Play all audios:
ABSTRACT BACKGROUND Two first-line (1L) bevacizumab trials showed the prognostic value of primary tumour location in metastatic colorectal cancer (mCRC). In this retrospective subgroup
analysis, further analysis of the predictive effect of bevacizumab is presented. METHODS Patients with sidedness information from two randomised phase III studies of bevacizumab +
chemotherapy (CT) vs CT as 1L mCRC treatment were analysed retrospectively. RESULTS Sidedness was determined in 1590 (27% right and 73% left) of 2214 patients. Progression-free survival was
improved with bevacizumab + CT vs CT in right-sided (HR = 0.75; 95% CI 0.61, 0.93; _p_ = 0.008) and left-sided (HR = 0.76; 95% CI 0.67, 0.86; _p_ < 0.001) mCRC (pooled analysis).
Similarly, overall survival was numerically improved with bevacizumab + CT vs CT in right-sided mCRC (HR = 0.82; 95% CI 0.65, 1.03; _p_ = 0.085), and significantly improved in left-sided
mCRC (HR = 0.85; 95% CI 0.74, 0.98; _p_ = 0.028). CONCLUSIONS This analysis indicates that the effect of bevacizumab is independent of tumour location in mCRC. SIMILAR CONTENT BEING VIEWED
BY OTHERS CLINICAL IMPACT OF PRIMARY TUMOUR LOCATION, EARLY TUMOUR SHRINKAGE, AND DEPTH OF RESPONSE IN THE TREATMENT OF METASTATIC COLORECTAL CANCER WITH FIRST-LINE CHEMOTHERAPY PLUS
CETUXIMAB OR BEVACIZUMAB Article Open access 13 November 2020 FIRST-LINE CETUXIMAB IMPROVES THE EFFICACY OF SUBSEQUENT BEVACIZUMAB FOR RAS WILD-TYPE LEFT-SIDED METASTATIC COLORECTAL CANCER:
AN OBSERVATIONAL RETROSPECTIVE STUDY Article Open access 23 July 2020 FOLFIRI PLUS CETUXIMAB OR BEVACIZUMAB FOR ADVANCED COLORECTAL CANCER: FINAL SURVIVAL AND PER-PROTOCOL ANALYSIS OF
FIRE-3, A RANDOMISED CLINICAL TRIAL Article Open access 06 November 2020 BACKGROUND Metastatic colorectal cancer (mCRC) is a heterogeneous disease increasingly characterised by chromosomal
and molecular variations.1 CRCs arising from the right or left side of the colon have markedly different clinical characteristics, incidence rates, and gene expression profiles.2,3,4,5
Right-sided tumours are more likely to be characterised by _BRAF_ mutations and high microsatellite instability, whereas left-sided tumours are commonly associated with chromosomal
instability and overexpression of epidermal growth factor receptor ligands.3 Right- and left-sided tumours also differ with regard to their microbiomes and host-related factors. Mucosal
microbiota organisation has been shown to be a distinct feature of right-sided tumours,6 and right-sided CRC is associated with older age and fewer surgeries for urgent indications.7 Recent
reports have focused on differences in prognosis and therapeutic outcome between tumours originating in the right and left side of the colon.1,8,9 In a report of two randomised phase III
mCRC trials with bevacizumab, overall survival (OS) was significantly longer in left-sided vs right-sided tumours (AVF2017g: hazard ratio (HR) = 0.55, 95% confidence interval (CI) =
0.43–0.70; NO16966: HR = 0.71, 95% CI = 0.62–0.82); these differences remained statistically significant within treatment subgroups (i.e., bevacizumab + chemotherapy (CT) or CT alone).8
Given the fundamental differences in colon tumour sidedness, further understanding of the influence of sidedness from pivotal mCRC studies may add value to our current knowledge of optimal
mCRC treatments. Here we present data on the benefit of adding bevacizumab to CT in patients with right- or left-sided tumours from the NO16966 and AVF2107g trials. MATERIALS AND METHODS
Patient populations, treatment, and outcomes have been described in the AVF2107g and NO16966 studies.10,11 Briefly, AVF2107g was a randomised, placebo-controlled trial of bevacizumab with
irinotecan, bolus fluorouracil, and leucovorin in patients with previously untreated mCRC.10 NO16966 was a randomised, noninferiority comparison of 5-fluorouracil, folinic acid, and
oxaliplatin (FOLFOX4) vs capecitabine plus oxaliplatin (XELOX), which was subsequently amended to a 2 × 2 factorial design with further randomisation to bevacizumab or placebo.11 The
aforementioned analysis by Loupakis et al.8 which included data from AVF2107g and NO16966, evaluated the association between tumour location and survival in mCRC. Thus, all evaluable
patients from AVF2107g and both part 1 (FOLOX4 vs XELOX) and part 2 (2 × 2 factorial plus bevacizumab) of NO16966 were included. However, since our objective was to compare the effect of
bevacizumab + CT vs CT in right- and left-sided tumours, our analysis focuses on patients who were concurrently randomised to bevacizumab + CT. Patients who received CT only (FOLFOX4 or
XELOX) from part 1 of NO16966 are excluded here. Sidedness of patients from NO16966 (NCT00069095) and AVF2107g (NCT00109070) was identified by Clinical Study Report information, including
surgery procedural reports, updated from Loupakis et al.8 Cancers proximal (i.e. occurring in the caecum, ascending colon, or transverse colon) or distal (i.e., occurring in the descending
colon, sigmoid colon, or rectum) to the splenic flexure were defined as right- or left-sided, respectively. Tumours occurring precisely at the splenic flexure were assigned to the left-sided
group. Patients with synchronous right- and left-sided tumours were excluded. Median progression-free survival (PFS) and OS, as well as corresponding 95% CIs, were estimated using
Kaplan-Meier methods. Interaction tests were performed to determine whether treatment outcomes with bevacizumab + CT vs CT were different for patients with right- vs left-sided mCRC tumours.
Unadjusted Cox proportional hazards models with right/left terms were used to compare survival outcomes between treatment groups and were correlated with efficacy endpoints; corresponding
HRs and 95% CIs were estimated. In an overall treatment comparison for all patients with right- or left-sided tumours, tumour location was included as a covariate in the Cox model and
interaction of treatment and tumour location was assessed. Overall response rate (ORR) was summarised as categorical data and compared between treatment groups by odds ratio (OR). RESULTS
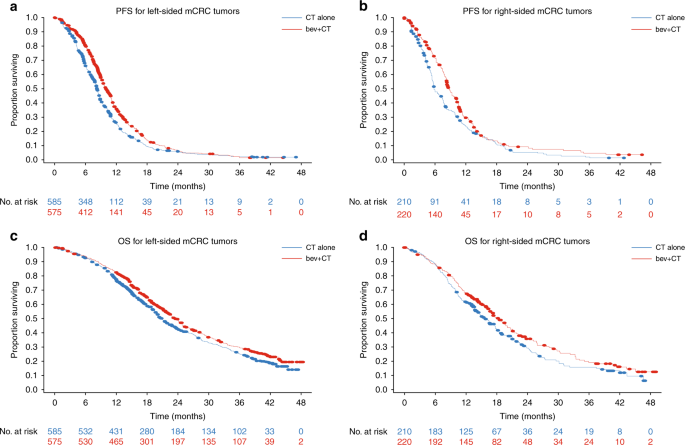
Sidedness was determined in 1590 patients (27% right and 73% left) of 2214 patients total (Table 1). PFS was superior in patients treated with bevacizumb + CT vs CT in both right- (median
PFS, 8.7 vs 5.8 months; HR = 0.75; 95% CI 0.61, 0.93; _p_ = 0.008) and left-sided (median PFS, 10.0 vs 8.2 months; HR = 0.76; 95% CI 0.67, 0.86; _p_ < 0.001) mCRC in this pooled analysis
of NO16966 and AVF2107g (Fig. 1a, b). OS was also numerically improved with bevacizumab + CT vs CT in right-sided mCRC (median OS, 18.3 vs 15.6 months; HR = 0.82; 95% CI 0.65, 1.03; _p_ =
0.085) and statistically significantly improved in left-sided mCRC (median OS, 23.5 vs 20.8 months; HR = 0.85; 95% CI 0.74, 0.98; _p_ = 0.028) (Fig. 1c, d). The magnitude of PFS and OS
benefit did not differ by tumour location. To determine survival outcomes by baseline characteristics, patients were stratified by sex, age, race, Eastern Cooperative Oncology Group
performance status, presence of colon cancer, prior adjuvant CT, and prior radiation therapy. Compared with CT, bevacizumab + CT was associated with greater PFS and OS benefits for both
right- and left-sided mCRC for most of these categories (Fig. 2). In an overall treatment comparison for all patients with right- or left-sided tumours, tumour location was included as a
covariate in the Cox model and interaction of treatment and tumour location was non-significant (PFS, _p_ = 0.898; OS, _p_ = 0.697). Cox model analysis also showed a non-significant (PFS,
_p_ = 0.980; OS, _p_ = 0.879) interaction of bevacizumab use and availability of sidedness information (right/left vs. not classified). Interaction tests for PFS (_p_ = 0.904) and OS (_p_ =
0.689) indicated that treatment effect did not differ between patients based on tumour location. ORRs were similar with bevacizumab + CT vs CT in both right-sided (40.9% vs 42.4%; OR, 0.94;
95% CI 0.64–1.38; _p_ = 0.770) and left-sided (54.4% vs 52.0%; OR, 1.10; 95% CI 0.88–1.39; _p_ = 0.410) mCRC. DISCUSSION Right-sided tumours have been widely associated with worse prognosis
compared with left-sided tumours in mCRC.8,12,13,14 Meta-analysis showed that left-sided CRC was associated with a 20% reduction in the risk of death vs right-sided CRC, independent of
disease stage, ethnicity, and study type.12 In our retrospective subgroup analysis of two randomised phase III mCRC studies, bevacizumab + CT improved survival vs CT alone, and the effect of
bevacizumab was independent of tumour location. Similar improvements in PFS and OS were observed when patients were stratified according to baseline characteristics, such as age or race. In
addition, OS was numerically improved with bevacizumab + CT vs CT in right-sided mCRC (median OS, 18.3 vs 15.6 months; _p_ = 0.085) and significantly improved in left-sided mCRC (median OS,
23.5 vs 20.8 months; _p_ = 0.028). However, it is likely that the smaller magnitude of the right-sided mCRC patient subset contributed to the non-significant _p_ value for OS; therefore,
these data suggest survival benefit overall and for both right- and left-sided tumours. This is the first analysis of two large phase III mCRC studies comparing bevacizumab + CT vs CT alone
by tumour location. As such, it represents a more comprehensive analysis of the effect of bevacizumab by tumour location than previous reports, which have been mixed.15,16,17 For instance, a
retrospective analysis of mCRC patients treated with XELOX with or without bevacizumab suggested that bevacizumab may primarily benefit patients with left-sided primary tumours.16 However,
in another study, PFS was superior with bevacizumab + CT vs CT regardless of tumour site (HR = 0.46).15 Our results build upon findings from the study by Loupakis et al.8 which showed that
primary tumour location has a strong prognostic effect on patients with mCRC irrespective of bevacizumab exposure. This study was a retrospective subgroup analysis, and was therefore limited
to the patient populations investigated in the AVF2017g and NO16966 trials.10,11 The tumour location of all patients was not identifiable; however, interaction tests showed no evidence of a
relationship between the ability to identify the side and outcomes (PFS interaction, _p_ = 0.980; OS interaction, _p_ = 0.879). Although both AVF2107g and NO16966 investigated bevacizumab,
the CT backbones were different, which may have influenced the pooled results in our study. Another limitation is that data on RAS mutation status could not be included in our analysis.
Since these data were not collected for NO16966, samples are not currently available for testing; for AVF2107g, sidedness information was only available for 180 out of 230 patients for whom
KRAS data were collected,18 making the sample size too small for a valid analysis of the impact of treatment and RAS status. However, KRAS status has previously been shown not to be
predictive for bevacizumab efficacy.19 Recent evidence points to the value of defining the sublocation of mCRC tumours, within the right- or left-sided classification method. In a study of
1876 CRC patients, the prevalence of mutations in _BRAFV600_, _TP53_, _PIK3CA_, and other genes differed by sublocation within right- and left-sided tumours.20 The authors concluded that the
sigmoid-rectal region of the left side appears unique, and the transverse colon is distinct from other right-sided locations. In our study, the lack of data on precise tumour location could
be seen as a limitation. However, given that bevacizumab efficacy is maintained in both right- and left-sided tumours, further analyses by tumour sublocation would likely not influence the
data presented here. This retrospective exploratory subgroup analysis of two pivotal studies indicates the effect of bevacizumab is independent of tumour location in a non-biomarker selected
mCRC population. REFERENCES * Arnold, D. et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy
and EGFR directed antibodies in six randomised trials. _Ann. Oncol._ 28, 1713–1729 (2017). Article CAS Google Scholar * Meza, R., Jeon, J., Renehan, A. G. & Luebeck, G. Colorectal
cancer incidence trends in the United States and United Kingdom: evidence of right- to left-sided biological gradients with implications for screening. _Cancer Res._ 70, 5419–5429 (2010).
Article CAS Google Scholar * Missiaglia, E. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. _Ann. Oncol._ 25, 1995–2001 (2014).
Article CAS Google Scholar * Benedix, F. et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and
survival. _Dis. Colon Rectum_ 53, 57–64 (2010). Article Google Scholar * Meguid, R. A., Slidell, M. B., Wolfgang, C. L., Chang, D. C. & Ahuja, N. Is there a difference in survival
between right- versus left-sided colon cancers? _Ann. Surg. Oncol._ 15, 2388–2394 (2008). Article Google Scholar * Dejea, C. M. et al. Microbiota organization is a distinct feature of
proximal colorectal cancers. _Proc. Natl Acad. Sci. USA_ 111, 18321–18326 (2014). Article CAS Google Scholar * Mik, M., Berut, M., Dziki, L., Trzcinski, R. & Dziki, A. Right- and
left-sided colon cancer—clinical and pathological differences of the disease entity in one organ. _Arch. Med. Sci._ 1, 157–162 (2017). Article Google Scholar * Loupakis, F. et al. Primary
tumor location as a prognostic factor in metastatic colorectal cancer. _J. Natl Cancer Inst._ 107, 1–9 (2015). Article Google Scholar * Tejpar, S. et al. Prognostic and predictive
relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. _JAMA Oncol._ 3, 194–201 (2017).
Article Google Scholar * Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. _N. Engl. J. Med._ 350, 2335–2342 (2004). Article
CAS Google Scholar * Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer; a randomized phase III study.
_J. Clin. Oncol._ 26, 2013–2019 (2008). Article CAS Google Scholar * Petrelli, F. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review
and meta-analysis. _JAMA Oncol._ 3, 211–219 (2017). Article Google Scholar * Modest, D. P. et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site
(midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). _Anticancer Drugs_ 25, 212–218 (2014). Article CAS Google Scholar * Yahagi, M., Okabayashi,
K., Hasegawa, H., Tsuruta, M. & Kitagawa, Y. The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. _J. Gastrointest. Surg._
20, 648–655 (2016). Article Google Scholar * Wong, H. L. et al. Impact of primary tumor site on bevacizumab efficacy in metastatic colorectal cancer. _Clin. Colorectal Cancer_ 15, e9–e15
(2016). Article Google Scholar * Boisen, M. K. et al. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. _Ann. Oncol._ 24, 2554–2559
(2013). Article CAS Google Scholar * Holch, J. W., In, Ricard, Stintzing, S., Modest, D. P. & Heinemann, V. The relevance of primary tumour location in patients with metastatic
colorectal cancer: a meta-analysis of first-line clinical trials. _Eur. J. Cancer_ 70, 87–98 (2017). Article Google Scholar * Hurwitz, H. I., Yi, J., Ince, W., Novotny, W. F. & Rosen,
O. The clinical benefit of bevacizumb in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously
untreated metastatic colorectal cancer. _Oncologist_ 14, 22–28 (2009). Article CAS Google Scholar * Hurwitz, H. I. et al. Efficacy and safety of bevacizumab in metastatic colorectal
cancer: pooled analysis from seven randomized controlled trials. _Oncologist_ 18, 1004–1012 (2013). Article Google Scholar * Loree, J. M. et al. Classifying colorectal cancer by tumor
location rather than sidedness highlights a continuum in mutation profiles and Consensus Molecular Subtypes. _Clin. Cancer Res._ 24, 1062–1072 (2018). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS Linda Yau, PhD, of Genentech, Inc. contributed to the statistical analysis and provided critical feedback during early drafts of this manuscript. Medical writing
assistance was provided by Sachi Yim of CodonMedical, an Ashfield Business, part of UDG Healthcare PLC, and supported by F Hoffmann-La Roche/Genentech. AUTHOR INFORMATION Author notes *
Herbert I. Hurwitz Present address: Genentech, Inc., 1 DNA Way, South San Francisco, CA, 94080, USA AUTHORS AND AFFILIATIONS * Istituto Oncologico Veneto (IRCCS), Via Gattamelata, 64, 35128,
Padova, PD, Italy Fotios Loupakis * Duke University Medical Center, 10 Duke Medicine Circle, Durham, NC, 27710, USA Herbert I. Hurwitz * Memorial Sloan Kettering Cancer Center, 1275 York
Avenue, New York, NY, 10021, USA Leonard Saltz * Asklepios Tumorzentrum Hamburg, AK Altona, Paul-Ehrlich-Str. 1, 22763, Hamburg, Germany Dirk Arnold * Mayo Clinic, 200 1st St SW, Rochester,
MN, 55902, USA Axel Grothey * F. Hoffmann-La Roche, Ltd., Grenzacherstrasse 124, CH-4070, Basel, Switzerland Quynh Lan Nguyen, Stuart Osborne, Jonathan Talbot & Stefanie Srock *
University of Southern California, Norris Comprehensive Cancer Center, Keck School of Medicine, 1441 Eastlake Ave, Los Angeles, CA, 90033, USA Heinz-Josef Lenz Authors * Fotios Loupakis View
author publications You can also search for this author inPubMed Google Scholar * Herbert I. Hurwitz View author publications You can also search for this author inPubMed Google Scholar *
Leonard Saltz View author publications You can also search for this author inPubMed Google Scholar * Dirk Arnold View author publications You can also search for this author inPubMed Google
Scholar * Axel Grothey View author publications You can also search for this author inPubMed Google Scholar * Quynh Lan Nguyen View author publications You can also search for this author
inPubMed Google Scholar * Stuart Osborne View author publications You can also search for this author inPubMed Google Scholar * Jonathan Talbot View author publications You can also search
for this author inPubMed Google Scholar * Stefanie Srock View author publications You can also search for this author inPubMed Google Scholar * Heinz-Josef Lenz View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors participated in the data collection, data analysis, and drafting of the manuscript. All authors also
approved the final version of the manuscript to be submitted. CORRESPONDING AUTHOR Correspondence to Heinz-Josef Lenz. ETHICS DECLARATIONS COMPETING INTERESTS F.L. served as a
consultant/advisor for Roche, Amgen, and Bayer, and had travel, accommodations, and/or expenses paid for by Roche, Amgen, Merck Serono, and Bayer. H.I.H. is a current employee of
Genentech/Roche and previously served on the advisory board of Genentech/Roche. L.S. received research funding from Taiho. D.A. received honoraria from, and served as a consultant or advisor
for, BMS, Roche, Bayer, Merck, and EMD, and received research funding from BMS, Roche, Bayer, Merck, EMD, Incyte, and Taiho. Q.L.N., S.O., and J.T. are employees of Roche. S.S. is an
employee of Roche and has a leadership role, owns stock, served as a consultant/advisor, received research funding, had travel/accommodations expenses paid, and had other relationships with
Roche. H.-J.L. served on the advisory board for Bayer, Lilly, Merck, Roche, Sanofi, Servier, Sirtex, and Terumo; served as a lecturer for Bayer, Biocompatibles, Lilly, Merck, MSD, Roche,
Sanofi, Servier, and Sirtex; and received research funding from Mologen, Roche, and Sanofi. A.G. declares no competing interests. ETHICS APPROVAL This study was conducted in accordance with
International Conference on Harmonisation Good Clinical Practice regulations and the Declaration of Helsinki. The informed consent document and protocol were reviewed and approved by the
Independent Ethics Committees of each study centre. All patients provided written informed consent before enrolment. DATA AVAILABILITY Qualified researchers may request access to individual
patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here
(https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access
to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm). FUNDING This study
was supported by F Hoffmann-La Roche/Genentech. LS was funded in part by P30-17 CA008748. ADDITIONAL INFORMATION Note: This work is published under the standard license to publish agreement.
After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0). RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Loupakis, F., Hurwitz, H.I., Saltz,
L. _et al._ Impact of primary tumour location on efficacy of bevacizumab plus chemotherapy in metastatic colorectal cancer. _Br J Cancer_ 119, 1451–1455 (2018).
https://doi.org/10.1038/s41416-018-0304-6 Download citation * Received: 26 January 2018 * Revised: 28 August 2018 * Accepted: 24 September 2018 * Published: 29 November 2018 * Issue Date: 11
December 2018 * DOI: https://doi.org/10.1038/s41416-018-0304-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative