Play all audios:
ABSTRACT Ovarian cancer is one of the most common gynecological malignant tumors with insidious onset, strong invasiveness, and poor prognosis. Metabolic alteration, particularly aerobic
glycolysis, which is tightly regulated by transcription factors, is associated with the malignant behavior of OC. We screened FOXK2 in this study as a key transcription factor that regulates
glycolysis in OC. FOXK2 is overly expressed in OC, and poor prognosis is predicted by overexpression. FOXK2 promotes OC cell proliferation both in vitro and in vivo and cell migration in
vitro. Further studies showed that PDK2 directly binds to the forkhead-associated (FHA) domain of FOXK2 to phosphorylate FOXK2 at Thr13 and Ser30, thereby enhancing the transcriptional
activity of FOXK2. FOXK2 transcriptionally regulates the expression of PDK2, thus forming positive feedback to sustain glycolysis in OC cells. SIMILAR CONTENT BEING VIEWED BY OTHERS FBP1
REGULATES PROLIFERATION, METASTASIS, AND CHEMORESISTANCE BY PARTICIPATING IN C-MYC/STAT3 SIGNALING AXIS IN OVARIAN CANCER Article Open access 06 August 2021 UPREGULATION OF FAM83F BY C-MYC
PROMOTES CERVICAL CANCER GROWTH AND AEROBIC GLYCOLYSIS VIA WNT/Β-CATENIN SIGNALING ACTIVATION Article Open access 16 December 2023 PER1 SUPPRESSES GLYCOLYSIS AND CELL PROLIFERATION IN ORAL
SQUAMOUS CELL CARCINOMA VIA THE PER1/RACK1/PI3K SIGNALING COMPLEX Article Open access 15 March 2021 INTRODUCTION Ovarian cancer (OC) is the leading cause of death for gynecologic
malignancies worldwide, mainly due to diagnosis at an advanced stage and chemotherapy resistance after high-rate recurrence [1]. Due to receiving nonstandard treatment or no treatment at all
and chemotherapy resistance, some patients still fail to achieve desirable progression-free survival (PFS) [2]. Clarifying the mechanism of OC tumorigenesis and exploring potential
therapeutic targets are essential to improve the prognosis of patients with OC. The reprogramming of energy metabolism has been considered as a new hallmark of cancers while aerobic
glycolysis has been regarded as the major metabolic phenotype [3]. Altered metabolic pathways in ovarian cancer facilitate cancer cell survival and proliferation while also influencing the
ability to metastasize, acquiring resistance to chemotherapy, maintaining the cancer stem cell phenotype and escaping from the effects of antitumor immune defense [4]. Cancers and other
proliferating cells exhibit increased expression of many glycolytic enzymes, which is consistent with their high utilization of glycolysis [5,6,7]. The glycolytic pathway contains 10
chemical reaction steps, each catalyzed by a specific enzyme. The overexpression of GLUT-1, which functions as a rate-limiting step in the flux of glucose for glycolysis, was associated with
growth and poor prognosis in several human tumors [8, 9]. High ALDOA expression has been shown to boost glycolysis to promote the proliferation and invasion of cancer cells [10]. Cancer
cells can significantly increase glucose uptake and utilization to enhance aerobic glycolysis, and thereby rapidly produce ATP and biosynthesis, ultimately promoting tumorigenesis and
metastasis [11]. Simultaneously, a series of signaling pathways, including the PI3K/AKT, mTOR, MAPK, Wnt and AMPK signaling pathway, are involved in regulating aerobic glycolysis in cancer
cells [12,13,14,15]. P53, c-myc, HIF-1 and SIX are transcription factors that play an important role in regulating the Warburg effect [16, 17]. C-myc enhances aerobic glycolysis by directly
regulating glycolytic genes, including GLUTs, HK, PFK, PGK, enolase, and LDHA [18]. HIF-1α induces the glycolytic enzymes, including HKII, PFK1, LDHA, aldolase, and GLUT-1/3, to switch the
glucose metabolism to the glycolytic pathway in hypoxic tumor cells [19]. The transcription factor-forkhead box K2 (FOXK2) belongs to the forkhead box (FOX) family, shares a conserved DNA
binding domain and regulates a wide spectrum of biological processes within the cell. Regarded as an interleukin enhancer binding factor, FOXK2 was initially confirmed as a nuclear factor of
an activated T-cell-like interleukin-binding factor. FOXK2 acts as a suppressor in breast cancer, renal clear cell carcinoma, non-small cell lung cancer, and glioma [20,21,22,23] but has
oncogenic roles in colon cancer, colorectal cancer, and hepatocellular carcinoma [24,25,26]. A recent study has found that FOXK2 promotes ovarian cancer stemness by regulating the unfolded
protein response pathway [27], and it regulate glycolysis in adipocyte [28], but the role of FOXK2 in glycolysis in OC is still unclear. In this study, we identified FOXK2 as a key
transcription factor regulating glycolysis in OC. FOXK2 was highly expressed in OC and played a tumor-promoting role. Pyruvate dehydrogenase Kinase2(PDK2) was shown in further studies to
promote the phosphorylation of FOXK2 at serine and threonine, and enhance the transcriptional activity of FOXK2. Additionally, FOXK2 directly transcriptionally regulates the expression of
PDK2. Therefore, PDK2-FOXK2 formed positive feedback to further increase the expression level of glycolytic enzymes and ultimately sustained glycolysis levels in OC cells. RESULTS FOXK2 IS
SCREENED AS A KEY TRANSCRIPTION FACTOR THAT REGULATES GLYCOLYSIS IN OC We analyzed normal ovarian data from The Genotype-Tissue Expression (_GTEx_) and OC data from The Cancer Genome Atlas
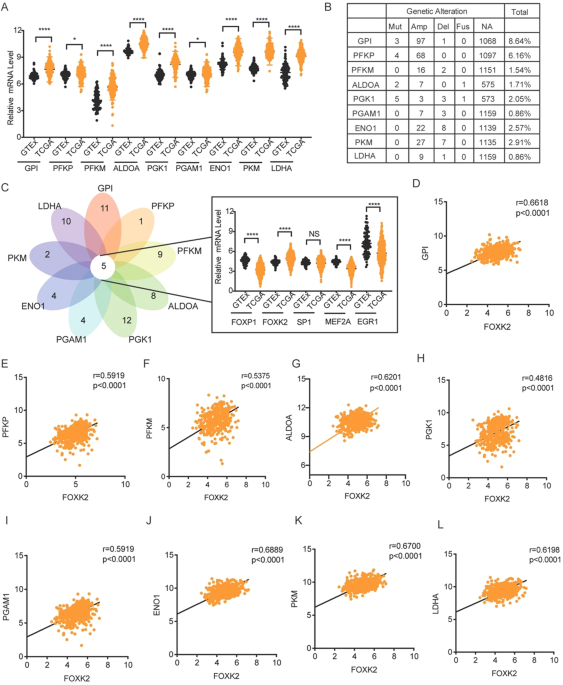
(TCGA) to investigate the expression of key glycolytic enzymes in OC. The nine key glycolytic enzymes exhibited high expression in OC compared with normal samples (Fig. 1A). We analyzed
their genetic alterations in OC samples using the cBio Cancer Genomics Portal (cBioPortal) to further explore the causes of the increased expression of key glycolytic enzymes. Except for
genomic alterations rates of GPI and PFKP exceeding 5%, the remaining 7 genes are all less than 3%. So, we speculated that their increased expression might be caused by transcription factors
(Fig. 1B). We used the JASPAR database to analyze all the respective transcription factors and took the top 50% of transcription factors for the intersection to determine which
transcription factors could regulate all nine key glycolytic enzymes. Finally, five transcription factors were screened to regulate nine key enzymes simultaneously, of which only FOXK2 was
highly expressed in OC (Fig. 1C, D). The correlation between FOXK2 and nine key glycolytic enzymes was further analyzed. As expected, FOXK2 was positively correlated with nine key glycolytic
enzymes (Fig. 1E). We believe that FOXK2 is a key transcription factor regulating ovarian cancer glycolysis based on the above results. FOXK2 IS UPREGULATED IN OC AND IS CLOSELY RELATED TO
POOR PROGNOSIS IN OC PATIENTS We first analyzed FOXK2 expression using data from multiple databases to determine FOXK2 expression in OC and found that FOXK2 expression was significantly
higher than that of the control individuals (Fig. 2A–E). We next performed Kaplan–Meier analysis to explore the relationship between FOXK2 expression and the prognosis of patients. The
results suggest that high FOXK2 expression is closely related to poor prognosis in OC patients, as the average progression-free survival (PFS) in patients with high FOXK2 expression patients
was shorter than that in patients with low FOXK2 expression (Fig. 2F). We analyzed the FOXK2 expression status with respect to various pathological parameters in 191 OC patients to further
investigate the clinical significance of FOXK2 in OC. The results indicated that FOXK2 expression was closely correlated with TNM stage and lymph node metastasis in OC tissues (Fig. 2G,
Table 1). Overall, these results demonstrated that OC-upregulated FOXK2 acted as an indicator of OC progression and predicted poor prognosis. INHIBITING FOXK2 EXPRESSION SUPPRESSES CELL
PROLIFERATION AND MIGRATION IN OC We first knocked down FOXK2 in OC cells and confirmed the efficiency (Fig. 3A, B) to determine the biological functions of FOXK2 in OC cells. Then we
investigated the effects of FOXK2 on OC cell proliferation. FOXK2 knockdown obviously inhibited cell growth in vitro (Fig. 3C, D). A subcutaneous xenograft model was established to explore
the impacts of FOXK2 expression on tumors in vivo. The results showed that knockdown of FOXK2 significantly reduced tumor growth (Fig. 3E). We additionally investigated the effects of FOXK2
on OC cell migration. FOXK2 knockdown apparently inhibited cell migration (Fig. 3F). These results indicate that FOXK2 promotes the malignant phenotypic transformation of OC, suggesting that
FOXK2 functions as an oncogene in OC. INHIBITING FOXK2 EXPRESSION REDUCES THE GLYCOLYSIS LEVELS IN OC We first investigated whether FOXK2 affects glycolytic capacity in OC. FOXK2 was
screened as a transcription factor that regulates several key enzymes of glycolysis. The OC samples in the TCGA database were divided into two groups by median FOXK2 expression. Striking
alterations in metabolic processes, including glycolysis, Myc targets and mTORC1 signaling (Fig. 4A), were shown using the hallmark gene sets of two group transcriptome data using Gene Set
Enrichment Analysis (GSEA). We knocked down FOXK2 to further verify the effect of FOXK2 on the expression levels of key enzymes in glycolysis and then detected the RNA and protein levels of
key enzymes. The results showed that the RNA and protein levels of 11 key enzymes were significantly reduced after FOXK2 knockdown (Fig. 4B–D). We examined the extracellular acidification
rate (ECAR; an indicator of glycolysis) with the Seahorse XF96 analyzer to verify the effect of FOXK2 on glycolysis levels in OC. As shown in Fig. 4E, F, we observed a reduction in the
glycolytic rate (ECAR) following the FOXK2-siRNA treatment of OVCAR8 and ES-2 cells. Taken together, these data suggest that the tumorigenic effect by activation of the FOXK2 may be largely
resulted from its enhancement of glycolysis. TRANSCRIPTION FACTOR FOXK2 DIRECTLY REGULATES 7 KEY ENZYMES OF GLYCOLYSIS IN OC To demonstrate whether FOXK2 could directly regulate the
expression of key enzymes in glycolysis, we used MEME software to predict the binding site of FOXK2 to key enzymes and found that FOXK2 had binding sites with the promoter regions of 7 key
enzymes, GPI, PKM2, PGK1, GAPDH, LDH, PFK and HK2. The databases of ChIP Seq also showed that these key enzymes were targets of FOXK2 (Supplementary Table S4). We constructed the primers
according to the promoters of the six key enzymes, and we performed ChIP PCR in OVCAR8 and ES-2 cells to evaluate whether FOXK2 could directly bind to the promoters. As expected, ChIP
analysis indicated that FOXK2 directly bound to specific regions in the 7 key enzyme promoters. (Fig. 5A–F and Supplementary Fig. S1). Luciferase reporter assays further confirmed that the
transcriptional activity of the GPI, PKM2, PGK1, GAPDH, LDH, PFK and HK2 promoters was significantly induced by FOXK2 (_P_ < 0.001) and was significantly decreased by the seven above
promoter mutations (Fig. 5A–F and Supplementary Fig. S1). These data suggest that FOXK2 directly regulates the expression of HK2, GPI, PKM2, PGK1, GAPDH, LDH, and PFK at the transcriptional
level in OC cells. PDK2 PHOSPHORYLATES FOXK2 AT SERINE AND THREONINE AND PROMOTES FOXK2 TRANSCRIPTIONAL ACTIVITY Protein phosphorylation is one of the most common, yet key posttranslational
modifications that induce conformational changes in proteins. By analyzing the data in PhosphoSitePlus, a comprehensive resource for investigating the structure and function of
experimentally determined post translational modifications in human and mice, we discovered many phosphorylation sites in the FOXK2 protein (Supplementary Fig. S2). We used Phosbind
acrylamide to separate phosphorylated and nonphosphorylated proteins and demonstrated the presence of phosphorylated of FOXK2 in OC cell proteins by western blotting (Fig. 6A) due to the
lack of FOXK2 phosphorylated antibodies. We queried the BioGRID interaction database to identify kinases that phosphorylate FOXK2 and found that the kinase PDK2 may interact with FOXK2. To
verify the interaction between FOXK2 and PDK2, we conducted CO-IP experiments in OVCAR8 and ES-2 cells. The results showed that FOXK2 could interact with PDK2 (Fig. 6B, C).
Immunofluorescence of FOXK2 and PDK2 additionally displayed obvious colocalization in the nucleus and a small amount in the cytoplasm (Fig. 6D). To characterize the effect of PDK2 on the
tumorigenicity of OC cells, we knocked down the expression of PDK2 in OVCAR8 and ES-2 cells (Fig. 6E). In contrast to control cells, the cell proliferation ability of PDK2-silenced cells was
significantly reduced (Fig. 6F, G). To determine whether PDK2 phosphorylates FOXK2, we first performed FOXK2 single transfection or FOXK2/PDK2 double transfection in OC cells to detect the
phosphorylation type of FOXK2 protein by PDK2. We performed immunoprecipitation on the protein extracts and found that the FOXK2 protein was phosphorylated by serine and threonine, but no by
tyrosine (Fig. 6H). Then, we performed in vitro kinase assay to determine whether PDK2 phosphorylates FOXK2 using their purified protein. The results showed that the amount of ATP remaining
in solution following a kinase reaction was only approximately 8% left (Supplementary Fig. S3). The serine/threonine band of FOXK2 was significantly increased in the presence of ATP (Fig.
6I), demonstrating that this reaction consumed a large amount of ATP and that PDK2 facilitated serine and threonine phosphorylation of FOXK2. These data suggest that FOXK2 is a novel protein
substrate of PDK2 and can be phosphorylated at serine and threonine residues. We previously reported that FOXK2 binds to the promoters of glycolysis-related genes. To further explore the
effect of PDK2 on the transcriptional activity of FOXK2, we performed luciferase reporter assays and found that overexpression of PDK2 enhanced GPI promoter activity (Fig. 6J). These data
suggest that PDK2 promotes FOXK2 transcriptional activity. FOXK2 THR13 AND SER30 PHOSPHORYLATION BY PDK2 INCREASES FOXK2 TRANSCRIPTIONAL ACTIVITY We truncated the protein domain of FOXK2 to
determine the specific binding site of PDK2 to FOXK2, and then co-immunoprecipitated PDK2 with the protein truncation of FOXK2. The results are shown in the Fig. 7A, B. PDK2 could interact
with the truncated proteins without the second or third domains but not with the truncated proteins without the first domain-FHA, indicating that PDK2 specifically bound the FHA domain of
FOXK2. Then we performed mass spectrometry (MS) analysis of the protein products after the kinase reaction to confirm the specific phosphorylation sites, and found three sites, Ser9, Thr13,
and Ser30, were significantly phosphorylated (Fig. 7C, Supplementary Table S5), and these three sites have been predicted on the website PhosphoSitePlus (Supplementary Fig. S2). Next, we
generated the original plasmid and phosphorylation site mutant plasmids and performed FOXK2 single transfection, FOXK2/PDK2 or FOXK2 mutant /PDK2 double transfection in OC. We performed
immunoprecipitation on the protein extracts and found that serine and threonine phosphorylation were significantly enhanced in FOXK2-PDK2 double-transfected cells but reduced in FOXK2
mutant-PDK2 double-transfected cells. These results indicate that PDK2 phosphorylates FOXK2 at Ser9, Thr13, and Ser30. We further examined whether phosphorylation of FOXK2 at Ser9, Thr13,
and Ser30 enhances ovarian cancer cell proliferation. The proliferation assay results showed that FOXK2 and PDK2 alone enhanced cell proliferation compared with the vector group; of note,
cell proliferation was inhibited by the Thr13 and Ser30 mutants compared with FOXK2, while the Ser9 mutant promoted cell proliferation at a similar level to FOXK2 (Fig. 7E). Next, we
investigated the effect of PDK2 on cancer cell growth through phosphorylation of FOXK2 at Ser9, Thr13 or Ser30 and found that FOXK2 or Ser9-mediated induction of cancer cell proliferation
could be induced by PDK2 overexpression in cells. However, such proliferation enhancement was attenuated in cells expressing Thr13 and Ser30 even in the presence of PDK2 overexpression (Fig.
7E). In addition, we overexpressed FOXK2 or mutants in cells that interfered with FOXK2, and the results showed that FOXK2 or Ser9A rescued the knockdown-induced suppression of
proliferation, but not in Ser30A or Thr13A (Supplementary Fig. S4A, B). At the same time, we examined the mRNA expression of FOXK2 targets in OC cells expressing FOXK2 or serine and
threonine mutants. As expected, the mRNA expression level of FOXK2 targets was increased upon FOXK2 or Ser9 mutant overexpression, whereas cells expressing phosphorylation Thr13 and Ser30
mutants could not upregulate FOXK2 targets (Fig. 7F). Next, we investigated the effect of PDK2 on the mRNA expression of FOXK2 targets and found that the mRNA expression level of FOXK2
targets could be induced by PDK2 overexpression in cells. However, this enhancement was attenuated in cells expressing Thr13 and Ser30 even in the presence of PDK2 overexpression (Fig. 7F).
These data indicate that PDK2 phosphorylates FOXK2 at Thr13 and Ser30 lead to an increase in the mRNA level of the target genes, which may be due to PDK2 promoting transcriptional activation
of FOXK2. FOXK2 THR-13 AND SER-30 PHOSPHORYLATION BY PDK2 PROMOTES THE GLYCOLYTIC ABILITY OF OC CELLS Through analysis of our RNA-seq data, we found that PDK2 may be the target of FOXK2.
Therefore, we knocked down or overexpressed FOXK2 to verify the effect of FOXK2 on the expression levels of PDK2 and found that the mRNA of PDK2 was significantly reduced after FOXK2
knockdown and was significantly increased after FOXK2 overexpression (Fig. 8A, B). To demonstrate whether FOXK2 directly regulate the PDK2 expression, we performed ChIP PCR and luciferase
reporter assays. ChIP analysis indicated that FOXK2 directly bound to the promoter region of PDK2 (Fig. 8C). Luciferase reporter assays further confirmed that the transcriptional activity of
the PDK2 promoter was significantly induced by FOXK2 and was significantly decreased by promoter mutation (Fig. 8D). To explore the effect of FOXK2 Thr-13 and Ser-30 phosphorylation on
glycolysis, we examined the ECAR with the Seahorse XF96 analyzer and found that the glycolysis level was increased upon FOXK2 or PDK2 overexpression, whereas cells expressing phosphorylated
mutants (T13A and S30A) could not upregulate the glycolysis level. Additional increase in PDK2 expression resulted in a further increase in glycolytic capacity in the FOXK2 group, while the
mutant group did not show a significant increase (Fig. 8E). In addition, we overexpressed FOXK2 or mutants in cells that interfered with FOXK2, and the results showed that FOXK2 or Ser9A
rescued the knockdown-induced suppression of glycolysis, but not in Ser30A or Thr13A (Supplementary Fig. S4C, D). These data indicate that PDK2 phosphorylates FOXK2 at Thr13 and Ser30 to
promote the glycolytic ability of OC cells. To explore whether phosphorylation sites at the Thr13 and Ser30 affect the translocation of FOXK2, we transfected FOXK2 and phosphorylated mutants
(T13A and S30A) into OC cells, and then performed nuclear and cytoplasmic protein extraction to detect subcellular localization changes of FOXK2 protein. The results showed that after Thr13
and Ser30 mutation, the localization of FOXK2 in the nucleus significantly decreased compared to that in the nonmutated FOXK2 expression group (Fig. 8F), indicating that the phosphorylation
of Thr13 and Ser30 was responsible for the translocation of FOXK2 into the nucleus for its effect on the transcription of glycolytic enzymes. In conclusion, our research found that FOXK2
directly regulates multiple genes of the glycolysis pathway through transcriptional regulation, thereby promoting the glycolysis in OC. Phosphorylation of FOXK2 at Thr13 and Ser30 by PDK2
promotes the nuclear translocation of FOXK2, thereby promoting its transcriptional regulation. In addition, high expression of FOXK2 also directly regulated the expression of PDK2 by
transcription, and thus formed positive feedback to sustain in OC cell glycolysis, thereby promoting OC progression (Fig. 8G). DISCUSSION The reprogramming of energy metabolism has been
considered as a new hallmark of cancers while aerobic glycolysis has been regarded as the major metabolic phenotype. Several enzymes in the glycolytic pathway, such as GLUT1, HK2, PKM2 and
LDHA, have been identified as promising therapeutic targets for anticancer intervention and numerous studies have shown that the proliferation and progression of OC cells are dependent on
glycolysis [29, 30]. In our study, we found that the expression of all enzymes was significantly increased in OC without apparent copy number alterations. We therefore considered that the
high expression of glycolytic genes may be caused by upstream transcription factors. Further study revealed that these glycolytic enzymes were all transcriptionally regulated by FOXK2. FOXK2
was identified as a key factor regulating glycolysis in OC. FOXK2 is a transcription factor belonging to the forkhead box (FOX) family. The function of FOXK2 in tumorigenesis has been
characterized in several studies. FOXK2 was shown to promote tumor progression by activating Wnt signaling in colon cancer [25] but suppressed ER–positive breast cancer cell proliferation by
interacting with transcriptional corepressor complexes [21]. Recently, it was reported that _FOXK2_ was upregulated in ovarian CSCs and in human ovarian tumors and implicated in the
regulation of the cellular stress response [27]. Our study suggests that FOXK2 is upregulated in the ovarian cancer, which was consistent with existing research results. Our further research
showed that FOXK2 promoted cell proliferation and migration and that FOXK2 expression was related to tumor stage and lymph node metastasis in our clinical samples. FOXK2 may be a novel
target in OC. PDK is one of the key regulators of glycolysis and oxidative phosphorylation. PDK2 suppresses the conversion of pyruvic acid to acetyl coenzyme A, which enters the TCA cycle in
mitochondria by inhibiting pyruvate dehydrogenase (PDH) through phosphorylation of Ser293 on the E1α subunit of PDH (PDHE1-alpha) [31]. PDK2 leads to cisplatin resistance in ovarian clear
cell carcinoma through suppression of mitochondrial function [32]. FOXK2 is structurally characterized by a forkhead-associated (FHA) domain, a FOX domain and a nuclear localization signal.
FHA domains are small phosphopeptide recognition modules found in eubacteria and eukaryotes that specifically recognize phosphorylated residues, particularly threonine and serine [33]. In
our study, we were surprised to find that FOXK2 interacts with the kinase PDK2 and that PDK2 directly binds to the FHA domain of FOXK2 to phosphorylate FOXK2 at threonine and serine and PDK2
enhances the transcriptional activity of FOXK2. Therefore, we speculate that PDK2 regulates FOXK2 transcriptional activity by phosphorylating FOXK2 at serine and threonine. The function of
FOXK2 Is affected by epigenetic modifications. Posttranslational modifications (PTMs) of proteins link cellular signals to the functional properties. A ubiquitously utilized mechanism to
transduce extracellular signals to the nucleus is rapidly reversible phosphorylation, which may affect transcription factor stability, location, structure, and/or the protein–interaction
network. FOXK2 SUMOylation at the K527 and K633 sites modulates its transcriptional activity in response to paclitaxel. Paclitaxel resistance is associated with the inability of FOXK2 to
bind target genes [34]. FOXK2 phosphorylation plays a role in defining FOXK2 function by regulating its stability and its activity as a transcriptional repressor protein at serine 368 and
423 sites by CDK [35]. Here, we reported PDK2 could phosphorylate FOXK2 at Thr13 and Ser30, and enhanced the transcriptional activity of FOXK2. In cells overexpressing FOXK2, additional
expression of PDK2 significantly increased proliferation and glycolytic ability, but there was no similar effect in the FOXK2 Thr13 and Ser30 mutation groups. In addition, high expression of
FOXK2 also directly regulates the expression of PDK2 by transcription. Moreover, after dephosphorylation mutation, the entry of FOXK2 into the nucleus is significantly reduced, affecting
its transcriptional activity. Ultimately leading to a decrease in glycolytic capacity. Indicating that the phosphorylation site of FOXK2 regulated by PDK2 sustained glycolysis in ovarian
cancer. In adipocytes, overexpression of FOXK1 or FOXK2 upregulates PDK1 and PDK4, and Ser232 on the E1α subunit of PDKs was significantly positively regulated by overexpression or knockdown
of FOXK2 [28]. In our study, we found the expression of PDK2 was significantly positively regulated by FOXK2, and FOXK2 transcriptionally regulates PDK2 directly. In ovarian cancer, the
expression of PDK1 and PDK4 were relatively low compared to PDK2, so we haven’t further explored whether they can phosphorylate FOXK2 [28]. In our study, we found the expression of PDK2 was
significantly positively regulated by FOXK2, and FOXK2 transcriptionally regulates PDK2 directly. In ovarian cancer, the expression of PDK1 and PDK4 were relatively low compared to PDK2, so
we haven’t further explored whether they can phosphorylate FOXK2. In conclusion, we identified FOXK2 as a key transcription factor that regulates OC glycolysis and provided a novel oncogenic
regulatory mechanism of phosphorylation by PDK2 in regulating FOXK2 transcriptional activity. PDK2-mediated FOXK2 phosphorylation at Thr13 and Ser30 upregulated the expression of FOXK2
downstream glycolytic genes. FOXK2 transcriptionally regulates glycolytic genes directly, and FOXK2 phosphorylation by PDK2 enhances its transcriptional activity leading to upregulation of
the expression of FOXK2 downstream genes, including glycolytic genes. In addition, high expression of FOXK2 also directly regulates the expression of PDK2 by transcription and thus orms
positive feedback to sustain in OC cell glycolysis, thereby promoting the OC occurrence. MATERIALS AND METHODS CLINICAL SAMPLES AND DATABASE The human ovarian cancer and normal ovarian
tissues used in this study were obtained from the Department of Obstetrics and Gynecology, Fengxian District Center Hospital and the Department of Obstetrics and Gynecology, The Affiliated
Changzhou No. 2 People’s Hospital of Nanjing Medical University. None of the patients had received radiotherapy, chemotherapy, or other related antitumor therapies before surgery. All human
tissues were obtained with informed consent, and the study was approved by the Research Ethics Committee of Fengxian District Center Hospital. We downloaded and analyzed the ovarian cancer
cohorts in The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/), GTEx (https://www.gtexportal.org/home/index.html) and the Gene Expression Omnibus (GEO,
https://www.ncbi.nlm.nih.gov/geo/). The RNA-seq level 4 gene expression data contain log2-transformed RNA-seq by expectation maximization (RSEM) values summarized at the gene level. Genetic
alteration data were downloaded from the cBio Cancer Genomics Portal (http://cbioportal.org/). Transcription factors of glycolytic genes were downloaded in JASPAR
(https://jaspar.genereg.net). CELL CULTURE AND REAGENTS The human ovarian cancer cell lines OVCAR8 (RRID:CVCL_1629), ES-2 (RRID:CVCL_3509),human embryonic kidney 293T (RRID:CVCL_0063) cells
were all preserved in Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. OVCAR8 cells were cultured in RPMI 1640 containing 10% fetal bovine serum
(FBS), 2 mM glutamine and 1% penicillin/streptomycin (P/S). ES-2 and HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) and 1% P/S. All
cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. All experiments were performed with mycoplasma-free cells. All the cells we used were purchased from ATCC with
STR certification. We conducted a cell quality inspection in 2019 and found that they were free of HIV, HBV, HCV, mycoplasma, bacterial, yeast, and fungal contamination. IMMUNOHISTOCHEMISTRY
(IHC) STAINING Immunohistochemical staining was performed as described. After treatment with diaminobenzidine and counterstaining with hematoxylin, all the sections were observed and
photographed with a microscope (Axio Imager: Carl Zeiss). Scoring was conducted according to the ratio and intensity of positive-staining cells: 0–5% scored 0; 6–35% scored 1; 36–70% scored
2; more than 70% scored 3. The final scores were designated as low or high expression groups as follows: low expression: score 0–1; high expression: score 2–3. These scores were assigned
independently and in a blinded manner by two senior pathologists. The primary antibodies used for FOXK2 detection are shown in Supplementary Table 2. SIRNA TRANSFECTION Cells were plated at
60–70% confluence in 60 mm dishes. OVCAR8 and ES-2 cells were transfected with si- FOXK2 or with a nontargeted siRNA as a control. The sequences of the siRNAs used were as follows:
si-FOXK2-1, sense (5′-3′): GCGAGUUCGAGUAUCUGAUTT, antisense (5′-3′): AUCAGAUACUCGAACUCGCTT; si-FOXK2-2, sense (5′-3′): CGGUGACCAUAGUACAACATT, antisense (5′-3′): UGUUGUACUAUGGUCACCGTT. SiRNA
oligos were produced by Gene Pharma (Shanghai, China). Transfection steps were performed according to the manufacturer’s protocols using Lipofectamine® RNAiMAX (Thermo Fisher Scientific,
Waltham, MA, USA). PLASMID TRANSFECTION The sequences of the short hairpin (sh)RNAs targeting FOXK2 were sh-1, 5′- GCGAGTTCGAGTATCTGATTT -3′ and sh-2, 5′- CGGTGACCATAGTACAACATT-3′. The
shRNA-containing plasmids and a negative control plasmid were purchased from GenePharma (Shanghai, China). All these plasmids were packaged into virus particles using HEK 293T cells and the
viral titers were determined. To establish stable FOXK2-knockdown cell lines, the target cells were infected with 1 × 108 lentivirus-transducing units with 6 μg/mL polybrene (Sigma-Aldrich,
St. Louis, MO, USA). The infected cells were then screened with 2.5 μg/mL puromycin after 72 h. The efficiency of the knockdown or overexpression was verified by western blotting. The
plasmids containing FOXK2 (NM_004514.3, 4002) and PDK2(NM_002611.4, A3968) and a negative control plasmid were obtained from FulenGen Ltd., Co. (Guangzhou, China). The sequences of FOXK2-HA,
FOXK2-Δ(54-128)-HA, FOXK2-Δ(129-171)-HA, FOXK2-Δ(300-371)-HA, and PDK2-Flag are shown in Supplementary Data. RNA ISOLATION AND QUANTITATIVE REAL-TIME PCR Total cellular RNA was extracted
using TRIzol reagent (Takara). A PrimeScript RT-PCR kit (Takara) was used to perform the RT according to the protocol. SYBR Premix Ex Taq (Takara) on a 7500 real-time PCR system (Applied
Biosystems) was used to determine the mRNA expression at the following cycling settings: one initial cycle at 95 °C for 10 s followed by 40 cycles of 5 s at 95 °C and 31 s at 60 °C. Data
were normalized to 18S RNA expression and represented as the average of three repeated experiments. Prime sequences used for FOXK2, GLUT1, HK2, GPI1, PFK1, ALDOA, GAPDH, PGK2, PGAM2, ENO1,
PKM2, LDHA, PDK2 and 18 s detection are shown in Supplementary Table 1. WESTERN BLOTTING Total cellular protein was extracted using a total protein extraction buffer (Beyotime, China)
according to the manufacturer’s instructions. Equal amounts of proteins were loaded onto 10% Tris-glycine sodium dodecyl sulfate‒polyacrylamide gel electrophoresis gels (Bio-Rad
Laboratories, CA, USA). Then the separated proteins were transferred onto nitrocellulose membranes (Millipore, MA, USA). After blocking with 10% nonfat milk, the membranes were incubated
with a primary antibody at 4 °C overnight. The membranes were further incubated with secondary antibody and protein signals were detected with the Odyssey imaging system (LI-COR Biosciences,
Lincoln, NE). Primary antibodies against FOXK2, GLUT1, HK2, GPI1, PFKL, ALDOA, GAPDH, PGK1, PGAM1, ENO1, PKM2, LDHA, PDK2 and β-Actin used for detection are shown in Supplementary Table 2.
The phos-tag gels contained 20 μM Phos-tag (APExBIO, F4002) and 40 μM MnCl2. Before transferring onto nitrocellulose membrane, the phos-tag gel was immersed in transmembrane buffer
containing 10 mM EDTA and washed to eliminate manganese ions. CELL VIABILITY ASSA_Y_ The cells were plated in 96-well plates at a density of 3000 cells per well with 100 μl of complete
culture medium and cultured for 2-5 days. Each group contained five wells. 10 μl Cell Counting Kit-8 (CCK-8, WST-8, Dojindo, Japan) solution was added to each well after 24 h, 48 h, 72 h and
96 h. CCK-8 was metabolized to produce a colorimetric dye that was read at 450 nm using a microplate reader. CELL MIGRATION In the migration assay, 2.5 × 104 cells were seeded into the
upper chamber of the Transwell plate (Millipore, USA). Cells were allowed to migrate for 24 h at 37 °C. The migrated cells were then fixed and stained with 0.1% crystal violet, six randomly
selected fields were photographed, and the cell numbers were counted. IN VIVO TUMOR XENOGRAFT MODEL Six-week-old female athymic nude (nu/nu) mice (SLAC, Shanghai, China) were randomly
divided into four groups and injected subcutaneously in the right flank with the stable single cell clones of OVCAR8-sh and control cells at 5 × 106 cells in 100 μl PBS medium for each nude
mouse. We measured tumor volume once a week. After the mice were killed. The tumors were dissected and fixed with phosphate-buffered neutral formalin for standard histologic examination. The
mice were manipulated and housed according to protocols approved by the East China Normal University Animal Care Commission. SEAHORSE ANALYSES The assays for extracellular acidification
rate (ECAR) and oxygen consumption rate (OCR) in the cultured cells were performed with the Seahorse XF96 Flux Analyzer (Seahorse Bioscience, Agilent) according to the manufacturer’s
instructions. Briefly, OVCAR8 and ES-2 cells were seeded in an XF96-well plate at a density of 1 × 104 per well with the indicated treatments. The medium was replaced with the assay medium 1
h before the assay. For the glycolytic stress test (Seahorse Cat. #103020-100), 10 mM glucose, 1 μM oligomycin and 50 mM 2-deoxyglucose (2-DG) were injected into the wells. For the
mitochondrial stress test (Seahorse Cat. #103015-100), 1 μM oligomycin, 1 μM FCCP, 0.5 μM rotenone and 0.5 μM antimycin A were added to the wells. The above experiments were performed in
triplicate and repeated twice. CHROMATIN IMMUNOPRECIPITATION (CHIP) ASSAY ChIP assays were performed using a Pierce Agarose ChIP Kit (Thermo, 26156) according to the manufacturer’s
instructions. Antibodies against FOXK2 (Abcam, ab5298, 4 μg per ChIP) were used for immunoprecipitation. Quantitative analysis of ChIP-derived DNA was performed by real-time qPCR analysis
(primers in Supplementary Table S3). The assays were performed in triplicate. PLASMID CONSTRUCTION AND DUAL-LUCIFERASE REPORTER ASSAY HK2, GPI, GAPDH, PGK1, PKM2, LDH and PDK2
promoter-luciferase reporter plasmids containing the promoter region were constructed in the pGL4.10/pGL3B plasmid. Wild-type and mutant promoter luciferase constructs were verified by DNA
sequencing. A dual luciferase reporter assay (Promega, WI, USA) was performed according to the manufacturer’s instructions. CO-IMMUNOPRECIPITATION (CO-IP) ASSAY Cells were lysed in IP buffer
(P0013, Beyotime) containing protease inhibitors (B14001, Selleck) and phosphatase inhibitors (B15001, Selleck). Protein A/G beads (Santa Cruz Biotechnology) were preincubated with antibody
and IgG for 30–60 min on a spinning wheel. The bead-antibody complexes were then suspended with protein lysate. All Co-IP was performed overnight on a spinning wheel at 4 °C. The beads were
washed 3 times with extraction buffer and were collected by centrifugation at 5000 rpm. The immunoprecipitants were subjected to western blotting. IMMUNOFLUORESCENCE (IF) Ovarian cancer
cells were planted in 8-well chambers (Ibidi, Germany) for IF. We fixed cells with 4% polyformaldehyde (30 min), permeabilized them with 0.1% Triton X-100 (10 min) and blocked with 10% BSA
(1 h) at room temperature. All cells were incubated with the primary antibodies at room temperature for 2 h and then labeled with Alexa Fluor-488-conjugated Alexa (1:400, Rabbit, Sigma, USA)
and Fluor-594-conjugated secondary antibodies (1:400, Mouse, Sigma, USA) at room temperature. DAPI was used to stain the nucleus for 5 min (Sigma, USA). Confocal microscopy (LSM 510,
METALaser scanning microscope, Zeiss) was used to acquire the images. The primary antibodies used for FOXK2 and PDK2 detection are shown in Supplementary Table 2. IN VITRO KINASE ASSAY
Immunoprecipitation (IP) of FOXK2 and PDK2 followed the same steps as the Co-IP assay to purify FOXK2 and PDK2 proteins. Equal aliquots of FOXK2 and PDK2 proteins were then subjected to
kinase reactions. Kinase reactions were performed in kinase buffer (Cell Signaling Technology, #9802), for 30 min at 30 °C, with 200 µM ATP (Cell Signaling Technology, #9804). Then we used
ADP-Glo™Kinase Assay (Promega, V6930) to measure kinase activity by quantifying the amount of ADP produced during a kinase reaction according to the manufacturer’s instructions. In addition,
reactions were then stopped by the addition of 4X SDS‒PAGE buffer and loaded for separation on a 10% SDS‒PAGE gel. LIQUID CHROMATOGRAPHY TANDEM MASS SPECTROMETRY (LC-MS/MS) Sample
Preparation: Gel pieces were cut from SDS PAGE, destained in 100 mM NH4HCO3 with 30% Acetonitrile and washed with Milli-Q water for10 min until the gels were destained. The spots were then
lyophilized in a vacuum centrifuge. The in-gel proteins were reduced with dithiothreitol (10 mM DTT/100 mM NH4HCO3) for 30 min at 56 °C, then alkylated with iodoacetamide (200 mM IAA/100 mM
NH4HCO3) in the dark at room temperature for 20 min. Gel pieces were briefly rinsed with 100 mM NH4HCO3 and I, respectively. Gel pieces were digested overnight in 12.5 ng/μl trypsin in 25 mM
NH4HCO3. The peptides were extracted three times with 60% I/0.1% TFA. The extracts were pooled and dried completely by a vacuum centrifuge. LC-MS/MS: The peptide of each sample was desalted
on C18 Cartridges (Empore™ SPE Cartridges, Sigma), then concentrated by vacuum centrifugation and reconstituted in 10 µl of 0.1% (v/v) Formic acid. MS experiments were performed on a Q
Exactive HF mass spectrometer that was coupled to Easy nLC (Thermo Scientific). Peptide was first loaded onto a trap column (100 μm*20 mm, 5 μm, C18) with 0.1% formic acid, then separated by
an analytical column (75 μm*100 mm, 3 μm, C18)) with a binary gradient of buffer A (0.1% Formic acid) and buffer B (84% acetonitrile and 0.1% Formic acid) at a flow rate of 300 nL/min over
60 min. The gradient was set as following: 2–7% buffer B from 0 min to 3 min, 7–35% buffer B from 3 min to 48 min, 35–90% buffer B from 48 min to 53 min, 90% buffer B kept till to 60 min.
After peptide segment separation, DDA (data dependent collection) mass spectrometry analysis was performed using a Q-Exactive Plus mass spectrometer (Thermo Scientific).MS data was acquired
using a data-dependent top20 method dynamically choosing the most abundant precursor ions from the survey scan (300–1800 m/z) for HCD fragmentation.The full MS scans were acquired at a
resolution of 70,000 at m/z 200, and 17,500 at m/z 200 for MS/MS scan. The maximum injection time was set to for 50 ms for MS and 50 ms for MS/MS. Normalized collision energy was 27 and the
isolation window was set to 2.0 Th. Database search: The MS data were analyzed using MaxQuant software version 1.6.1.0. MS data were searched against the UniProtKB Human database (173,343
total entries, downloaded 09/2019). The trypsin was selected as digestion enzyme. The maximal two missed cleavage sites and the mass tolerance of 4.5 ppm for precursor ions and 20 ppm for
fragment ions were defined for database search. Carbamidomethylation of cysteines was defined as fixed modification, while Oxidation (M), Acetyl (Protein N-term) and Phospho (STY) were set
as variable modifications for database searching. The database search results were filtered and exported with <1% false discovery rate (FDR) at peptide level and protein level,
respectively. STATISTICAL ANALYSIS Data are shown as means ± S.D. GraphPad Prism 10 was used to manipulate statistical analyses. Correlation of FOXK2 expression with categorical clinical
variables in patients with OC was evaluated by χ2 test (SPSS 20.0 statistical software). After testing the homogeneity of variance, two-tailed Student’s _t_ test was used to compare the
results from different groups. Spearman rank correlation test was used to analyze the correlation between FOXK2 and glycolysis related genes. All experiments with cell lines were done in at
least triplicates. _P_ > 0.05 = NS, *_P_ ≤ 0.05, **_P_ ≤ 0.01, ***_P_ ≤ 0.001, ****_P_ ≤ 0.0001. DATA AVAILABILITY All data and materials were available from the corresponding authors on
reasonable request. REFERENCES * Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–96. Article PubMed
PubMed Central Google Scholar * Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304.
Article PubMed Google Scholar * Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. Article CAS PubMed Google Scholar * Zhang Y, Wang Y, Zhao G,
Orsulic S, Matei D. Metabolic dependencies and targets in ovarian cancer. Pharmacol Ther. 2023;245:108413. Article CAS PubMed Google Scholar * Li XB, Gu JD, Zhou QH. Review of aerobic
glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac Cancer. 2015;6:17–24. Article CAS PubMed PubMed Central Google Scholar * Vander Heiden MG, Cantley LC,
Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. Article Google Scholar * Garber K. Energy deregulation:
licensing tumors to grow. Science. 2006;312:1158–9. Article CAS PubMed Google Scholar * Lu YY, Wu CH, Hong CH, Chang KL, Lee CH. GLUT-1 Enhances Glycolysis, Oxidative Stress, and
Fibroblast Proliferation in Keloid. Life. 2021;11:505. Article CAS PubMed PubMed Central Google Scholar * Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg
did not anticipate. Cancer Cell. 2012;21:297–308. Article CAS PubMed PubMed Central Google Scholar * Li X, Yu C, Luo Y, Lin J, Wang F, Sun X, et al. Aldolase A Enhances Intrahepatic
Cholangiocarcinoma Proliferation and Invasion through Promoting Glycolysis. Int J Biol Sci. 2021;17:1782–94. Article CAS PubMed PubMed Central Google Scholar * Jia D, Lu M, Jung KH,
Park JH, Yu L, Onuchic JN, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA. 2019;116:3909–18. Article CAS PubMed
PubMed Central Google Scholar * Irey EA, Lassiter CM, Brady NJ, Chuntova P, Wang Y, Knutson TP, et al. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing
expression of protumorigenic factors. Proc Natl Acad Sci USA. 2019;116:12442–51. Article CAS PubMed PubMed Central Google Scholar * Engelman JA, Luo J, Cantley LC. The evolution of
phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. Article CAS PubMed Google Scholar * Han J, Zhang L, Guo H, Wysham WZ, Roque DR,
Willson AK, et al. Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol. 2015;138:668–75. Article
CAS PubMed PubMed Central Google Scholar * Hibdon ES, Razumilava N, Keeley TM, Wong G, Solanki S, Shah YM, et al. Notch and mTOR Signaling Pathways Promote Human Gastric Cancer Cell
Proliferation. Neoplasia. 2019;21:702–12. Article CAS PubMed PubMed Central Google Scholar * Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional Regulation of the
Warburg Effect in Cancer by SIX1. Cancer Cell. 2018;33:368–85.e367. Article CAS PubMed Google Scholar * Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis -
the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–99. Article CAS PubMed PubMed Central Google Scholar * Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and
metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. Article CAS PubMed PubMed Central Google Scholar * Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid
tumour. Nat Rev Cancer. 2008;8:705–13. Article CAS PubMed Google Scholar * Chen S, Jiang S, Hu F, Xu Y, Wang T, Mei Q. Foxk2 inhibits non-small cell lung cancer epithelial-mesenchymal
transition and proliferation through the repression of different key target genes. Oncol Rep. 2017;37:2335–47. Article CAS PubMed Google Scholar * Liu Y, Ao X, Jia Z, Bai XY, Xu Z, Hu G,
et al. FOXK2 transcription factor suppresses ERalpha-positive breast cancer cell growth through down-regulating the stability of ERalpha via mechanism involving BRCA1/BARD1. Sci Rep.
2015;5:8796. Article CAS PubMed PubMed Central Google Scholar * Zhang F, Ma X, Li H, Zhang Y, Li X, Chen L, et al. FOXK2 suppresses the malignant phenotype and induces apoptosis through
inhibition of EGFR in clear-cell renal cell carcinoma. Int J Cancer. 2018;142:2543–57. Article CAS PubMed Google Scholar * Wang B, Zhang X, Wang W, Zhu Z, Tang F, Wang D, et al.
Forkhead box K2 inhibits the proliferation, migration, and invasion of human glioma cells and predicts a favorable prognosis. Onco Targets Ther. 2018;11:1067–75. Article PubMed PubMed
Central Google Scholar * Lin MF, Yang YF, Peng ZP, Zhang MF, Liang JY, Chen W, et al. FOXK2, regulted by miR-1271-5p, promotes cell growth and indicates unfavorable prognosis in
hepatocellular carcinoma. Int J Biochem Cell Biol. 2017;88:155–61. Article CAS PubMed Google Scholar * Wang W, Li X, Lee M, Jun S, Aziz KE, Feng L, et al. FOXKs promote Wnt/beta-catenin
signaling by translocating DVL into the nucleus. Dev Cell. 2015;32:707–18. Article PubMed PubMed Central Google Scholar * Qian Y, Xia S, Feng Z. Sox9 mediated transcriptional activation
of FOXK2 is critical for colorectal cancer cells proliferation. Biochem Biophys Res Commun. 2017;483:475–81. Article CAS PubMed Google Scholar * Zhang Y, Wang Y, Zhao G, Tanner EJ, Adli
M, Matei D. FOXK2 promotes ovarian cancer stemness by regulating the unfolded protein response pathway. J Clin Invest. 2022;132:e151591. Article CAS PubMed PubMed Central Google Scholar
* Sukonina V, Ma H, Zhang W, Bartesaghi S, Subhash S, Heglind M, et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature. 2019;566:279–83. Article CAS PubMed Google Scholar * Han
CY, Patten DA, Richardson RB, Harper ME, Tsang BK. Tumor metabolism regulating chemosensitivity in ovarian cancer. Genes Cancer. 2018;9:155–75. Article CAS PubMed PubMed Central Google
Scholar * Xintaropoulou C, Ward C, Wise A, Queckborner S, Turnbull A, Michie CO, et al. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a
strategy for ovarian cancer treatment. BMC Cancer. 2018;18:636. Article PubMed PubMed Central Google Scholar * Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in
cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2016;138:809–17. Article CAS PubMed Google Scholar * Kitamura S, Yamaguchi K,
Murakami R, Furutake Y, Higasa K, Abiko K, et al. PDK2 leads to cisplatin resistance through suppression of mitochondrial function in ovarian clear cell carcinoma. Cancer Sci.
2021;112:4627–40. Article CAS PubMed PubMed Central Google Scholar * Almawi AW, Matthews LA, Guarne A. FHA domains: Phosphopeptide binding and beyond. Prog Biophys Mol Biol.
2017;127:105–10. Article CAS PubMed Google Scholar * Nestal de Moraes G, Ji Z, Fan LY, Yao S, Zona S, Sharrocks AD, et al. SUMOylation modulates FOXK2-mediated paclitaxel sensitivity in
breast cancer cells. Oncogenesis. 2018;7:29. Article PubMed PubMed Central Google Scholar * Marais A, Ji Z, Child ES, Krause E, Mann DJ, Sharrocks AD. Cell cycle-dependent regulation of
the forkhead transcription factor FOXK2 by CDK.cyclin complexes. J Biol Chem. 2010;285:35728–39. Article CAS PubMed PubMed Central Google Scholar Download references FUNDING This work
was supported by the National Natural Science Foundation of China [Grant numbers 81974407 and 81672587 to RZ; 82173242 to TYS; 82203228 to DXL]. AUTHOR INFORMATION Author notes * These
authors contributed equally: Cancan Zhang, Yinyin Xu, Xinyue Zhu. AUTHORS AND AFFILIATIONS * Fengxian Hospital, The Third School of Clinical Medicine, Southern Medical University, Shanghai,
China Cancan Zhang, Yinyin Xu, Xinyue Zhu, Fengmian Wang, Huan Lu, Chunlin Tao & Rong Zhang * Shanghai Geriatric Medical Center, Shanghai, 201104, China Cancan Zhang & Rong Zhang *
Department of Obstetrics and Gynecology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China Cancan Zhang * State Key Laboratory of Oncogenes and Related
Genes, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China Xueli Zhang, Lipeng Hu, Zhigang Zhang & Dongxue Li * Department of
Gynecologic Oncology, Zhongshan Hospital, Fudan University, Shanghai, China Kai Xu & Tingyan Shi Authors * Cancan Zhang View author publications You can also search for this author
inPubMed Google Scholar * Yinyin Xu View author publications You can also search for this author inPubMed Google Scholar * Xinyue Zhu View author publications You can also search for this
author inPubMed Google Scholar * Xueli Zhang View author publications You can also search for this author inPubMed Google Scholar * Fengmian Wang View author publications You can also search
for this author inPubMed Google Scholar * Lipeng Hu View author publications You can also search for this author inPubMed Google Scholar * Huan Lu View author publications You can also
search for this author inPubMed Google Scholar * Chunlin Tao View author publications You can also search for this author inPubMed Google Scholar * Kai Xu View author publications You can
also search for this author inPubMed Google Scholar * Zhigang Zhang View author publications You can also search for this author inPubMed Google Scholar * Dongxue Li View author publications
You can also search for this author inPubMed Google Scholar * Tingyan Shi View author publications You can also search for this author inPubMed Google Scholar * Rong Zhang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS RZ, TYS, DXL and CCZ conceived and supervised the study, CCZ, YYX and XYZ designed and performed the
experiments. FMW, HL and CLT assisted in conducting IHC experiments and the bioinformatics analyses. XLZ, LPH, KX and ZGZ provided insightful suggestions and experimental materials. CCZ.
Wrote the manuscript, RZ, TYS, DXL, Z.Y.W and C.C.Z edited the manuscript. All authors contributed to the discussion and approved the manuscript. CORRESPONDING AUTHORS Correspondence to
Dongxue Li, Tingyan Shi or Rong Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIALS FIGURE S1 FIGURE S2 FIGURE S3 FIGURE S4 TABLE
S1 TABLE S2 TABLE S3 TABLE S4 TABLE S5 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, C., Xu, Y., Zhu, X. _et al._ Phosphorylation of FOXK2 at Thr13 and Ser30 by PDK2 sustains glycolysis through a positive feedback
manner in ovarian cancer. _Oncogene_ 43, 1985–1999 (2024). https://doi.org/10.1038/s41388-024-03052-x Download citation * Received: 02 November 2023 * Revised: 20 April 2024 * Accepted: 24
April 2024 * Published: 11 May 2024 * Issue Date: 24 June 2024 * DOI: https://doi.org/10.1038/s41388-024-03052-x SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative