Play all audios:
ABSTRACT BACKGROUND Extremely low birth weight (ELBW) infants comprise a fragile population at risk for neurodevelopmental disabilities (NDD). Systemic steroids were previously associated
with NDD, but more recent studies suggest hydrocortisone (HCT) may improve survival without increasing NDD. However, the effects of HCT on head growth adjusted for illness severity during
NICU hospitalization are unknown. Thus, we hypothesize that HCT will protect head growth, accounting for illness severity using a modified neonatal Sequential Organ Failure Assessment
(M-nSOFA) score. METHODS We conducted a retrospective study that included infants born at 23–29 weeks gestational age (GA) and < 1000 g. Our study included 73 infants, 41% of whom
received HCT. RESULTS We found negative correlations between growth parameters and age, similar between HCT and control patients. HCT-exposed infants had lower GA but similar normalized
birth weights; HCT-exposed infants also had higher illness severity and longer lengths of hospital stay. We found an interaction between HCT exposure and illness severity on head growth,
such that infants exposed to HCT had better head growth compared to those not exposed to HCT when adjusted for illness severity. CONCLUSION These findings emphasize the importance of
considering patient illness severity and suggest that HCT use may offer additional benefits not previously considered. IMPACT * This is the first study to assess the relationship between
head growth and illness severity in extremely preterm infants with extremely low birth weights during their initial NICU hospitalization. * Infants exposed to hydrocortisone (HCT) were
overall more ill than those not exposed, yet HCT exposed infants had better preserved head growth relative to illness severity. * Better understanding of the effects of HCT exposure on this
vulnerable population will help guide more informed decisions on the relative risks and benefits for HCT use. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS PROPHYLACTIC EARLY LOW-DOSE HYDROCORTISONE AND SURVIVAL WITHOUT BRONCHOPULMONARY DYSPLASIA AMONG EXTREMELY PRETERM INFANTS BORN AT 22–27 WEEKS’ GESTATION
Article 08 March 2024 THE BENEFICIAL EFFECT OF PROPHYLACTIC HYDROCORTISONE TREATMENT IN EXTREMELY PRETERM INFANTS IMPROVES UPON ADJUSTMENT OF THE BASELINE CHARACTERISTICS Article Open access
31 August 2023 POST-NATAL STEROID EXPOSURE IN VERY LOW BIRTHWEIGHT NEONATES AND ASSOCIATIONS WITH ACUTE KIDNEY INJURY Article Open access 23 May 2024 INTRODUCTION Infants born extremely
premature ( < 29 weeks gestation) with extremely low birth weight (ELBW, < 1000 grams) are a population at high risk for disrupted brain development.1 While advances over the last few
decades have led to decreased mortality, there still exists significant long-term neurodevelopmental morbidity and disability in this population.2,3 For example, the ELGAN (Extremely Low
Gestational Age Newborns) study found that > 50% of their ELBW cohort had moderate to severe neurocognitive deficits by 10 years of age,4 likely affected by impaired brain growth and
altered connectivity. In support of this conclusion, brain MRI studies of ELBW infants at term-equivalent age show smaller brain volumes compared to full-term infants at the same corrected
gestational age (GA), and these differences persist into adolescence.5 Moreover, among extremely premature ELBW infants, smaller brain volumes are associated with worse developmental
outcomes.6 Many genetic and maternal-fetal-postnatal factors have been implicated in increasing risk for neurodevelopmental deficits in extremely premature ELBW infants. ELBW infants
routinely require invasive life-sustaining therapies, such as mechanical ventilation and medications that pose additional risks.7 One such commonly utilized therapy within this population is
systemic corticosteroids for treatment of hypotension at early ages and for treatment of chronic lung disease (CLD) dependent on mechanical ventilation at later ages.8,9 There is evidence
that steroids facilitate earlier extubation and reduce risk of CLD as well as improve overall survival.10 However, studies in the early 2000s associated use of steroids, largely
dexamethasone, in preterm infants with increased risk of neurodevelopmental disability and cerebral palsy.11,12 Dexamethasone has been additionally associated with reduced intracranial,
cerebral tissue, cortical gray matter, and cerebellar volumes.13 More recent studies suggest that steroid type along with factors such as dosing and timing affect the relative risk-benefit
ratio.14,15,16,17 In a small randomized control trial among a small cohort of ELBW infants, Parikh et al. (2013)18 showed no difference in brain volume as measured by MRI between infants
treated with hydrocortisone (HCT) and controls. Furthermore, a recent randomized control trial of 800 ELBW infants randomized to a 10-day HCT or placebo treatment for BPD, notably with
open-label usage of dexamethasone in both groups, showed no difference in head circumference z-scores at 36wga.17 These studies have subsequently shifted clinical use of systemic
corticosteroids away from dexamethasone and towards HCT, which may be beneficial for survival without adverse effects on neurodevelopmental outcomes. Several limitations of these previous
studies still need to be addressed. First, the earliest brain MRIs were typically obtained at near term-equivalent age, which, for extremely premature ELBW infants, can be as late as four
months after birth. Thus, conclusions regarding head growth over the period between birth and term-equivalent age are not feasible, but this is when these infants are often most critically
ill and most likely to be exposed to corticosteroids. As such, longitudinal assessment of head growth is needed to determine risk factors for impaired brain growth and neurodevelopmental
deficits among ELBW infants over time. Second, prior studies have not adequately accounted for illness severity beyond GA and birth weight (BW).19,20 Compared to brain MRI measurements,
occipitofrontal circumference (OFC) measurement provides the added benefit of being able to be easily assessed early during the hospital course of extremely premature ELBW infants, when
these patients are often too unstable to obtain brain MRIs, and repeated measurements can be obtained over the entire hospital course. Multiple studies have also found that head size and
head growth correlate with neurodevelopmental outcomes.21,22 Therefore, OFC is an apt proxy for brain growth, particularly during the initial hospital course in patients without
ventriculomegaly or hydrocephalus. To date, no study has examined the effect of systemic HCT on the rate of head growth while at the same time adjusting for illness severity. We hypothesize
that HCT interacts with the relationship between head growth and illness severity. METHODS PATIENTS We conducted a retrospective cohort study that included infants recruited into the Adult
Biomarkers in Neonatal Injury and Development study. Infants were born between 23- and 29-weeks of gestation and < 1000 g, and were admitted to the level 4 neonatal intensive care unit
(NICU) at the Johns Hopkins Hospital (JHH), Baltimore, MD from 7/20/2016-8/19/2019. Infants with genetic syndromes, chromosomal abnormalities, and major congenital anomalies were not
approached for consent. The study received institutional review board approval (JHH IRB 00026068), and signed parental informed consent was obtained for each participant within the infant’s
first 30 days of life and prior to NICU discharge. The study included access to maternal and obstetrics data as well as neonatal data collected throughout their NICU hospitalization that
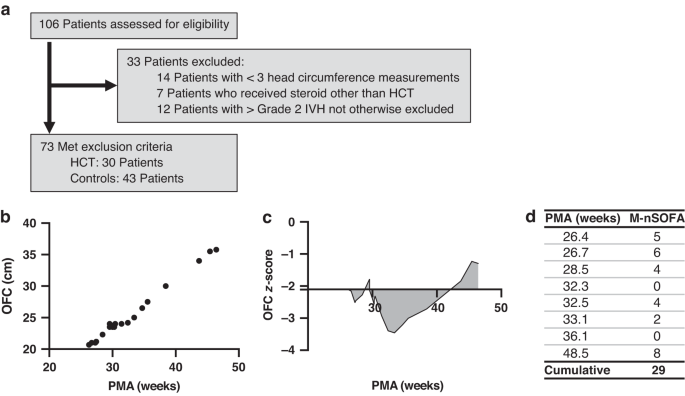
were obtained from electronic medical records. For our study purposes, exclusion criteria included: 1) less than 3 total OFC measurements, 2) use of a steroid other than HCT, and 3) patients
with larger than Grade 2 IVH (Fig. 1a). NUTRITION All patients received nutrition as per our unit protocol (Supplementary Table 1) and had similar proportions of breastmilk to formula feeds
(Supplementary Table 2). GROWTH MEASUREMENTS Growth measurements were made according to our standardized NICU protocol. This includes: 1) weighing infants naked on an infant scale prior to
feeds in the first set of cares daily, 2) measuring length from the top of their head to the bottom of one of their heels with extended lower extremities on the length board on admission and
then weekly, and 3) measuring OFC using a flexible and non-stretchable measuring tape around the most prominent part on the occiput and over the supraorbital ridge, on admission and then
weekly. Weight, length, and OFC measurements from birth to 50 weeks post menstrual age (PMA) were normalized to generate z-scores that account for GA and sex using the Fenton 2013 preterm
growth chart data.23 Normalized data were not available for length or head circumference of infants < 23.5 weeks GA, so z-scores for length or head circumference at younger ages were not
calculated. Outliers, defined as z-scores <-5 or > 3, were excluded, as these values were likely measurement or data entry errors. Only weights with corresponding lengths measured on
the same day were used. If multiple measurements were made on the same day, only the first value was included for analysis. Area under the curve (AUC) of z-scores were calculated using
linear interpolation using the composite trapezoid rule, relative to z-score at birth, such that increases in z-scores above the z-score at birth were calculated as positive areas and
decreases in z-scores were calculated as negative areas added to the total AUC. As such, a value of zero would reflect growth along the same percentile as their birth size, positive values
reflect greater than birth percentile growth, and negative values reflect lower than birth percentile growth. An example for a single patient is shown in Fig. 1b, c. CLINICAL SCORES FOR
MEASURING SEVERITY OF ILLNESS Patient illness severity at birth was calculated using the Clinical Risk Index for Babies (CRIB II) score.24 Subsequent illness severity was calculated using
the neonatal sequential organ failure assessment (nSOFA) score, which has previously been shown to be an effective predictor of adverse outcomes and mortality for ELBW infants.20,25,26,27 As
we are studying the effects of HCT, the nSOFA score was modified to exclude the steroids component, which had a binary score range of 0–1 within the inotropic support category in the
original nSOFA score, hereon referred to as the modified-nSOFA score (M-nSOFA, Table 1). M-nSOFA scores were assessed at times of critical illness, identified as times when blood cultures
were obtained until 50 weeks post-menstrual age (PMA). To do so, M-nSOFA scores were computed at 0, 6, 12, 24, and 48 h before and after each blood culture draw for a total of 9 timepoints
per period. Then, the maximum M-nSOFA scores from each of these periods were summated to calculate a cumulative maximum M-nSOFA score per patient. An example of this calculation for a single
patient is shown in Fig. 1d. HEAD ULTRASOUND MEASUREMENTS Head ultrasounds (HUS) were obtained for every patient at approximately 1 month of age. Biparietal diameters and ventricle/brain
(V/B) ratios from coronal slices were measured by a pediatric neuroradiologist as previously described28 and depicted in Fig. 2a. V/B ratios were calculated using ventricular indices (VI),
where V/B = [VI(left) + VI(right)]/biparietal diameter. OFC measurements taken closest to the date of the HUS within one week were used for correlations between HUS and OFC measurements.
When comparing effects between groups we excluded those patients in the HCT group who had not yet been exposed to HCT at the time of the HUS. STATISTICAL ANALYSIS Statistical analysis was
performed using GraphPad Prism (GraphPad Software) and R Statistical Software (v5.0; R Core Team 2021). Correlations were fitted with a linear regression and assessed for significance using
Spearman correlation. Repeated measures correlation was conducted using the R package rmcorr29 and, where applicable, is reported as the correlation coefficient rrm. Comparisons of
correlations were made using ANCOVA. For group comparisons, normality was first assessed using the Shapiro-Wilk test. Parametric tests were used for normal distributions. In these cases,
pairwise statistical tests were performed using unpaired two-tailed _t_ tests, and multiple comparisons were performed by ANOVA. If distributions were not normally distributed, the
Mann-Whitney U test was used for pairwise comparisons, and the Kruskal-Wallis test was used for multiple comparisons. Comparisons of categorical variables were made using the Fisher’s exact
test for small sample sizes or Chi-square test for larger sample sizes. RESULTS PATIENT DEMOGRAPHICS – YOUNGER ELBW INFANTS WERE MORE LIKELY TO RECEIVE SYSTEMIC HCT We assessed 106 patients
for inclusion eligibility. Of those, 33 patients were excluded: 14 patients who had less than 3 OFC measurements due to death, 7 patients who had received a steroid other than HCT (in all
cases, dexamethasone), and 12 patients with severe intraventricular hemorrhage (IVH > Grade 230). In total, 73 patients met inclusion criteria. Of these 30 patients (41%) had received
HCT, and 43 control patients had received no steroids. HCT-treated infants were notably born at an earlier GA than controls (U = 318, _p_ < 0.001). While the HCT group had lower BW (_t_ =
5.26, df = 71, _p_ < 0.0001), BW z-scores were not significantly different (_t_ = 0.31, df = 71, _p_ = 0.76), suggesting the difference in BW was driven by the difference in GA. The
remainder of maternal and birth characteristics as well as other clinical characteristics were similar between groups (Table 2, Supplementary Table 2). Among the HCT group, HCT exposure
first occurred at a median age of 3 weeks of life, IQR = 2.39 (1.32, 3.71). 7/30 patients received HCT within the first week of life. HCT dose varied from 1 to 10 mg/kg/day with durations
varying from 1 to 36 days for a median cumulative dose of 74.88 mg/kg, IQR = 300.94 (24.38, 325.31). Per our NICU protocols, HCT was administered for the following indications and
accompanying doses: (1) physiologic replacement 7–9 mg/m2 per day in 2–3 doses; (2) stress dose 20–50 mg/m2 per day in 2–3 doses; (3) hypotension 1 mg/kg followed by 0.5 mg/kg q6-12 h; (4)
ventilator dependent CLD 72.5 mg/kg over 22-day course.31 Amongst the patients who received HCT, 12/30 (40%) received HCT for physiologic replacement, 10/30 (33%) as stress dose HCT, 12/30
(40%) for hypotension, and 18/30 (60%) for CLD. Several of these patients received HCT for multiple of these indications at different times during their initial NICU hospitalization: 6/30
(20%) received HCT for 2 of the above indications, 5/30 (17%) received HCT for 3 indications, and 2/30 (7%) received HCT for all 4 indications. As such, there was variability in the total
dosage of HCT given throughout the initial NICU admission. OFC MEASUREMENTS OF HEAD SIZE STRONGLY CORRELATED WITH HUS MEASUREMENTS OF BRAIN SIZE To evaluate the validity of our OFC
measurements, we compared them to measurements taken from HUS. HUS were obtained for every patient at approximately 1 month into the NICU hospitalization (mean ± SD = 33±8 days-of-life, 31 ±
2 weeks PMA). As expected, there was a robust positive correlation between OFC and biparietal diameter on HUS (r2 = 0.57, _p_ < 0.0001, Fig. 2b) and a significant negative correlation
between OFC and V/B ratio (r2 = 0.15, _p_ < 0.001, Fig. 2c). There were no significant differences in these relationships between HCT and control groups, (OFC vs BPD: F(1, 63) = 0.05, _p_
= 0.82; OFC vs V/B: F(1, 63) = 3.411, _p_ = 0.07). This supports the idea that head size as measured by OFC closely reflects underlying brain size. On review of HUS clinical impressions by
our pediatric neuroradiologists, 4 patients in the HCT group and 1 patient in the control group were noted to have increased white matter echogenicity. We compared this data with available
near-term MRIs, which were performed on 17/73 of included patients, of which 71% (12/17) received HCT, and 29% (5/17) did not. On review of MRI clinical impressions, within the HCT group,
42% (5/12) were unremarkable, 42% (5/12) demonstrated evidence of remote IVH, 17% (2/12) had mild ventriculomegaly, one had white matter abnormalities, and one had moderate ventriculomegaly.
Within the control group, 60% (3/5) were unremarkable, 40% (2/5) had white matter abnormalities, and one had mild ventriculomegaly. A complete summary of MRI interpretations is available in
Supplemental Table 3. Due to the limited sample size of available MRIs, we were not able to make group comparisons using this data. DEFICIENT POSTNATAL GROWTH DURING NICU HOSPITALIZATION
While there was significant growth with age, as measured by weight-to-length ratio (Fig. 3a, rrm(911) = 0.98, 95% CI [0.978, 0.984], _p_ < 0.001), normalized weight and length, and
z-scores showed significant negative correlations with PMA (Fig. 3b–d, rrm, weight(911) = −0.29, 95% CI[−0.350, −0.231], _p_ < 0.001; rrm, length(911) = −0.49, 95% CI[−0.540, −0.441], _p_
< 0.001). Normalized OFC z-scores did not significantly correlate with PMA (rrm(972) = 0.06, 95% CI[−0.005, 0.120], p0.072). We further assessed overall growth for each patient during
this period by calculating the weight, length, and OFC z-score AUC as a function of the chronological age of the patient when the last measurements included were taken, which reflects the
length of hospitalization. In all growth domains, there was a negative association between AUC and the last measurement age (weight R2 = 0.13, _p_ < 0.01; length R2 = 0.41, _p_ <
0.0001; OFC R2 = 0.05, _p_ = 0.06). No differences in these relationships were observed between HCT and control groups (Fig. 3a–g right panels, statistics in Supplemental Table 4). This data
suggests that patients had difficulty attaining appropriate weight and length gain during NICU hospitalization with relatively preserved head growth. HCT EXPOSED PATIENTS HAD HIGHER
SEVERITY OF ILLNESS THAN CONTROLS Illness severity was assessed at birth using CRIB II scores.24 The HCT group had significantly higher CRIB II scores than the control group, suggesting
higher illness severity at birth (Table 2). We measured subsequent illness severity during hospitalization using M-nSOFA scores. The HCT group had higher illness severity as measured by
cumulative M-nSOFA score (U = 124, _p_ < 0.0001; Fig. 4a), which likely reflects the clinical rationale for use of HCT in the first place. This in part may be due to the HCT group being
born at younger GAs than the control group, as we found a negative correlation between GA and cumulative M-nSOFA scores (R2 = 0.21, _p_ < 0.0001; Fig. 4b), suggesting that being born at
younger GA was associated with higher illness severity. Accordingly, HCT patients also had significantly longer lengths of stay (LOS) with a median LOS of 120 days compared to control
patients at 56 days (U = 167, _p_ < 0.0001; Fig. 4c). This, again, is likely in part due to younger gestational ages at birth (R2 = 0.23, _p_ < 0.0001; Fig. 4d) and greater illness
severity as measured by cumulative M-nSOFA score (R2 = 0.65, _p_ < 0.0001; Fig. 4e). HCT EXPOSURE MODULATES THE EFFECT OF ILLNESS SEVERITY ON HEAD GROWTH Given that HCT and control
patients had similar growth trajectories throughout hospitalization despite notably different degrees of illness severity, we then sought to understand whether HCT exposure may modulate the
effect of illness severity on growth, particularly head growth. We first evaluated the correlations between overall growth, measured by weight, length, and OFC AUCs, and illness severity,
measured by cumulative M-nSOFA scores for all study patients (Fig. 5a–c left panels). We found negative correlations between growth and illness severity. While the correlation between length
AUC and cumulative M-nSOFA scores (R2 = 0.11, _p_ < 0.01) as well as the correlation between OFC AUC and cumulative M-nSOFA scores (R2 = 0.10, _p_ < 0.01) were statistically
significant, the correlation between weight AUC and cumulative M-nSOFA scores showed only a trend (R2 = 0.04, _p_ = 0.09). Interestingly, we found that this relationship differed for
patients exposed to HCT compared to controls (Fig. 5a–c right panels, statistics in Supplementary Table 5). Specifically, there was a significant negative correlation between length AUC and
cumulative M-nSOFA scores in the HCT group (R2 = 0.18, _p_ < 0.05) but not in the control group (R2 = 0.01, _p_ = 0.57, Fig. 4b), though direct comparison of these correlations was not
statistically significant (F(1,69) = 0.26, _p_ = 0.61). Meanwhile there remained no significant correlation between weight AUC and cumulative M-nSOFA scores in either HCT or control groups
(HCT R2 = 0.06, _p_ = 0.20; Control R2 = 0.06, _p_ = 0.12; Fig. 5a). This suggests that HCT exposure may slightly worsen the effect of illness severity on growth in length but not weight.
Moreover, there was a significant negative correlation between OFC AUC and cumulative M-nSOFA scores only for the control group (R2 = 0.26, _p_ < 0.001), not the HCT group (R2 = 0.04, _p_
= 0.32; Fig. 5c). Direct comparison of these correlations was statistically significant (F(1,69) = 5.79, _p_ < 0.05), suggesting that HCT exposure is associated with an attenuated effect
of illness severity on head growth. Within the HCT group, we found that cumulative HCT dosage did not have a significant interaction with the effect of illness severity on head growth
(Supplementary Table 6). DISCUSSION The use of steroids for extremely premature ELBW infants in the NICU remains controversial despite multi-center randomized clinical trials.14,15,16 Thus,
better understanding of the risks and benefits of steroid use is necessary to guide informed clinical decision-making for this vulnerable population. This study of extremely premature ELBW
infants is the first to report the relationship between HCT exposure and growth for whom individualized patient level severity of illness was calculated during the initial NICU
hospitalization. In our cohort, the patients who received HCT were significantly more premature and had significantly greater severity of illness from birth to discharge, as demonstrated by
higher CRIB II scores at admission, increased LOS, and higher cumulative maximum M-nSOFA scores. We found that in terms of growth, there were weak but significant negative correlations
between weight, length, but not OFC z-scores with age, suggesting overall subpar growth with relative preservation of head growth over the time course of hospitalization. This was not
different between HCT and control groups. These data are consistent with larger population studies, though it should be noted that these larger studies included patients who received
dexamethasone or other glucocorticoids in addition to HCT and utilized different dosing and duration of HCT treatment.16,17,32 We also found significant negative correlations between length
AUC and OFC AUC but not weight AUC with illness severity. Interestingly, we found that HCT exposure interacts with these relationships. Specifically, HCT exposure may mitigate the effect of
severity of illness on head growth, suggesting that it may provide support for better brain growth in this population. While we did not find cumulative HCT dose to be a significant mediator
of illness severity on head growth amongst HCT exposed patients, we acknowledge that we may lack the statistical power or spread in our data to identify a more subtle effect. As we found a
very strong correlation between head size measured by OFC and brain size measured by HUS, consistent with prior studies,21 we expect that these finding for head growth reflect trajectories
of underlying brain tissue growth. These findings emphasize the importance of considering patient illness severity and suggest that HCT use may offer additional benefits not previously
considered, although larger studies are needed to confirm these associations. Additionally, larger studies may also allow us to better understand whether the indication for HCT use may be
associated with different effects. Further investigation is necessary to better understand the mechanisms by which HCT may modulate effects of illness severity on head growth, as well as
what differentiates HCT and dexamethasone. One possibility is that HCT and dexamethasone have differential effects directly on neuronal survival and maturation. In animal models, endogenous
steroids have been shown to affect neurogenesis, neuronal survival, and neuronal morphology.33,34 This may in part be due to its interactions with TrkB receptors to mediate brain derived
neurotrophic factor (BDNF)-dependent pathways of glutamatergic activity and neuroprotection.35,36 Anti-inflammatory effects of steroids may also protect against excitotoxicity.37 Exogenous
steroid administration have also been shown to affect synaptic maintenance through dendritic spine turnover.38,39 Another possibility is that HCT and dexamethasone have differential
secondary effects, such as the ability to promote improved cardiorespiratory physiology, that in turn better facilitates brain perfusion and oxygenation. Akin to intrauterine growth
restricted (IUGR) infants who have preferential perfusion to vital organs during fetal development (i.e., brain vs. gastrointestinal tract), extremely premature, ELBW infants with high
illness severity (i.e. those requiring HCT) may have preferential perfusion of their brain (reflected in their OFC) over their gastrointestinal tract (reflected in their weight) or skeletal
system (reflected in their length).40 Past studies have implicated steroids in upregulating expression of cardiovascular adrenergic receptors, which may be downregulated during critical
illness.41 Steroids may also inhibit catecholamine metabolism, release vasoactive factors, and increase intracellular calcium availability in cardiac and vascular smooth muscles that
increase responsiveness to catecholamines.42 This study has limitations inherent to all single-center, retrospective, pilot analyses. First, for these extremely premature ELBW infants, we
did not have longer term follow-up data available at the time of analysis. Second, institution-specific practices, including use of systemic steroids and unit specific feeding guidelines,
may modify both severity of illness metrics and growth, thus affecting the generalizability of our results. Moreover, as the HCT and control groups were not matched, and the HCT cohort was
significantly more premature than the control group, there are likely other confounds for which we could not account. For instance, the effects of HCT were noted in surviving patients, so
they may represent a subgroup of patients who were inherently more responsive to steroids. Nevertheless, the proportion of our cohort exposed to steroids and the differential characteristics
between HCT and control groups were comparable to larger, multi-center cohort studies regarding this population.43 Our results highlight the need for a more nuanced understanding of factors
that modulate risk for neurodevelopmental outcomes in extremely premature ELBW infants. Ultimately, additional studies that account for individual patient level severity of illness relative
to therapies, such as HCT, are needed to better tailor treatments to patients in the era of precision medicine. DATA AVAILABILITY The datasets generated and analyzed during the current
study are available from the corresponding author on reasonable request. REFERENCES * Volpe, J. J. Brain injury in premature infants: A complex amalgam of destructive and developmental
disturbances. _Lancet Neurol._ 8, 15 (2009). Article Google Scholar * Montagna, A. & Nosarti, C. Socio-emotional development following very preterm birth: Pathways to psychopathology.
_Front. Psychol._ 7, 80 (2016). Article PubMed PubMed Central Google Scholar * Delobel-Ayoub, M. et al. Behavioral problems and cognitive performance at 5 years of age after very preterm
birth: The EPIPAGE study. _PEDIATRICS_ 123, 1485–1492 (2009). Article PubMed Google Scholar * Joseph, R. M. et al. Neurocognitive and Academic outcomes at age 10 years of extremely
preterm newborns. _Pediatrics_ 137, e20154343 (2016). Article PubMed PubMed Central Google Scholar * Thompson, D. K. et al. Tracking regional brain growth up to age 13 in children born
term and very preterm. _Nat. Commun._ 11, 696 (2020). Article CAS PubMed PubMed Central Google Scholar * Keunen, K. et al. Brain tissue volumes in preterm infants: prematurity,
perinatal risk factors and neurodevelopmental outcome: A systematic review. _J. Matern. Fetal Neonatal Med._ 25, 89–100 (2012). Article PubMed Google Scholar * Yates, N., Gunn, A. J.,
Bennet, L., Dhillon, S. K. & Davidson, J. O. Preventing brain injury in the preterm infant—current controversies and potential therapies. _Int. J. Mol. Sci._ 22, 1671 (2021). Article
CAS PubMed PubMed Central Google Scholar * Ibrahim, H., Sinha, I. P. & Subhedar, N. V. Corticosteroids for treating hypotension in preterm infants. _Cochrane Database Syst. Rev._
2011, CD003662 (2011). PubMed PubMed Central Google Scholar * Doyle, L. W., Ehrenkranz, R. A. & Halliday, H. L. Late (> 7 days) postnatal corticosteroids for chronic lung disease
in preterm infants. _Cochrane Database Syst. Rev_. https://doi.org/10.1002/14651858.CD001145.pub3. (2014). * Halliday, H. L. Update on postnatal steroids. _Neonatology_ 111, 415–422 (2017).
Article PubMed Google Scholar * Barrington, K. J. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. _BMC Pediatr._ 1, 1
(2001). Article CAS PubMed PubMed Central Google Scholar * Yeh, T. F. et al. Outcomes at School Age after Postnatal Dexamethasone Therapy for Lung Disease of Prematurity. _N. Engl. J.
Med._ 350, 1304–1313 (2004). Article CAS PubMed Google Scholar * Parikh, N. A. et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants.
_Pediatrics_ 119, 265–272 (2007). Article PubMed Google Scholar * Cummings, J. J., Pramanik, A. K., & COMMITTEE ON FETUS AND NEWBORN. Postnatal Corticosteroids to Prevent or Treat
Chronic Lung Disease Following Preterm Birth. _Pediatrics_ e2022057530. https://doi.org/10.1542/peds.2022-057530. (2022). * Baud, O. et al. Effect of early low-dose hydrocortisone on
survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. _Lancet_ 387, 1827–1836 (2016).
Article CAS PubMed Google Scholar * Onland, W. et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm
infants receiving mechanical ventilation: A randomized clinical trial. _JAMA_ 321, 354 (2019). Article CAS PubMed PubMed Central Google Scholar * Watterberg, K. L. et al. Hydrocortisone
to improve survival without bronchopulmonary dysplasia. _N. Engl. J. Med._ 386, 1121–1131 (2022). Article CAS PubMed PubMed Central Google Scholar * Parikh, N. A., Kennedy, K. A.,
Lasky, R. E., McDavid, G. E. & Tyson, J. E. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. _J. Pediatr._
162, 685–690.e1 (2013). Article CAS PubMed Google Scholar * Aziz, K. B. et al. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight
infants. _J. Perinatol._ https://doi.org/10.1038/s41372-021-01030-9 (2021). * Lavilla, O. C. et al. Hourly kinetics of critical organ dysfunction in extremely preterm infants. _Am. J.
Respir. Crit. Care Med._ 205, 75–87 (2022). Article PubMed Google Scholar * Cheong, J. L. Y. et al. Head growth in preterm infants: correlation with magnetic resonance imaging and
neurodevelopmental outcome. _PEDIATRICS_ 121, e1534–e1540 (2008). Article PubMed Google Scholar * Raghuram, K. et al. Head growth trajectory and neurodevelopmental outcomes in preterm
neonates. _Pediatrics_ 140, e20170216 (2017). Article PubMed Google Scholar * Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for
preterm infants. _BMC Pediatr._ 13, 59 (2013). Article PubMed PubMed Central Google Scholar * Parry, G., Tucker, J. & Tarnow-Mordi, W. CRIB II: an update of the clinical risk index
for babies score. _Lancet_ 361, 1789–1791 (2003). Article PubMed Google Scholar * Aziz, K. B., Schles, E. M., Makker, K. & Wynn, J. L. Frequency of acute kidney injury and association
with mortality among extremely preterm infants. _JAMA Netw. Open_ 5, e2246327 (2022). Article PubMed PubMed Central Google Scholar * Fleiss, N. et al. Evaluation of the neonatal
sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. _JAMA Netw. Open_ 4, e2036518 (2021). Article PubMed PubMed Central Google Scholar *
Wynn, J. L. & Polin, R. A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. _Pediatr. Res._ 88,
85–90 (2020). Article PubMed Google Scholar * Dorner, R. A., Burton, V. J., Allen, M. C., Robinson, S. & Soares, B. P. Preterm neuroimaging and neurodevelopmental outcome: A focus on
intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. _J. Perinatol._ 38, 1431–1443 (2018). Article PubMed PubMed Central Google Scholar * Bakdash, J.
Z. & Marusich, L. R. Repeated Measures Correlation. _Front. Psychol_. 8, (2017). https://doi.org/10.3389/fpsyg.2017.00456. * Papile, L. A., Burstein, J., Burstein, R. & Koffler, H.
Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. _J. Pediatr._ 92, 529–534 (1978). Article CAS PubMed
Google Scholar * Morris, I. P., Goel, N. & Chakraborty, M. Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: A
systematic review and meta-analysis. _Eur. J. Pediatr._ 178, 1171–1184 (2019). Article CAS PubMed PubMed Central Google Scholar * Williams, E. E., Dassios, T., Mann, M. & Greenough,
A. The effect of postnatal corticosteroids on growth parameters in infants with bronchopulmonary dysplasia. _J. Perinat. Med._ 49, 1141–1144 (2021). Article CAS PubMed Google Scholar *
Spanswick, S. C., Epp, J. R. & Sutherland, R. J. Time-course of hippocampal granule cell degeneration and changes in adult neurogenesis after adrenalectomy in rats. _Neuroscience_ 190,
166–176 (2011). Article CAS PubMed Google Scholar * Gould, E., Woolley, C. S. & McEwen, B. S. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the
adult dentate gyrus. _Neuroscience_ 37, 367–375 (1990). Article CAS PubMed Google Scholar * Numakawa, T. et al. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered
PLC-gamma signaling for glutamate release via a glutamate transporter. _Proc. Natl Acad. Sci. USA_ 106, 647–652 (2009). Article CAS PubMed PubMed Central Google Scholar * Jeanneteau,
F., Garabedian, M. J. & Chao, M. V. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. _Proc. Natl Acad. Sci. USA_ 105, 4862–4867 (2008).
Article CAS PubMed PubMed Central Google Scholar * Bellavance, M.-A. & Rivest, S. The neuroendocrine control of the innate immune system in health and brain diseases. _Immunol.
Rev._ 248, 36–55 (2012). Article PubMed Google Scholar * Liston, C. & Gan, W.-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. _Proc.
Natl Acad. Sci._ 108, 16074–16079 (2011). Article CAS PubMed PubMed Central Google Scholar * Liston, C. et al. Circadian glucocorticoid oscillations promote learning-dependent synapse
formation and maintenance. _Nat. Neurosci._ 16, 698–705 (2013). Article CAS PubMed PubMed Central Google Scholar * Aucott, S. W., Donohue, P. K. & Northington, F. J. Increased
morbidity in severe early intrauterine growth restriction. _J. Perinatol. J. Calif. Perinat. Assoc._ 24, 435–440 (2004). Google Scholar * Ullian, M. E. The role of corticosteroids in the
regulation of vascular tone. _Cardiovasc. Res._ 41, 55–64 (1999). Article CAS PubMed Google Scholar * Lösel, R. & Wehling, M. Nongenomic actions of steroid hormones. _Nat. Rev. Mol.
Cell Biol._ 4, 46–55 (2003). Article PubMed Google Scholar * Puia-Dumitrescu, M. et al. Dexamethasone, Prednisolone, and Methylprednisolone Use and 2-Year Neurodevelopmental Outcomes in
Extremely Preterm Infants. _JAMA Netw. Open_ 5, e221947 (2022). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the families of the
participants and the staff at Johns Hopkins Hospital and laboratory for their willingness to participate in this study. FUNDING Supported by National Institutes of Health RO1HD086058 (A.E.,
F.J.N.); R01 HD110091 (F.J.N., A.D.E., K.Z., R.C-V.); RO1HD070996, AG061643, and NS109029, HD074593-07 (F.J.N.); KO8NS096115 (R.C-V.), and the Thomas Wilson Foundation (R.-V.). AUTHOR
INFORMATION Author notes * These authors contributed equally: Haiwen Chen, Khyzer B. Aziz. AUTHORS AND AFFILIATIONS * Division of Pediatric Neurology, Department of Neurology, Johns Hopkins
University School of Medicine, Baltimore, MD, USA Haiwen Chen & Carl E. Stafstrom * Division of Neonatology – Neuroscience Intensive Care Nursery, Johns Hopkins University School of
Medicine, Baltimore, MD, USA Khyzer B. Aziz, Harisa Spahic, Sarah Miller, Alison Kilborn, Frances J. Northington, Carl E. Stafstrom & Raul Chavez-Valdez * Division of Pediatric
Neuroradiology, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Melike Guryildirim * Division of Neonatology, Johns Hopkins All Children’s Hospital,
St. Petersburg, FL, USA Austin Sellers & Sandra Brooks * Division of Pediatric Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Allen D. Everett Authors *
Haiwen Chen View author publications You can also search for this author inPubMed Google Scholar * Khyzer B. Aziz View author publications You can also search for this author inPubMed Google
Scholar * Harisa Spahic View author publications You can also search for this author inPubMed Google Scholar * Sarah Miller View author publications You can also search for this author
inPubMed Google Scholar * Melike Guryildirim View author publications You can also search for this author inPubMed Google Scholar * Austin Sellers View author publications You can also
search for this author inPubMed Google Scholar * Sandra Brooks View author publications You can also search for this author inPubMed Google Scholar * Alison Kilborn View author publications
You can also search for this author inPubMed Google Scholar * Allen D. Everett View author publications You can also search for this author inPubMed Google Scholar * Frances J. Northington
View author publications You can also search for this author inPubMed Google Scholar * Carl E. Stafstrom View author publications You can also search for this author inPubMed Google Scholar
* Raul Chavez-Valdez View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.C., K.B.A., R.C-V. conceived and designed the study, analyzed the
data, and drafted the article. H.C., K.B.A, H.S., S.M., A.S., S.B., A.D.E., F.J.N and R.C-V. contributed to acquisition of the data. M.G. and A.K. performed the HUS measurements. H.C.,
K.B.A., A.S., S.B., A.D.E., C.E.S., and R.C-V. provided critical revisions. All authors provided final approval of the version to be published. CORRESPONDING AUTHOR Correspondence to Raul
Chavez-Valdez. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICAL APPROVAL The study received institutional review board approval (JHH IRB 00026068),
and signed parental informed consent was obtained for each participant within the infant’s first 30 days of life and prior to NICU discharge. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES RIGHTS AND PERMISSIONS
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Chen, H., Aziz, K.B., Spahic, H. _et al._ Interaction of hydrocortisone and illness severity on head growth in cohort of ELBW infants. _Pediatr Res_ 94, 1958–1965 (2023).
https://doi.org/10.1038/s41390-023-02689-w Download citation * Received: 21 December 2022 * Revised: 06 May 2023 * Accepted: 15 May 2023 * Published: 20 June 2023 * Issue Date: December 2023
* DOI: https://doi.org/10.1038/s41390-023-02689-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative