Play all audios:
ABSTRACT Metastasis remains the major obstacle to improved survival for breast cancer patients. Downregulation of FOXO3a transcription factor in breast cancer is causally associated with the
development of metastasis through poorly understood mechanisms. Here, we report that FOXO3a is functionally related to the inhibition of VEGF-A/NRP1 signaling and to the consequent
suppression of breast cancer metastasis. We show that FOXO3a directly induces miR-29b-2 and miR-338 expression. Ectopic expression of miR-29b-2/miR-338 significantly suppresses EMT,
migration/invasion, and in vivo metastasis of breast cancer. Moreover, we demonstrate that miR-29b-2 directly targets VEGF-A while miR-338 directly targets NRP1, and show that regulation of
miR-29b-2 and miR-338 mediates the ability of FOXO3a to suppress VEGF-A/NRP1 signaling and breast cancer metastasis. Clinically, our results show that the
FOXO3a-miR-29b-2/miR-338-VEGF-A/NRP1 axis is dysregulated and plays a critical role in disease progression in breast cancer. Collectively, our findings propose that FOXO3a functions as a
metastasis suppressor, and define a novel signaling axis of FOXO3a-miRNA-VEGF-A/NRP1 in breast cancer, which might be potential therapeutic targets for breast cancer. SIMILAR CONTENT BEING
VIEWED BY OTHERS CRISPR INTERFERENCE AND ACTIVATION OF THE MICRORNA-3662-HBP1 AXIS CONTROL PROGRESSION OF TRIPLE-NEGATIVE BREAST CANCER Article 02 November 2021 MICRORNA-488 INHIBITS
PROLIFERATION AND MOTILITY OF TUMOR CELLS VIA DOWNREGULATING FSCN1, MODULATED BY NOTCH3 IN BREAST CARCINOMAS Article Open access 24 October 2020 MICRORNA-203A INHIBITS BREAST CANCER
PROGRESSION THROUGH THE PI3K/AKT AND WNT PATHWAYS Article Open access 27 February 2024 INTRODUCTION Breast cancer is the most common cancer diagnosed and the second leading cause of
cancer-related deaths among women worldwide [1]. Despite progress in treating primary breast cancers with chemotherapy, radiation, surgery, and targeted therapies, metastasis and recurrence
remain the major obstacles to improved survival for breast cancer patients [2]. Elucidating the molecular mechanisms of breast cancer metastasis/recurrence and searching for effective target
therapies is a promising pathway for improving survival. FOXO3a, a transcription factor of the Forkhead box O (FOXO) family, controls diverse biological processes by regulating the
expression of gene involved in cell-cycle progression, apoptosis, differentiation, metabolism, or stress resistance [3]. FOXO3a is frequently downregulated in various cancers and functions
as a tumor suppressor because it induces cell-cycle arrest and apoptosis [4,5,6]. Moreover, recent studies demonstrated that inactivation of FOXO3a can induce epithelial-to-mesenchymal
transition (EMT) and subsequently promote cancer cell invasion and dissemination, indicating that FOXO3a can act as a potential biomarker for the prediction and therapy of cancer metastasis
[7,8,9,10,11]. However, the biological function and exact mechanism of FOXO3a in regulating breast cancer metastasis are not completely understood. Various previous studies identified some
key signaling transduction cascades that are implicated in the progression, invasion, and metastasis of breast cancer [12]. Vascular endothelial growth factor (VEGF)-A, a main supervisor of
angiogenesis, is a dimeric glycoprotein secreted by many kinds of cells, including cancer cells [13]. VEGF-A binds to and activates both VEGFR-1 and VEGFR-2, promoting angiogenesis [14].
Increased signaling through the VEGF-A/VEGFRs axis has been suggested to promote cancer cell invasiveness and metastasis and is associated with poor prognosis [15,16,17]. Specifically,
neuropilin-1 (NRP1), a coreceptor of VEGF-A, forms complexes with VEGFRs to enhance the binding of VEGF-A to VEGFRs, thus promoting VEGF-A-mediated breast cancer cells EMT and metastasis
[18,19,20]. It has been reported that VEGF-A is negatively regulated by FOXO3a, and FOXO3a-mediated repression of VEGF-A may be involved in transcriptional repression [21]. However, it is
not clear whether other mechanisms may also account for this negative correlation between FOXO3a and VEGF-A. Specifically, no information is available as to whether microRNAs (miRNAs) play a
role in the FOXO3a-mediated inhibition of VEGF-A/NRP1 signaling. miRNAs are a class of small noncoding RNAs that regulate gene expression, either by inhibiting translation or by causing
degradation through binding to the 3′-untranslated regions (UTRs) of target messenger RNAs [22]. Emerging evidence has revealed that miRNAs play key roles in multiple biological processes of
cancer cells, including cancer cell proliferation, apoptosis, tumorigenesis, and metastasis [23]. Recently, several reports focused on the interaction of miRNAs and FOXO3a and confirmed the
involvement of miRNAs in the FOXO3a-mediated regulation of cancer progression and metastasis [7, 24], suggesting that FOXO3a might use miRNAs to make cell fate decisions. In the current
study, we demonstrate that FOXO3a is downregulated in breast cancer, and overexpression of FOXO3a suppresses VEGF-A/NRP1 signaling and breast cancer invasion and metastasis. We further show
that FOXO3a induces miR-29b/miR-338 expression by interacting with the miR-29b/miR-338 promoter and that miR-29b and miR-338 directly targets the VEGF-A and NRP1 3′-UTR, respectively. As a
new member of the FOXO3a regulatory network, miR-29b/miR-338 may play a critical role in the posttranscriptional regulation of the VEGF-A/NRP1 axis and breast cancer metastasis. Therefore,
our study suggests an important role of FOXO3a in inhibiting breast cancer metastasis and provides a previously undescribed mechanism of FOXO3a-mediated repression of VEGF-A/NRP1. RESULTS
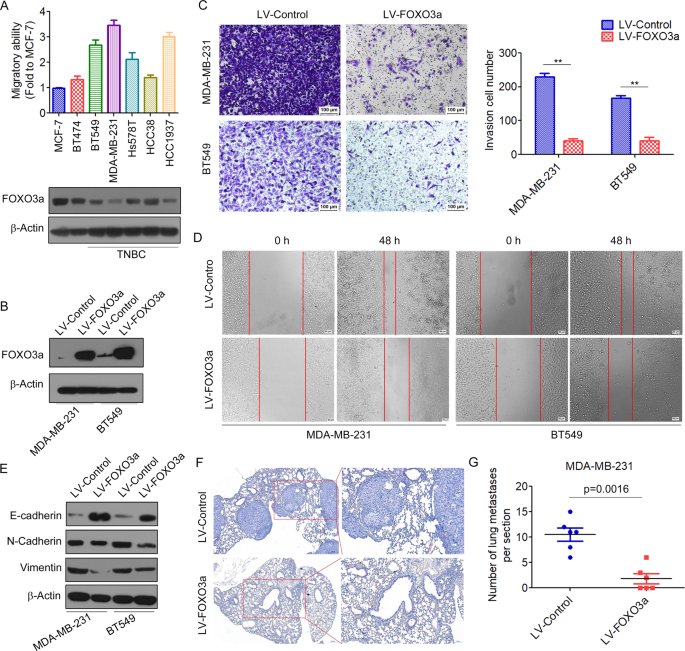
FOXO3A INHIBITS INVASION AND METASTASIS OF BREAST CANCER We previously observed a negative correlation between FOXO3a expression and lymph node metastasis in breast cancer tissues
(Supplementary Table 1) [25]. To determine the association between FOXO3a and tumor metastatic ability, we analyzed the protein expression of FOXO3a in several breast cancer cell lines. We
found that the invasion ability of breast cancer cells was inversely correlated with FOXO3a expressions (Fig. 1A). We then performed a transwell assay to investigate the effects of FOXO3a on
the invasive behaviors of breast cancer cells in vitro. The results demonstrated that overexpression of FOXO3a (Fig. 1B and Supplementary Fig. 1) significantly inhibited the invasion of
MDA-MB-231 and BT549 cells (Fig. 1C). A wound-healing assay also demonstrated that overexpression of FOXO3a decreased the migration capacity of breast cancer cells (Fig. 1D). EMT, a highly
conserved genetic program that enables epithelial tumor cells to migrate from the existing cell layer into surrounding tissues, is a key step in tumor metastasis [26]. We then investigated
the effects of FOXO3a on the expression of EMT markers in breast cancer cells. We found that overexpression of FOXO3a inhibited EMT in breast cancer cells, which was evidenced by increased
E-cadherin expression in conjunction with a concomitant decreased in the expression of mesenchymal markers vimentin and N-cadherin (Fig. 1E and Supplementary Fig. 2). To further investigate
the inhibition of in vivo tumor metastasis by FOXO3a, we implanted MDA-MB-231/LV-FOXO3a cells that were stably expressing FOXO3a or control cells into nude mice through the lateral tail
vein. Lung metastasis of breast cancer was apparent in mice injected with MDA-MB-231/LV-control cells. In contrast, few metastatic tumors were detected in mice injected with
MDA-MB-231/LV-FOXO3a cells (Fig. 1F, G). Together, these results suggested that FOXO3a might function as a metastasis suppressor in breast cancer cells. FOXO3A INHIBITS VEGF-A/NRP1 SIGNALING
IN BREAST CANCER CELLS The tumor microenvironment is increasingly recognized as an important contributor to tumor metastasis [27]. Cancer cells support their own progression by releasing
multiple growth factors that interact with themselves directly through both autocrine and paracrine manners [28]. To gain further insight into how FOXO3a suppresses breast cancer metastasis,
we examined the effect of FOXO3a on many microenvironmental genes, including TGF-β, TNF-α, EGF, FGF, PDGF, VEGF, and IGF, which have been implicated in promoting metastasis (Supplementary
Fig. 3A). Importantly, we found that overexpression of FOXO3a significantly inhibited VEGF-A expression (Fig. 2A, B and Supplementary Fig. 3A) and secretion (Fig. 2C). Previous studies
suggested that increased signaling through the VEGF-A/VEGFRs axis promotes cancer cell invasiveness and metastasis and is associated with poor prognosis [15,16,17]. We then investigated
whether FOXO3a regulates VEGF-A receptor expression. We found that overexpression of FOXO3a had no effect on VEGFR-1 and VEGFR-2 expression, but significantly decreased the expression of
NRP1 (Fig. 2A, B). In contrast, knockdown of FOXO3a by either of two short hairpin RNAs (shRNAs) significantly decreased the expression of VEGF-A and NRP1 (Supplementary Fig. 3B). Previous
studies suggested that activation of PI3K/AKT and ERK signaling could induce phosphorylation and nuclear exclusion of FOXO3a, thereby inhibiting FOXO3a transcriptional activity [4,5,6]. We
found that treatment with TIC10, a dual inhibitor of Akt and ERK [29], significantly increased the expression of FOXO3a, but decreased the expression of VEGF-A and NRP1 in MDA-MB-231 and
BT549 cells (Supplementary Fig. 4). We further compared FOXO3a, VEGF-A, and NRP1 expression in a tissue microarray containing 100 independent primary breast tumor samples by
immunohistochemistry. We observed a significant inverse correlation between FOXO3a and VEGF-A/NRP1 expression levels, and a significant positive correlation between VEGF-A and NRP1 in the
breast cancer tissue set (Fig. 2D, E). The correlation between FOXO3a and VEGF-A/NRP1 expression levels was further validated at the mRNA level in the cases from the cBioPortal database
(Supplementary Fig. 5). Moreover, we found that high levels of VEGF-A and NRP1 were correlated with lymph node metastasis (Supplementary Table 2). Furthermore, we examined whether the levels
of FOXO3a, VEGF-A, and NRP1 were associated with the survival of patients with breast cancer. Kaplan–Meier survival analyses revealed that patients with low FOXO3a expression had poorer
overall survival than patients with high FOXO3a expression, whereas patients with high VEGF-A and NRP1 expression had poorer overall survival than patients with low VEGF-A or NRP1 expression
(Fig. 2F). We next analyzed the correlation of FOXO3a, VEGF-A, and NRP1 expression with the prognosis of breast cancer patients with lymph node metastasis in the cases from the Kaplan–Meier
plotter data set. Lower levels of FOXO3a and higher levels of VEGF-A or NRP1 were correlated with shorter survival in the lymph node metastasis positive subgroup (Supplementary Fig. 6A).
However, no significant difference in prognosis was observed between lymph node metastasis negative breast cancer patients with high or low NRP1 expression (Supplementary Fig. 6B). Taken
together, these findings indicated that FOXO3a negatively regulates VEGF-A/NRP1 and that dysregulated FOXO3a/VEGF-A/NRP1 signaling plays a critical role in disease progression in breast
cancer. FOXO3A INDUCES MIR-29B AND MIR-338 EXPRESSION IN BREAST CANCER CELLS Previous studies suggested that FOXO3a directly binds to the VEGF-A promoter and inhibits its expression [21].
However, the NRP1 promoter lacked clear FOXO3a-binding sites, and FOXO3a did not decrease the activity of a luciferase reporter containing the NRP1 promoter (data not shown). We therefore
speculated that FOXO3a might regulate VEGF-A/NRP1 indirectly through miRNAs. To identify differentially expressed miRNAs regulated by FOXO3a in breast cancer, we compared the miRNA profiles
of the FOXO3a-overexpressing MDA-MB-231 cells and control cells by miRNA microarray analysis. Unsupervised clustering of significantly deregulated miRNAs is presented in Fig. 3A. We found
that thirty-six miRNAs were significantly upregulated (greater than twofold) in MDA-MB-231/FOXO3a cells compared with MDA-MB-231/Control cells. In addition, forty-three miRNAs were
downregulated (<50%) in MDA-MB-231/FOXO3a cells. The target mRNAs of differentially expressed miRNAs were predicted using TargetScan. We found that miR-29b-2, which targets VEGF-A, and
miR-338 and miR-211, which targets NRP1 were upregulated in MDA231-FOXO3a cells (Fig. 3B). qRT-PCR analysis further confirmed that overexpression of FOXO3a significantly increased the
expression level of miR-29b-2 and miR-338, but not miR-211, in both MDA-MB-231 and BT549 cells (Fig. 3C). In contrast, knockdown of FOXO3a significantly decreased the expression of miR-29b-2
and miR-338 in MCF-7 and BT474 cells (Supplementary Fig. 7). A sequence analysis of the promoter regions revealed that the miR-29b-2 promoter contains two FOXO3a-binding sites (FHRE-1,
FHRE-2) and the miR-338 promoter contains one FOXO3a-binding site (FHRE) (Fig. 3D). To validate a direct binding of FOXO3a to the promoter region, we conducted a chromatin
immunoprecipitation (ChIP)-qPCR assay using anti-FOXO3a antibody. The results demonstrated strong enrichment of FOXO3a in the miR-29-2 promoter regions and miR-338 promoter regions
corresponding to the predicted FOXO3a-binding sites (Fig. 3E). To further demonstrate the regulation of the promoter regions of miR-29b-2 and miR-338 by FOXO3a, we performed a luciferase
reporter assay. Our results showed that FOXO3a significantly increased the activity of both miR-29b-2 and miR-338 promoter reporter (Fig. 3F), and deleting the FHRE sites diminished
FOXO3a-mediated reporter induction, demonstrating that these sites are necessary and functional (Fig. 3F). MIR-29B-2 DIRECTLY TARGETS VEGF-A WHILE MIR-338 DIRECTLY TARGETS NRP1 TargetScan
analyses revealed that the 3′-UTR of VEGF-A mRNA contains two highly conserved miR-29b-2 binding sites, whereas the 3′-UTR of NRP1 mRNA contains one highly conserved miR-338 binding site
(Fig. 4A). We then tested whether VEGF-A and NRP1 are direct targets of miR-29b-2 and miR-338, respectively. Dual-luciferase reporter analysis showed that transfection of miR-29b-2 mimics
significantly inhibited the activity of firefly luciferase that carried the wild-type but not mutant 3′UTR of VEGF-A (Fig. 4B). We also found that miR-338 mimics repressed the activity of
firefly luciferase that carried the wild-type but not mutant 3′UTR of NRP1 (Fig. 4B). Furthermore, ectopic expression of miR-29b-2 in MDA-MB-231 and BT549 cells resulted in a marked decrease
in VEGF-A mRNA and protein levels. Similarly, ectopic expression of miR-338 also significantly suppressed the mRNA and protein expression of NRP1 in breast cancer cells (Fig. 4C, D).
Together, these data supported direct inhibition of VEGF-A by miR-29b-2 and NRP1 by miR-338. MIR-29B-2 AND MIR-338 SYNERGISTICALLY INHIBIT BREAST CANCER INVASION AND METASTASIS Using a data
set of syngeneic cell lines with varying metastatic capabilities, we found that the expression levels of miR-29b-2 and miR-338 were lowest in cells with the highest metastatic capacities
(Fig. 5A). The result suggested metastasis suppressor properties of miR-29b-2 and miR-338 in breast cancer cells. Therefore, functional analysis is required to clearly assign potential
cancer-inhibition effects to one specific miR or the cooperation of both. To this end, we constructed lentiviral vectors for efficient and stable expression of miR-29b-2 or miR-338 or
coexpression of miR-29b-2 and miR-338 in MDA-MB-231, BT549, and HCC1937 cells (Supplementary Fig. 8). A wound-healing assay demonstrated that stable expression of miR-29b-2 or miR-338
resulted in decreased migration and invasion. Importantly, coexpression of miR-29b-2 and miR-338 significantly decreased migration and invasion compared to those observed with stable
expression of miR-29b-2 or miR-338 alone (Fig. 5B). Moreover, the transwell assay results also showed that overexpression of miR-29b-2/miR-338 significantly inhibited the migration and
invasion of MDA-MB-231, BT549, and HCC1937 cells (Fig. 5C, D). In addition, we analyzed the impact of miR-29b-2/miR-338 on the EMT. In accordance with the effects of FOXO3a on EMT (Fig. 1),
stable expression of miR-29b-2/miR-338 resulted in an increase in E‐cadherin but decreased levels of vimentin and N-cadherin (Fig. 5E). To further investigate the inhibition of in vivo tumor
metastasis by miR-29b/miR-338, we implanted MDA-MB-231/miR-29b-2, MDA-MB-231/miR-338, or MDA-MB-231/miR-29b-2/miR-338 cells into nude mice through the lateral tail vein. We found that
stable expression of miR-29b-2/miR-338 significantly decreased lung metastasis of breast cancer compared with that in control cells (Fig. 5F, G). Taken together, these results suggested that
miR-29b-2 and miR-338 synergistically inhibits breast cancer invasion and metastasis. REGULATION OF MIR-29B-2 AND MIR-338 MEDIATES THE ABILITY OF FOXO3A TO SUPPRESS VEGF-A/NRP1 SIGNALING
AND BREAST CANCER METASTASIS To demonstrate the role of miR-29b-2 and miR-338 in the FOXO3a-mediated repression of VEGF-A/NRP1 and breast cancer metastasis, we determined whether the
FOXO3a-mediated repression of VEGF-A/NRP1 can be reversed by knockdown of miR-29b-2 and miR-338. Indeed, although overexpression of FOXO3a reduced the VEGF-A level, this reduction was
blocked by anti-miR-29b-2 in MDA-MB-231/FOXO3a and BT549/FOXO3a cells (Fig. 6A and Supplementary Fig. 9). Similarly, knockdown of miR-338 abrogated the inhibitory effect of FOXO3a on NRP1
(Fig. 6A). We then investigated whether FOXO3a-mediated metastasis suppression requires miR-29b and miR-338. We found that loss of miR-29-2 or miR-338 in MDA-MB-231/FOXO3a and BT549/FOXO3a
cells caused a mesenchymal morphology accompanied by an increase in the levels of vimentin but decrease in the level of E-cadherin (Fig. 6A), whereas transfected with miR-29b-2 or/and
miR-338 reversed the effect of FOXO3a shRNA on the expression of E-cadherin and vimentin in MCF-7 cells (Supplementary Fig. 10). Furthermore, overexpression of FOXO3a inhibited cell
invasion, and concomitant loss of miR-29b-2 and miR-338 abrogated the effects of FOXO3a, resulting in increased cell invasion (Fig. 6B, C). Significantly, we found that knockdown of
miR-29b-2 and miR-338 reversed FOXO3a-mediated suppression of in vivo tumor metastasis (Fig. 6D, E). Consistently, overexpression of FOXO3a decreased the expression of VEGF-A and NRP1 but
increased the expression of E-cadherin, whereas inhibition of miR-29b-2 and miR-338 reversed the effect of FOXO3a on the expression of VEGF-A, NRP1, and E-cadherin in the metastatic lesions
(Supplementary Fig. 11). Together, our results demonstrated that miR-29b-2 and miR-338 are important downstream factors of FOXO3a that control VEGF-A/NRP1 signaling, ultimately leading to
metastasis suppression. FOXO3A-MIRNA-VEGF-A/NRP1 SIGNALING IS DYSREGULATED IN HUMAN BREAST CANCER To further confirm that FOXO3a-miRNA-VEGF-A/NRP1 signaling is dysregulated in human breast
cancer, expression levels of FOXO3a, miR-29b-2, miR-338, VEGF-A, and NRP1 were quantified in total RNA derived from 34 breast cancer tissues and 20 normal breast tissues. We found that the
mRNA expression levels of FOXO3a, miR-29b-2, and miR-338 were significantly decreased in tumor tissues, whereas those of VEGF-A and NRP1 were significantly increased in tumor tissues (Fig.
7A). Next, using mRNA expression data from 34 primary patient tumor samples for which hormone receptor and HER2 expression data were available, we found that VEGF-A and NRP1 mRNA expression
levels were significantly elevated in triple-negative tumors compared with hormone receptor-positive or HER2-positive tumors (Fig. 7B). In contrast, FOXO3a, miR-29b, and miR-338 had the
lowest expression levels in triple-negative tumors. Furthermore, our results generally showed a negative correlation between FOXO3a and VEGF-A or NRP1 levels, a positive correlation between
FOXO3a and miR-29b or miR-338 levels (Fig. 7C, D), and a negative correlation between VEGF-A and miR-29b levels or between NRP1 and miR-338 levels (Fig. 7E). Taken together, our findings
suggested that FOXO3a-miRNA-VEGF-A/NRP1 signaling is dysregulated and plays a critical role in disease progression of breast cancer. DISCUSSION Metastasis is the process by which tumor cells
spread from the primary tumor to distant organs and form secondary tumors [30]. Metastatic dissemination of breast cancer cells remains the major obstacle to effective therapy [31]. The
loss of function of metastasis suppressor genes is a major rate-limiting step in breast cancer progression that prevents the formation of new colonies at distal sites [32]. Several lines of
evidence demonstrated that downregulation of FOXO3a affects phenotypes such as cell proliferation, EMT, and stemness that contribute to breast cancer progression and poor response to
therapies [10, 25, 33, 34]. In the current study, our findings demonstrated that FOXO3a expression is inversely correlated with the migration ability of breast cancer cells and
overexpression of FOXO3a suppresses breast cancer invasion and metastasis. In addition, we found that FOXO3a-driven miR-29b/miR-338 expression plays a critical role in the
posttranscriptional regulation of VEGF-A/NRP1 signaling and breast cancer metastasis. The role of FOXO3a in the regulation of cancer cell invasion and metastasis has been investigated in
different cellular models [7,8,9,10, 35]. For example, FOXO3 downregulation increased the expression of Twist1 and cell motility in urothelial cancer cells [36]. FOXO3a expression is also
decreased in renal cell carcinoma and is associated with metastasis-free survival of patients [9]. Our previous studies also demonstrated that FOXO3a modulates WNT/β-catenin signaling and
suppresses EMT in prostate cancer cells [7]. To date, the role of FOXO3a in breast cancer metastasis remains controversial. Most studies have defined FOXO3a proteins as a metastasis
suppressor, as it induces E-cadherin expression and represses EMT-inducing transcription factors, which in turn reversed the invasive phenotype of breast cancer cells [10]. In contrary,
FOXO3a was demonstrated as a MMP-9 and MMP-13 inducer promoting the invasion of HeLa and MDA-MB-435 cell lines [37]. In this study, we found that overexpression of FOXO3a significantly
suppressed the migration and invasion of breast cancer cells in vitro. Moreover, the antimetastatic function of FOXO3a was further confirmed using a nude mouse xenograft model. Therefore, we
propose that FOXO3a as a metastasis suppressor and that loss of function of FOXO3a might promote breast cancer progression. Interestingly, a recent study suggested that either activation or
loss of FOXO3a function suppressed breast cancer growth and metastasis. A potential explanation might be that metastasis is delayed in both situations because primary tumor growth is also
delayed and a tumor needs to reach a certain size or stage before it can metastasize [38]. Moreover, Stephan et al. suggested that high nuclear β-catenin content might subvert FOXO3a to
promote metastasis by enhancing the expression of many FOXO3a target genes that are involved in cytoskeleton remodeling and motility in colon cancer [39]. It would therefore be interesting
to study the role of FOXO3a in suppressing or supporting breast cancer cells with diverse biological properties and various amounts of nuclear β-catenin or some factors. Our study provides
evidence that the antimetastatic effect of FOXO3a could be partially caused by VEGF-A/NRP1 repression. VEGF-A is one of the major inducers of angiogenesis and is highly correlated with tumor
progression, invasion, and metastasis in breast cancer [40]. A previous study demonstrated that treatment with Lapatinib resulted in nuclear translocation and activation of FOXO3a, followed
by a reduction in VEGF expression in BT474 and SKBR3 cells [21]. Similarly, we also found that overexpression of FOXO3a significantly inhibits VEGF-A expression and secretion, as well as
the expression of its coreceptor NRP1. Moreover, we observed a significant inverse correlation between FOXO3a and VEGF-A/NRP1 expression levels, and a significant positive correlation
between VEGF-A and NRP1 in the breast cancer tissues. Lower levels of FOXO3a, and higher levels of VEGF-A or NRP1 were correlated with shorter survival in the lymph node metastasis positive
subgroup. Thus, our results indicated that FOXO3a is a negative regulator of VEGF-A/NRP1 and that dysregulated FOXO3a/VEGF-A/NRP1 signaling contributes to disease progression of breast
cancer. As a master regulator of gene expression, FOXO3a has been shown to directly or indirectly regulate numerous protein-coding genes [3]. It has been reported that FOXO3a-mediated
repression of VEGF-A might be involved in transcriptional repression [21]. However, it is not clear whether other mechanisms may also account for the negative correlation between FOXO3a and
VEGF-A. An important strength of our current work is that we define the role of miR-29b in the posttranscriptional regulation of VEGF-A by FOXO3a and suggest that, as a new member of the
FOXO3a regulatory network, miR-29b provides a direct link between FOXO3a and VEGF-A in this gene regulatory network. To date, only a very limited number of miRNAs has been shown to be direct
targets of FOXO3a. For example, miR-622 overexpression mediated by FOXO3a was shown to repress the invasiveness of lung tumor cells by inhibition of HIF-1α mediated by ERK inactivation in
U0126-treated A549 cells [41]. Our previous study also demonstrated that miR-34b/c is directly regulated by FOXO3a and that FOXO3a-mediated transactivation of miR-34b/c inhibits
WNT/β-catenin signaling and suppresses β-catenin-dependent EMT in prostate cancer [7]. Therefore, the identification of miR-29b/miR-338 as a direct FOXO3a target in this study expands the
repertoire of FOXO3a-regulated genes. miR-29b targets a network of pro-metastatic regulators involved in EMT, collagen remodeling, and proteolysis, including Snail, MMPs, Mcl-1, COL1A1,
TGF-β, VEGF, and PDGF [42, 43]. As about miR-338, it also exerts tumor suppressive effects by targeting a variety of oncogenes and upregulating tumor suppressors [44, 45]. In accordance with
these previous studies, we found that miR-29b and miR-338 activated by FOXO3a directly targets VEGF-A and NRP1, respectively. Moreover, miR-29b cooperated with miR-338 to inhibit breast
cancer invasion and metastasis in both in vitro and in vivo models. In particular, we found that the expression of miR-29b and miR-338 was significantly reduced in human breast cancer
tissues. Furthermore, our results generally showed a negative correlation between FOXO3a and VEGF-A or NRP1 levels, a positive correlation between FOXO3a and miR-29b or miR-338 levels, and a
negative correlation between VEGF-A and miR-29b levels or between NRP1 and miR-338 levels. Therefore, we propose that a reduction in miR-29b/miR-338 expression elicited by FOXO3a
inactivation results in alleviation of miR-29b/miR-338-mediated targeting of VEGF-A/NRP1, thus contributing to the aggressive behavior of breast cancers. In conclusion, our observations
support the hypothesis that FOXO3a is a metastasis suppressor that performs its effect by inhibiting EMT, invasion, and metastatic progression of breast cancers. Our study reveals a novel
mechanistic insight for breast cancer metastasis mediated by the FOXO3a-miRNA-VEGF/NRP1 signaling axis and highlights the potential of targeting FOXO3a-miR-29b/miR-338 as a novel therapeutic
strategy for breast cancer (Fig. 7F). MATERIALS AND METHODS CELL CULTURE The human breast cancer cell line MCF-7, BT474, BT549, MDA-MB-231, Hs578T, HCC38, and HCC1937 were obtained from the
American Type Culture Collection and authenticated by short tandem repeat analysis every 6 months after used in our laboratory. All cell lines were maintained with Dulbecco’s Modified Eagle
Medium (HyClone, Logan, UT, USA) containing 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified incubator of 5% CO2 at 37 °C. PATIENTS AND SPECIMENS Primary tumor
specimens were obtained from 100 patients diagnosed with breast cancer who underwent complete resection in the Affiliated Tumor Hospital of Guangzhou Medical University between 2004 and
2008. Follow-up information was obtained from review of the patients’ medical record. Furthermore, 56 of fresh primary breast cancer tissues and 20 of normal breast tissues used in this
study were collected from the Affiliated Tumor Hospital of Guangzhou Medical University. Written informed consent was obtained from all study participants. This study was approved by the
Ethics Committee of Guangzhou Medical University and written informed consent was provided by all patients based on the Declaration of Helsinki. LENTIVIRUS PRODUCTION AND TRANSDUCTION FOXO3a
ORF was cloned into the lentiviral vector GV358 (GENECHEM, Shanghai, China). FOXO3a shRNAs or Control shRNA sequences were cloned into the EGFP-labeled lentiviral vector GV248 (GENECHEM).
The target sequences selected are shown in Supplementary Table 3. Lentivirus was generated using the packaging plasmids pHelper 1.0 and pHelper 2.0 (GENECHEM). Recombinant lentiviruses
containing hsa-mir-29b-2, hsa-mir-338, or scrambled sequences were purchased from GENECHEM. Anti-miR-29b-2 and anti-miR-338 were obtained from GeneCopoeia Inc. (Guangzhou, China).
Lentiviruses production was added to cells with Polybrene, and stably transduced cells were selected in puromycin for at least 5 days. MIRNA MICROARRAY ANALYSIS Total RNA, containing miRNA,
was extracted from MDA-MB-231/FOXO3a cells and MDA-MB-231/control cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The miRNA expression
profiles were generated by using the Affymetrix GeneChip miRNA Array v. 4.0 (Affymetrix). Briefly, the flashTag Biotin RNA Labeling Kit (Affymetrix) was used to label 1 μg of total RNA,
followed by the hybridization overnight according to the manufacturer’s instructions. Images were scanned using the GeneChip Scanner 3000 and image analysis was done with the GeneChip
Operating Software. REAL-TIME RT-PCR For mRNA detection, total RNAs were extracted using the E.Z.N.A.® HP Total RNA Kit (Omega Bio-tek, Doraville, GA, USA), followed by reverse-transcription
using the PrimeScript® RT reagent Kit (TakaRa, Shiga, Japan), and quantitative PCR using the SYBR Green qRT-PCR according to the manufacturer’s instructions (Applied Biosystems). The primer
pairs used are shown in Supplementary Table 4. For miRNA detection, the reverse transcribed cDNA was synthesized with the All-in-One™ miRNA First-Strand cDNA Synthesis Kit (GeneCopoeia,
Rockville, MD, USA). miR-29b-2 and miR-338 expression was determined with the All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD, USA). WESTERN BLOT Western blot analysis
was performed as previously described. Briefly, total protein was isolated using RIPA buffer (Beyotime Biotechnology, China) in the presence of protease inhibitor cocktail. The protein
concentration of lysates was measured using a BCA Protein Assay Kit (Thermo Scientific). Equivalent amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane. The
membranes were subsequently blocked in 5% nonfat milk and then incubated with primary antibodies. After extensive washing, immunoreactivity was detected using secondary antibody-conjugated
horseradish peroxidase. Signals were captured using ECL and x-ray film. The antibodies used for Western blotting assays are shown in Supplementary Table 5. ANIMAL STUDIES All animal work was
performed in accordance with protocols approved by the Animal Experimentation Ethics Committee of Guangzhou medical University. 1 × 106 cells were injected into the tail vein of 6-week-old
female nude mice (_N_ = 6 per group). After 6 weeks, mice were sacrificed. Their lungs were fixed in 4% paraformaldehyde, paraffin-embedded and sliced. Lung sections were stained by
hematoxylin and eosin (H&E) and immunohistochemical assay. The numbers of micrometastases in the lungs per tissue section in individual mice were determined from morphological
observation of H&E-stained sections. Wound-healing assay, transwell assay, ELISA assay, ChIP, Dual-luciferase reporter assays, and immunohistochemical assay are described in the
Supplementary Experimental Procedure. STATISTICAL ANALYSIS Statistical analyses were conducted using the SPSS16.0 software. Comparisons between groups were analyzed by the _t_ test and _χ_2
test. Overall survival curves were plotted according to the Kaplan–Meier method with the log-rank test applied for comparison. Survival was measured from the day of surgery. Variables with
values of _P_ < 0.05 by univariate analysis were used in subsequent multivariate analysis based on the Cox proportional hazards model. The differences were considered statistically
significant at _P_ < 0.05. REFERENCES * Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. PubMed Google Scholar * Harbeck N, Penault-Llorca F,
Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66. PubMed Google Scholar * Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning
forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–95. CAS PubMed Google Scholar * Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription
factors in cancer. Clin Cancer Res. 2009;15:752–7. CAS PubMed PubMed Central Google Scholar * Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by
inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. CAS PubMed PubMed Central Google Scholar * Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical
role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104. PubMed PubMed Central Google Scholar * Liu H, Yin J, Wang H, Jiang G, Deng M, Zhang G, et al. FOXO3a modulates WNT/beta-catenin
signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cell Signal. 2015;27:510–8. CAS PubMed Google Scholar * Luo M, Wu C, Guo E, Peng S, Zhang L, Sun W,
et al. FOXO3a knockdown promotes radioresistance in nasopharyngeal carcinoma by inducing epithelial-mesenchymal transition and the Wnt/beta-catenin signaling pathway. Cancer Lett.
2019;455:26–35. CAS PubMed Google Scholar * Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, et al. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20:1779–90. CAS PubMed Google Scholar * Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, et al. AMPK
reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783–95. CAS PubMed PubMed Central Google Scholar * Ma Z, Xin Z,
Hu W, Jiang S, Yang Z, Yan X, et al. Forkhead box O proteins: crucial regulators of cancer EMT. Semin Cancer Biol. 2018;50:21–31. CAS PubMed Google Scholar * Weigelt B, Peterse JL, van ‘t
Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. CAS PubMed Google Scholar * Karaman S, Leppanen VM, Alitalo K. Vascular endothelial growth factor
signaling in development and disease. Development. 2018;145:dev151019. PubMed Google Scholar * Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr
Rev. 2004;25:581–611. CAS PubMed Google Scholar * Bando H, Weich HA, Brokelmann M, Horiguchi S, Funata N, Ogawa T, et al. Association between intratumoral free and total VEGF, soluble
VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br J Cancer. 2005;92:553–61. CAS PubMed PubMed Central Google Scholar * Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp
RL, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol.
2008;39:1835–43. CAS PubMed PubMed Central Google Scholar * Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis.
Trends Mol Med. 2011;17:347–62. CAS PubMed Google Scholar * Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol.
2012;226:50–60. CAS PubMed Google Scholar * Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell
migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766–76. CAS PubMed PubMed Central Google Scholar * Luo M, Hou
L, Li J, Shao S, Huang S, Meng D, et al. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and
beta-catenin. Cancer Lett. 2016;373:1–11. CAS PubMed Google Scholar * Karadedou CT, Gomes AR, Chen J, Petkovic M, Ho KK, Zwolinska AK, et al. FOXO3a represses VEGF expression through
FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2012;31:1845–58. CAS PubMed Google Scholar * Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function.
Cell. 2004;116:281–97. CAS Google Scholar * Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. PubMed PubMed Central Google Scholar *
Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J Biol Chem. 2010;285:16958–66. CAS PubMed PubMed Central Google Scholar * Liu H, Song Y, Qiu H, Liu Y, Luo K,
Yi Y, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966–83. CAS PubMed Google Scholar * Yeung KT, Yang
J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39. PubMed Google Scholar * Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical
microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–8. CAS PubMed PubMed Central Google Scholar * Quail DF, Joyce JA. Microenvironmental regulation of
tumor progression and metastasis. Nat Med. 2013;19:1423–37. CAS PubMed PubMed Central Google Scholar * Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, et al. Dual
inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5:171ra17. PubMed PubMed Central Google
Scholar * Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. CAS PubMed Google Scholar * Lu J, Steeg PS, Price JE,
Krishnamurthy S, Mani SA, Reuben J, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69:4951–3. CAS PubMed Google Scholar * Stafford LJ, Vaidya KS, Welch
DR. Metastasis suppressors genes in cancer. Int J Biochem Cell Biol. 2008;40:874–91. CAS PubMed Google Scholar * Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G,
Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. CAS PubMed Google Scholar
* Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, et al. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast
Cancer Res. 2008;10:R21. PubMed PubMed Central Google Scholar * Li J, Yang R, Dong Y, Chen M, Wang Y, Wang G. Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes
metastasis of pancreatic ductal adenocarcinoma by activation of the beta-catenin/TCF4 pathway through SPRY2. J Exp Clin Cancer Res. 2019;38:38. PubMed PubMed Central Google Scholar *
Shiota M, Song Y, Yokomizo A, Kiyoshima K, Tada Y, Uchino H, et al. Foxo3a suppression of urothelial cancer invasiveness through Twist1, Y-box-binding protein 1, and E-cadherin regulation.
Clin Cancer Res. 2010;16:5654–63. CAS PubMed Google Scholar * Storz P, Doppler H, Copland JA, Simpson KJ, Toker A. FOXO3a promotes tumor cell invasion through the induction of matrix
metalloproteinases. Mol Cell Biol. 2009;29:4906–17. CAS PubMed PubMed Central Google Scholar * Hornsveld M, Smits LMM, Meerlo M, van Amersfoort M, Koerkamp MJAG, van Leenen D, et al.
FOXO transcription factors both suppress and support breast cancer progression. Cancer Res. 2018;78:2356–69. CAS PubMed Google Scholar * Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I,
Arques O, Landolfi S, et al. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. CAS PubMed
Google Scholar * Su JC, Mar AC, Wu SH, Tai WT, Chu PY, Wu CY, et al. Disrupting VEGF-A paracrine and autocrine loops by targeting SHP-1 suppresses triple negative breast cancer metastasis.
Sci Rep. 2016;6:28888. CAS PubMed PubMed Central Google Scholar * Cheng CW, Chen PM, Hsieh YH, Weng CC, Chang CW, Yao CC, et al. Foxo3a-mediated overexpression of microRNA-622 suppresses
tumor metastasis by repressing hypoxia-inducible factor-1alpha in ERK-responsive lung cancer. Oncotarget. 2015;6:44222–38. PubMed PubMed Central Google Scholar * Ru P, Steele R, Newhall
P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012;11:1166–73. CAS PubMed
Google Scholar * Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell
Biol. 2013;15:201–13. CAS PubMed PubMed Central Google Scholar * Sun F, Yu MC, Yu J, Liu ZJ, Zhou XY, Liu YQ, et al. miR-338-3p functions as a tumor suppressor in gastric cancer by
targeting PTP1B. Cell Death Dis. 2018;9:522. PubMed PubMed Central Google Scholar * Jin Y, Zhao M, Xie Q, Zhang HY, Wang Q, Ma QJ. MicroRNA-338-3p functions as tumor suppressor in breast
cancer by targeting SOX4. Int J Oncol. 2015;47:1594–602. CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation
of China (81772825, 81402497, 81672616, and 81272450), supported by grants from Guangdong Natural Science Funds for Distinguished Young Scholars (No.2016A030306003), Supported by Guangdong
Special Support Program (2017TQ04R809), grants from Guangdong Natural Science Funds (2017A030313500 and 2017A030313867), the Science and Technology Program of Guangzhou (201707010381 and
201707010354), Clinical Key Specialty Project of Guangzhou Medical University (No. 2020-05), and Guangzhou key medical discipline construction project fund. AUTHOR INFORMATION Author notes *
These authors contributed equally: Ying Song, Shanshan Zeng, Guopei Zheng AUTHORS AND AFFILIATIONS * Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou Key
Laboratory of “Translational Medicine on Malignant Tumor Treatment”, Guangzhou, 510095, PR China Ying Song, Shanshan Zeng, Guopei Zheng, Danyang Chen, Pan Li, Mingqiang Yang, Kai Luo, Jiang
Yin, Yixue Gu, Zhijie Zhang, Xiaoting Jia, Ni Qiu, Zhimin He, Hongsheng Li & Hao Liu Authors * Ying Song View author publications You can also search for this author inPubMed Google
Scholar * Shanshan Zeng View author publications You can also search for this author inPubMed Google Scholar * Guopei Zheng View author publications You can also search for this author
inPubMed Google Scholar * Danyang Chen View author publications You can also search for this author inPubMed Google Scholar * Pan Li View author publications You can also search for this
author inPubMed Google Scholar * Mingqiang Yang View author publications You can also search for this author inPubMed Google Scholar * Kai Luo View author publications You can also search
for this author inPubMed Google Scholar * Jiang Yin View author publications You can also search for this author inPubMed Google Scholar * Yixue Gu View author publications You can also
search for this author inPubMed Google Scholar * Zhijie Zhang View author publications You can also search for this author inPubMed Google Scholar * Xiaoting Jia View author publications You
can also search for this author inPubMed Google Scholar * Ni Qiu View author publications You can also search for this author inPubMed Google Scholar * Zhimin He View author publications
You can also search for this author inPubMed Google Scholar * Hongsheng Li View author publications You can also search for this author inPubMed Google Scholar * Hao Liu View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS HL and ZH designed and performed experiments, analyzed data, and wrote the paper; YS and SZ performed
experiments and analyzed the data. GZ, DC, PL, KL, and JY performed some of the experiments. YG and ZZ analyzed the data. H. Li provided the patient samples for clinical data analysis. XJ
and NQ provide assistance in the study; HL, GZ, and ZH initiated the study, organized, designed, and wrote the paper. All authors read and approved the final manuscript. CORRESPONDING
AUTHORS Correspondence to Zhimin He, Hongsheng Li or Hao Liu. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1-11
SUPPLEMENTARY TABLE 1-5 SUPPLEMENTARY EXPERIMENTAL PROCEDURES RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Song, Y., Zeng, S., Zheng, G. _et al._ FOXO3a-driven miRNA signatures suppresses VEGF-A/NRP1 signaling and breast cancer metastasis.
_Oncogene_ 40, 777–790 (2021). https://doi.org/10.1038/s41388-020-01562-y Download citation * Received: 10 January 2020 * Revised: 19 October 2020 * Accepted: 11 November 2020 * Published:
01 December 2020 * Issue Date: 28 January 2021 * DOI: https://doi.org/10.1038/s41388-020-01562-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative