Play all audios:
ABSTRACT BACKGROUND Many drugs are used off-label or unlicensed in neonates. This does not mean they are used without evidence or knowledge. We aimed to apply and evaluate the Grading and
Assessment of Pharmacokinetic–Pharmacodynamic Studies (GAPPS) scoring system for the level of evidence of two commonly used anti-epileptic drugs. METHODS Midazolam and phenobarbital as
anti-epileptics were evaluated with a systematic literature search on neonatal pharmacokinetic (PK) and/or pharmacodynamic [PD, (amplitude-integrated) electroencephalography effect] studies.
With the GAPPS system, two evaluators graded the current level of evidence. Inter-rater agreement was assessed for dosing evidence score (DES), quality of evidence (QoE), and strength of
recommendation (REC). RESULTS Seventy-two studies were included. DES scores 4 and 9 were most frequently used for PK, and scores 0 and 1 for PD. Inter-rater agreements on DES, QoE, and REC
ranged from moderate to very good. A final REC was provided for all PK studies, but only for 25% (midazolam) and 33% (phenobarbital) of PD studies. CONCLUSIONS There is a reasonable level of
evidence concerning midazolam and phenobarbital PK in neonates, although using a predefined target without integrated PK/PD evaluation. Further research is needed on midazolam use in term
neonates with therapeutic hypothermia, and phenobarbital treatment in preterms. IMPACT * There is a reasonable level of evidence concerning pharmacotherapy of midazolam and phenobarbital in
neonates. Most evidence is however based on PK studies, using a predefined target level or concentration range without integrated, combined PK/PD evaluation. * Using the GAPPS system, final
strength of recommendation could be provided for all PK studies, but only for 25% (midazolam) to 33% (phenobarbital) of PD studies. * Due to the limited PK observations of midazolam in term
neonates with therapeutic hypothermia, and of phenobarbital in preterm neonates these subgroups can be identified for further research. You have full access to this article via your
institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS PHARMACOMETRIC APPROACH TO ASSIST DOSAGE REGIMEN DESIGN IN NEONATES UNDERGOING THERAPEUTIC HYPOTHERMIA Article Open access 07
September 2021 EFFICACY AND SAFETY OF DEXMEDETOMIDINE FOR ANALGESIA AND SEDATION IN NEONATES: A SYSTEMATIC REVIEW Article 16 October 2023 ASSOCIATION BETWEEN ANTI-SEIZURE MEDICATION AND
OUTCOMES IN INFANTS Article 20 October 2021 INTRODUCTION Preterm and critically ill neonates are often treated with drugs not registered for use in this population or administered in a
different dose, mode of administration or for an alternative indication.1 This off label and unlicensed pharmacotherapy results from the fact that most drugs were not studied in the neonatal
population during their registration process. Meanwhile vulnerable newborns needed drug therapy to improve their outcome and clinicians started to use drugs outside of registrations. The
registration process to label medication for specific use in newborns with different gestational ages (GAs) is both time consuming and needs a significant amount of resources. The changes in
legislation by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) that intended to promote the registration of drugs in pediatric patients, have mainly increased
knowledge on new drugs. However, in Neonatal Intensive Care Units (NICUs) many old drugs continue to be used, which will not be re-evaluated for labeling. Consequently, for these compounds
only limited impact of this legislation is anticipated and most drugs will therefore continue to be used off label.2 For efficacy and safety reasons off-label use is a highly unfavorable
situation that requires action. However, “off label use” does not necessarily mean “off knowledge use.” Most drugs administered to neonates have been studied to inform health care providers
to a certain extent, ranging from retrospective observational studies up to high quality prospective trials that may mimic drug registration trials. No clear overview on the amount of
knowledge and level of evidence for individual drugs, their age specific dosing, effectiveness and safety is currently available. An important step to improve neonatal care is to identify
the knowledge gaps by grading the current level of evidence for each (group of) drug(s) and its indication in the neonatal population, including specific subpopulations (e.g., extreme low
birth weight neonates, critically ill asphyxiated neonates receiving therapeutic hypothermia). Over the past 20 years, the understanding of appropriate dosing and developmental pharmacology
has largely improved. Drug dosing was initially extrapolated from the available knowledge in adults. Different body composition, maturation of pharmacokinetic (PK) and pharmacodynamic (PD)
processes necessitate for most drugs a dosing recommendation taking these maturational factors (e.g., GA, postnatal age (PNA)) into account.3,4 In addition, also non-maturational factors can
impact PK and PD in neonates. These include pharmacogenetics, critical illness, inflammation, therapeutic hypothermia, or extracorporeal support systems. The optimal way to improve dosing
guidelines in special populations with large inter- and intra-individual variability is by performing dose-finding studies, development of population PK models, model-informed dosing
regimens and its consequent validation in clinical practice.5,6 However, these consecutive steps are often not performed. The Grading of Recommendations, Assessment, Development and
Evaluation (GRADE) system, introduced in 2004, is a widely used tool adopted by many organizations, for grading the quality of scientific evidence and for making recommendations.7 However,
for evaluation of PK/PD evidence of pharmacotherapy this framework is less appropriate. Recently, Gastine et al. suggested a critical appraisal system to quantify the strength of each PK/PD
assessment and rate the study quality of published articles.8 This Grading and Assessment of Pharmacokinetic–Pharmacodynamic Studies (GAPPS) scoring system, was developed and applied to
pediatric antibiotic PK/PD studies.8 Although we are aware the authors stated that the system needs further development and validation, and that only PK related studies were included
(studies on therapeutic drug monitoring or reporting drug plasma concentrations without any PK parameters calculated were excluded) in their paper selection, we consider this recent system
as promising due to the sound methodological development, and the lack of validated grading systems for neonatal pharmacotherapy. Therefore, we aimed to quantify the level of evidence of PK
and/or PD for another drug class, more specifically anti-epileptic drugs (AEDs; or anti-seizure medication (ASM)) in neonates, by using the GAPPS system. For this purpose, midazolam (a
benzodiazepine) and phenobarbital (a barbiturate), frequently used AEDs in neonates,1 were selected as proof of principle compounds. METHODS The GAPPS critical appraisal system,8 was applied
to evaluate level of evidence for two AEDs. The compounds were selected in an interactive meeting of the members of the European Society for Pediatric Research (ESPR) Pharmacology section.
This proof of principle was limited to two frequently used drugs in NICUs,1 to guarantee feasibility. The selected drugs needed to have clear indications and a clear effect parameter. For
this analysis, we therefore agreed to focus on midazolam and phenobarbital. The scoring system consists of a three-step sequential assessment (Fig. 2). First, the _dosing evidence score_
(DES) evaluates the analytical strength of the methods used to achieve the PK parameters. This contains the analytical approach (e.g., individual PK, population PK/PD study), the model
appraisal and validation, and the consistency of the model. In this step, a numeric composite score is obtained (<5: weak, 5–8 intermediate, >8 strong). Second, the _quality of
evidence level_ (QoE) scores the study design. This design can go from a case study with PK or therapeutic drug monitoring data (lowest level, 4), towards a full meta-analysis of PK data
(highest level, 1a). Third, both previous scores are combined to result in a _recommendation level_ (strength of recommendation, REC: weak, intermediate, strong) as indicated in Fig. 2. For
more details on the content and use of the GAPPS score, we refer to the original publication and its supplement.8 For the current study, the PD focus was limited to drug effects assessed on
(amplitude integrated) electroencephalography ((a)EEG) as effect parameter. To select PK and/or PD studies for midazolam and phenobarbital in neonates, the following systematic searches were
performed in Pubmed in December 2020: SEARCH 1—MIDAZOLAM PK IN NEONATES: ((“Midazolam”[Mesh] OR midazolam[tiab]) AND (“Infant, Newborn”[Mesh] OR newborn[tiab] OR newborns[tiab] OR
neonat*[tiab])) AND (“Pharmacokinetics”[Mesh] pharmacokinetic*[tiab] OR disposition[tiab] OR “pharmacokinetics” [Subheading]) NOT (review[pt]) SEARCH 2—MIDAZOLAM PD [LIMITED TO (A)EEG
EFFECTS] IN NEONATES: (((“Midazolam”[Mesh] OR midazolam[tiab]) AND (“Infant, Newborn”[Mesh] OR newborn*[tiab] OR neonat*[tiab])) AND (“Pharmacology”[Mesh] OR “pharmacology” [Subheading] OR
pharmacodynamic*[tiab])) AND (“Electroencephalography”[Mesh] OR Electroencephalograph*[tiab] OR EEG[tiab] OR aEEG[tiab]) NOT review[pt] SEARCH 3—PHENOBARBITAL PK IN NEONATES:
((“Phenobarbital”[Mesh] OR phenobarbital[tiab]) AND (“Infant, Newborn”[Mesh] OR newborn*[tiab] OR neonat*[tiab])) AND (“Pharmacokinetics”[Mesh] OR “pharmacokinetics” [Subheading] OR
pharmacokinetic*[tiab] OR disposition[tiab]) NOT review[pt] SEARCH 4—PHENOBARBITAL PD [LIMITED TO (A)EEG EFFECTS] IN NEONATES: (((“Phenobarbital”[Mesh] OR phenobarbital[tiab]) AND (“Infant,
Newborn”[Mesh] OR newborn*[tiab] OR neonat*[tiab])) AND (“Pharmacology”[Mesh] OR “pharmacology” [Subheading] OR pharmacodynamics*[tiab])) AND (“Electroencephalography”[Mesh] OR EEG[tiab] OR
“Electroencephalograph*“[tiab] OR aEEG[tiab]) NOT review[pt] Only studies in humans were included. Articles of which the full text was not available or neither accessible online, were
excluded. For each search, eligible articles were selected based on the title and abstract, and subsequently the full text was assessed by two evaluators/co-authors, who were all members of
the ESPR Pharmacology section. In case of inconsistency, the article was discussed by the entire group until a decision on inclusion/exclusion was reached. Subsequently, the GAPPS system was
applied to each paper, by two evaluators/co-authors independently. Data were analyzed using descriptive statistics and reported as median (range) or incidence. For each drug, the DES and
REC scoring distribution of the included PK and/or PD studies are graphically presented. Inter-rater variability for the obtained scores was assessed as a measure of reproducibility. First,
for the DES scores of each pair of evaluators (i.e., for all included papers of each of four literature searches), Pearson’s correlation was obtained. Second, inter-rater agreement (Kappa)
was assessed for DES, QoE and for the strength of recommendation (REC). Observations rated as “not applicable” were not included for inter-rater agreement. To take the degree of disagreement
into account, Weighted Kappa (linear weights) was applied.9 The Kappa value with accompanying strength of agreement, was interpreted as follows: <0.20 (poor), 0.21–0.40 (fair), 0.41–0.60
(moderate), 0.61–0.80 (good), 0.81–1.00 (very good agreement).10 Analysis was performed using MedCalc® Statistical Software version 20.014 (MedCalc Software Ltd, Ostend, Belgium). A _p_
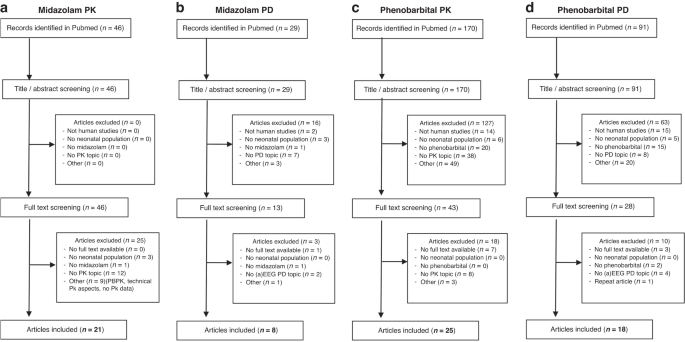
value <0.05 was considered statistically significant. RESULTS The flowcharts of the four separate literature searches on PK/PD of midazolam and phenobarbital in neonates are presented in
Fig. 1. The GAPPS system applied is provided in Fig. 2.8 An overview of the included papers on midazolam PK/PD, and phenobarbital PK/PD is presented in Supplementary Tables S1–S4,
respectively. Therapeutic indications in the PD papers mainly covered seizures, and sedation in some papers, while therapeutic indication in the PK articles was more variable (mainly
sedation for midazolam, seizures for phenobarbital). MIDAZOLAM PK AND PD The literature search revealed 46 papers concerning midazolam PK. In the full-text screening process, 25 papers were
excluded because they were off topic or presented data about physiology-based (PB) PK, technical PK aspects, did not provide human data, or contained no PK data. Therefore, 21 papers were
selected for the GAPPS grading evaluation (Fig. 1a) and were scored as follows for DES (Supplementary Table S1): two studies scored a 10 for dosing evidence and 7 other studies received a
score above 5. The other 12 studies had a score of 4 (_N_ = 7) or lower. For four studies, there was a one-point disagreement between the DES scores. High QoE was scored for 6 studies (level
1a _N_ = 1, level 1b _N_ = 6). Other studies were scored QoE at either level 2 (_N_ = 3), level 3 (_N_ = 10), or level 4 (_N_ = 1). For only 1 study, there was disagreement if the study was
a level 2a or 2c QeE study. The strength of recommendation for the midazolam PK studies was scored strong for 8 studies, intermediate for 11 studies and weak for 1 article. In the judgment
of 1 study, there was disagreement whether this study should receive either a strong or intermediate recommendation (Supplementary Table S1). We identified 29 studies reporting on midazolam
PD. Based on title, abstract, and full-text screening, we selected 8 papers suitable for GAPPS grading evaluation (Fig. 1b). The study features and scores are summarized in Supplementary
Table S2. We were only able to apply the GAPPS grading system to 2 out of 8 papers: one study was rated with a DES score of 8, providing a strong QoE, while the second one received a DES
score of 1, with a low QoE. For the other 6 papers, it was not feasible to apply the grading system. PHENOBARBITAL PK AND PD For phenobarbital PK, the initial literature search revealed 170
abstracts in Pubmed. After screening of 43 full-text articles, 25 studies were eligible for GAPPS assessment (Fig. 1c). Of the 25 analyzed studies (Supplementary Table S3), 7 studies scored
either a 9 or 10 on the DES score. Two studies retrieved a score 7 or 8, whereas the DES score of 16 studies was below 5. For most studies (_N_ = 19) there was full agreement about the DES
score. Disagreement of 1 or 2 points difference in DES score was found for 5 and 1 studies, respectively. The QoE level scored was level 2 for 8 different studies (Supplementary Table S3).
There was only some disagreement in scoring for the QoE for 4 different studies. The strength of the recommendation for the phenobarbital PK studies was strong for 2 studies, intermediate
for 17 studies and weak for 4 studies. For 3 studies there was disagreement in the strength of recommendation between strong and intermediate in 1 study and between intermediate and weak in
2 other studies (Supplementary Table S3). With regards to phenobarbital PD, the initial search resulted in 91 Pubmed records. After exclusion of 63 records based on title/abstract screening,
and another 10 after full-text screening, a total of 18 articles were included for GAPPS assessment (Fig. 1d). Of these studies, all were scored for DES, 6 for QoE and for strength of
recommendation. Twelve studies could not be put through the entire GAPPS grading tool. Only 1 paper had a DES score of 7–8, with QoE 1b resulting in a strength of recommendation classified
as strong by both evaluators. The remaining 17 papers had a DES below 5, scored by both evaluators. Two papers ended with an intermediate and 3 with a weak strength of recommendation by both
evaluators. INTEGRATED PK/PD ANALYSIS In the PK searches, 6 and 8 papers were described as population PK/PD studies for midazolam and phenobarbital, respectively (Supplementary Tables S1
and S3). All PK/PD studies used simulation-based dosing recommendations and according to the GAPPS grading methodology. They were all labeled as “_target identification with simulation-based
dosing recommendations_,” also if the target was not identified in the same study but reported by others or if the target concerned the effect on a different indication than the indication
for which the study was performed (Supplementary Tables S1 and S3). NEONATAL SUBPOPULATIONS Midazolam PK in preterm neonates was mainly studied for the indication of sedation, and final
strength of recommendation is intermediate to strong for this subgroup, often classified as “_target identification with simulation-based dosing recommendations_.” The same findings hold
true for midazolam PK in term neonates. Within the term group, two midazolam PK papers focused on sedation during therapeutic hypothermia, one with intermediate and one with strong strength
of recommendation. No studies with seizures as indication for midazolam were retained. In preterm neonates, phenobarbital PK was studied for seizures, with intermediate-to-weak strength of
recommendation. The number of phenobarbital PK observations for term neonates is larger. Study indication was also seizures, and six studies contained the subpopulation of asphyxiated
neonates receiving therapeutic hypothermia (strength of recommendation intermediate). INTER-RATER AGREEMENTS AND APPLICABILITY OF THE GAPPS SYSTEM The DES scores of both evaluators for the
papers of the literature searches on midazolam PK, phenobarbital PK and phenobarbital PD were significantly correlated (Table 1). The search of midazolam PD contained insufficient data to
assess this correlation (Table 1). In Table 2, inter-rater agreements of DES, QoE and strength of recommendation are provided for both raters involved in the 4 literature searches. To
evaluate the applicability of the GAPPS system to papers retrieved from the PK searches versus the PD searches, the relative frequencies of the DES scores (Fig. 3, midazolam and
phenobarbital pooled), and of the final strength of recommendations (Fig. 4, midazolam and phenobarbital pooled) of evaluator 1 versus evaluator 2 are visually presented. For the papers of
the PK searches, a peak at DES scores 4 and 9 can be distinguished for both evaluators (Fig. 3a, b), while for the papers of the PD searches most of DES scores were low (i.e., 0 and 1) (Fig.
3c, d). Final strength of recommendation for the papers of the PK searches was most frequently rated as intermediate (Fig. 4a, b), while for most of the PD searches a final recommendation
could not be provided (Fig. 4c, d). DISCUSSION The aim of this study was to use and apply the GAPPS grading system to map the available knowledge on PK and PD for midazolam and
phenobarbital, two frequently used AEDs in neonates. Overall, it is most important for off label used drugs to quantify the level of knowledge and evidence for each indication and each
specific (sub)population. Also, for multiple on label drugs this need exists, such as neonatal dosing of ibuprofen, caffeine and midazolam (on label in preterm neonates for sedation, but not
for convulsions) which appeared to be suboptimal over time.11,12,13 The results of our current analysis would also provide an overview on the knowledge gaps and the future research agenda.
In addition, the current approach could also be used to inform neonatal formularies.14 Our study showed that DES scores 4 (i.e., <5, weak quality of evidence) and 9 (i.e., >8, strong
quality of evidence) were most frequently used for the papers of the PK searches, which is slightly higher than the most frequently reported DES score of 3 (i.e., <5, weak quality of
evidence) in the assessment of antibiotic drugs by Gastine et al.8 However, most frequently used DES scores for the papers of the PD searches were extremely low (0 and 1). Neonatal PD
studies were often hampered by the use of multiple drugs. The GAPPS tool seems mainly applicable for PK studies, and its applicability for evaluation of PD papers is limited, and in part
subjective. We are aware on the differences in inclusion criteria compared to Gastine at al., who only included PK studies.8 The current study also included searches for PD papers, which
resulted in a somewhat artificial application of the GAPPS system. The fact that the tool is less suitable for PD papers can be derived from Supplementary Table S2 (midazolam) and
Supplementary Table S4 (phenobarbital), in which respectively 6/8 and 12/18 QoE scores were lacking. This is also obvious from Table 2, in which the inter-rater agreements (PK versus PD) are
provided. A final strength of recommendation of both evaluators was obtained for 100% of the included PK papers, while for the PD papers only 25% (midazolam) to 33% (phenobarbital) ended
with a final strength of recommendation. Both intrinsic limitations of the GAPPS grading system for its use in PD setting, as well as the quality of the studies included in the search, may
contribute to the low applicability. Furthermore, the PD papers often report on drug effects, without integration of (already available) PK models or drug exposure. The drug effects are
underrepresented in the GAPPS system, whereas efficacy, safety and toxicity are of main importance for appropriate clinical applicability of a drug. The wide heterogeneity of study designs,
especially in the definition of PD endpoints emerged as a major issue. It is challenging to establish an efficacy target, especially in neonates as it may change across different populations
(prematurity, asphyxia, critical state) or over the course of the disease.4 Moreover, this search pointed out several low-level of evidence papers (i.e., case reports, case series,
retrospective analysis) for both PD knowledge of midazolam and phenobarbital. Four of the 18 PD phenobarbital studies were case reports. The current study also illustrates that performing PK
studies is feasible, but that a shift towards PK/PD assessment is needed. This lack in combined analyses to assess PK/PD is not unique to neonates, but still also is present in many other
special populations. Furthermore, an increase in the research on developmental PD and tool development for this specific PD field are requested. For each drug, the indication and
subpopulation specific PD endpoints including efficacy and safety need to be determined. Integrated PK/PD studies (see PK searches) for both selected AEDs in neonates were rare. They all
used simulation-based dosing recommendations that qualified all as “_target identification with simulation-based dosing recommendations_,” also if the target was not identified in the same
cohort but reported by others or if it concerned the effect on a different indication. The GAPPS system offered limited options to adequately score the PK/PD design. An important clinical
question would be if a clear PK/PD relationship was found or if a predetermined target exposure was used to calculate potential dosing schemes. Barker et al. reported on how the use of e.g.,
targeted pharmacometrics strategies can support the conduct of high-quality PK/PD studies.15 Although all PK/PD studies for midazolam and phenobarbital with dosing simulations have been
graded “_target identification with simulation-based dosing recommendations_,” none of them really identified the target for e.g., seizures in the same cohort as to study PK, except from one
study by Van den Broek et al. concerning PK/PD of phenobarbital in term asphyxiated neonates.16 To summarize final recommendations, evidence on midazolam PK in preterm and term neonates is
strong to intermediate but lacks seizure target identification. Evidence on phenobarbital PK in term neonates is more substantial and stronger than for preterm neonates. Due to the limited
PK observations of midazolam in term neonates treated with therapeutic hypothermia, and of phenobarbital in preterm neonates these subgroups warrant further research. As mentioned by Gastine
et al., the GAPPS system has been derived from expert consensus and needs further development and validation before use in practice.8 We hereby provide some additional reflections and
suggestions for further improvement. One critical issue relates to the inter-individual subjectivity in the evaluation of some DES criteria, maybe due to the way the criteria are defined.
Terms such as “acceptable” or “robustness” may be interpreted differently, based on the expertise or background of the evaluator. While extreme level (high and low) quality manuscripts may
receive more consistent evaluations, we speculate that for middle-quality manuscripts discrepancy may be more common. Multidisciplinary input of clinicians, pharmacists, but also
pharmacometricians on definitions to adequately use the scoring tool (e.g., to define robustness of a model, validation of a model) is of utmost importance to further improve the tool. The
need and interest to develop grading tools for neonatal pharmacotherapy is apparent from the literature. Recently, Nijstad et al. published on the pharmacological evidence of cytotoxic drug
dosing in neonates and infants.17 They applied a level of evidence, and grade of recommendation provided on a consensus basis and inspired by the Oxford Centre for Evidence-Based Medicine
system, to PK studies of chemotherapeutic agents in neonates, infants, and children up to 4 years.17 As another illustration, in the Netherlands a Dutch framework was developed to provide
pediatric dosing guidelines based on the best available evidence and consensus (Dutch Pediatric Formulary (DPF)).18 This initiative not only improves local uniformity in drug prescribing,
but is also a basis for development and implementation of a pediatric formulary in other countries.19 This group recently showed that the level of evidence for off-label pediatric
pharmacotherapy is low. Of all off-label records used for the DPF, only 14% were supported by high quality evidence.20 Based on a consensus approach, Kanji et al. developed the ClinPK
checklist, defining minimal criteria for transparent and complete reporting of PK studies. The tool is not specifically for a special population but considered more general.21 Furthermore,
only one term for “target identification” was used. The grading system would benefit from more stratification and details, e.g., target identification (i) in the same cohort as used to study
PK or in a different cohort but same neonatal subpopulation; or (ii) in a different neonatal subpopulation. In addition, (iii) the target can be studied for the same or for a different
indication. Thus, a ranking score (top–down) could be included considering the population in which the target is identified and the drug indication. Supplementary Fig. S1 provides a proposal
for further stratification of the specific setting “_target identification with simulation-based dosing recommendations_.” These suggestions raise the question if one generic grading tool
should be developed which is applicable for PK/PD of all drug classes (in neonates), instead of development of separate tools for different drug classes. In fact, neonatology and pediatrics
would highly benefit from one grading system for drugs that is integrated within the generally accepted evidence-based medicine GRADE system. This is required because drugs can already be
studied in randomized controlled trials (RCTs) and receive high ratings in the grade system, whereas appropriate dose finding and PK/PD evidence is lacking. An interesting example is
caffeine that has been very well studied in a placebo controlled RCT with long-term evaluation of efficacy and safety,22,23 but is currently used in much higher dosages supported by
population PK studies.12,24 In fact, this integrated grading system would need equal levels of knowledge and evidence as are used in new drug development and registration processes for both
dosing and PK/PD, as well as for efficacy and long-term safety studies with the appropriate dose. The latter is currently often neglected in meta-analyses or literature reviews. Again, these
need to be sub-defined for specific indications and specific populations. An open access online, updated system that provides the current level of knowledge for all drugs used in the
neonatal population including planned and ongoing studies and trials would also provide a great overview on the current knowledge gaps and the required research agenda. Overall, our
manuscript illustrates that the quality of evidence of PK and/or PD of two predefined AEDs for use in neonates needs further improvement, and that an optimal grading tool is essential. This
is however the case for all drug classes used in neonates. To further improve rational medicines use, more data, and tools to assess efficacy and safety in neonates are required.25,26 This
is reflected by the complexity to grade PD papers in the current study. Rational use of AED requires (i) the development of more advanced tools to improve accurate seizure diagnosis, and
(ii) increased knowledge in pathophysiology and pathways involved in neonatal seizures to make AED treatment more personalized.25 Seizure detection has shifted over time from clinical to
(a)EEG driven. At present, continuous video EEG (cvEEG) is considered as gold standard for seizure detection in neonates, and should be the standard biomarker to assess PD of AEDs in
clinical trials, and likely also in clinical care. This is in line with the recommendations of the International Neonatal Consortium for the design of therapeutic trials for neonatal
seizures.27 In this recommendation paper, the authors mention that both FDA and EMA recognize (i) the superiority of multichannel cvEEG over reduced devices like aEEG for accurate detection
of neonatal seizures, and (ii) the need for cvEEG monitoring to determine PD in neonatal seizure trials to obtain regulatory (FDA/EMA) approval.27 Although long-term neurodevelopmental
outcome data of, e.g., phenobarbital are limited, the compound is used as a first-line drug for neonatal seizures.28 In case of resolution of acute symptomatic neonatal seizures,
discontinuation of antiseizure medication prior to hospital discharge is currently advised for most cases. This is based on a recent comparative effectiveness study showing no difference in
neurodevelopment or epilepsy at 24 months of age in cases with discontinuation versus maintenance of pharmacotherapy after discharge.29 Based on a meta-analysis, Kumar et al. indicated
phenobarbital is at least as efficacious and safe as other AED (e.g., phenytoin, levetiracetam), and there is insufficient evidence to advise other compounds instead of phenobarbital.30
Recently (November 2022) phenobarbital received FDA approval for the treatment of neonatal seizures in term and preterm infants.31 The approved dosing recommendation contains a loading dose
of 20 mg/kg (with a second loading dose if clinically indicated, of 10 or 20 mg/kg for preterms and 20 mg/kg for term infants), followed by a total daily maintenance dose of 4.5 mg/kg/day
(administered as 1.5 mg/kg every 8 h or 2.25 mg/kg every 12 h) up to 5 days.32 The results of the NEOLEV2 trial, a multicenter, randomized, blinded, controlled phase IIb trial, indicating
phenobarbital was more effective than levetiracetam for the treatment of neonatal seizures, contributed to this approval decision.33 This paper was not retained in our phenobarbital PD
search, which might be explained by the fact that our search (proof-of-principle study) was too limited (predefined search terms mentioned above). A full PD evaluation of a specific drug
requires a broader literature search containing additional concepts like efficacy on other outcome variables, or safety, and (be it both PK and PD related) drug-drug interactions.
Multicenter and multidisciplinary collaboration, prospective validation studies, and long-term follow-up might contribute to improve knowledge towards optimal and individualized neonatal
pharmacotherapy.4 Learned societies like ESPR can play an important role in such a multidisciplinary collaboration, which needs input not only from academia, industry, regulatory agencies,
and health-care workers, but also patients and parent organizations. Within the NICU population, 88% of neonates receive at least one off-label or unlicensed drug prescription.34 Even
observations up to 96% of neonates exposed to off-label drugs are reported.35 On the other hand, the label of midazolam has been expanded in 2016 with a dosing regimen for (pre)term neonates
with the indication sedation at intensive care. Despite this registration, Voller et al. have illustrated using simulations that these registered dosages result in extreme differences in
achieved steady-state concentrations for preterms with various gestational and postnatal age.13 For specific subpopulations like extreme preterm infants, intrauterine growth restriction, or
asphyxiated neonates, evidence on validated individualized drug dosing guidelines remains very limited. In conclusion, there is a reasonable level of evidence about the off-label used AEDs
midazolam and phenobarbital in neonates. Most of the evidence is however based on PK studies, using a predefined target level without integrated, combined PK/PD evaluation. Using the GAPPS
system, final strength of recommendation could be provided for all PK studies, but only for 25% (midazolam) to 33% (phenobarbital) of PD studies. A proposal for an adapted grading system is
provided. Due to the limited PK observations of midazolam in term neonates with therapeutic hypothermia, and of phenobarbital in preterm neonates these subgroups can be identified for
further research. DATA AVAILABILITY All data generated or analyzed during this study are included in this published article and its Supplementary Information files. For additional
information concerning these data, the corresponding author can be contacted. REFERENCES * Flint, R. B. et al. Large differences in neonatal drug use between nicus are common practice: time
for consensus? _Br. J. Clin. Pharmacol._ 84, 1313–1323 (2018). Article PubMed PubMed Central Google Scholar * Slater, R., Moultrie, F., Bax, R., van den Anker, J. & Bhatt, A. Preterm
health: time to bridge the evidence gap. _Lancet_ 396, 872–873 (2020). Article PubMed Google Scholar * van den Anker, J., Reed, M. D., Allegaert, K. & Kearns, G. L. Developmental
changes in pharmacokinetics and pharmacodynamics. _J. Clin. Pharmacol._ 58, S10–S25 (2018). PubMed Google Scholar * Smits, A. et al. Current knowledge, challenges and innovations in
developmental pharmacology: a combined Conect4children Expert Group and European Society for Developmental, Perinatal and Paediatric Pharmacology White Paper. _Br. J. Clin. Pharmacol_. 88,
4965–4984 (2022). * Smits, A. et al. Prospective evaluation of a model-based dosing regimen for amikacin in preterm and term neonates in clinical practice. _Antimicrob. Agents Chemother._
59, 6344–6351 (2015). Article CAS PubMed PubMed Central Google Scholar * Smits, A., Kulo, A., van den Anker, J. & Allegaert, K. The amikacin research program: a stepwise approach to
validate dosing regimens in neonates. _Expert Opin. Drug Metab. Toxicol._ 13, 157–166 (2017). Article CAS PubMed Google Scholar * Atkins, D. et al. Grading quality of evidence and
strength of recommendations. _BMJ_ 328, 1490 (2004). Article PubMed Google Scholar * Gastine, S. et al. GAPPS (Grading and Assessment of Pharmacokinetic-Pharmacodynamic Studies) a
critical appraisal system for antimicrobial PKPD studies - development and application in pediatric antibiotic studies. _Expert Rev. Clin. Pharmacol._ 12, 1091–1098 (2019). Article CAS
PubMed Google Scholar * Cohen, J. Weighted Kappa: nominal scale agreement with provision for scaled disagreement or partial credit. _Psychol. Bull._ 70, 213–220 (1968). Article CAS
PubMed Google Scholar * Altman, D. G. _Practical Statistics for Medical Research_ (Chapman & Hall/CRC, 1991). Google Scholar * Engbers, A. G. J. et al. Enantiomer specific
pharmacokinetics of ibuprofen in preterm neonates with patent ductus arteriosus. _Br. J. Clin. Pharmacol._ 86, 2028–2039 (2020). Article CAS PubMed PubMed Central Google Scholar *
Engbers, A. G. J. et al. The pharmacokinetics of caffeine in preterm newborns: no influence of doxapram but important maturation with age. _Neonatology_ 118, 106–113 (2021). Article CAS
PubMed Google Scholar * Voller, S. et al. Recently registered midazolam doses for preterm neonates do not lead to equal exposure: a population pharmacokinetic model. _J. Clin. Pharmacol._
59, 1300–1308 (2019). Article PubMed PubMed Central Google Scholar * Shaniv, D. et al. Neonatal drug formularies-a global scope. _Children_ 10, 848 (2023). * Barker, C. I. S. et al.
Pharmacokinetic studies in children: recommendations for practice and research. _Arch. Dis. Child._ 103, 695–702 (2018). PubMed Google Scholar * van den Broek, M. P. et al.
Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. _Clin. Pharmacokinet._ 51, 671–679 (2012). Article
PubMed Google Scholar * Nijstad, A. L. et al. Clinical pharmacology of cytotoxic drugs in neonates and infants: providing evidence-based dosing guidance. _Eur. J. Cancer_ 164, 137–154
(2022). Article CAS PubMed PubMed Central Google Scholar * van der Zanden, T. M. et al. Developing a paediatric drug formulary for the Netherlands. _Arch. Dis. Child._ 102, 357–361
(2017). Article PubMed Google Scholar * van der Zanden, T. M. et al. Extending the Dutch Paediatric Formulary across Europe: successful development of country specific, parallel,
paediatric drug formularies. Abstract ESDPPP Congres Basel, Switserland. _Arch. Dis. Child._ 104, e59–e60 (2019). Google Scholar * van der Zanden, T. M. et al. Off-label, but on-evidence? A
review of the level of evidence for pediatric pharmacotherapy. _Clin. Pharmacol. Ther._ 112, 1243–1253 (2022). * Kanji, S. et al. Reporting guidelines for clinical pharmacokinetic studies:
the ClinPK Statement. _Clin. Pharmacokinet._ 54, 783–795 (2015). Article PubMed Google Scholar * Schmidt, B. et al. Long-term effects of caffeine therapy for apnea of prematurity. _N.
Engl. J. Med._ 357, 1893–1902 (2007). Article CAS PubMed Google Scholar * Schmidt, B. et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of
prematurity. _JAMA_ 307, 275–282 (2012). Article CAS PubMed Google Scholar * Koch, G. et al. Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm
neonates. _J. Pediatr._ 191, 50.e1–56.e1 (2017). Article Google Scholar * Allegaert, K. Rational use of medicines in neonates: current observations, areas for research and perspectives.
_Healthcare_ 6, 115 (2018). * Allegaert, K., Smits, A., Simons, S. & den Anker, J. V. Perspectives in neonatal pharmacology: drug discovery, knowledge integration and structured
prioritization. _Curr. Pharm. Des._ 24, 4839–4841 (2018). Article CAS PubMed Google Scholar * Soul, J. S. et al. Recommendations for the design of therapeutic trials for neonatal
seizures. _Pediatr. Res._ 85, 943–954 (2019). Article PubMed Google Scholar * Young, L., Berg, M. & Soll, R. Prophylactic barbiturate use for the prevention of morbidity and mortality
following perinatal asphyxia. _Cochrane Database Syst. Rev._ 2016, CD001240 (2016). PubMed PubMed Central Google Scholar * Glass, H. C. et al. Safety of early discontinuation of
antiseizure medication after acute symptomatic neonatal seizures. _JAMA Neurol._ 78, 817–825 (2021). Article PubMed Google Scholar * Kumar, J., Meena, J., Yadav, J. & Saini, L.
Efficacy and safety of phenobarbitone as first-line treatment for neonatal seizure: a systematic review and meta-analysis. _J. Trop. Pediatr._ 67, fmab008 (2021). * FDA. U.S. Food and Drug
Administration. NDA approval letter phenobarbital sodium. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215910Orig1s000ltr.pdf (2022). * Sun Pharmaceutical Industries. Sezaby
(phenobarbital sodium) for injection. https://sezaby.com/ (2023). * Sharpe, C. et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. _Pediatrics_
145, e20193182 (2020). * Cuzzolin, L. & Agostino, R. Off-label and unlicensed drug treatments in neonatal intensive care units: an Italian multicentre study. _Eur. J. Clin. Pharmacol._
72, 117–123 (2016). Article CAS PubMed Google Scholar * Costa, H., Costa, T. X., Martins, R. R. & Oliveira, A. G. Use of off-label and unlicensed medicines in neonatal intensive
care. _PLoS ONE_ 13, e0204427 (2018). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The research activities of A.S. are supported by the Clinical
Research and Education Council of the University Hospitals Leuven. FUNDING No funding was used for the development of this paper. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * University
Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK Liam Mahoney * Department of Clinical Sciences and Community Health, Università Degli Studi Di Milano, Milan, Italy Genny
Raffaeli * Neonatal Intensive Care Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy Genny Raffaeli & Giacomo Cavallaro * Section of Neonatology, Department
of Pediatrics, School of Medicine, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey Serdar Beken * Department of Neonatology, Ankara Etlik City Hospital, University of Health
Sciences, Ankara, Turkey Sezin Ünal * Department of Women’s and Children’s Health, University of Liverpool, Liverpool Health Partners, Liverpool, UK Charalampos Kotidis * University of
Liverpool, Liverpool Womens Hospital, Liverpool, UK Charalampos Kotidis * Clínica Universidad de Navarra, Madrid, Spain Felipe Garrido * Department of Paediatrics, University of Oxford,
Oxford, OX3 9DU, UK Aomesh Bhatt * INFANT Research Centre, University College Cork, Cork, Ireland Eugene M. Dempsey * Department of Neonatology, Cork University Maternity Hospital, Cork,
Ireland Eugene M. Dempsey * Department of Paediatrics and Child Health, University College Cork, Cork, Ireland Eugene M. Dempsey * Department of Hospital Pharmacy, Erasmus MC, Rotterdam, the
Netherlands Karel Allegaert & Robert B. Flint * Department of Development and Regeneration, KU Leuven, Leuven, Belgium Karel Allegaert & Anne Smits * Department of Pharmaceutical
and Pharmacological Sciences, KU Leuven, Leuven, Belgium Karel Allegaert * Division of Neonatology, Department of Neonatal and Pediatric Intensive Care, Erasmus University Medical Center -
Sophia Children’s Hospital, Rotterdam, The Netherlands Sinno H. P. Simons & Robert B. Flint * Neonatal Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium Anne Smits
Authors * Liam Mahoney View author publications You can also search for this author inPubMed Google Scholar * Genny Raffaeli View author publications You can also search for this author
inPubMed Google Scholar * Serdar Beken View author publications You can also search for this author inPubMed Google Scholar * Sezin Ünal View author publications You can also search for this
author inPubMed Google Scholar * Charalampos Kotidis View author publications You can also search for this author inPubMed Google Scholar * Giacomo Cavallaro View author publications You
can also search for this author inPubMed Google Scholar * Felipe Garrido View author publications You can also search for this author inPubMed Google Scholar * Aomesh Bhatt View author
publications You can also search for this author inPubMed Google Scholar * Eugene M. Dempsey View author publications You can also search for this author inPubMed Google Scholar * Karel
Allegaert View author publications You can also search for this author inPubMed Google Scholar * Sinno H. P. Simons View author publications You can also search for this author inPubMed
Google Scholar * Robert B. Flint View author publications You can also search for this author inPubMed Google Scholar * Anne Smits View author publications You can also search for this
author inPubMed Google Scholar CONSORTIA ON BEHALF OF THE ESPR PHARMACOLOGY SECTION CONTRIBUTIONS Concept and design: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A.,
S.H.P.S., A.S. Acquisition of data: L.M., G.R., S.B., R.B.F., S.Ü., G.C., F.G., K.A., S.H.P.S., A.S. Analysis and interpretation of data: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G.,
A.B., E.M.D., K.A., S.H.P.S., A.S. Drafting the article: L.M., G.R., R.B.F., G.C., F.G., K.A., S.H.P.S., A.S. Critical revision of the article: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C.,
F.G., A.B., E.M.D., K.A., S.H.P.S., A.S. Final approval of the version to be published: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A., S.H.P.S., A.S. CORRESPONDING
AUTHOR Correspondence to Sinno H. P. Simons. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE No patient consent
was applicable for this paper. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mahoney, L., Raffaeli, G., Beken, S. _et al._ Grading the level of evidence of neonatal
pharmacotherapy: midazolam and phenobarbital as examples. _Pediatr Res_ 95, 75–83 (2024). https://doi.org/10.1038/s41390-023-02779-9 Download citation * Received: 08 February 2023 * Revised:
07 July 2023 * Accepted: 17 July 2023 * Published: 26 September 2023 * Issue Date: January 2024 * DOI: https://doi.org/10.1038/s41390-023-02779-9 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative