Play all audios:
ABSTRACT Sepsis, a dysregulated host response to infection, has been difficult to accurately define in children. Despite a higher incidence, especially in neonates, a non-specific clinical
presentation alongside a lack of verified biomarkers has prevented a common understanding of this condition. Platelets, traditionally regarded as mediators of haemostasis and thrombosis, are
increasingly associated with functions in the immune system with involvement across the spectrum of innate and adaptive immunity. The large number of circulating platelets (approx. 150,000
cells per microlitre) mean they outnumber traditional immune cells and are often the first to encounter a pathogen at a site of injury. There are also well-described physiological
differences between platelets in children and adults. The purpose of this review is to place into context the platelet and its role in immunology and examine the evidence where available for
its role as an immune cell in childhood sepsis. It will examine how the platelet interacts with both humoral and cellular components of the immune system and finally discuss the role the
platelet proteome, releasate and extracellular vesicles may play in childhood sepsis. This review also examines how platelet transfusions may interfere with the complex relationships between
immune cells in infection. IMPACT * Platelets are increasingly being recognised as important “first responders” to immune threats. * Differences in adult and paediatric platelets may
contribute to differing immune response to infections. * Adult platelet transfusions may affect infant immune responses to inflammatory/infectious stimuli. SIMILAR CONTENT BEING VIEWED BY
OTHERS MARKERS OF PLATELET ACTIVATION FOR IDENTIFICATION OF LATE ONSET SEPSIS IN INFANTS: PARENT STUDY PROTOCOL Article 27 September 2023 CHANGES IN INFLAMMATORY PROTEINS FOLLOWING PLATELET
TRANSFUSION IN A NEONATAL POPULATION Article Open access 13 July 2023 MECHANISMS AND MODULATION OF SEPSIS-INDUCED IMMUNE DYSFUNCTION IN CHILDREN Article 24 December 2021 INTRODUCTION Sepsis,
a dysregulated host response to infection, has been recognised as a global threat to both adults and children by the United Nations World Health assembly and a priority for the World Health
Organisation to address.1 Despite this, accurately defining sepsis in children is fraught with difficulty. It has been challenging to generate paediatric definitions that adequately combine
the unique paediatric pathophysiological response with adult Sepsis 3 criteria, and attempts to do so have yet to be validated outside of an intensive care setting.1 In neonates this
difficulty is compounded by a high incidence of culture-proven sepsis with non-specific clinical presentations.2 Globally there is an estimated 3 million cases of neonatal sepsis and 1.2
million cases of paediatric sepsis a year.3 This leads to a situation where many paediatricians and neonatologists treat suspected sepsis empirically, as delayed diagnosis is associated with
worse outcomes.4 Despite decades of interest, no single or set of validated biomarkers have been developed which accurately diagnose sepsis over a significant bacterial infection in the
absence of a dysregulated host response.5 Anucleate platelets, derived from megakaryocytes, traditionally function in haemostasis and thrombosis. However an appreciation of the wider role of
platelets as “first responders” in host defence has emerged recently with important roles in wound healing, innate and adaptive immunity described.6 Normal platelet production is driven by
the production of thrombopoietic factors which stimulate megakaryocytopoiesis by increasing the number of megakaryocyte progenitors. These then differentiate, mature and ultimately release
platelets into the bloodstream.7 The interest in platelets as mediators of an inflammatory cascade has an impact in how we view both neonatal and paediatric sepsis. Neonatal platelets have
previously been shown to have a different functional and transcriptomic profile to their adult counterparts, suggesting their response to infection is likely to differ.8,9,10 How long this
platelet “hyporesponsiveness” persists into childhood is not yet fully elucidated with almost adult response to agonists demonstrated at 14 days of life but with documented differences up
until 15 years of age.11,12 Additionally, how this interacts with other parts of the immune system, which also demonstrate age-specific responses, is poorly described. The purpose of this
review is to place into context the role of paediatric platelets in the development of fulminant sepsis. To approach this we will examine what features of the platelet may contribute to its
role outside of thrombosis, investigate the use of traditional platelet indices in paediatric sepsis and infection, and explore emerging molecular mechanisms underpinning platelet response
to infection and why these might differ in both the neonatal period and childhood. Finally we will look at how this understanding could contribute to the development of a universal
definition of sepsis through novel platelet derived extracellular vesicles (EVs) and proteomics, and examine gaps in the current literature which need to be addressed in order to fully
understand platelets as key immune regulators in sepsis. PLATELET INDICES AND PAEDIATRIC SEPSIS Several platelet indices have been utilised as non-specific means of both prognostication and
diagnosis in paediatric sepsis.13,14 Thrombocytopenia is a well-recognised component of both neonatal sepsis and severe paediatric sepsis.13,15,16,17 Thrombocytosis is a rarer finding in the
septic child, and may represent a rebound phenomenon following a period of relative thrombocytopenia.18 Metrics such as platelet distribution width (PDW), which describes the range in
platelet size, and mean platelet volume (MPV), the measure of average platelet size, have been used to predict outcomes in paediatric and neonatal sepsis.13,19 Changes in these parameters
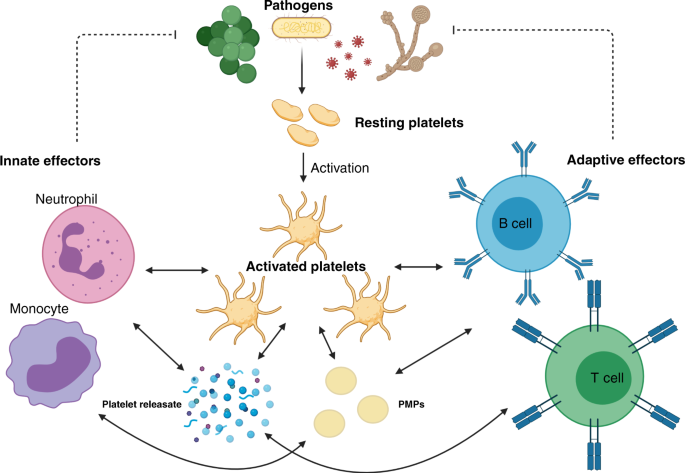
during sepsis offer a crude insight into the multiple roles played by platelets in sepsis pathophysiology (Fig. 1). DEVELOPMENT OF THROMBOCYTOPENIA IN PAEDIATRIC AND NEONATAL SEPSIS A low
platelet count is described in a large number of PICU and NICU admissions with sepsis16,20 A number of pathological mechanisms are thought to contribute to this process. One of the best
understood is the development of a consumptive coagulopathy, i.e., disseminated intravascular coagulation (DIC), where platelets are inappropriately activated due to widespread vascular
inflammation leading to the development of intravascular microthrombotic events throughout the systemic circulation. These consume both platelets and coagulation factors, leading
paradoxically to increased bleeding and clotting. These events likely occur on a spectrum even in the absence of fulminant DIC, leading to a reduction of platelet count without necessarily
associated signs of inappropriate bleeding or clotting.21 The bone marrow as the site of thrombopoiesis becomes both exhausted and suppressed as part of the dysregulated host response in
sepsis.22 Septic neonates produce an excess of thrombopoietin (TPO), and the eventual development of thrombocytopenia may represent an “exhaustion” of marrow resident megakaryocytes and
their precursors rather than a depression.23,24 This is particularly important as the neonatal megakaryocyte precursors, while responsive to TPO, tend to increase in number but not in size
in response to stimulation. MPV tends to increase in response to TPO stimulation as larger, more immature platelets are released, leading to more rapid depletion of the rapidly
differentiated, small, megakaryocytes.7 The association of increased MPV and thrombocytopenia with mortality in one study of sepsis in a PICU setting suggested that in septic children, bone
marrow exhaustion may represent an almost preterminal event.14 Another measure of megakaryocytopoiesis which has been examined in neonatal sepsis and necrotising enterocolitis is immature
platelet fraction (IPF). This is a measure of larger and more fluorescent platelets suggesting immaturity. IPF was shown to be higher prior to diagnosis of NEC or sepsis compared to
previously reported healthy neonates and then declined reaching a nadir 3–5 days following diagnosis. This again supports a process of initial stimulation, possibly before the development of
clinical signs, followed by bone marrow exhaustion.25 Developmental factors in paediatric patients may cloud this issue, as factors such as MPV and PDW may be partly age-dependent (see
Table 1).26,27 A morphological transition from relatively small uniform megakaryocytes to a heterogeneous mixture of sizes commences from around 24 months with a typical “adult” appearance
not achieved until 4 years of age.28 The functional consequences of this in the context of neonatal, infant and pre-school age children who are septic is as of yet undescribed. Bone marrow
suppression due to the stimulation of Toll-like receptor 4 (TLR 4) and downstream signalling through Myeloid differentiation primary response 88 (Myd88) and TIR domain inducing adaptor
inducing interferon β (TRIF) is well-described.22,29 Experimental models using lipopolysaccharide (LPS) or animal models of sepsis have permitted elucidation of this pathway in
haematopoietic stem cells, megakaryocytes and platelets.30 While the latter are primed by TLR 4 stimulation and thus consumed in clot formation, controversy exists over possible further
roles for TLR 4 in platelet immune function. Megakaryocytes and their progenitors are suppressed by Myd88/TRIF.22 Granulopoiesis, or the production of neutrophils, is favoured in the septic
bone marrow by TLR 4/TRIF signalling to produce granulocyte colony-stimulating factor (G-CSF), leading to depletion of megakaryocytes and their precursors.31 In severe sepsis this leads to
unchecked proliferation of monocytes from granulocyte/macrophage progenitor cells. These consume common megakaryocyte-erythroid precursors, erythrocytes and platelets in a phenomenon known
as haemophagocytosis, which further exacerbates septic thrombocytopenia.32,33 PLATELETS AND PATHOGENS Platelets interact directly with pathogen-associated molecular patterns (PAMPs). LPS,
found in Gram-negative organisms, activates a number of Toll-like receptors (TLRs) including TLR 4, TLR 6 and TLR 9 (ref. 34). This leads to sequestration and aggregation of platelets in the
microvasculature of the liver and lungs.35 Interactions between stimulated platelets and neutrophils lead to neutrophil extracellular traps (NETs) formation, which capture circulating
bacteria but also promote platelet aggregation, further reducing the circulating platelet count in sepsis.36 A number of pathogens have been shown to interact with platelets directly.
Bacteria such as _Streptococcus pyogenes_ and _Staphylococcus aureus_ have specific virulence factors that target platelets and impair their function.37,38,39,40 Fungal interactions with
platelets have been described given the long association between invasive fungal infection and thrombocytopenia.41 _Candida_ species appear to inhibit platelet responses while _Aspergillus_
spp. are potent activators of platelets which may contribute to the characteristic necrosis accompanying these infections.41,42,43 These virulence factors may have evolved as a means of
pathogen circumvention of platelet facilitation of the immune response. PLATELETS AS ASSOCIATE INNATE IMMUNE CELLS Platelets possess a suite of pattern recognition receptors (PRRs) including
members of the TLR family, C-type lectin receptors (CLRs), NOD or nucleoside oligomerisation domain-like receptors (NLRs), and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs)
(Fig. 2). While these contribute to sequestration and consumption of platelets in the septic child and neonate, their function is to allow platelets to lead the initial response to noxious
stimuli through rapid degranulation and release of directly antimicrobial effectors.44,45 Platelets also facilitate the recruitment of other innate immune effectors such as granulocytes,
monocytes and innate lymphoid cells (ILCs) including natural killer (NK) cells.46 These functions act in concert in both adult and paediatric sepsis, but important differences in these cell
lines in children contribute to an altered host response. PRRS ON PLATELETS: SIGNALLING AND ROLES TLRs are among the best described signalling pathways as they are both highly conserved and
widespread across different cell types.47 Nevertheless, the initial discovery of TLRs on the surface of platelets was unexpected as traditional TLR signalling pathways terminate in nuclear
receptors leading to transcriptional alterations, e.g., nuclear factor κB (NFκB), which should not be possible in an anucleate cell.34 In platelets, the mechanism of action appears to be
driven by altered transcription of pre-packaged mRNA. This is likely driven by signals from endogenous spliceosome components.48 Similar signalling pathways are utilised by other PRR
classes. Potential barriers to TLR signalling in platelets include the absence of co-receptors on the platelet surface. The co-receptor for TLR 4, CD14, is not present on platelets, but
their response to a TLR 4-driven stimulus is potentiated in the presence of soluble CD14, an acute phase reactant increased in the presence of infection and inflammation.34,49 Preterm
neonates have lower expression of TLR 4 and MyD88 on monocytes, and reduced response to LPS and lipoteichoic acid in vitro, which may contribute to the high rate of severe bacterial
infections and sepsis in this cohort.50 There is a paucity of data on similar expression in other myeloid cell lineages including preterm and neonatal platelets. Among the best known NLR
receptors is NLRP3 (NOD-, LRR-, Pyrin-domain-containing protein 3). This cytosolic sensor identifies a wide range of signals including PAMPs and signs of cellular damage, referred to as
danger-associated molecular patterns (DAMPs). It then forms the NLRP3 inflammasome, a complex of the NLRP3, ASC (apoptosis-associated speck like protein containing a CARD) and pro caspase 1,
which drives production of IL-1β and IL-18 and pyroptosis.51 While several papers have demonstrated the presence of NLRP3 in platelets using flow cytometry techniques, with colocalization
occurring in activated platelets, other transcriptomic methods failed to show evidence of the NLRP3 inflammasome components and argued against its presence within platelets.52,53,54,55 The
study using transcriptomic methods noted that platelet co-culture with macrophage, monocytes and neutrophils appeared to drive increased NLRP3 activation in those cell lines, acting as a
potentiator of the innate inflammatory response.55 Another study characterised platelet and NLRP3 activation in a rat model of polymicrobial sepsis (caecal ligation and puncture), showing
that greater platelet NLRP3 activation was associated with end organ dysfunction.52 In neonatal studies, NLRP3 has been studied predominantly in the context of hypoxic ischaemic
encephalopathy and chronic lung disease rather than sepsis and in serum as opposed to in platelets.56,57 Other inflammasomes have received even less attention.58 The presence of NLRP3 in
platelets is likely to be a topic of for future debate. Age-dependent functional differences particularly in septic patients remain poorly described. The presence of C-type Lectin-like
receptors (CLRs) is well established on the surface of platelets, with numerous studies describing C-Type lectin Receptor 2 (CLEC 2) and dendritic cell (DC)-specific intracellular adhesion
molecule 3 grabbing non-integrin (DC-SIGN) and their functions.59,60 CLEC 2 interacts with podoplanin, expressed by activated monocytes. A study which characterised _Salmonella typhi_
infection in a mouse model reported increased thrombosis as a consequence of this interaction particularly in the liver using a mixture of immunohistology and flow cytometry,61 whereas
another (using two separate mouse models of sepsis) showed an immunoregulatory role with reduction in inflammatory monocyte activity without thrombosis.62 It is unclear whether these
differences reflect an organism-specific interaction, a context-dependent reaction according to other interacting cell types, or that a prothrombotic and antinflammatory effect occur
simultaneously or at different points in the course of human sepsis. CLEC 2 has been examined alongside glycoprotein VI (GPVI), which signals through the same intracellular pathway in both
preterm and term neonates, where agonist rhodocytin was used to activate these receptors. Expression of activated integrin αIIbβ3 was used to characterise responsiveness of platelets. This
study showed both reduced expression of CLEC 2, GPVI and αIIbβ3 at transcriptional level and diminished response.63 It was suggested that this might represent a more generalised reduction in
responsiveness to immunotyrosine-based activation motif (ITAM)-containing receptors. The GPVI-spleen tyrosine kinase (SYK) signalling cascade has been shown recently to be downregulated in
critically ill adult patients with sepsis, hinting towards a key role in sepsis pathophysiology.64 Given the known hyporesponsiveness of TLR 4 in monocytes from this age group, and the fact
that SYK is involved in both pathways, it is tempting to hypothesise that this represents a key intracellular step in the relative immunodeficiency in neonates.65 DC-SIGN is a CLR which
binds to mannose or lewis x carbohydrate structures in order to recognise a wide range of pathogens. It has been most studied for its role in immune evasion by HIV and TB.66 Platelets have
been shown to express DC SIGN, and its role is widely understood in the context of viral host defence59,67; however, there is limited information available on its role in bacterial
infections, or on any differences between paediatric and adult platelet expression or signalling. Similarly RLRs research has predominately examined viral infection in megakaryocytes,
without investigation of any maturational changes in different age groups.68 In summary, platelets express a wide variety of PRRs allowing recognition of PAMPs and DAMPs, which are
associated with a range of immunological insults including sepsis (Fig. 2). While early efforts have been made to describe differences between these pathways in adults and neonates, there
remains a gap in the knowledge regarding how and when these pathways mature, and how maturation affects the differing pathophysiology of sepsis from childhood to adulthood. PLATELET
INTERACTIONS WITH OTHER INNATE IMMUNE EFFECTOR CELLS Platelets also interact with other innate effector cells through direct contact and through the release of a myriad of paracrine and
autocrine signalling molecules through their platelet releasate.69,70,71 Interactions between platelets and other immune effector cells are therefore complex and context dependent, with many
gaps in the current literature particularly with respect to paediatric and neonatal disease processes. The neutrophil–platelet interaction is among the best understood, due to the
phenomenon of NETs and their prominent role in the innate immune response.72 Previous reviews have described the plethora of signalling pathways resulting in NETosis, the release of a DNA
and protein web which captures pathogens and prevents their dissemination.72 TLR 4 has been shown to play a key role in the initial platelet–neutrophil interaction by activating both cell
types when stimulated.36 Activated platelets express P-selectin interacts with P-selectin glycoprotein ligand 1 (PSGL-1), expressed on the surface of neutrophils and other leucocytes. This
facilitates the interaction of high mobility group protein B1 (HMGB1) from platelets with the receptor for advanced glycosylation end-products (RAGE) by spatial approximation of the two cell
types. An intracellular signalling cascade within neutrophils is then triggered, which produces reactive oxygen species (ROS) and promotes neutrophil elastase translocation to the nucleus,
where it digests chromatin. This in turn results in the release of free DNA for incorporation into NETs alongside granular antibacterial proteins. Intracellular regulators then produce
either lytic NETosis, through death of the neutrophil, or the more rapid non-lytic NETosis.72 Animal models have shown this to be a key component of the inflammatory response in sepsis.73
Evidence of NETosis has also been demonstrated in clinical studies examining meningococcal sepsis in a PICU setting.74 While NET formation is a key point in neutrophil–platelet interactions
it is important to note that while platelets are important in augmenting NET formation they are not necessary for this to occur.72 Term and preterm infants not only exhibit hyporeactive
platelets and reduced PRR signalling (particularly TLR 4), but reduced NETosis has also been demonstrated in these patients in some studies, which could contribute to the high rate of sepsis
in the newborn period.75 Reduction of NETosis in the immediate newborn period has been ascribed to a series of related peptides released from the placenta including neonatal NET inhibitory
factor.76 However, conversely, NET production was increased in an “infant” mouse model of sepsis and associated with worse organ injury and greater pro-inflammatory cytokine production.77
Whether this represents a difference between species, or species-specific immune development, is difficult to ascertain from existing literature. While NETs are hypothesised to reduce the
spread of infection they are also associated with the development of DIC and microvascular damage.73 Determining the contribution of platelet–neutrophil interactions to the neutrophil
hypofunction of the neonatal period has yet to be described. Additionally, the timing of when a more “mature” effector neutrophil function develops has yet to be elucidated. Beyond the
formation of NETs, the neutrophil–platelet interaction is characterised by both chemical and biomechanical crosstalk. Platelets can slowly migrate and “bundle” invading bacteria so they are
more localised and vulnerable to neutrophil phagocytosis/NET formation.78 Similarly, neutrophils “scan” for activated platelets to indicate where injury, inflammation or infection is
occurring.79 Relevant interactions occur not only through the P-Selectin–PSGL-1 complex but through a wide range of integrins. These include LFA 1 (CD11a/CD18), which interacts with ICAM 1
expressed on the platelet surface, and Mac 1 (CD11b/CD18), which binds GPIb.80,81 Platelets themselves tend to be pushed towards vessel walls due to their relatively small size compared with
erythrocytes.82 This gives them ample opportunity to activate and interact with endothelial cells, increasing the expression/translocation of integrins from weibel-palade bodies.83 These
mechanical interactions allow platelets to facilitate and enhance the neutrophil functions of adhesion, rolling and transmigration through the endothelial wall to sites of inflammation and
infection. Neonatal neutrophils exhibit reduced expression of Mac 1, which likely impairs their ability to effectively localise to sites of inflammation/infection.84,85,86 This may explain
the neonatal increased susceptibility to sepsis, as they mount a relatively weak innate response to localised infections. A number of major components of platelet α granules also facilitate
neutrophil activation and chemotaxis. Notably, the chemokines platelet factor 4 (PF4/CXCL4), RANTES (CCL5) and neutrophil-activating peptide-2 (NAP-2/CXCL7) are involved in endothelial
adhesion, chemotaxis and transmigration.80 These signals are in turn regulated by chemical signalling from the neutrophil itself with cathepsin G, a neutrophil-derived serine protease,
potentiating these reactions. Signals including phospholipids (such as platelet activating factor/PAF), nitric oxide, ROS and other cell surface proteins are contribute to this complex
interplay between platelets, neutrophils and the endothelium.80,81 These interactions are both site-specific and context dependent. For example, acidosis, a common finding in the perinatal
period and neonatal sepsis, deviates platelets away from traditional haemostatic and thrombotic roles and towards increased immunological activity.87 How these factors contribute to the
neonatal response to sepsis is not yet known, and there is even less information available about how maturation of this cellular interplay contributes to the pathophysiology of sepsis in
later infancy and early childhood. More information has been elucidated about platelet–neutrophil interactions than other models of traditional immune cell and platelet crosstalk, but there
is substantial evidence that similar communication mechanisms exist with other immune cells. Type 2 ILCs have been shown to express PSGL-1 and are involved in polymicrobial sepsis
responses.88,89 A number of interactions have been also noted between NK cells and platelets in the oncological states.90 Although both cell types play key roles in sepsis, and exhibit
maturational, age-dependent changes from childhood to adulthood, no research looking at the relationship between NK cells and platelets in childhood sepsis was found.91,92 Platelets also
have numerous interactions with specialised antigen-presenting cells, which act as a bridge between the innate and adaptive immune systems. Phagocytosis of platelet precursors and platelets
in bone marrow is a feature of sepsis and other inflammatory disease (including the sepsis mimic haemophagocytic lymphohistocytosis/HLH).33,93 However, a more nuanced relationship between
these cell types also exists. Monocyte–platelet complexes are formed using many of the same receptors utilised in neutrophil–platelet interactions, with P-selectin on activated platelets and
PSGL-1 on monocytes being key initiators of this reaction. These are then strengthened with multiple enforcing reactions through HMGB-1-RAGE, CLEC 2-Podoplanin and glycoprotein Ib
(GPIb)-CD11b interactions.94 The resulting complex function is situation-dependent, and is most commonly described with a pro-inflammatory profile, but with substantial evidence of immune
regulatory/anti-inflammatory profiles in certain situations.94,95 When pro-inflammatory, it leads to an increase in pro-inflammatory cytokines and chemokines, tumour necrosis factor α
(TNF-α), IL-1β, monocyte chemoattractant protein 1 (MCP-1/CCL2) and IL-8 among others.94 This phenotype has predominated in the sepsis literature to date, with increased platelet monocyte
aggregates associated with in Gram positive as opposed to Gram-negative bacteraemia, and a similar increase in aggregates is linked to a higher mortality in adult sepsis.94 No studies have
attempted to replicate these findings in children or neonates, despite known difference in the monocytic response to sepsis in children and neonates.96,97,98 The P-Selectin–PSGL-1
interaction acts as a key checkpoint in the maturation of monocytes into DCs.99 Activated platelets also express CD40L, an important stimulator of DC function which is required for an
effective adaptive immune response.100,101 Kupffer cells, the resident macrophages of the liver, “capture” circulating platelets in liver sinusoids and use them to encase bacteria using the
GPIb–CD11b interaction.102 This contributes to the propensity for clot formation in the liver in the context of sepsis. Platelets also influence antigen presentation through their
interaction with the complement cascade. This essential component of humoral immunity acts by separate pathways to facilitate direct lysis of threats, through the formation of the membrane
attack complex or by “tagging” threats for phagocytosis (opsonisation).103 Studies have shown that this system is underdeveloped during the initial neonatal period, with a distinct
developmental trajectory shown for the classical and alternative complement pathways and differences in lytic activity over the first 24 months of life.104 Deficiency in C9 in particular has
been associated with hyporesponsiveness to Gram-negative organisms.105 Platelets contain complement factors (C1, C1 inhibitor, factor H) and chondroitin sulfate, a glycosaminoglycan that
can trigger complement activation.103 How the neonatal platelet interacts with the underdeveloped complement system of infancy is as of yet unclear. Platelets themselves can present antigens
to adaptive immune cells utilising the major histocompatibility class I mechanism, possessing all the necessary molecular machinery to stimulate a CD8+ T cell response.106 Platelets play a
key role in the coordination of all components of the innate immune system, producing an initial response in their own right and interacting with myeloid, lymphoid and humoral components of
the initial response to infection. While much work has demonstrated that neonates in particular have deficiencies in generating appropriate immune response to infections, enormous gaps
remain in the understanding of innate immune system ontogenesis. Further understanding of how platelets interact in this nascent innate immune system, in different contexts and in response
to different threats, will be key to understanding the nature of neonatal and paediatric sepsis. PLATELETS AND THE ADAPTIVE IMMUNE RESPONSE IN PAEDIATRIC SEPSIS Platelets also shape the
adaptive response to infective stimuli. However, the relationships between platelets and the adaptive immune effector cells are less well defined. As discussed above, platelets express
surface markers which are key for the differentiation (p-selectin) and activation (CD40L) of DCs.99,100 A key step in the formation of an adaptive response is the formation of a germinal
centre. This is where DCs presenting an antigen interact with T and B cells possessing a cognate receptor, allowing for clonal expansion and somatic hypermutation of B cells and the
generation of long lasting adaptive immunity.107 While the formation of both memory B cells and high affinity IgG antibody-producing plasma cells is traditionally thought of as T cell
dependent, the rapid presence of high affinity immunoglobins following initial exposure to a pathogen in vivo argues against this.108 Platelets have been hypothesised to act during this
first phase of the development of humoral immunity. A number of features of platelet physiology strongly support this hypothesis. They are plentiful in circulation, and have been shown to
interact with both pathogens and innate immune effectors including DCs. A study conducted in a C57BL/6 mouse model demonstrated that CD40L-expressing platelets were key to generating a
robust germinal centre response provided there was also functional CD4+ T cells present.109 There is an altered CD4+ T cell phenotype in the neonatal period and infancy, with an initial skew
towards a T helper 2(Th2) anti-inflammatory phenotype, which slowly falls during childhood and is replaced by a T helper 1 (Th1) pro-inflammatory response. This has mostly been investigated
in the context of the early life development of allergic disease; however, this “tolerogenic” phenotype has also been postulated to contribute to the high incidence of sepsis in the
neonatal population.110 While the specific role of neonatal platelets in the formation of early life germinal matrix and in T helper cell maturation has not been assessed formally, the
hyporeactive phenotype of neonatal platelets may lend itself to a Th2 skewed adaptive immune response as a result of the absence of Th1 inducing signals.111 This is an important
consideration, as a large number of septic neonates and infants will receive (adult derived) platelet transfusions due to sepsis-related thrombocytopenia. When taken in the context of the
PlaNet-2 trial, which demonstrated a liberal platelet transfusion threshold resulted in worse outcomes over a restrictive threshold, consideration should be given to the generation of a
pro-inflammatory Th1 phenotype secondary to an exogenous mature platelet infusion.112 Adult platelets have been shown to be powerful regulators of both a Th1 and T helper 17 (Th17) response
in the context of atherosclerosis, but less impact on Th2 type responses.113 Research conducted in a paediatric intensive care setting suggests that typically a more liberal transfusion
threshold is applied, often in infant (<1 year old cohort) and that children who received platelet transfusions were more likely to suffer multiorgan failure.114 While a cautious
interpretation of this retrospective study is warranted, especially as thrombocytopenia in itself is a predictor of a worse outcome, careful consideration of the clinical requirement for
platelet transfusion in this vulnerable population may be justified until stronger prospective evidence is gathered.14 Given the frequency of use of platelet transfusions not only in
neonatal but also in early childhood septic illness, further scientific and clinical investigations are warranted into how platelets and T and B cells interact in the context of paediatric
septic illness in the formation of germinal centres. PLATELET-DERIVED EVS AND THE PLATELET PROTEOME/SECRETOME IN SEPSIS EVs are endogenously produced nanoparticles released by a range of
cell types. These EVs allow communication by packaging cellular contents (nucleic acids, proteins, lipid mediators) and allowing them to be taken up by distant cells.115 Platelet-derived EVs
(EVs) are among the highest concentration in peripheral circulation.116 EVs contain many of the immune mediators, including cytokines, chemokines, complement factors and surface receptors
such CD40L.117,118,119,120 They may act as important “warning” signals in sepsis, being released from a site of localised infection and priming a systemic immune response.117 Two major
subsets of EVs exist, microparticles and exosomes. Microparticles (alternatively referred to as ectosomes/PMPs) are larger (0.1–1 µM) and are generated by major cytoskeletal rearrangements
resulting in “shedding”. Exosomes are smaller particles (30–90 nM) and are released from platelets in substantial numbers as part of their “releasate”.121,122 Both vesicular categories have
been associated with immune functions. PMPs from healthy volunteers have shown to be immunologically active, downregulating NK cell function, DC function and macrophage activation.123,124
Platelet-derived exosomes have been shown to contribute to myocardial dysfunction and NET formation.125,126 Interestingly, exosome production by platelets was tightly associated with IκB
kinase (IKK) in the context of sepsis linking production of these EVs to PRR signalling.126 A recent paper showed that neonatal EVs from umbilical cord blood are different in size,
concentration and in their protein content from adult EVs, with an increased expression of prothrombotic proteins and a lower expression of immunoglobulins.127 This supports previous work
from our group examining size and concentration of neonatal EVs and how this alters in the first 3 days in premature infants.128 In addition to EVs, degranulation is associated with the
release of a range of proteins termed the platelet releasate. Recent work has shown changes in platelet proteome and transcriptome in septic and non-septic patients and animal models.129,130
Expression of heparinase increases in the platelets of septic patients as opposed to healthy, matched controls. Increasing heparinase expression is associated with worse clinical outcome,
possibly due to loss of the protective vascular glycocalyx leading to oedema and vascular injury.131 Studies using the platelet releasate from healthy volunteers induced an anti-inflammatory
phenotype in DCs similar to that elucidated by PMPs.132 Given the changes in the proteome and transcriptome in the context of sepsis, it would be interesting to examine whether this
represents a context-dependent effect or is a general feature of the platelet releasate. CONCLUSIONS There is an increasing focus on platelets as key regulators of the immune system. The
infant immune system is characterised by a hyporesponsive, tolerogenic phenotype that changes over time during the paediatric period to a pro-inflammatory phenotype in adulthood. The exact
ontogeny of individual components of the immune system, and how they interact with platelets to drive the response to infectious agents, is of particular importance in understanding
paediatric sepsis and a range of other important inflammatory conditions. Understanding how platelets act as components of the immune system may be key in developing a common definition of
sepsis across age groups. The clinical assumption that paediatric platelets and transfused adult platelets possess a similar phenotype is likely flawed, and underestimates the impact that
the more reactive, adult platelet may have on the infant immune response. Thrombocytopenia is a common occurrence in sepsis in children and neonates, with trial data suggesting that
increased platelet transfusions result in worse outcomes in neonates.112 This is postulated to be due to a “developmental haemostatic mismatch risk”, where adult platelets compromise the
delicate homoeostasis of neonatal bleeding.133 Platelet transfusions with relatively hyperactive platelets and immunologically active EVs may also pose a “developmental immunological
mismatch risk” which, in the presence of an inflammatory stimulus like sepsis, may inadvertently impair outcomes. High quality, prospectively collected data on how best to support neonates,
infants and children with low platelet counts is urgently required. EVs are demonstrably different between neonates and adults. Understanding the normal range of EV release, EV content in
sepsis versus health and the roles of EVs versus platelets themselves may represent an important source of sepsis biomarker discovery in the future. REFERENCES * Schlapbach, L. J. &
Kissoon, N. Defining pediatric sepsis. _JAMA Pediatr._ 172, 313–314 (2018). Article Google Scholar * Cailes, B. et al. Epidemiology of UK neonatal infections: the neonin infection
surveillance. _Netw. Arch. Dis. Child Fetal Neonatal Ed._ 103, F547–F553 (2018). Article Google Scholar * Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal
sepsis: a systematic review. _Lancet Respir. Med._ 6, 223–230 (2018). Article PubMed Google Scholar * Weiss, S. L. et al. Delayed antimicrobial therapy increases mortality and organ
dysfunction duration in pediatric sepsis. _Crit. Care Med._ 42, 2409–2417 (2014). Article CAS PubMed PubMed Central Google Scholar * Marshall, J. C., Reinhart, K. & Forum, F. T. I.
S. Biomarkers of sepsis. _Crit. Care Med._ 37, 2290–2298 (2009). Article CAS PubMed Google Scholar * Smyth, S. S. et al. Platelet functions beyond hemostasis. _J. Thrombosis Haemost._ 7,
1759–1766 (2009). Article CAS Google Scholar * Sola-Visner, M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the
potential impact of therapies. _Hematology_ 2012, 506–511 (2012). Article PubMed Google Scholar * Caparrós-Pérez, E. et al. Comprehensive comparison of neonate and adult human platelet
transcriptomes. _PLoS ONE_ 12, e0183042 (2017). Article PubMed PubMed Central Google Scholar * Stokhuijzen, E. et al. Differences between platelets derived from neonatal cord blood and
adult peripheral blood assessed by mass spectrometry. _J. Proteome Res._ 16, 3567–3575 (2017). Article CAS PubMed Google Scholar * Saxonhouse, M. A. & Sola, M. C. Platelet function
in term and preterm neonates. _Clin. Perinatol._ 31, 15–28 (2004). Article PubMed Google Scholar * Bednarek, F. J., Bean, S., Barnard, M. R., Frelinger, A. L. & Michelson, A. D. The
platelet hyporeactivity of extremely low birth weight neonates is age-dependent. _Thrombosis Res._ 124, 42–45 (2009). Article CAS Google Scholar * Hvas, A.-M. & Favaloro, E. J.
Platelet function testing in pediatric patients. _Expert Rev. Hematol._ 10, 281–288 (2017). Article CAS PubMed Google Scholar * Akarsu, S. et al. The effects of different infectious
organisms on platelet counts and platelet indices in neonates with sepsis: is there an organism-specific response? _J. Trop. Pediatr._ 51, 388–391 (2005). Article PubMed Google Scholar *
Sayed, S. Z., Mahmoud, M. M., Moness, H. M. & Mousa, S. O. Admission platelet count and indices as predictors of outcome in children with severe sepsis: a prospective hospital-based
study. _BMC Pediatr._ 20, 387 (2020). Article PubMed PubMed Central Google Scholar * Claushuis, T. A. et al. Thrombocytopenia is associated with a dysregulated host response in
critically Ill sepsis patients. _Blood_ 127, 3062–3072 (2016). Article CAS PubMed Google Scholar * Gunnink, S. F. et al. Neonatal thrombocytopenia: etiology, management and outcome.
_Expert Rev. Hematol._ 7, 387–395 (2014). Article CAS PubMed Google Scholar * Guida, J. D., Kunig, A. M., Leef, K. H., McKenzie, S. E. & Paul, D. A. Platelet count and sepsis in very
low birth weight neonates: is there an organism-specific response? _Pediatrics_ 111, 1411–1415 (2003). Article PubMed Google Scholar * Denton, A. & Davis, P. Extreme thrombocytosis
in admissions to paediatric intensive care: no requirement for treatment. _Arch. Dis. Child_ 92, 515–516 (2007). Article PubMed PubMed Central Google Scholar * O’Connor, T. A., Ringer,
K. M. & Gaddis, M. L. Mean platelet volume during coagulase-negative Staphylococcal sepsis in neonates. _Am. J. Clin. Pathol._ 99, 69–71 (1993). Article PubMed Google Scholar *
Carcillo, J. A. et al. Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. _Pediatr. Crit. Care Med._ 18, 513–523 (2017).
Article PubMed PubMed Central Google Scholar * Levi, M., Toh, C. H., Thachil, J. & Watson, H. G. Guidelines for the diagnosis and management of disseminated intravascular
coagulation. British Committee for Standards in Haematology. _Br. J. Haematol._ 145, 24–33 (2009). Article CAS PubMed Google Scholar * Zhang, H. et al. Sepsis induces hematopoietic stem
cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. _Stem Cell Rep._ 6, 940–956 (2016). Article CAS Google Scholar * Colarizi, P. et al. Circulating
thrombopoietin levels in neonates with infection. _Acta Paediatr._ 88, 332–337 (1999). Article CAS PubMed Google Scholar * Eissa, D. S. & El-Farrash, R. A. New insights into
thrombopoiesis in neonatal sepsis. _Platelets_ 24, 122–128 (2013). Article CAS PubMed Google Scholar * Cremer, M. et al. Low immature platelet fraction suggests decreased
megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. _J. Perinatol._ 33, 622–626 (2013). Article CAS PubMed Google Scholar * Arad, I. D., Alpan, G., Sznajderman, S. D.
& Eldor, A. The mean platelet volume (MPV) in the nonatal period. _Am. J. Perinatol._ 3, 1–3 (1986). Article CAS PubMed Google Scholar * Patrick, C. H., Lazarchick, J., Stubbs, T.
& Pittard, W. B. Mean platelet volume and platelet distribution width in the neonate. _Am. J. Pediatr. Hematol. Oncol._ 9, 130–132 (1987). Article CAS PubMed Google Scholar * Fuchs,
D. A. et al. Developmental differences in megakaryocyte size in infants and children. _Am. J. Clin. Pathol._ 138, 140–145 (2012). Article PubMed Google Scholar * Bugl, S. et al.
Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. _Blood_ 121, 723–733 (2013). Article CAS PubMed Google Scholar * van Lieshout, M. H. P. et al. Differential roles
of MYD88 and TRIF in hematopoietic and resident cells during murine Gram-negative pneumonia. _J. Infect. Dis._ 206, 1415–1423 (2012). Article PubMed Google Scholar * Rodriguez, S. et al.
Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. _Blood_ 114, 4064–4076 (2009). Article CAS PubMed PubMed Central Google
Scholar * François, B. et al. Thrombocytopenia in the sepsis syndrome: role of hemophagocytosis and macrophage colony-stimulating factor. _Am. J. Med._ 103, 114–120 (1997). Article PubMed
Google Scholar * Strauss, R. et al. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients—a postmortem
clinicopathologic analysis. _Crit. Care Med._ 32, 1316–1321 (2004). Article PubMed Google Scholar * Vallance, T. M., Zeuner, M.-T., Williams, H. F., Widera, D. & Vaiyapuri, S.
Toll-like receptor 4 signalling and its impact on platelet function, thrombosis, and haemostasis. _Mediat. Inflamm._ 2017, 9605894 (2017). Article Google Scholar * Sharron, M. et al.
Platelets induce apoptosis during sepsis in a contact-dependent manner that is inhibited by GPIIB/IIIA blockade. _PLoS ONE_ 7, e41549 (2012). Article CAS PubMed PubMed Central Google
Scholar * Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. _Nat. Med._ 13, 463–469 (2007). Article CAS PubMed Google
Scholar * Palm, F., Sjöholm, K., Malmström, J. & Shannon, O. Complement activation occurs at the surface of platelets activated by Streptococcal M1 protein and this results in
phagocytosis of platelets. _J. Immunol._ 202, 503–513 (2019). Article CAS PubMed Google Scholar * Waller, A. K. et al. Staphylococcus aureus lipoteichoic acid inhibits platelet
activation and thrombus formation via the Paf receptor. _J. Infect. Dis._ 208, 2046–2057 (2013). Article CAS PubMed PubMed Central Google Scholar * Powers, M. E., Becker, R. E. N.,
Sailer, A., Turner, J. R. & Bubeck, W. J. Synergistic action of Staphylococcus aureus α-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. _Cell Host Microbe_ 17,
775–787 (2015). Article CAS PubMed PubMed Central Google Scholar * O´Brien, L. et al. Multiple mechanisms for the activation of human platelet aggregation by _Staphylococcus aureus_:
roles for the clumping factors Clfa and Clfb, the serine–aspartate repeat protein SdrE and protein A. _Mol. Microbiol._ 44, 1033–1044 (2002). Article Google Scholar * Yang, Y. C. &
Mao, J. Value of platelet count in the early diagnosis of nosocomial invasive fungal infections in premature infants. _Platelets_ 29, 65–70 (2018). Article CAS PubMed Google Scholar *
Rødland, E. K. et al. Activation of platelets by _Aspergillus fumigatus_ and potential role of platelets in the immunopathogenesis of aspergillosis. _Infect. Immun._ 78, 1269–1275 (2010).
Article PubMed Google Scholar * Schultz, C. M. et al. Stepping up to the plate(let) against Candida albicans. _Infect. Immun._ 88, e00784–00719 (2020). Article PubMed PubMed Central
Google Scholar * Blair, P. & Flaumenhaft, R. Platelet alpha-granules: basic biology and clinical correlates. _Blood Rev._ 23, 177–189 (2009). Article CAS PubMed PubMed Central
Google Scholar * Tang, Y. Q., Yeaman, M. R. & Selsted, M. E. Antimicrobial peptides from human platelets. _Infect. Immun._ 70, 6524–6533 (2002). Article CAS PubMed PubMed Central
Google Scholar * Rossaint, J., Margraf, A. & Zarbock, A. Role of platelets in leukocyte recruitment and resolution of inflammation. _Front. Immunol._ 9, 1–13 (2018). * Akira, S.,
Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. _Nat. Immunol._ 2, 675–680 (2001). Article CAS PubMed Google Scholar * Denis, M.
M. et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. _Cell_ 122, 379–391 (2005). Article CAS PubMed PubMed Central Google Scholar *
Damien, P. et al. Lps stimulation of purified human platelets is partly dependent on plasma soluble CD14 to secrete their main secreted product, soluble-CD40-ligand. _BMC Immunol._ 16, 3
(2015). Article PubMed PubMed Central Google Scholar * Sadeghi, K. et al. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor
signaling. _J. Infect. Dis._ 195, 296–302 (2007). Article CAS PubMed Google Scholar * Swanson, K. V., Deng, M. & Ting, J. P. Y. The NLRP3 inflammasome: molecular activation and
regulation to therapeutics. _Nat. Rev. Immunol._ 19, 477–489 (2019). Article CAS PubMed PubMed Central Google Scholar * Cornelius, D. C. et al. NLRP3 inflammasome activation in
platelets in response to sepsis. _Physiol. Rep._ 7, e14073 (2019). Article PubMed PubMed Central Google Scholar * Yang, Y., Wang, H., Kouadir, M., Song, H. & Shi, F. Recent advances
in the mechanisms of NLRP3 inflammasome activation and its inhibitors. _Cell Death Dis._ 10, 128 (2019). Article PubMed PubMed Central Google Scholar * Qiao, J. et al. NLRP3 regulates
platelet Integrin ΑIIBΒ3 outside-in signaling, hemostasis and arterial thrombosis. _Haematologica_ 103, 1568–1576 (2018). Article CAS PubMed PubMed Central Google Scholar * Rolfes, V.
et al. Platelets fuel the inflammasome activation of innate immune cells. _Cell Rep._ 31, 1–17 (2020). * Omer, M. et al. Emerging role of the NLRP3 inflammasome and interleukin-1β in
neonates. _Neonatology_ 117, 545–554 (2020). Article CAS PubMed Google Scholar * Kelly, L. A. et al. Altered inflammasome activation in neonatal encephalopathy persists in childhood.
_Clin. Exp. Immunol._ 205, 89–97 (2021). * Guo, H., Callaway, J. B. & Ting, J. P. Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. _Nat. Med._ 21, 677–687
(2015). Article PubMed PubMed Central Google Scholar * Chaipan, C. et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. _J. Virol._ 80, 8951–8960
(2006). Article CAS PubMed PubMed Central Google Scholar * May, F. et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. _Blood_ 114, 3464–3472
(2009). Article CAS PubMed Google Scholar * Hitchcock, J. R. et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. _J. Clin. Invest._ 125, 4429–4446
(2015). Article PubMed PubMed Central Google Scholar * Rayes, J. et al. The Podoplanin-CLEC-2 axis inhibits inflammation in sepsis. _Nat. Commun._ 8, 2239 (2017). Article PubMed PubMed
Central Google Scholar * Hardy, A. T. et al. Significant hypo-responsiveness to GPVI and CLEC-2 agonists in pre-term and full-term neonatal platelets and following immune
thrombocytopenia. _Thromb. Haemost._ 118, 1009–1020 (2018). Article PubMed PubMed Central Google Scholar * Weiss, L. J. et al. Acquired platelet Gpvi receptor dysfunction in
critically-ill patients with sepsis. _Blood_ 137, 3105–3115 (2021). * Miller, Y. I., Choi, S. H., Wiesner, P. & Bae, Y. S. The SYK side of TLR4: signalling mechanisms in response to LPS
and minimally oxidized LDL. _Br. J. Pharmacol._ 167, 990–999 (2012). Article CAS PubMed PubMed Central Google Scholar * van Kooyk, Y. & Geijtenbeek, T. B. H. DC-SIGN: escape
mechanism for pathogens. _Nat. Rev. Immunol._ 3, 697–709 (2003). Article PubMed Google Scholar * Boukour, S., Massé, J. M., Bénit, L., Dubart-Kupperschmitt, A. & Cramer, E. M.
Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. _J. Thromb. Haemost._ 4, 426–435 (2006). Article CAS PubMed Google Scholar * Kell, A. M., Hemann, E.
A., Turnbull, J. B. & Gale, M. Jr. Rig-I-like receptor activation drives type I IFN and antiviral signaling to limit Hantaan orthohantavirus replication. _PLoS Pathog._ 16, e1008483
(2020). Article PubMed PubMed Central Google Scholar * Danese, S. et al. Cutting edge: T cells trigger Cd40-dependent platelet activation and granular RANTES release: a novel pathway for
immune response amplification. _J. Immunol._ 172, 2011–2015 (2004). Article CAS PubMed Google Scholar * Cloutier, N. et al. Platelets release pathogenic serotonin and return to
circulation after immune complex-mediated sequestration. _Proc. Natl Acad. Sci._ 115, E1550–E1559 (2018). Article CAS PubMed PubMed Central Google Scholar * Rex, S. et al. Immune versus
thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. _Thromb. Haemost._ 102, 97–110
(2009). Article CAS PubMed PubMed Central Google Scholar * Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. _Nat. Rev. Immunol._ 18, 134–147 (2018). Article
CAS PubMed Google Scholar * McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. _Blood_ 129, 1357–1367
(2017). Article CAS PubMed PubMed Central Google Scholar * Hoppenbrouwers, T. et al. Neutrophil extracellular traps in children with meningococcal sepsis. _Pediatr. Crit. Care Med._
19, e286–e291 (2018). Article PubMed Google Scholar * Yost, C. C. et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates.
_Blood_ 113, 6419–6427 (2009). Article CAS PubMed PubMed Central Google Scholar * Yost, C. C. et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular
trap formation. _J. Clin. Invest._ 126, 3783–3798 (2016). Article PubMed PubMed Central Google Scholar * Colón, D. F. et al. Neutrophil extracellular traps (NETs) exacerbate severity of
infant sepsis. _Crit. Care_ 23, 113 (2019). Article PubMed PubMed Central Google Scholar * Gaertner, F. et al. Migrating platelets are mechano-scavengers that collect and bundle
bacteria. _Cell_ 171, 1368–1382.e1323 (2017). Article CAS PubMed Google Scholar * Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. _Science_
346, 1234–1238 (2014). Article CAS PubMed PubMed Central Google Scholar * Page, C. & Pitchford, S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment
and activation. _Int. Immunopharmacol._ 17, 1176–1184 (2013). Article CAS PubMed Google Scholar * Pitchford, S., Pan, D. & Welch, H. C. Platelets in neutrophil recruitment to sites
of inflammation. _Curr. Opin. Hematol._ 24, 23–31 (2017). Article CAS PubMed PubMed Central Google Scholar * Aarts, P. A. et al. Blood platelets are concentrated near the wall and red
blood cells, in the center in flowing blood. _Arteriosclerosis_ 8, 819–824 (1988). Article CAS PubMed Google Scholar * Dole, V. S., Bergmeier, W., Mitchell, H. A., Eichenberger, S. C.
& Wagner, D. D. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. _Blood_ 106, 2334–2339 (2005). Article CAS PubMed PubMed
Central Google Scholar * Anderson, D. C. et al. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. _Blood_
70, 740–750 (1987). Article CAS PubMed Google Scholar * Abughali, N., Berger, M. & Tosi, M. F. Deficient total cell content of Cr3 (CD11B) in neonatal neutrophils. _Blood_ 83,
1086–1092 (1994). Article CAS PubMed Google Scholar * Anderson, D. C., Rothlein, R., Marlin, S. D., Krater, S. S. & Smith, C. W. Impaired transendothelial migration by neonatal
neutrophils: abnormalities of Mac-1 (CD11B/CD18)-dependent adherence reactions. _Blood_ 76, 2613–2621 (1990). Article CAS PubMed Google Scholar * Etulain, J. et al. Acidosis
downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. _Thromb. Haemost._ 107, 99–110 (2012). Article CAS PubMed Google
Scholar * Chun, T. T. et al. Group 2 innate lymphoid cells (ILC2s) are key mediators of the inflammatory response in polymicrobial sepsis. _Am. J. Pathol._ 188, 2097–2108 (2018). Article
CAS PubMed PubMed Central Google Scholar * Karta, M. R. et al. Platelets attach to lung type 2 innate lymphoid cells (ILC2s) expressing P-Selectin glycoprotein Ligand 1 and influence
ILC2 function. _J. Allergy Clin. Immunol._ 144, 1112–1115.e1118 (2019). Article CAS PubMed PubMed Central Google Scholar * Clar, K. L., Hinterleitner, C., Schneider, P., Salih, H. R.
& Maurer, S. Inhibition of NK reactivity against solid tumors by platelet-derived Rankl. _Cancers_ 11, 277 (2019). Article CAS PubMed Central Google Scholar * Almeida-Oliveira, A. et
al. Age-related changes in natural killer cell receptors from childhood through old age. _Hum. Immunol._ 72, 319–329 (2011). Article CAS PubMed Google Scholar * Guo, Y., Patil, N. K.,
Luan, L., Bohannon, J. K. & Sherwood, E. R. The biology of natural killer cells during sepsis. _Immunology_ 153, 190–202 (2018). Article CAS PubMed Google Scholar * Filipovich, A. H.
Hemophagocytic lymphohistiocytosis (HLH) and related disorders. _Hematology_ 2009, 127–131 (2009). Article Google Scholar * Fu, G., Deng, M., Neal, M. D., Billiar, T. R. & Scott, M.
J. Platelet-monocyte aggregates: understanding mechanisms and functions in sepsis. _Shock_ 55, 156–166 (2021). Article CAS PubMed PubMed Central Google Scholar * Zamora, C. et al.
Inverse association between circulating monocyte-platelet complexes and inflammation in ulcerative colitis patients. _Inflamm. Bowel Dis._ 24, 818–828 (2018). Article PubMed Google Scholar
* Skrzeczyñska, J., Kobylarz, K., Hartwich, Z., Zembala, M. & Pryjma, J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies.
_Scand. J. Immunol._ 55, 629–638 (2002). Article PubMed Google Scholar * Arenson, E. B., Epstein, M. B. & Seeger, R. C. Monocyte subsets in neonates and children. _Pediatrics_ 64,
740–743 (1979). Article CAS PubMed Google Scholar * Mahdi, M. & Maródi, L. Monocytes in neonatal immunity. _NeoReviews_ 11, e558–e565 (2010). Article Google Scholar * Han, P. et
al. Platelet P-Selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. _Sci. Adv._ 6, eaaz1580 (2020). Article CAS PubMed PubMed Central Google
Scholar * Inwald, D. P., McDowall, A., Peters, M. J., Callard, R. E. & Klein, N. J. Cd40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation.
_Circulation Res._ 92, 1041–1048 (2003). Article CAS PubMed Google Scholar * Ma, D. Y. & Clark, E. A. The role of CD40 and CD154/CD40L in dendritic cells. _Semin. Immunol._ 21,
265–272 (2009). Article CAS PubMed PubMed Central Google Scholar * Wong, C. H., Jenne, C. N., Petri, B., Chrobok, N. L. & Kubes, P. Nucleation of platelets with blood-borne
pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. _Nat. Immunol._ 14, 785–792 (2013). Article CAS PubMed PubMed Central Google Scholar *
Eriksson, O., Mohlin, C., Nilsson, B. & Ekdahl, K. N. The human platelet as an innate immune cell: interactions between activated platelets and the complement system. _Front. Immunol._
10, 1–16 (2019). * Ferriani, V. P., Barbosa, J. E. & de Carvalho, I. F. Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. _Acta Paediatr.
Scand._ 79, 322–327 (1990). Article CAS PubMed Google Scholar * McGreal, E. P., Hearne, K. & Spiller, O. B. Off to a slow start: under-development of the complement system in term
newborns is more substantial following premature birth. _Immunobiology_ 217, 176–186 (2012). Article CAS PubMed Google Scholar * Chapman, L. M. et al. Platelets present antigen in the
context of MHC class I. _J. Immunol._ 189, 916–923 (2012). Article CAS PubMed Google Scholar * Stebegg, M. et al. Regulation of the germinal center response. _Front. Immunol._ 9 1–13
(2018). * Baumgarth, N. A two-phase model of B-cell activation. _Immunol. Rev._ 176, 171–180 (2000). Article CAS PubMed Google Scholar * Elzey, B. D. et al. Cooperation between
platelet-derived CD154 and CD4+T cells for enhanced germinal center formation. _J. Leukoc. Biol._ 78, 80–84 (2005). Article CAS PubMed Google Scholar * Levy, O. Innate immunity of the
newborn: basic mechanisms and clinical correlates. _Nat. Rev. Immunol._ 7, 379–390 (2007). Article CAS PubMed Google Scholar * Zhu, J., Yamane, H. & Paul, W. E. Differentiation of
effector CD4 T cell populations (*). _Annu. Rev. Immunol._ 28, 445–489 (2010). * Curley, A. et al. Randomized trial of platelet-transfusion thresholds in neonates. _N. Engl. J. Med._ 380,
242–251 (2018). Article PubMed Google Scholar * Gerdes, N. et al. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. _Thromb. Haemost._ 106, 353–362 (2011).
Article CAS PubMed Google Scholar * Du Pont-Thibodeau, G., Tucci, M., Robitaille, N., Ducruet, T. & Lacroix, J. Platelet transfusions in pediatric intensive care. _Pediatr. Crit.
Care Med_. 17, e420–e429 (2016). Article PubMed Google Scholar * Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the
International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. _J. Extracell. Vesicles_ 7, 1535750 (2018). Article PubMed PubMed Central Google Scholar *
Aatonen, M. T. et al. Isolation and characterization of platelet-derived extracellular vesicles. _J. Extracell. Vesicles_ 3, 24692 (2014). Article Google Scholar * Iba, T. & Ogura, H.
Role of extracellular vesicles in the development of sepsis-induced coagulopathy. _J. Intensive Care_ 6, 68 (2018). Article PubMed PubMed Central Google Scholar * Karasu, E., Eisenhardt,
S. U., Harant, J. & Huber-Lang, M. Extracellular vesicles: packages sent with complement. _Front. Immunol._ 9, 1–15 (2018). * Fitzgerald, W. et al. A system of cytokines encapsulated in
extracellular vesicles. _Sci. Rep._ 8, 8973 (2018). Article PubMed PubMed Central Google Scholar * Mobarrez, F. et al. CD40L expression in plasma of volunteers following LPS
administration: a comparison between assay of CD40L on platelet microvesicles and soluble CD40L. _Platelets_ 26, 486–490 (2015). Article CAS PubMed Google Scholar * Toth, B. et al.
Microparticles and exosomes: impact on normal and complicated pregnancy. _Am. J. Reprod. Immunol._ 58, 389–402 (2007). Article CAS PubMed Google Scholar * Parsons, M. E. M. et al.
Platelet releasate proteome profiling reveals a core set of proteins with low variance between healthy adults. _Proteomics_ 18, 1800219 (2018). Article Google Scholar * Sadallah, S. et al.
Platelet-derived ectosomes reduce NK cell function. _J. Immunol._ 197, 1663–1671 (2016). Article CAS PubMed Google Scholar * Sadallah, S., Eken, C., Martin, P. J. & Schifferli, J.
A. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. _J. Immunol._ 186, 6543–6552 (2011). Article CAS PubMed
Google Scholar * Azevedo, L. C. P. et al. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. _Crit. Care_ 11, R120 (2007). Article PubMed PubMed Central
Google Scholar * Jiao, Y. et al. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. _Crit. Care_ 24, 380 (2020). Article PubMed PubMed
Central Google Scholar * Peñas-Martínez, J. et al. Qualitative and quantitative comparison of plasma exosomes from neonates and adults. _Int. J. Mol. Sci._ 22, 1–20 (2021). * O’Reilly, D.
et al. The population of circulating extracellular vesicles dramatically alters after very premature delivery—a previously unrecognised postnatal adaptation process? _Blood_ 132, 1129–1129
(2018). Article Google Scholar * Middleton, E. A. et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. _Blood_ 134, 911–923 (2019). Article
CAS PubMed PubMed Central Google Scholar * Hu, J. Y., Li, C. L. & Wang, Y. W. Altered proteomic pattern in platelets of rats with sepsis. _Blood Cells Mol. Dis._ 48, 30–35 (2012).
Article PubMed Google Scholar * Eustes, A. S. et al. Heparanase expression and activity are increased in platelets during clinical sepsis. _J. Thromb. Haemost._ 19, 1319–1330 (2021). *
Saris, A. et al. Inhibition of dendritic cell activation and modulation of T cell polarization by the platelet secretome. _Front. Immunol._ 12, 1–12 (2021). * Davenport, P. &
Sola-Visner, M. Hemostatic challenges in neonates. _Front. Pediatr._ 9, 1–14 (2021). * Forestier, F., Daffos, F., Catherine, N., Renard, M. & Andreux, J. P. Developmental hematopoiesis
in normal human fetal blood. _Blood_ 77, 2360–2363 (1991). Article CAS PubMed Google Scholar * Sitaru, A. G. et al. Neonatal platelets from cord blood and peripheral blood. _Platelets_
16, 203–210 (2005). Article CAS PubMed Google Scholar * Sola-Visner, M., Sallmon, H. & Brown, R. New insights into the mechanisms of nonimmune thrombocytopenia in neonates. _Semin.
Perinatol._ 33, 43–51 (2009). Article PubMed PubMed Central Google Scholar * Hézard, N. et al. Unexpected persistence of platelet hyporeactivity beyond the neonatal period: a flow
cytometric study in neonates, infants and older children. _Thromb. Haemost._ 90, 116–123 (2003). Article PubMed Google Scholar * Grosshaupt, B., Muntean, W. & Sedlmayr, P.
Hyporeactivity of neonatal platelets is not caused by preactivation during birth. _Eur. J. Pediatr._ 156, 944–948 (1997). Article CAS PubMed Google Scholar * Rajasekhar, D., Barnard, M.
R., Bednarek, F. J. & Michelson, A. D. Platelet hyporeactivity in very low birth weight neonates. _Thromb. Haemost._ 77, 1002–1007 (1997). Article CAS PubMed Google Scholar *
Rajasekhar, D. et al. Neonatal platelets are less reactive than adult platelets to physiological agonists in whole blood. _Thromb. Haemost._ 72, 957–963 (1994). Article CAS PubMed Google
Scholar * Larkin, C. M., Santos-Martinez, M.-J., Ryan, T. & Radomski, M. W. Sepsis-associated thrombocytopenia. _Thrombosis Res._ 141, 11–16 (2016). Article CAS Google Scholar * Ree,
I. M. C. et al. Thrombocytopenia in neonatal sepsis: incidence, severity and risk factors. _PLoS ONE_ 12, e0185581 (2017). Article PubMed PubMed Central Google Scholar * Gao, Y. et al.
The impact of various platelet indices as prognostic markers of septic shock. _PLoS ONE_ 9, e103761–e103761 (2014). Article PubMed PubMed Central Google Scholar Download references
FUNDING C.A.M. is funded by a grant from the National Children’s Research Centre, Dublin, Ireland. Open Access funding provided by the IReL Consortium. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Neonatology, Rotunda Hospital, Dublin, Ireland Daniel O’Reilly, Claire A. Murphy, Afif El-Khuffash & Naomi Mc Callion * Conway-SPHERE Research Group, Conway
Institute, University College Dublin, Dublin, Ireland Daniel O’Reilly, Claire A. Murphy, Patricia B. Maguire & Fionnuala Ni Ainle * Department of Paediatrics, Royal College of Surgeons
in Ireland, Dubin, Ireland Claire A. Murphy, Afif El-Khuffash & Naomi Mc Callion * Clinical Innovation Unit, Rotunda Hospital, Dublin, Ireland Richard Drew * Irish Meningitis and Sepsis
Reference Laboratory, Children’s Health Ireland at Temple Street, Dublin, Ireland Richard Drew * Department of Clinical Microbiology, Royal College of Surgeons in Ireland, Dublin, Ireland
Richard Drew * School of Biomolecular & Biomedical Science, University College Dublin, Dublin, Ireland Patricia B. Maguire & Fionnuala Ni Ainle * Department of Haematology, Mater
Misericordiae University Hospital, Dublin, Ireland Fionnuala Ni Ainle * Department of Haematology, Rotunda Hospital, Dublin, Ireland Fionnuala Ni Ainle * School of Medicine, University
College Dublin, Dublin, Ireland Fionnuala Ni Ainle Authors * Daniel O’Reilly View author publications You can also search for this author inPubMed Google Scholar * Claire A. Murphy View
author publications You can also search for this author inPubMed Google Scholar * Richard Drew View author publications You can also search for this author inPubMed Google Scholar * Afif
El-Khuffash View author publications You can also search for this author inPubMed Google Scholar * Patricia B. Maguire View author publications You can also search for this author inPubMed
Google Scholar * Fionnuala Ni Ainle View author publications You can also search for this author inPubMed Google Scholar * Naomi Mc Callion View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS D.O.R., C.A.M., P.B.M., F.N.A., and N.M.C. conceived and drafted this article. R.D. and A.E.-K. revised critically for intellectual
important content. All authors reviewed draft prior to submission. CORRESPONDING AUTHOR Correspondence to Daniel O’Reilly. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE O’Reilly, D.,
Murphy, C.A., Drew, R. _et al._ Platelets in pediatric and neonatal sepsis: novel mediators of the inflammatory cascade. _Pediatr Res_ 91, 359–367 (2022).
https://doi.org/10.1038/s41390-021-01715-z Download citation * Received: 15 April 2021 * Revised: 14 July 2021 * Accepted: 16 August 2021 * Published: 28 October 2021 * Issue Date: January
2022 * DOI: https://doi.org/10.1038/s41390-021-01715-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative