Play all audios:
ABSTRACT Acute anxiety impacts cognitive performance. Inhalation of air enriched with carbon dioxide (CO2) in healthy humans provides a novel experimental model of generalised anxiety, but
has not previously been used to assess cognition. We used inhalation of 7.5% CO2 to induce acute anxiety and autonomic arousal in healthy volunteers during neuropsychological tasks of
cognitive flexibility, emotional processing and spatial working memory in a single-blind, placebo-controlled, randomized, crossover, within-subjects study. In Experiment 1 (_n_ _=_ 44),
participants made significantly more extra-dimensional shift errors on the Cambridge Neuropsychological Test Automated Battery (CANTAB) Intra-Extra Dimensional Set Shift task under CO2
inhalation compared with ‘normal’ air. Participants also had slower latencies when responding to positive words and made significantly more omission errors for negative words on the CANTAB
Affective Go/No-go task. In Experiment 2 (_n_ = 28), participants made significantly more total errors and had poorer heuristic search strategy on the CANTAB Spatial Working Memory task. In
both experiments, CO2 inhalation significantly increased negative affect; state anxiety and fear; symptoms of panic; and systolic blood pressure/heart rate. Overall, CO2 inhalation produced
robust anxiogenic effects and impaired fronto-executive functions of cognitive flexibility and working memory. Effects on emotional processing suggested a mood-congruent slowing in
processing speed in the absence of a negative attentional bias. State-dependent effects of anxiety on cognitive-emotional interactions in the prefrontal cortex warrant further investigation.
SIMILAR CONTENT BEING VIEWED BY OTHERS METHYLPHENIDATE MODULATES INTERACTIONS OF ANXIETY WITH COGNITION Article Open access 21 October 2021 CHARACTERISING THE ANXIOGENIC NETWORK FROM
FUNCTIONAL CONNECTIVITY ANALYSIS OF THE CO2 CHALLENGE MODEL Article Open access 26 November 2024 WORKING MEMORY, CORTICAL DOPAMINE TONE, AND FRONTOPARIETAL BRAIN RECRUITMENT IN
POST-TRAUMATIC STRESS DISORDER: A RANDOMIZED CONTROLLED TRIAL Article Open access 12 July 2021 INTRODUCTION Emotion and cognition are closely integrated phenomena, such that emotions
influence, and are influenced by, cognitive processes1,2,3. Executive functions heavily rely on the frontal lobes and are necessary for optimal selection, organisation and monitoring of
actions for attaining goals4,5,6. However, negative emotional states, such as anxiety, increase arousal and corresponding autonomic responses and can also bias cognitive processes in favour
of selectively prioritising negative information7,8. Anxiety in particular enhances vigilance when detecting emotionally-salient information in the environment, but disrupts working
memory9,10. Although anxiety can mediate adaptive behaviour in response to threat, it can also impair core aspects of cognition through its profound influence on prefrontal executive
functions11. Deficits in executive functions have been found in anxiety disorders12,13,14. Acute anxiety impairs cognitive flexibility, the ability to adapt one’s behaviour in response to
rapid changes in the environment15. Individuals with high-trait anxiety favour recently acquired responses, even when they are no longer relevant16. Reduced cognitive flexibility has been
proposed to be undermined by interference from irrelevant stimuli, in which anxiety prioritises stimulus-driven (bottom-up) attention over and above goal-directed (top-down)
attention16,17,18. Emotionally-salient cues are known to bias attention, such that high-trait anxious individuals will selectively attend to negative information that matches and exacerbates
their emotional state19. One measure of emotional processing is the Affective Go/No-go task20,21. Using this task, patients with depression have shown to make more omission errors when
responding to happy than to sad words and respond more quickly to sad targets22. Patients with depression have also shown an inability to shift their attention from one affective valence to
another, further supporting mood-congruent processing of negative stimuli20. In healthy volunteers, findings support an affective bias for positive information, as shown by faster responses
for happy faces23. Effects of acute anxiety on working memory have been mixed, with some studies showing anxiety-inducing impairments, e.g., see refs. 24,25,26,27. Discrepant findings are
likely due to different paradigms being used for manipulating emotional states, including variations in delivery and length of delay between the acute stressor and cognitive assessment28.
Negative affect has been hypothesized to selectively deplete processing resources required for adequate working memory performance27,29,30. Classic findings demonstrate that anxiety improves
performance on simpler and well-rehearsed tasks, but impair performance on tasks that require complex, flexible thinking31. More generally, behavioural performance improves with low levels
of arousal, but decreases with higher levels through deleterious effects on cognitive processes such as working memory. One safe, reliable and robust method for inducing acute anxiety and
autonomic arousal is through the inhalation of a gas mixture with an enhanced concentration of carbon dioxide32,33. Air enriched with CO2 has previously shown to evoke anxiety-related
symptoms in healthy volunteers34,35,36 and in patients with anxiety disorders37,38. Acute administration of the benzodiazepine agonist lorazepam and chronic administration of the selective
serotonin re-uptake inhibitor (SSRI) paroxetine has been shown to attenuate the effects of CO2 inhalation on state anxiety in healthy volunteers39, thus providing an experimental model of
generalised anxiety40. However, the effects of CO2 inhalation on cognitive performance are less well characterised. Only one laboratory study has used an emotional antisaccade task to show
that inhalation of 7.5% CO2 selectively increases attention to threat in healthy humans41. The aim of our study was to characterise impairments in fronto-executive functions in healthy
humans using an experimental manipulation analogous to generalised anxiety. We report two experiments investigating the effects of 7.5% CO2 inhalation on cognitive flexibility, emotional
processing and spatial working memory in healthy human volunteers. Tasks were selected based on previous studies showing prefrontal and amygdalar dysfunction in highly anxious individuals
and in patients with generalised anxiety disorder (GAD)42,43. We hypothesized that compared with a ‘normal air’ control condition; CO2 inhalation would impair cognitive flexibility and
spatial working memory and induce mood-congruent processing of negative information. We further hypothesized, consistent with the literature34,39,40,41, that CO2 inhalation would increase
negative affect; state anxiety and fear; symptoms of panic; and cardiovascular measures associated with somatic anxiety. MATERIALS AND METHODS PARTICIPANTS Seventy-two healthy volunteers
were recruited via mailing lists, posted flyers and from the Behavioural and Clinical Neuroscience Institute research database. Inclusion criteria were no current or past medical,
psychiatric or neurological conditions or substance abuse. Exclusion criteria were pregnancy, currently smoking and having a first-degree relative diagnosed with a panic disorder.
Participants were free of regular medication intake, but use of the oral contraceptive pill was accepted. These criteria were screened using the Mini-International Neuropsychiatric
Inventory44; and by telephone interview. Invited participants were asked to abstain from alcohol consumption 36 h prior to the experiment as well as caffeinated drinks from the midnight
before testing. All participants provided written informed consent. GAS MIXTURE Air enriched with CO2 (7.5% CO2, 21% O2, 71.5% N2) was used to evoke somatic anxiety34 and was stored in 10 L
cylinders compressed with 200 bar. The air condition (control) consisted of approximately 0.0016% CO2, 21% O2 and 78% N2. NEUROPSYCHOLOGICAL MEASURES We tested cognitive tasks in two cohorts
of healthy participants. Participants performed parallel versions of cognitive tasks from the computerised Cambridge Neuropsychological Test Automated Battery (CANTAB;
www.cambridgecogntion.com) in the two gas inhalation sessions. Tasks were performed over two experiments given the limited time window to safely assess cognitive performance during the CO2
challenge. Inhalation of CO2 for up to 20 min is the maximal inhalation duration known to be safely tolerated without serious side effects (e.g., see refs. 34,37,39,40,41). EXPERIMENT 1
CANTAB INTRA-DIMENSIONAL/EXTRA-DIMENSIONAL SET-SHIFTING TASK (IDED) The CANTAB IDED task45 is a measure of rule acquisition and reversal. It features visual discrimination, attentional set
formation, maintenance of attention, set shifting and flexibility. Two artificial dimensions are used: colour-filled shapes and white lines. Simple stimuli are made of just one of these
dimensions, whereas compound stimuli are made of both, namely white lines overlying colour-filled shapes. Participants must use feedback to work out a rule that determines which stimulus is
correct. After six correct responses, the stimuli and/or rule changes. Initially the task will involve simple stimuli which are made up of just one of the dimensions. Later on, compound
stimuli are used. The shifts in rule are firstly ‘intra-dimensional’ and then secondly ‘extra-dimensional.’ Outcome measures include the total errors made; errors made in the critical stages
of intra-dimensional set shift; errors made in the critical stages of extra-dimensional set shift; and the number of errors made prior to the extra-dimensional shift of the task. CANTAB
AFFECTIVE GO/NO-GO TASK (AGN) The CANTAB AGN task20 is a measure of information-processing biases for positive and negative stimuli. The task consists of several blocks, each of which
presents a series of words from two of three different affective categories: positive (e.g. joyful), negative (e.g. burden) or neutral (e.g. pause). The participant is given a target
category and is asked to select a word when it matches this category. Outcome measures include errors of commission (an incorrect response to a distractor stimulus on ‘No/go’ trials) and
omission (no response to a target stimulus on ‘Go’ trials) and latency (speed of response). EXPERIMENT 2 CANTAB SPATIAL WORKING MEMORY TASK (SWM) The CANTAB SWM46 is a measure of ability to
retain spatial information and to manipulate remembered items in working memory. It is a self-ordered task, which also assesses heuristic strategy. A number of coloured boxes are first shown
on the screen. Participants are instructed to find a blue token in each box, using a process of elimination, and to use them to fill up an empty column on the right side of the screen. The
colour and position of the boxes are changed from trial to trial (with the number of boxes increasing). Outcome measures include total errors (selecting boxes that have already been found to
be empty and revisiting boxes which have already been found to contain a token) and strategy (a predetermined sequence by beginning with a specific box and then, once a blue token has been
found, to return to that box to start the new search sequence). QUESTIONNAIRE MEASURES Administered trait measures included Spielberger’s Trait-Anxiety Inventory [STAI-T; ref. 47], Beck’s
Depression Inventory [BDI; ref. 48] and the Intolerance of Uncertainty Scale [IUS; ref. 49]. The STAI-T is a 20-item self-report measure of trait anxiety, with higher scores indicating
higher anxiety levels (range 20–80); the BDI is a 21-item self-report measure of depression, with higher scores indicating higher levels of depression severity (range 0–63); and the IUS is a
12-item self-report measure of responses to uncertainty, ambiguous situations and the future (e.g. ‘When it’s time to act, uncertainty paralyses me’), with higher scores indicating less
tolerance (range 12–60). State measures included the Negative Affect subscale of the Positive and Negative Affect Schedule [PANAS; ref. 50; a 10-item measure of current negative affect, with
items such as irritability, distress and nervousness enabling a more comprehensive measure of negative emotionality induced by CO2 inhalation41; range 10–50]; the Acute Panic Inventory
[API; ref. 51; a 17-item measure of the severity of symptoms that typically occur during spontaneous panic attacks, with scores ranging from 0 = symptom not experienced to 3 = severe
experience of symptom; e.g. ‘Do you have rapid or difficulty breathing’; range 0–51]; and 10 cm visual analogue scales of state anxiety, fear and happiness (higher scores indicate a greater
emotional response). PROCEDURE This study received full ethical approval from the University of Cambridge Psychology Research Ethics committee (Pre.2013.98). This study was a single-blind,
placebo-controlled, randomised, within-subject crossover design. All participants provided written informed consent. For the gas sessions, the experimenter installed the mask used for
inhaling the mixture on the participant’s face. Participants were asked to breathe through a soft silicon rubber nasal-oral mask, which was attached to a tube that led to a 100 L Douglas
bag. Participants faced a screen, with the gas bag positioned behind it. In the control condition, participants breathed room air and pre-recorded sounds of gas being released from the
cylinder were played in the background. In the CO2 condition, the valve of the gas bag was switched, such that the participant breathed the gas mixture from the Douglas bag. A cylinder with
a compressed gas mixture was used to keep the bag filled. For safety reasons, the participant was accompanied by at least two researchers and a carbon dioxide safety monitor was used to
monitor the CO2 concentration in the testing room throughout the entire experiment. Systolic blood pressure (mmHG) and heart rate (beats per minute) were measured using a digital blood
pressure monitor. Baseline trait (anxiety, depression, intolerance of uncertainty), panic (API) and cardiovascular (heart rate, blood pressure) measures were first collected in this fixed
order pre-inhalation (5 min). Each gas session then comprised cognitive testing, state (panic, affect, mood) and cardiovascular (as above) measures, which lasted a maximum duration of 20
min. A 5–10-min break was given in between the inhalation sessions. Participants in Experiment 1 completed the CANTAB IDED and AGN tasks and participants in Experiment 2 completed the CANTAB
SWM test. Gas administration was counterbalanced separately by gender using two orders (CO2/air, air/CO2) randomly generated across experiments. Participants were given a 5–10-min rest
period between the inhalation sessions. After the two inhalation sessions, participants were debriefed about their gas administration order. All participants were paid £8/h and thanked for
their time. STATISTICAL ANALYSES With alpha set at 0.05 and 80% power, an a priori power analysis based on a previous study41 found that 15 participants would be sufficient to detect a
within-subjects effect of CO2 inhalation on negative affect (_η_p2 = 0.31). Paired samples _t_-tests were used to investigate within-subject differences in cognitive performance under CO2
and ‘normal’ air inhalation. To test the effect of the gas manipulation on negative affect, panic symptoms and autonomic arousal, repeated-measures ANOVAs were used with ‘Time’ as the
within-subjects factor with three levels (baseline/pre-inhalation, CO2, and air). Main effects were further compared between mean scores under CO2 and mean scores at baseline and under air
separately, adjusting confidence intervals using Bonferroni correction for multiple comparisons. Paired samples _t_-tests were performed to investigate state anxiety, fear and happiness
under CO2 and air. Correlational and regression analyses were used to examine potential associations between cognitive performance and baseline trait measures, the degree of anxiety/panic
symptoms reported and cardiovascular effects experienced under CO2. Due to the high number of neuropsychological test outcome measures, the Benjamini-Hochberg procedure52 was applied at _q_
< 0.05 to control for false discovery; significant _p_-values remained (all two-sided). RESULTS PARTICIPANT CHARACTERISTICS Experiment 1 was composed of 22 males and 22 females with a
mean age of 29.25 years (SD = 10.66) and Experiment 2 was composed of 13 males and 14 females with a mean age of 26.78 years (SD = 9.94). All participants had completed at least
pre-University education at the time of testing with an average of 18.25 years in education (SD = 1.26) in Experiment 1 and 17.17 years in education (SD = 2.57) in Experiment 2. As expected,
mean trait-anxiety and depression scores were within normal ranges for healthy adults (STAI-T: Experiment 1 = 34.98, SD = 6.24; Experiment 2 = 31.86, SD = 7.70; normative score = 33.0, SD =
9.4; ref. 53; and BDI: Experiment 1 = 4.63, SD = 4.75; Experiment 2 = 2.68, SD = 3.08; minimal range = 0–13). Furthermore, mean intolerance of uncertainty scores (Intolerance of Uncertainty
Scale) were 27.51 (SD = 8.27) in Experiment 1 and 26.32 (SD = 7.38) in Experiment 2. Age, gender, years in education, trait-anxiety, depression and intolerance of uncertainty were not
significantly different between experiments (all _p_’s > 0.06). In both samples, baseline (pre-inhalation) measures of trait- and state anxiety, negative affect and panic symptoms were
not significantly different between the two gas administration orders (all _p_’s > 0.09). Furthermore, gas order did not interact with the effects of CO2 on measures of state anxiety and
fear, negative affect and panic (all _p_’s between 0.07 and 0.75) in either experiment. NEGATIVE AFFECT AND MOOD STATE Means and standard deviations for negative affect at each time point
are presented in Table 1. In Experiment 1, there was a main effect of Time, _F_(2, 40) = 9.40, _p_ < 0.001, _η_p2 = 0.32, such that negative affect was significantly higher under CO2
compared with baseline, _p_ < 0.001 (mean difference = 2.81) and air, _p_ = 0.003 (mean difference = 2.64). Similarly in Experiment 2, there was a main effect of Time, _F_(2, 26) = 17.79,
_p_ < 0.001, _η_p2 = 0.56, with negative affect again being significantly higher under CO2 compared with baseline, _p_ = 0.005 (mean difference = 5.34) and air, _p_ < 0.001 (mean
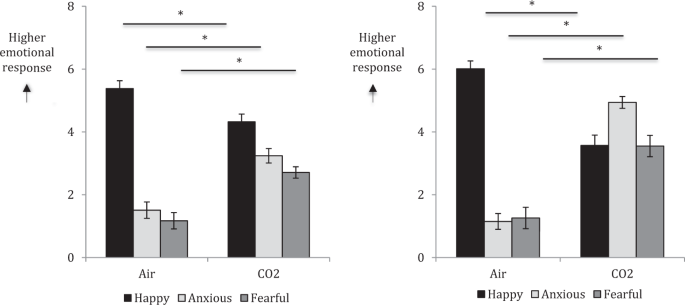
difference = 7.04). In both experiments, state anxiety and fear were significantly increased under CO2 compared with air (all _p_’s < 0.001), whereas state happiness was significantly
decreased under CO2 compared with air (all _p_’s < 0.001) (Fig. 1). In both experiments, women gave significantly higher ratings for state fear than men (_p_ = 0.03 and 0.007,
respectively) during CO2 inhalation. PANIC SYMPTOMS AND AUTONOMIC AROUSAL Means and standard deviations for panic and arousal measures at each time point are presented in Table 1. In
Experiment 1, there was a main effect of Time for the API, _F_(2, 40) = 41.35, _p_ < 0.001, _η_p2 = 0.68. As expected, mean panic symptoms were significantly higher under CO2 compared
with baseline, _p_ < 0.001 (mean difference = 8.57) and air, _p_ < 0.001 (mean difference = 7.17). CO2 inhalation also increased cardiovascular measures of arousal. There were main
effects of Time for both systolic blood pressure, _F_(2, 41) = 34.52, _p_ < 0.001, _η_p2 = 0.63 and heart rate, _F_(2, 41) = 10.47, _p_ < 0.001, _η_p2 = 0.34. Mean systolic blood
pressure was significantly higher under CO2 compared with baseline, _p_ < 0.001 (mean difference = 11.58) and air, _p_ < 0.001 (mean difference = 14.23). However, mean heart rate under
CO2 only significantly differed from mean heart rate at baseline, _p_ < 0.001 (mean difference = 6.51). In Experiment 2, there was a main effect of Time for the API, _F_(2, 26) = 50.67,
_p_ < 0.001, _η_p2 = 0.80. Mean panic symptoms were significantly higher under CO2 compared with baseline, _p_ < 0.001 (mean difference = 15.25) and air, _p_ < 0.001 (mean
difference = 14.68). Again, there were main effects of Time for systolic blood pressure, _F_(2, 25) = 27.08, _p_ < 0.001, _η_p2 = 0.68 and heart rate, _F_(2, 25) = 18.07, _p_ < 0.001,
_η_p2 = 0.59. Mean systolic blood pressure was significantly higher under CO2 compared with baseline, _p_ < 0.001 (mean difference = 21.48) and air, _p_ < 0.001 (mean difference =
24.15). Mean heart rate was also significantly higher under CO2 compared with baseline, _p_ < 0.001 (mean difference = 19.52) and air, _p_ < 0.001 (mean difference = 18.04). COGNITIVE
MEASURES EXPERIMENT 1 COGNITIVE FLEXIBILITY (CANTAB IDED) A paired samples _t_-test revealed that participants (Fig. 2) made significantly more total errors under CO2 compared with air,
_t_(43) = 2.69, _p_ = 0.01, _d_ = 0.41 (CO2 mean = 17.89, SD = 15.09; air mean = 13.00, SD = 9.70). Participants also made significantly more extra-dimensional shift errors under CO2
compared with air, _t_(43) = 2.63, _p_ = 0.01, _d_ = 0.40 (CO2 mean = 7.07, SD = 9.08; air mean = 3.77, SD = 5.30). Pre-ED errors were not significantly different, _t_(43) = 0.62, _p_ = 0.54
(CO2 mean = 8.39, SD = 9.08; air mean = 7.39, SD = 7.14). Furthermore, the mean number of intra-dimensional shift errors under CO2 and air was not significantly different, _t_(43) = 1.86,
_p_ = 0.07 (CO2 mean = 0.75, SD = 0.84; air mean = 0.43, SD = 0.55. AFFECTIVE BIAS (CANTAB AGN) Commission errors were not significantly (Fig. 3) different, _t_(43) = 0.03, _p_ = 0.98 (CO2
mean = 7.34, SD = 6.27; air mean = 7.34, SD = 6.21). However, participants made significantly more omission errors under CO2 compared with air, _t_(43) = 3.31, _p_ = 0.002, _d_ = 0.50 (mean
CO2 = 6.95, SD = 5.85; mean air = 5.00, SD = 4.29). Follow-up paired samples _t_-tests revealed significantly more omission errors for negative words under CO2 compared with air, _t_(43) =
2.96, _p_ = 0.005, _d_ = 0.45 (mean CO2 = 3.41, SD = 3.65; mean air = 2.23, SD = 2.34), with mean omission errors for positive words not reaching significance, _t_(43) = 1.87, _p_ = 0.07
(mean CO2 = 3.55, SD = 3.10; mean air = 2.77, SD = 2.55. Mean latencies for correct responses were significantly slower for positive words under CO2 compared with air, _t_(43) = 2.67, _p_ =
0.01, _d_ = 0.04 (mean CO2 = 591.65, SD = 65.33; mean air = 573.16, SD = 67.64), but not negative words, _t_(43) = 1.65, _p_ = 0.11 (mean CO2 = 600.29, SD = 76.80; mean air = 586.80, SD =
66.45). EXPERIMENT 2 SPATIAL WORKING MEMORY (CANTAB SWM) Participants made significantly more total (Fig. 4) errors under CO2 compared with air, _t_(27) = 3.40, _p_ = 0.002, _d_ = 0.63 (CO2
mean = 33.89, SD = 21.97; air mean = 22.14, SD = 18.01). Follow-up within-subject comparisons at each level of difficulty revealed that participants made significantly more errors at the
hardest task level (ten-box stage) under CO2 compared with air, _t_(27) = 3.19, _p_ = 0.004, _d_ = 0.60 (CO2 mean = 24.39, SD = 15.06; air mean = 15.11, SD = 11.45). Within-subject
performance at lower levels of difficulty (eight-, six-, and four-box stages) was not significantly different (all _p_’s > 0.16). Participants showed an inferior heuristic search strategy
under CO2 compared with air, _t_(27) = 2.38, _p_ = 0.03, _d_ = 0.45 (CO2 mean = 23.36, SD = 7.39; air mean = 21.50, SD = 6.73). WHOLE SAMPLE ANALYSES (PERFORMED SEPARATELY BY TASK)
Correlational analyses revealed that outcome measures from the IDED and SWM tasks were not significantly associated with symptoms of panic, state anxiety/fear or cardiovascular measures
during CO2 inhalation (all _p_’s ≥ 0.05). However, on the AGN task, significant associations were found between total commission errors and negative affect (_r_ = 0.47, _p_ = 0.001), panic
symptoms (_r_ = 0.37, _p_ = 0.01) and state fear (_r_ = 0.32, _p_ = 0.04), and total omission errors and negative affect (_r_ = 0.41, _p_ = 0.006) during CO2 inhalation. A regression
analysis revealed that baseline intolerance of uncertainty significantly predicted omission errors for negative words under CO2, _β_ = 0.32, _t_ = 2.15, _p_ _=_ 0.04 (the model accounted for
32% of the variance and was significant, _F_(1, 42) = 4.62, _p_ = 0.04). Lastly, post hoc power analyses revealed that Experiment 1 achieved 74% power and Experiment 2 achieved 86% power
based on key within-group effects of CO2 on IDED extra-dimensional shift errors and SWM errors made at the ten-box stage, respectively. DISCUSSION We investigated the effects of
experimentally induced acute anxiety and autonomic arousal on core fronto-executive functions in healthy humans as a model of those underlying generalised anxiety. As hypothesized, we found
that compared with baseline and ‘normal’ air, inhalation of air enriched with 7.5% CO2 significantly increased negative affect, state anxiety/fear, symptoms of panic, and cardiovascular
measures of systolic blood pressure and heart rate. Conversely and as expected, CO2 inhalation also significantly reduced state happiness. On the CANTAB IDED task, CO2 inhalation
significantly increased the number of extra-dimensional, but not intra-dimensional, shift errors. Participants also made significantly more total errors and had an inferior heuristic search
strategy on the CANTAB SWM task. Evidence of a mood-congruent processing bias was mixed: on the CANTAB AGN task, we found that participants responded more slowly to positive words, but made
more omission errors for negative words. Correlational analyses revealed significant associations between impairments on the AGN task and negative affect, panic symptoms and state fear under
CO2, suggesting that cognitive effects were influenced by emotional rather than cardiovascular changes. Throughout both experiments, the impact of CO2 was highly present during its
inhalation, but disappeared immediately upon cessation, thereby demonstrating that anxiety induction was acute rather than chronic and with good reproducibility and safety. Studies to date
have shown evidence of anxiety-induced impairments on cognitive flexibility54,55,56. Participants in our study generally made very few, if any, intra-dimensional shift errors, indicating
rather specific effects on attentional set shifting rather than discrimination learning per se57. Impaired extra-dimensional shifting is characteristic of adult patients with
obsessive-compulsive disorder (OCD) and their unaffected first-degree relatives, suggesting that cognitive flexibility is a candidate endophenotype that exists even in the absence of
clinically significant symptoms58. Our data raise the possibility that cognitive flexibility may also be impaired in generalised anxiety disorder (GAD), which might reflect specific
dysfunction in prefrontal cortical regions. Indeed, poor attentional flexibility for extra-dimensional shifting is sensitive to frontal lobal injury59 and related to distinctly weakened
functional connectivity between the caudate and the ventrolateral prefrontal cortex60. The effects of CO2 inhalation on emotional processing revealed slower responding to positive words, but
significantly more omission errors for negative words. However, both total omission and commission errors were highly associated with negative emotions and panic under CO2 across the
samples of each experiment separately. Healthy individuals are characterised by a positive attentional bias and have previously been shown to make more omission errors in response to sad
stimuli than to happy stimuli, whereas patients with depression show the reverse pattern of responding20,22. Our data provide some evidence of an affective bias _congruent with_ negative
mood induction, such that healthy participants showed a slowing of response to positive words under CO2. Although CO2 inhalation is an emotional manipulation, an overall adaptive ‘healthy’
attentional bias may confer resilience against negative emotional processing. This may in part explain why less intolerance of uncertainty, a key construct impaired in GAD, OCD, panic and
other emotional disorders, significantly predicted omission errors for negative words only (e.g. rejection of uncertainty/ambiguity protects against the processing of negative emotional
information). Theoretical and neurophysiological perspectives suggest that anxiety impairs working memory by competing for processing resources via modulation of prefrontal cortical network
functioning29. As expected, we found that participants committed significantly more total errors in our spatial working memory task during CO2 inhalation compared with ‘normal’ air
inhalation. Furthermore, this effect was driven by a significant difference at the hardest level of task difficulty (i.e. the ten-box stage). Participants under CO2 inhalation also exhibited
a significantly worse ‘strategy’ score, meaning they more frequently started a new trial by searching for a token in a new box rather than following a more systematic search strategy46.
Others have shown that increased cortisol or adrenergic activity impairs working memory61 and that alpha-1 receptor agonists can impair the spatial delayed response task in rhesus monkeys62.
In general, alpha-1 activity tend to promote relatively automatic conditioned avoidance behaviours and habitual or ritualistic behaviours62,63. Our data have important clinical
implications. They generally contribute to the existing literature on the interaction between anxiety and cognitive processes using an experimental model that readily translates between
animals and humans. More specifically, CO2 inhalation safely and quickly induces a maladaptive level of anxiety and arousal that impacts executive functioning similar to psychiatric
disorders. As such, this model can be used to translate the efficacy of novel compounds from preclinical models to healthy human volunteers prior to clinical trials in patients (e.g.
evaluation of new anxiolytic treatments such as beta-adrenergic agonists for anxiety disorders35). However, it should be noted that mood and panic symptoms in the present sample were below
the values typically seen in clinical populations, making it difficult to generalise to clinical levels of anxiety. Others have suggested that higher concentrations of CO2 may provide a
better model of panic disorder (e.g. 35%)38,64, although similar effects on panic-like symptoms have been observed using 7.5%34,37. Future work could investigate the potential differential
effects of anxiety types (e.g. somatic vs. psychic; central vs. peripheral) on cognitive performance during CO2 inhalation as well as the neural circuitry implicated in the underlying
mechanisms of anxiety when performing complex executive tasks (e.g. dorsolateral prefrontal cortex and lateral parietal cortex)65. Other research outside of the scope of the present study
could disentangle psychological/emotional effects, interference from physical sensations and physiological changes in respiratory or autonomic function when interpreting cognitive changes.
With respect to emotional processing, it has been previously shown that threat is associated with over-activation of the amygdala in highly anxious individuals41. Although our data showed
that ratings of anxiety and fear significantly increased during CO2 inhalation (with ratings of happiness significantly decreasing), it is important to note that the stimuli used in the
present study were generally negative rather than threatening. Achieving selective attentional effects may therefore require amygdala reactivity following anticipation or provocation of
threat not induced by CO2 inhalation. Limitations include single-blind administration of the gas manipulation (for safety reasons) and unequal sample sizes between experiments (although both
achieved adequate power). Prior to debriefing, most participants reported that the CO2 inhalation session was a relatively intense and/or unpleasant experience, which may have increased
demand characteristics of the air inhalation session, although sessions were counterbalanced to reduce this and gas administration order did not interact with key measures of subjective
anxiety and panic. Finally, our sample mostly comprised young adults, which may not represent the wider population, but does capture a group in which anxiety disorders are increasingly
prevalant. Although a gender difference was only found on subjective ratings of state fear during CO2 inhalation, more detailed investigation of their effects on cognitive/emotional
responses to acute anxiety are warranted in larger samples. Overall, the present study demonstrated state-dependent effects of acute anxiety and autonomic arousal on fronto-executive
functions in healthy humans, including impaired cognitive flexibility and working memory. Effects on emotional processing showed a mood-congruent slowing of response in the absence of a
negative attentional bias. Identification of resilience factors that protect against acute anxiety may help promote cognitive performance needed for achieving optimal behavioural
performance. 7.5% CO2 inhalation in healthy humans also provides a robust model of generalised anxiety that could be used to test new drug therapies for its treatment. REFERENCES * Yiend,
J., Mackintosh, B. & Savulich, G. in _Cognitive Psycholog_y (eds Braisby, N. & Gellatly, A) 507–545 (Oxford University Press, Oxford, 2012). * Taylor Tavares, J. V., Drevets, W. C.
& Sahakian, B. J. Cognition in mania and depression. _Psychol. Med._ 33, 959–967 (2003). Article CAS PubMed Google Scholar * Robinson, O. J., Roiser, J. P. & Sahakian, B. J. in
_Cognitive Impairment in Major Depressive Disorder: Clinical Relevance, Biological Substrates, and Treatment Opportunities_ (ed. McIntyre, R. S.) 69 (Cambridge University Press, Cambridge,
2016). * Fuster, J. M. Executive frontal functions. _Exp. Brain Res._ 133, 66–70 (2000). Article CAS PubMed Google Scholar * Miller, E. K. The prefrontal cortex and cognitive control.
_Nat. Rev. Neurosci._ 1, 59–65 (2000). Article CAS PubMed Google Scholar * Dalley, J. W., Cardinal, R. N. & Robbins, T. W. Prefrontal executive and cognitive functions in rodents:
neural and neurochemical substrates. _Neurosci. Biobehav Rev._ 28, 771–784 (2004). Article CAS PubMed Google Scholar * Yiend, J. & Mackintosh, B. in _Cognition, Emotion and
Psychopathology_ (ed. Yiend, J.) 190–210 (Cambridge University Press, Cambridge, 2004). * Savulich, G., Shergill, S. & Yiend, J. Biased cognition in psychosis. _J. Exp. Psychopathol._ 3,
514–536 (2012). Article Google Scholar * Clarke, R. & Johnstone, T. Prefrontal inhibition of threat processing reduces working memory interference. _Front Hum. Neurosci._ 7, 228
(2013). Article PubMed PubMed Central Google Scholar * Robinson, O. J., Vytal, K., Cornwell, B. R. & Grillon, C. The impact of anxiety upon cognition: perspectives from human threat
of shock studies. _Front Hum. Neurosci._ 7, 203 (2013). PubMed PubMed Central Google Scholar * Okon-Singer, H., Hendler, T., Pessoa, L. & Shakman, A. J. The neurobiology of
cognition-emotion interactions: fundamental questions and strategies for future research. _Front Hum. Neurosci._ 9, 58 (2015). Article PubMed PubMed Central Google Scholar * Gulpers, B.,
Lugtenburg, A., Zuidersma, M., Verhey, F. R. J. & Voshaar, R. C. O. Anxiety disorders and figural fluency: a measure of executive function. _J. Affect Disord._ 234, 38–44 (2018).
Article CAS PubMed Google Scholar * Ajilchi, B. & Nejati, V. Executive functions in students with depression, anxiety and stress symptoms. _Basic Clin. Neurosci._ 8, 223–232 (2017).
Article PubMed PubMed Central Google Scholar * Olley, A., Malhi, G. & Sachdev, P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. _J. Affect
Disord._ 104, 15–23 (2007). Article PubMed Google Scholar * Klanker, M., Feenstra, M. & Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. _Front
Neurosci._ 7, 201 (2013). Article PubMed PubMed Central Google Scholar * Wilson, C. G., Nusbaum, A. T., Whitney, P. & Hinson, J. M. Trait anxiety impairs cognitive flexibility when
overcoming a task acquired response and a preexisitng bias. _PLoS ONE_ 13, e0204694 (2018). Article PubMed PubMed Central CAS Google Scholar * Eysenck, M. W. & Derakshan, N. New
perspectives in attentional control theory. _Personal. Individ. Differences._ 50, 955–960 (2011). Article Google Scholar * Eysenck, M. W., Derakshan, B., Santos, R. & Calvo, M. G.
Anxiety and cognitive performance: attentional control theory. _Emotion_ 7, 336–353 (2007). Article PubMed Google Scholar * Yiend, J. The effects of emotion on attention: a review of
attentional processing of emotional information. _Cognition Emot._ 24, 3–47 (2010). Article Google Scholar * Murphy, F. C. et al. Emotional bias and inhibitory control processes in mania
and depression. _Psychol. Med._ 29, 1307–1321 (1999). Article CAS PubMed Google Scholar * Elliott, R., Rubinsztein, J. S., Sahakian, B. J. & Dolan, R. J. The neural basis of
mood-congruent processing biases in depression. _Arch. Gen. Psychiatry_ 59, 597–604 (2002). Article PubMed Google Scholar * Erikson, K. et al. Mood-congruent bias in affective go/no-go
performance of unmedicated patients with major depressive disorder. _Am. J. Psychiatry_ 162, 2171–2173 (2005). Article Google Scholar * Schulz, K. P. et al. Does the emotional go/no-go
task really measure behavioral inhibition? Convergence with measures of a non-emotional analog. _Arch. Clin. Neuropsychol._ 22, 151–160 (2007). Article PubMed Google Scholar * Oei,
Everaerd, Elzinga & van Well, Bermond Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. _Stress_ 9, 133–141 (2006).
Article CAS PubMed Google Scholar * Schoofs, D., Preuss, D. & Wolf, O. T. Psychosocial stress induces working memory impairments in an n-back paradigm. _Psychoeuroendocrinology_ 33,
643–653 (2008). Article CAS Google Scholar * Schoofs, D., Wolf, O. T. & Smeets, T. Cold pressor stress impairs performance o working memory tasks requiring executive functions in
healthy young men. _Behav. Neursci._ 123, 1066–1075 (2009). Article Google Scholar * Shackman, A. J. et al. Anxiety selectively disrupts visuospatial working memory. _Emotion_ 6, 40–61
(2006). Article PubMed Google Scholar * Smeets, T. et al. Introducing the Masstrict Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and
glucocorticoid stress responses. _Psychoneuroendocrinology_ 37, 1998–2008 (2012). Article CAS PubMed Google Scholar * Eysenck, M. W. & Calvo, M. G. Anxiety and performance: the
processing efficiency theory. _Cognition Emot._ 6, 409–434 (1992). Article Google Scholar * Lavie, N., Hirst, A., de Fockert, J. W. & Viding, E. Load theory of selective attention and
cognitive control. _J. Exp. Psychol. Gen._ 133, 339–354 (2004). Article PubMed Google Scholar * Broadbent, D. E. _Decision and Stress_. (Academic Press, 1971). * Gorman, J. M. et al.
Response to hyperventilation in a group of patients with panic disorder. _Am. J. Psychiatry_ 141, 857–861 (1984). Article CAS PubMed Google Scholar * Van den Hout, M. A. & Griez, E.
Panic symptoms after inhalation of carbon dioxide. _Br. J. Psychiatry_ 144, 503–507 (1984). Article PubMed Google Scholar * Bailey, J. E., Argyropoulos, S. V., Kendrick, A. H. & Nutt,
D. J. Behavioural and cardiovascular effects of 7.5% CO2 inhalation in human volunteers. _Depress Anxiety_ 21, 18–25 (2005). Article PubMed Google Scholar * Poma, S. et al.
Characterisation of a 7% carbon dioxide inhalation paradigm to evoke anxiety symptoms n healthy subjects. _J. Psychopharmacol._ 19, 494–503 (2005). Article PubMed Google Scholar * Atwood,
A. S., Catling, J. C., Kwong, A. S. & Munafò, M. R. Effects of 7.5% cardon dioxide (CO2) inhalation and ethnicity on face memory. _Physiol. Behav._ 147, 97–101 (2015). Article CAS
Google Scholar * Seddon, K. et al. Effects of 7.5% CO2 challenge in generalized anxiety disorder. _J. Psychopharmacol._ 24, 43–51 (2011). Article CAS Google Scholar * Perna, G., Barbini,
B., Cocchi, S., Bertani, A. & Gasperini, M. 35% CO2 challenge in panic and mood disorders. _J. Affect Disord._ 33, 189–194 (1995). Article CAS PubMed Google Scholar * Bailey, J. E.,
Kendrick, A., Diaper, A., Potokar, J. P. & Nutt, D. J. A validation of the 7.5% Behavioural and cardiovascular effects of 7.5% CO2 model of GAD using paroxetine and lorazepam in healthy
volunteers. _J. Psychopharmacol._ 21, 42–49 (2007). Article CAS PubMed Google Scholar * Bailey, J. E., Dawson, G. R., Dourish, C. T. & Nutt, D. J. Validating the inhalation of 7.5%
CO2 in healthy volunteers as a human experimental medicine: a model of generalized anxiety disorder (GAD). _J. Psychopharmcol._ 25, 1192–1198 (2011). Article CAS Google Scholar * Garner,
M., Atwood, A., Baldwin, D. S., James, A. & Munafò, M. R. Inhalation of 7.5% carbon dioxide increases threat processing in humans. _Neuropsychopharmacology_ 36, 1557–1562 (2011). Article
CAS PubMed PubMed Central Google Scholar * Bishop, S., Duncan, J., Brett, M. & Lawrence, A. D. Prefrontal cortical function and anxiety: controlling to threat-related stimuli.
_Nat. Neurosci._ 7, 184–188 (2004). Article CAS PubMed Google Scholar * Bishop, S. J., Duncan, J. & Lawrence, A. D. State anxiety modulation of the amygdala response to unattended
threat-related stimuli. _J. Neurosci._ 24, 10364–10368 (2004). Article CAS PubMed PubMed Central Google Scholar * Lecrubier, Y. et al. The Mini International Neuropsychiatric Inventory
(MINI). A short diagnostic structured interview: reliability and validity according to CIDI. _Eur. Psychiatry_ 12, 224–231 (1998). Article Google Scholar * Downes, J. J. et al. Impaired
extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: evidence for a specific attentional dysfunction. _Neuropsychologia_ 27, 1329–1343 (1989). Article CAS
PubMed Google Scholar * Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E. & Robbins, T. W. Planning and spatial working memory following frontal lobe lesion in man.
_Neuropsychologia_ 28, 1021–1034 (1990). Article CAS PubMed Google Scholar * Spielberger, C. D., Gorsuch, R. L., Lushene, P. R., Vagg, P. R. & Jacobs, A. G. _Manual for the
State-Trait Anxiety Inventory (Form Y)._ (Consulting Psychologists Press, Palo Alto, 1983). Google Scholar * Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory
for measuring depression. _Arch. Gen. Psychiatry_ 4, 5614–5671 (1961). Article Google Scholar * Carleton, R. N., Norton, M. P. J. & Asmundson, G. J. Earing the unknown: a short
version of the intolerance of uncertainy scale. _J. Anxiety Disord._ 21, 105–117 (2007). Article PubMed Google Scholar * Watson, D., Clark, L. A. & Tellegan, A. Development and
validation of brief measures of positive and negative affect: the PANAS scales. _J. Pers. Sco. Psychol._ 54, 1063–1070 (1988). Article CAS Google Scholar * Dillon, D. J., Gorman, J. M.,
Liebowitz, M. R., Fryer, A. J. & Klein, D. F. Measurement of lactate-induced panic and anxiety. _Psychiatry Res._ 20, 97–105 (1987). Article CAS PubMed Google Scholar * Benjamini, Y.
& Hochberg, Y. Controlling the false discovery rate: A practical and powerful tool approach to multiple testing. _J. R. Stat. Soc. B._ 57, 289–300 (1995). Google Scholar * Del Carlo,
A. et al. Different measures of impulsivity in patients with anxiety disorders: a case control study. _Psychiatry Res._ 197, 231–236 (2012). Article PubMed Google Scholar * Plessow, F.,
Fischer, R., Kirschbaum, C. & Goschke, T. Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility
with increasing time lag to stressor. _J. Cogn. Neurosci._ 23, 3218–3227 (2011). Article PubMed Google Scholar * Alexander, J. K., Hillier, A., Smith, R. M., Tivarus, M. E. &
Beversdorf, D. Q. Beta-adrenergic modulation of cognitive flexibility during stress. _J. Cogn. Neuro._ 19, 468–478 (2007). Article Google Scholar * Laredo, S. A. et al. Effects of defeat
stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. _Eur. J. Neurosci._ 41, 434–441 (2015). Article PubMed PubMed Central Google Scholar *
Robbins, T. W. Dissociating executive functions of the prefrontal cortex. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 35, 1463–1470 (1996). Google Scholar * Chamberlain, S. R. et al.
Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. _Am. J. Psychiatry_ 164, 335–338 (2007). Article
PubMed PubMed Central Google Scholar * Owen, A. M., Roberts, A. C., Polkey, C. E., Sahakian, B. J. & Robbins, T. W. Extra-dimensional versus intra-dimensional set shifting performance
following frontal lobe excisions, temporal lobe excisions or amygalo-hyppocampectomy in man. _Neuropsychologia_ 29, 993–1006 (1991). Article CAS PubMed Google Scholar * Vaghi, M. M. et
al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity.
_Biol. Psychiatry_ 81, 708–717 (2017). Article PubMed PubMed Central Google Scholar * Elzinga, B. M. & Roelofs, K. Cortisol-induced impairments of working memory require acute
sympathetic activation. _Behav. Neurosci._ 119, 98–103 (2005). Article CAS PubMed Google Scholar * Arnsten, A. F. T. Stress signalling pathways that impair prefrontal cortex structure
and function. _Nat. Rev. Neurosci._ 10, 410–422 (2009). Article CAS PubMed PubMed Central Google Scholar * Smeets, T., van Ruitenbeek, P. & Hartogsveid, B. Quaedflieg CWEM.
Stress-induced reliance on habitual behaviour is moderated by cortisol reactivity. _Brain Cogn._ S0278-2626, 30046–0 (2018). Google Scholar * Colasanti, A. et al. Carbon dioxide-induced
emotion and respiratory symptoms in healthy volunteers. _Neuropsychopharmacology_ 33, 3013–3110 (2008). Article Google Scholar * Sheilds, G. S., Sazma, M. A. & Yonelinas, A. P. The
effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. _Neurosci. Biobehav Rev._ 68, 651–668 (2016). Article Google Scholar Download references
ACKNOWLEDGEMENTS This study was funded by a Wellcome Trust Senior Investigator Award to T.W.R. (104631/Z/14/Z/) and carried out in the Behavioural and Clinical Neuroscience Institute
supported by a joint award from the Medical Research Council (G1000183) and Wellcome Trust (Strategic Award 093875/Z/10/Z). G.S. was funded by The Wallitt Foundation and Eton College, with
support from the NIHR Cambridge Biomedical Research Centre (BRC) Mental Health theme. F.H. is supported by a Cambridge Trust Vice-Chanchellor’s Award and Fitzwilliam College scholarship and
was previously supported by an Erasmus scholarship. B.J.S. receives funding from the NIHR Cambridge Biomedical Research Centre (BRC) Mental Health Theme. AUTHOR INFORMATION Author notes *
These authors contributed equally: George Savulich, Frank H. Hezemans AUTHORS AND AFFILIATIONS * Department of Psychiatry, University of Cambridge, School of Clinical Medicine, Cambridge, UK
George Savulich & Barbara J. Sahakian * Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK George Savulich, Frank H. Hezemans, Sophia van Ghesel
Grothe, Jessica Dafflon, Norah Schulten, Annette B. Brühl, Barbara J. Sahakian & Trevor W. Robbins * MRC Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK Frank
H. Hezemans * Department of Psychology, University of Cambridge, Cambridge, UK Trevor W. Robbins Authors * George Savulich View author publications You can also search for this author
inPubMed Google Scholar * Frank H. Hezemans View author publications You can also search for this author inPubMed Google Scholar * Sophia van Ghesel Grothe View author publications You can
also search for this author inPubMed Google Scholar * Jessica Dafflon View author publications You can also search for this author inPubMed Google Scholar * Norah Schulten View author
publications You can also search for this author inPubMed Google Scholar * Annette B. Brühl View author publications You can also search for this author inPubMed Google Scholar * Barbara J.
Sahakian View author publications You can also search for this author inPubMed Google Scholar * Trevor W. Robbins View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Trevor W. Robbins. ETHICS DECLARATIONS CONFLICT OF INTEREST B.J.S. consults for Cambridge Cognition, Peak and Mundipharma. T.W.R. consults for
Cambridge Cognition, Mundipharma and Unilever and receives Royalties for CANTAB. The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Savulich, G., Hezemans, F.H., van Ghesel Grothe, S. _et al._ Acute anxiety and
autonomic arousal induced by CO2 inhalation impairs prefrontal executive functions in healthy humans. _Transl Psychiatry_ 9, 296 (2019). https://doi.org/10.1038/s41398-019-0634-z Download
citation * Received: 08 July 2019 * Revised: 08 October 2019 * Accepted: 20 October 2019 * Published: 12 November 2019 * DOI: https://doi.org/10.1038/s41398-019-0634-z SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative