Play all audios:
ABSTRACT In IBD patients, integration between a hyper-activated immune system and epithelial cell plasticity underlies colon cancer development. However, molecular regulation of such a
circuity remains undefined. Claudin-1 (Cld-1), a tight-junction integral protein deregulation alters colonic epithelial cell (CEC) differentiation, and promotes colitis severity while
impairing colitis-associated injury/repair. Tumorigenesis is a product of an unregulated wound-healing process and therefore we postulated that upregulated Cld-1 levels render IBD patients
susceptible to the colitis-associated cancer (CAC). Villin Cld-1 mice are used to carryout overexpressed studies in mice. The role of deregulated Cld-1 expression in CAC and the underlying
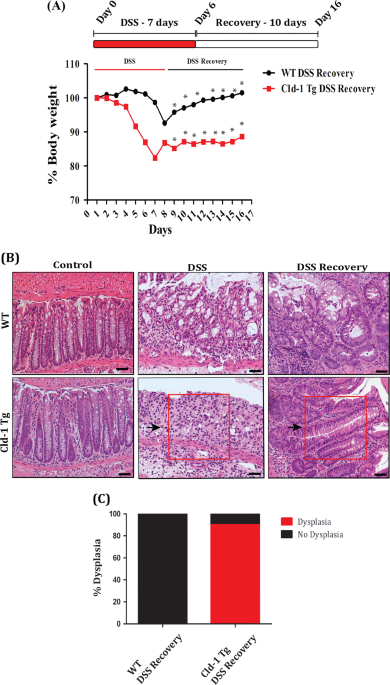
mechanism was determined using a well-constructed study scheme and mouse models of DSS colitis/recovery and CAC. Using an inclusive investigative scheme, we here report that upregulated
Cld-1 expression promotes susceptibility to the CAC and its malignancy. Increased mucosal inflammation and defective epithelial homeostasis accompanied the increased CAC in Villin-Cld-1-Tg
mice. We further found significantly increased levels of protumorigenic M2 macrophages and β-cateninSer552 (β-CatSer552) expression in the CAC in Cld-1Tg vs. WT mice. Mechanistic studies
identified the role of PI3K/Akt signaling in Cld-1-dependent activation of the β-CatSer552, which, in turn, was dependent on proinflammatory signals. Our studies identify a critical role of
Cld-1 in promoting susceptibility to CAC. Importantly, these effects of deregulated Cld-1 were not associated with altered tight junction integrity, but on its noncanonical role in
regulating Notch/PI3K/Wnt/ β-CatSer552 signaling. Overall, outcome from our current studies identifies Cld-1 as potential prognostic biomarker for IBD severity and CAC, and a novel
therapeutic target. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TNFAIP8 PROTEIN FUNCTIONS AS A TUMOR SUPPRESSOR IN INFLAMMATION-ASSOCIATED COLORECTAL TUMORIGENESIS Article Open access 06
April 2022 EPITHELIAL PBLD ATTENUATES INTESTINAL INFLAMMATORY RESPONSE AND IMPROVES INTESTINAL BARRIER FUNCTION BY INHIBITING NF-ΚB SIGNALING Article Open access 31 May 2021 DECREASED NHE3
EXPRESSION IN COLON CANCER IS ASSOCIATED WITH DNA DAMAGE, INCREASED INFLAMMATION AND TUMOR GROWTH Article Open access 30 August 2022 REFERENCES * Sengupta N, Yee E, Feuerstein JD. Colorectal
cancer screening in inflammatory bowel disease. Dig Dis Sci. 2016;61:980–9. Article Google Scholar * Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC. et al. Tight junctions in
inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117–26. Article CAS Google Scholar * Gunzel D, Yu AS. Claudins and
the modulation of tight junction permeability. Physiol Rev. 2013;93:525–69. Article Google Scholar * Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK. et al. Claudin-1
regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–34. Article CAS Google Scholar * Dhawan P, Singh AB, Deane NG, No Y, Shiou SR,
Schmidt C. et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–76. Article CAS Google Scholar * Pope JL, Ahmad R, Bhat
AA, Washington MK, Singh AB, Dhawan P. et al. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis. Mol
Cancer. 2014;13:167 Article Google Scholar * Ouban A. Claudin-1 role in colon cancer: an update and a review. Histol Histopathol. 2018;33:1013–19. PubMed Google Scholar * Liu L, Rao JN,
Zou T, Xiao L, Smith A, Zhuang R. et al. Activation of Wnt3a signaling stimulates intestinal epithelial repair by promoting c-Myc-regulated gene expression. Am J Physiol Cell Physiol.
2012;302:C277–85. Article CAS Google Scholar * Deitrick J, Pruitt WM. Wnt/beta catenin-mediated signaling commonly altered in colorectal cancer. Prog Mol Biol Transl Sci. 2016;144:49–68.
Article CAS Google Scholar * Sabatino L, Pancione M, Votino C, Colangelo T, Lupo A, Novellino E. et al. Emerging role of the beta-catenin-PPARgamma axis in the pathogenesis of colorectal
cancer. World J Gastroenterol. 2014;20:7137–51. Article Google Scholar * Goretsky T, Bradford EM, Ryu H, Tahir M, Moyer MP, Gao T. et al. A cytosolic multiprotein complex containing
p85alpha is required for beta-Catenin activation in colitis and colitis-associated cancer. J Biol Chem. 2016;291:4166–77. Article CAS Google Scholar * Miwa N, Furuse M, Tsukita S, Niikawa
N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–76. Article
CAS Google Scholar * Shiou S-R, Singh AB, Moorthy K, Datta PK, Washington MK, Beauchamp RD. et al. Smad4 regulates Claudin-1 expression in a transforming growth factor-β-independent manner
in colon cancer cells. Cancer Res. 2007;67:1571–9. Article CAS Google Scholar * Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising
model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–9. Article CAS Google Scholar * Liu Y, Cao X. The origin and function of tumor-associated
macrophages. Cell Mol Immunol. 2015;12:1–4. Article Google Scholar * Waniczek D, Lorenc Z, Snietura M, Wesecki M, Kopec A, Muc-Wierzgon M. Tumor-associated macrophages and regulatory T
cells infiltration and the clinical outcome in colorectal cancer. Arch Immunol Ther Exp (Warsz). 2017;65:445–54. Article CAS Google Scholar * Hermiston ML, Gordon JI. Inflammatory bowel
disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–7. Article CAS Google Scholar * Sebio A, Kahn M, Lenz HJ. The potential of targeting
Wnt/beta-catenin in colon cancer. Expert Opin Ther Targets. 2014;18:611–5. Article CAS Google Scholar * Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB. et al.
Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–81.81 e1–9. Article CAS
Google Scholar * Villegas SN, Gombos R, Garcia-Lopez L, Gutierrez-Perez I, Garcia-Castillo J, Vallejo DM. et al. PI3K/Akt cooperates with oncogenic Notch by inducing nitric oxide-dependent
inflammation. Cell Rep. 2018;22:2541–9. Article CAS Google Scholar * Breynaert C, Vermeire S, Rutgeerts P, Van Assche G. Dysplasia and colorectal cancer in inflammatory bowel disease: a
result of inflammation or an intrinsic risk?. Acta Gastroenterol Belg. 2008;71:367–72. CAS PubMed Google Scholar * Low D, Mino-Kenudson M, Mizoguchi E. Recent advancement in understanding
colitis-associated tumorigenesis. Inflamm Bowel Dis. 2014;20:2115–23. Article Google Scholar * Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology.
2010;138:2101–14 e5. Article CAS Google Scholar * Dave M, Loftus EV Jr. Mucosal healing in inflammatory bowel disease—a true paradigm of success? Gastroenterol Hepatol (NY). 2012;8:29–38.
Google Scholar * Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. Article CAS Google Scholar * Stidham RW, Higgins PDR. Colorectal cancer
in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31:168–78. Article Google Scholar * Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing—a literature review. Bras
Dermatol. 2016;91:614–20. Article Google Scholar * Neal MD, Richardson WM, Sodhi CP, Russo A, Hackam DJ. Intestinal stem cells and their roles during mucosal injury and repair. J Surg Res.
2011;167:1–8. Article CAS Google Scholar * Ormanns S, Neumann J, Horst D, Kirchner T, Jung A. WNT signaling and distant metastasis in colon cancer through transcriptional activity of
nuclear beta-Catenin depend on active PI3K signaling. Oncotarget. 2014;5:2999–3011. Article Google Scholar * Brown JB, Cheresh P, Goretsky T, Managlia E, Grimm GR, Ryu H. et al. Epithelial
phosphatidylinositol-3-kinase signaling is required for beta-catenin activation and host defense against Citrobacter rodentium infection. Infect Immun. 2011;79:1863–72. Article CAS Google
Scholar * Parang B, Kaz AM, Barrett CW, Short SP, Ning W, Keating CE. et al. BVES regulates c-Myc stability via PP2A and suppresses colitis-induced tumourigenesis. Gut. 2017;66:852–62.
Article CAS Google Scholar * Ahmad R, Kumar B, Chen Z, Chen X, Muller D, Lele SM. et al. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy
by hyperactivating Wnt/beta-catenin signaling. Oncogene. 2017;36:6592–604. Article CAS Google Scholar * Mendes RD, Canté-Barrett K, Pieters R, Meijerink JPP. The relevance of PTEN-AKT in
relation to NOTCH1-directed treatment strategies in T-cell acute lymphoblastic leukemia. Haematologica. 2016;101:1010–7. Article CAS Google Scholar * Wong GW, Knowles GC, Mak TW, Ferrando
AA, Zúñiga-Pflücker JC. HES1 opposes a PTEN-dependent check on survival, differentiation, and proliferation of TCRβ-selected mouse thymocytes. Blood. 2012;120:1439–48. Article CAS Google
Scholar * He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA. et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. Article CAS Google
Scholar * Singh A, Sharma A, Smith J, Krishnan M, Chen X, Eschrich S. et al. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells.
Gastroenterology. 2011;141:2140–53. Article CAS Google Scholar * Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I. et al. Activation of Notch1 signaling is required for
β-catenin–mediated human primary melanoma progression. J Clin Investig. 2005;115:3166–76. Article CAS Google Scholar * Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I. et al.
Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–48. Article CAS Google Scholar *
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 1525 Google Scholar * Snider AJ, Bialkowska
AB, Ghaleb AM, Yang VW, Obeid LM, Hannun YA. Murine model for colitis-associated cancer of the colon. Methods Mol Biol. 2016;1438:245–54. Article CAS Google Scholar * Erben U,
Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat M. et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol.
2014;7:4557–76. PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by BX002086 (VA merit), CA216746 (NIH/NCI) and a pilot project award
from Fred and Pamela Buffet Cancer Center, which is funded by a National Cancer Institute Cancer Center Support Grant under award number P30 CA036727 to P.D. and DK088902 (NIH/NIDDK) and
BX002761 (VA merit) to A.B.S. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, USA Saiprasad
Gowrikumar, Rizwan Ahmad, Srijayaprakash Babu Uppada, Amar B. Singh & Punita Dhawan * Department of Pathology, Vanderbilt University Medical Center, Nashville, TN, USA Mary K. Washington
& Chanjuan Shi * VA Nebraska-Western Iowa Health Care System, Omaha, NE, USA Amar B. Singh & Punita Dhawan * Buffet Cancer Center, University of Nebraska Medical Center, Omaha, NE,
USA Amar B. Singh & Punita Dhawan Authors * Saiprasad Gowrikumar View author publications You can also search for this author inPubMed Google Scholar * Rizwan Ahmad View author
publications You can also search for this author inPubMed Google Scholar * Srijayaprakash Babu Uppada View author publications You can also search for this author inPubMed Google Scholar *
Mary K. Washington View author publications You can also search for this author inPubMed Google Scholar * Chanjuan Shi View author publications You can also search for this author inPubMed
Google Scholar * Amar B. Singh View author publications You can also search for this author inPubMed Google Scholar * Punita Dhawan View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Punita Dhawan. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENT FIGURE LEGENDS SUPPLEMENT FIG. 1 SUPPLEMENT FIG. 2 SUPPLEMENT FIG. 3 SUPPLEMENT FIG. 4 SUPPLEMENT FIG. 5 SUPPLEMENT FIG. 6 SUPPLEMENT FIG. 7 RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gowrikumar, S., Ahmad, R., Uppada, S.B. _et al._ Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin
phosphorylation and activation in Notch/p-AKT-dependent manner. _Oncogene_ 38, 5321–5337 (2019). https://doi.org/10.1038/s41388-019-0795-5 Download citation * Received: 21 October 2018 *
Revised: 21 December 2018 * Accepted: 31 January 2019 * Published: 10 April 2019 * Issue Date: 27 June 2019 * DOI: https://doi.org/10.1038/s41388-019-0795-5 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative