complementary Med. 2012;18:662–7. Article Google Scholar * Sharma P, Yadav RK, Khadgawat R, Dada R. Transcriptional modulation of inflammation, and aging in Indian obese adults following a
12-week yoga-based lifestyle intervention: A randomized controlled trial. Front Med (Lausanne). 2022;9:898293. Article PubMed Google Scholar * Samtiya M, Aluko RE, Dhewa T, Moreno-Rojas
JM. Potential health benefits of plant food-derived bioactive components: An overview. Foods. 2021;10:1–25. Article Google Scholar * Yahfoufi N, Alsadi N, Jambi M, Matar C. The
immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1–23. Article Google Scholar * Sharma H, Zhang X, Dwivedi C. The effect of ghee (clarified butter) on serum
lipid levels and microsomal lipid peroxidation. Ayu. 2010;31:134–40. Article PubMed PubMed Central Google Scholar * Ulven SM, Holven KB, Gil A, Rangel-Huerta OD. Milk and Dairy Product
Consumption and Inflammatory Biomarkers: An Updated Systematic Review of Randomized Clinical Trials. Adv Nutr. 2019;10:S239–50. Article PubMed PubMed Central Google Scholar * Nieman KM,
Anderson BD, Cifelli CJ. The Effects of Dairy Product and Dairy Protein Intake on Inflammation: A Systematic Review of the Literature. J Am Coll Nutr. 2021;40:571–82. Article CAS PubMed
Google Scholar * Al-Waili NS. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: comparison with
dextrose and sucrose. J Med Food. 2004;7:100–7. Article CAS PubMed Google Scholar * Shambaugh P, Worthington V, Herbert JH. Differential effects of honey, sucrose, and fructose on blood
sugar levels. J Manipulative Physiol Ther. 1990;13:322–5. CAS PubMed Google Scholar * Samanta A, Burden AC, Jones GR. Plasma glucose responses to glucose, sucrose, and honey in patients
with diabetes mellitus: an analysis of glycaemic and peak incremental indices. Diabet Med. 1985;2:371–3. Article CAS PubMed Google Scholar * Al-Waili NS, Boni NS. Natural honey lowers
plasma prostaglandin concentrations in normal individuals. J Med Food. 2003;6:129–33. Article CAS PubMed Google Scholar * van den Berg AJJ, van den Worm E, van Ufford HCQ, Halkes SBA,
Hoekstra MJ, Beukelman CJ. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17:172–4. 176-178. Article PubMed Google
Scholar * Münstedt K, Sheybani B, Hauenschild A, Brüggmann D, Bretzel RG, Winter D. Effects of basswood honey, honey-comparable glucose-fructose solution, and oral glucose tolerance test
solution on serum insulin, glucose, and C-peptide concentrations in healthy subjects. J Med Food. 2008;11:424–8. Article PubMed Google Scholar * Sharma R, Martins N, Chaudhary A, Garg N,
Sharma V, Kuca K, et al. Adjunct use of honey in diabetes mellitus: A consensus or conundrum?. Trends Food Sci Technol. 2020;106:254–74. Article CAS Google Scholar * Yari Z, Cheraghpour
M, Alavian SM, Hedayati M, Eini-Zinab H, Hekmatdoost A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: an open-labeled randomized controlled trial. Eur J Clin
Nutr [Internet]. 2021;75:99–111. Article CAS PubMed Google Scholar * Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J
Clin Nutr. 2011;94:422–30. Article CAS PubMed PubMed Central Google Scholar * Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Grønbaek M, Rimm EB. Whole grains, bran, and germ in
relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr. 2006;83:275–83. Article CAS PubMed Google Scholar * Souza RGM, Gomes AC, Naves MMV,
Mota JF. Nuts and legume seeds for cardiovascular risk reduction: scientific evidence and mechanisms of action. Nutr Rev. 2015;73:335–47. Article PubMed Google Scholar * Mousavi SM,
Jalilpiran Y, Karimi E, Aune D, Larijani B, Mozaffarian D, et al. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response
Meta-analysis of Prospective Cohort Studies. Diabetes Care. 2021;44:2173–81. Article CAS PubMed Google Scholar * Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE.
Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res. 2013;33:367–75. Article CAS PubMed Google Scholar *
Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective
studies. Eur J Epidemiol. 2017;32:363–75. Article PubMed PubMed Central Google Scholar * Hertzler SR, Lieblein-Boff JC, Weiler M, Allgeier C. Plant proteins: Assessing their nutritional
quality and effects on health and physical function. Nutrients. 2020;12:1–27. Article Google Scholar * Rao PV, Gan SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement
Altern Med. 2014;2014:642942. Article Google Scholar * Li W, Zhi W, Zhao J, Li W, Zang L, Liu F, et al. Cinnamaldehyde attenuates atherosclerosis via targeting the IκB/NF-κB signaling
pathway in high fat diet-induced ApoE(-/-) mice. Food Funct. 2019;10:4001–9. Article CAS PubMed Google Scholar * Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a
low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med. 2014;46:182–7. Article CAS PubMed Google
Scholar * Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. Article Google Scholar * Kim CH, Park J, Kim M. Gut
Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014;14:277. Article PubMed PubMed Central Google Scholar * Nelson JB. Mindful eating: The art of
presence while you eat. Diabetes Spectr. 2017;30:171–4. Article PubMed PubMed Central Google Scholar * Liu YZ, Wang YX, Jiang CL. Inflammation: The common pathway of stress-related
diseases. Front Hum Neurosci. 2017;11:1–11. Article Google Scholar Download references ACKNOWLEDGEMENTS Authors thank TIFAC-CORE, the Government of India; BUILDER, DBT, the Government of
India (BT/INF/22/SP43065/2021) and Manipal Academy of Higher Education for infrastructure. The authors thank the Ministry of Science & Technology, Department of Science and Technology,
Government of India (DST/SATYAM/2020/247 (G)) for funding. FUNDING Open access funding provided by Manipal Academy of Higher Education, Manipal. AUTHOR INFORMATION Author notes * These
authors contributed equally: Anupama Vallazhath, Pooja Yedehalli Thimmappa. AUTHORS AND AFFILIATIONS * Department of Ageing Research, Manipal School of Life Sciences, Manipal Academy of
Higher Education, Manipal, 576104, India Anupama Vallazhath, Pooja Yedehalli Thimmappa & Manjunath B. Joshi * Division of Ayurveda, Centre for Integrative Medicine and Research, Manipal
Academy of Higher Education, Manipal, 576104, India Harshit B. Joshi, Krishna Raghava Hebbar, Anupama Nayak & Basavaraj S. Hadapad * Department of Medicine, Dr. T.M.A. Pai Hospital,
576101, Udupi, India Shashikiran Umakanth * Swami Vivekananda Yoga Anusandhana Samsthana, Bangalore, 560105, Karnataka, India Apar Avinash Saoji & Nandi Krishnamurthy Manjunath * Centre
for Ayurveda Biology, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, 576104, India Manjunath B. Joshi Authors * Anupama Vallazhath View author publications
You can also search for this author inPubMed Google Scholar * Pooja Yedehalli Thimmappa View author publications You can also search for this author inPubMed Google Scholar * Harshit B.
Joshi View author publications You can also search for this author inPubMed Google Scholar * Krishna Raghava Hebbar View author publications You can also search for this author inPubMed
Google Scholar * Anupama Nayak View author publications You can also search for this author inPubMed Google Scholar * Shashikiran Umakanth View author publications You can also search for
this author inPubMed Google Scholar * Apar Avinash Saoji View author publications You can also search for this author inPubMed Google Scholar * Nandi Krishnamurthy Manjunath View author
publications You can also search for this author inPubMed Google Scholar * Basavaraj S. Hadapad View author publications You can also search for this author inPubMed Google Scholar *
Manjunath B. Joshi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS MBJ conceptualized and supervised the study. AV, PYT, HBJ wrote the
manuscript. KRH, AN, SU, AAS, NKM and BSH provided critical comments and contributed to writing the manuscript. CORRESPONDING AUTHOR Correspondence to Manjunath B. Joshi. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Vallazhath, A., Thimmappa, P.Y., Joshi, H.B. _et al._ A comprehensive review on the implications of Yogic/_Sattvic_ diet in reducing inflammation in type 2
diabetes. _Nutr. Diabetes_ 15, 14 (2025). https://doi.org/10.1038/s41387-025-00371-0 Download citation * Received: 23 May 2024 * Revised: 25 March 2025 * Accepted: 28 March 2025 * Published:
11 April 2025 * DOI: https://doi.org/10.1038/s41387-025-00371-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative