Play all audios:
ABSTRACT BACKGROUND Surgery for renal cell carcinoma (RCC) with venous tumour thrombus (VTT) extension into the renal vein (RV) and/or inferior vena cava (IVC) has high peri-surgical
morbidity/mortality. NAXIVA assessed the response of VTT to axitinib, a potent tyrosine kinase inhibitor. METHODS NAXIVA was a single-arm, multi-centre, Phase 2 study. In total, 20 patients
with resectable clear cell RCC and VTT received upto 8 weeks of pre-surgical axitinib. The primary endpoint was percentage of evaluable patients with VTT improvement by Mayo level on MRI.
Secondary endpoints were percentage change in surgical approach and VTT length, response rate (RECISTv1.1) and surgical morbidity. RESULTS In all, 35% (7/20) patients with VTT had a
reduction in Mayo level with axitinib: 37.5% (6/16) with IVC VTT and 25% (1/4) with RV-only VTT. No patients had an increase in Mayo level. In total, 75% (15/20) of patients had a reduction
in VTT length. Overall, 41.2% (7/17) of patients who underwent surgery had less invasive surgery than originally planned. Non-responders exhibited lower baseline microvessel density (CD31),
higher Ki67 and exhausted or regulatory T-cell phenotype. CONCLUSIONS NAXIVA provides the first Level II evidence that axitinib downstages VTT in a significant proportion of patients leading
to reduction in the extent of surgery. CLINICAL TRIAL REGISTRATION NCT03494816. SIMILAR CONTENT BEING VIEWED BY OTHERS NEOADJUVANT TORIPALIMAB PLUS AXITINIB FOR CLEAR CELL RENAL CELL
CARCINOMA WITH INFERIOR VENA CAVA TUMOR THROMBUS: NEOTAX, A PHASE 2 STUDY Article Open access 04 October 2024 EFFICACY AND SAFETY OF NEOADJUVANT THERAPY WITH TISLELIZUMAB PLUS AXITINIB FOR
NONMETASTATIC RENAL CELL CARCINOMA WITH INFERIOR VENA CAVA TUMOR THROMBUS: A RETROSPECTIVE STUDY Article Open access 17 January 2025 COMPARATIVE ANALYSIS OF ONCOLOGIC OUTCOMES IN SURGICALLY
TREATED PATIENTS WITH RENAL CELL CARCINOMA AND RENAL VEIN THROMBOSIS BY PATHOLOGIC SUBTYPES Article Open access 07 May 2025 INTRODUCTION Venous tumour thrombus (VTT) extension into the renal
vein (RV) and/or inferior vena cava (IVC) occurs in 4–15% cases of renal cell cancer (RCC) [1]. Peri-surgical mortality is high (5–15%) and increases with the height of the VTT [1, 2].
Following this extensive surgery, the cure is possible with 5-year survival rates of ~40–65% for patients with non-metastatic RCC [3, 4]. The concept of using targeted therapies, to
downstage VTT prior to surgery is appealing. It is hypothesised that by reducing the level of the VTT and the extent of surgery, morbidity and mortality would be reduced. There is no Level I
or II evidence of pre-surgical targeted therapy in non-metastatic or metastatic RCC VTT. Four retrospective studies focused on mixed groups of vascular endothelial growth factor receptor
(VEGFR) tyrosine kinase inhibitors (TKI) [5,6,7,8]: sunitinib [9, 10], axitinib [11] and pazopanib [12]. VTT level decreased in a median of 22.6% patients (range 14.9–32.9%), remained stable
in 73.6% (64.1–81.4%) and increased in 7.2% (3.4–14.3%). Results were most favourable for sunitinib and axitinib [5, 7, 11]. There are several prospective studies on VEGFR TKIs in the
pre-nephrectomy setting [13,14,15], but none specifically addresses the question of surgical downstaging of vein-involved local extension. Wood et al. reported on four patients with IVC VTT
but reported no change in surgical management, and did not report specifically about change in the extent of venous involvement [13]. In a Phase II trial of 12 weeks of neoadjuvant axitinib
in clear cell RCC (ccRCC; all patients were cT3a), the median reduction in primary tumour diameter was 28% [15]. Most of the reduction in tumour size had occurred within 7 weeks of axitinib
treatment. The results of these small studies in non-metastatic RCC patients suggest that neoadjuvant VEGFR TKI treatment of RCC patients is safe and reduces tumour size. However, the effect
of these drugs on the extent of the VTT and the effect on the surgical approach has not been confirmed. The objective of NAXIVA was to determine safety, efficacy and effect of neoadjuvant
axitinib on VTT. PATIENTS AND METHODS STUDY DESIGN NAXIVA was a single-arm, single agent, open-label, multi-site, UK-based, Phase II feasibility study of 8 weeks axitinib treatment in M0 and
M1 patients with resectable ccRCC primary tumours with VTT. NAXIVA was prospectively, publicly registered (ISCRTN96273644; EudraCT Number 2017-000619-17; NCT03494816) and approved by an
independent ethics committee (REC reference: 17/EE/0240). See Appendix for the full study protocol. ENDPOINTS The primary endpoint was the percentage of evaluable patients with a reduction
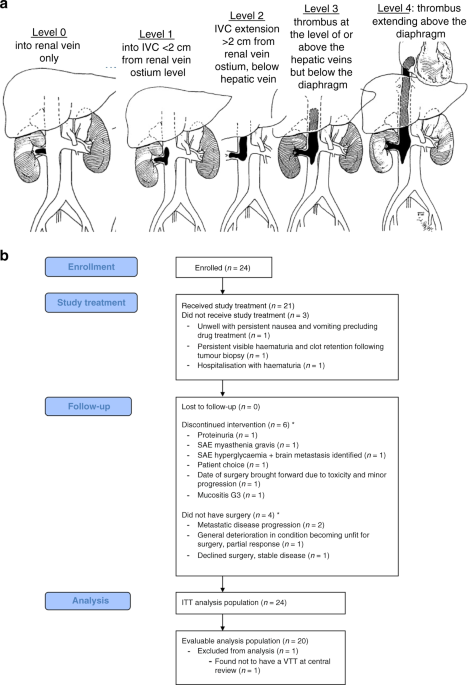
in the extent of VTT by Mayo level after 8 weeks of axitinib therapy. Definitions of the Mayo level (levels are ordered by increasing extensiveness; Fig. 1a) as previously described [2]: *
Level 0: thrombus limited to the renal vein (RV); * Level 1: into IVC < 2 cm from RV ostium level; * Level 2: IVC extension >2 cm from RV ostium but below hepatic veins; * Level 3:
thrombus at the level of or above the hepatic veins but below the diaphragm; * Level 4: thrombus extending above the diaphragm. Secondary endpoints were percentage change in surgical
approach, the percentage change in VTT length, response rate by RECIST version 1.1, and evaluation of surgical morbidity assessed by Clavien–Dindo classification [16]. Exploratory endpoints
were translational studies correlating changes in molecular markers with the response to axitinib in the VTT and primary tumour. PARTICIPANTS/ELIGIBILITY CRITERIA Key inclusion criteria were
RV (cT3a) or IVC VTT (cT3b/c), N0/1, M0/1, biopsy-proven ccRCC, over 18 years of age, suitable for immediate surgical resection of the primary tumour. Participants had to be Eastern
Cooperative Oncology Group (ECOG) performance status <2; have urinalysis <2+ protein, urinary protein <2 g/24 h or protein:creatinine ratio (PCR) < 200 mg/mmol; and serum
creatinine ≤1.5 × ULN or estimated creatinine clearance ≥30 mL/min calculated using the Cockcroft–Gault equation. Key exclusion criteria were Memorial Sloan Kettering Cancer Center (MSKCC)
poor-risk disease (M1 participants) and recent history of cardiac or vascular events. DRUG TREATMENT The starting dose of axitinib was 5 mg BD, escalated to 7 mg BD and then 10 mg BD every 2
weeks, as tolerated. Dose reductions were allowed. Patients stopped axitinib a minimum of 36 h and a maximum of 7 days prior to surgery in week 9. ASSESSMENTS Patients had clinical and
safety assessments according to the Schedule of Assessments (see Protocol in the Appendix). Axitinib-related toxicity was assessed using Common Terminology Criteria for Adverse Events
version 4 (CTCAEv4) criteria. MRI scans were performed before treatment, during week 3 and before surgery (see Supplementary Methods for MRI protocol). CT scans were performed before
treatment, week 3 (M0 patients only to assess for development of chest metastases), week 9- and 3-months post surgery. SURGERY Surgeons were asked to report their planned approach to the VTT
after reviewing the baseline MRI scan and record the performed approach after axitinib therapy, plus planned and performed adjuvant venous procedures. RV/IVC level of control
planned/performed intraoperatively was recorded: * Thrombus milked into RV and side clamped; * Infrahepatic IVC clamping with no liver mobilisation; * Retrohepatic IVC clamping below hepatic
veins, with liver mobilisation; * Retrohepatic IVC clamping above hepatic veins, with liver mobilisation; * Suprahepatic, infradiaphragmatic clamping; * Suprahepatic, supradiaphragmatic
clamping. OUTCOME MEASURES Mayo level and VTT length were assessed using the baseline and week-9 MRI scans, if no week-9 scan was undertaken, the week 3 scan (if available) was used;
calculation details are provided in Supplementary Methods. In order to minimise reporter bias due to the inability to the blind, the primary and relevant secondary endpoint data was based on
a consensus by two central uroradiologists’ (SU and FAG) review of the MRI images. The response rate was determined at local sites using RECIST version 1.1 comparing the screening
(baseline) and pre-surgical CT scans. Primary tumour measurements were included in RECISTv1.1 measurements in all patients. Surgical morbidity was assessed by Clavien–Dindo classification
[16]. METHOD OF CALCULATING PRIMARY ENDPOINTS The definition of an improvement varied according to the patient’s Mayo level as captured at screening. For patients presenting at screening
with a Mayo level 1 or above, an improvement in disease was represented by a reduction in their Mayo level at week 9. For patients presenting at the screening with Mayo-level 0, an
improvement in disease was represented by either: a change of VTT from the main renal vein to branches of the renal vein (on the right); or a change of VTT from main renal vein to the renal
vein lateral to the gonadal vein (on the left), or if the VTT was lateral to the gonadal vein at screening, a change from the main renal vein lateral to the gonadal vein to the branches of
the renal vein. This response designation for RV-only patients was developed as such changes would enable minimally invasive surgery to be undertaken. The number and percentage of patients
with no change in VTT status or extension of the VTT into the inferior vena cava between screening and week 9 was recorded. METHOD OF CALCULATING SECONDARY ENDPOINT OF PERCENTAGE CHANGE IN
VTT LENGTH Percentage change in VTT length was calculated using the following methodology for each timepoint as follows: * 1. Calculate the sum of (i) length of RV thrombus; (ii) the length
of IVC tumour thrombus ABOVE RV (measured from midpoint of the ostium of RV + IVC to tip of tumour thrombus); (iii) the length of IVC tumour thrombus BELOW RV (measured from midpoint of the
ostium of RV + IVC to the tip of tumour thrombus) at timepoint T. Note that in RV-only patients only distance (i) is measurable; * 2. Calculate the percentage reduction at each timepoint T
as follows: 1-(_Sum__T_/_Sum_0), where _Sum__T_ is the sum calculated as in Step 1 for timepoint T, and _Sum_0 is the sum calculated as in Step 1 at baseline. METHOD OF CALCULATING SECONDARY
ENDPOINT OF PERCENTAGE CHANGE IN SURGICAL APPROACH Percentage change in surgical approach was determined by comparing the surgeon-reported planned vs performed surgical approaches using
three pieces of data: * 1. Change from “Open Surgery” to “Minimally invasive surgery”; * 2. Change from a more invasive open to a less invasive open surgical approach (between that planned
by surgeons based on the baseline MRI scan and that actually performed); * 3. Less extensive surgical incision used. STATISTICAL PLAN A Simon two-stage minimax design [17] was used to
distinguish a ≤5% from a ≥25% cohort improvement in the Mayo level this required 20 evaluable patients (90% power, 10% one-sided). For the clinical trial to be considered a success, at least
three evaluable patients would demonstrate an improvement in disease on treatment between screening and week 9. In the two-stage design, 13 patients were to be recruited in the first stage.
If no patients demonstrated an improvement in their Mayo level between screening and week 9, accrual to the clinical trial would stop. If one or more patients demonstrated an improvement in
the Mayo level between screening and week 9, the final seven patients would be recruited. The intention-to-treat (ITT) population included all patients registered in the study. The
evaluable and safety populations included all patients in the ITT population who received at least one dose of the study drug (including any patients who were enrolled in error, received the
study drug and/or were subsequently found to be ineligible). In all, 80% two-sided confidence intervals (to correspond to the 10% one-sided sample size calculation) for the proportions
relevant to the efficacy endpoints were calculated using the approach of Koyama and Chen [18]. All analyses were carried out using R v3.5.1 and reporting was heavily supported by the CTutils
package (https://github.com/LisaHopcroft/CTutils). The trial data upload to EudraCT was enabled, in part, by the EudraCT package (https://eudract-tool.medschl.cam.ac.uk/). BIOSAMPLING
Blood, urine and tissue (fresh frozen and formalin-fixed paraffin-embedded (FFPE)) samples for translational studies were taken prior to, during and after therapy to evaluate biomarkers of
treatment response according to the Schedule of Assessments in the Protocol; see Appendix). Samples were processed and stored according to the NAXIVA Laboratory Manual (see Appendix).
IMMUNOFLUORESCENCE Formalin-fixed paraffin-embedded sections were dewaxed in xylene and rehydrated in graded alcohols prior to antigen retrieval in Tris-EDTA pH9. Slides were blocked and
incubated with primary antibodies at 4 °C overnight (CD31 (JC/70A, Abcam), Ki67 (EPR3610, Abcam), CD8 (SP16, Invitrogen), Granzyme B (NCL-L-GRAN-B, Leica), PD-1 (AF1086, RnD Systems), CD4
(EPR6855, Abcam), FOXP3 (236A/E7, Abcam), SMA (ab5694, Abcam), CD68 (KP1, Invitrogen)). Samples were washed and incubated in fluorescently conjugated secondary antibodies; nuclei were
counterstained with DAPI. Whole slides were scanned at ×40 magnification on the Zeiss Axio Scan Z1 system. Image analysis was performed using HALO Software (Indica Labs, analysis algorithms:
HighPlex FL v3.1.0, Object Colocalization FL v1.0, Area Quantification FL v2.1.5). CTDNA ANALYSIS ctDNA analysis was carried out as published previously [19]. Briefly, cell-free DNA was
extracted from blood and urine using the QIASymphony platform (QIAGEN). Libraries were prepared from DNA using the Thruplex Tag-Seq protocol (Takara) and sequenced on the Illumina HIseq4000
platform. Sequence data were analysed using an ‘in-house’ pipeline that consists of the following: paired-end sequence reads were aligned to the human reference genome (GRCh37) after
removing any contaminant adapter sequences. Duplicate reads or reads of with low mapping quality/secondary alignments were excluded from downstream analysis. Data were analysed with the
ichorCNA algorithm, version 0.2.0, using default parameters [20]. Samples were deemed to have ‘detected ctDNA’ if the predicted tumour fraction score was >0.025, and visual inspection of
copy number plots confirmed somatic copy number aberrations. RESULTS PATIENT CHARACTERISTICS Figure 1b and Table 1 detail patients recruited between December 2017 and January 2020. In total,
21 participants at five centres made up the evaluable population. On central review of imaging, one of the 21 patients was found not to have a VTT, making 20 patients who were both eligible
and evaluable and in whom the study endpoints are reported. PRIMARY ENDPOINT-REDUCTION IN MAYO LEVEL Of the 20 eligible and evaluable patients, 37.5% (6/16) IVC VTT patients had a reduction
in Mayo level and 25% (1/4) patients with RV-only VTT responded (Fig. 2). Hence, the overall response rate in evaluable and eligible patients with VTT was 35.0% (7/20). The remaining 13
patients had a stable Mayo level (65%), and none had an increase in Mayo level. By the inference procedures for Simon's two-stage minimax design there was a response rate of 32.8% [80%
CI 20.7%, 46.7%]. This was a statistically significant result (_P_ = 3.395 × 10−5), where the null hypothesis that the true response rate is <5% can be rejected in favour of the
alternative hypothesis of a 'good' (>25%) response. SECONDARY ENDPOINT-PERCENTAGE CHANGE IN VENOUS TUMOUR THROMBUS LENGTH Although 65% (13/20) patients had a stable Mayo level
(Fig. 2; classed as ‘non-responders’), seven of these 13 patients had a percentage reduction in the VTT length after 8 weeks of axitinib, therefore 15 of 20 patients (75%) had any degree of
reduction in VTT length (range 2–51%) (Fig. 3). One patient (5%) had no change in VTT length. At week 3, four patients (20%) had an increase in VTT length, two had surgery expedited as
detailed below. For all patients, the direction of change in VTT on the week 3 safety MRI was predictive of the response at 9 weeks (Figs. 2 and 3). There was a 15.2% (range −41% to 41%;
negative numbers indicating an increase in length) and 27.2% (range −20% to 51%) median reduction in VTT length at weeks 3 and 9, respectively. ABSOLUTE CHANGES IN VTT LENGTH The percentage
change in VTT length, equated to an absolute median reduction in VTT length at weeks 3 and 9 of 10 mm (range −12 to 56 mm) and 20 mm (range −34 to 68 mm), respectively. In four patients who
had an increase in length of VTT at 3 weeks, increases were 1 mm, 9 mm, 11 mm and 12 mm and at 9 weeks for the two patients with an increase in VTT these were 8 mm and 34 mm. IVC VTT was
identified and measured both above and below the ostium with the RV in 14 of 16 patients with IVC VTT (Supplementary Fig. S2). Changes in IVC VTT length on axitinib below the RV ostium
trended with the changes of VTT above the RV ostium. SECONDARY ENDPOINT-RECIST RESPONSE At week 3, one patient (5% of those having scan) had a RECIST-defined partial response (PR), 19
patients (95%) had stable disease (SD) and data were missing for one patient (N0601) who failed to attend the MRI (Supplementary Table S1). By week 9, 3 patients (16.7%) had a PR, 13 (72.2%)
had SD, 2 (11.1%) had PD, and data were missing for three patients as they had exited the trial. None of the M0 patients became M1 during the trial. At week 9, 7 of 17 patients (41.2%) had
a PR in their VTT (i.e. >30% reduction in length) (Fig. 3b, c). SECONDARY ENDPOINT-SURGICAL APPROACH In total, 17 patients underwent surgery. Despite an inclusion criterion for NAXIVA
being suitability for surgery, four patients did not have surgery (19.0%; three M1 and one M0). Of the M1 patients, reasons for not having surgery were the progression of metastatic disease
despite axitinib (_n_ = 2) and partial response but a general performance status decline resulting in becoming unfit for surgery (_n_ = 1). One M0 patient had stable disease at week 9 but
declined surgery. Surgery was brought forward in two patients from the planned surgery date of week 9. One patient stopped drug after 16 days and another after 33 days. Improvement in the
‘level of control’ of IVC/renal vein was observed in five out of 17 (29.4%) patients (Supplementary Table S2). No patients had deterioration in ‘level of control’ of IVC/renal vein performed
relative to that planned. Two patients had a change of approach from planned open to performed minimally invasive surgery (one also had an improved, lower venous ‘level of control’). One
additional patient had a substantially smaller incision (planned thoracoabdominal & midline laparotomy to perform subcostal and midline laparotomy). Therefore, 7/17 (41.1%) patients had
a less extensive surgery performed than was planned prior to axitinib treatment. Four Mayo ‘responders’ also had a reduction in extent of surgery. In 16 patients, the VTT tissue was
macroscopically cleared. PLANNED AND PERFORMED SURGERY Supplementary Tables S2 and S3 detail the planned and performed surgery in terms of correlation between Mayo change and change in
surgery. Four Mayo ‘responders’ also had a reduction in the extent of surgery (N0205, N0101, N0105 and N0201). Two Mayo responders did not have change in surgery (N0905 and N0606); these
were both reduction from level 2 to level 1 and for both the surgeon predicted and performed ‘Infrahepatic (IVC clamping with no liver mobilisation)’. Cardiac surgery and performing a
Pringle manoeuvre are both morbid and in NAXIVA two patients (N0101 and N0205) had supradiaphragmatic surgery and/or hypothermic cardiac arrest predicted and both had a reduction to
infradiaphragmatic surgery performed (N0205 to Retrohepatic (liver mobilisation and clamping below hepatic veins; N0101 to Suprahepatic (infradiaphragmatic)). There were no
suprahepatic/infradiaphragmatic cases predicted at baseline. One patient was planned to have a venovenous bypass and one to have hypothermic cardiac arrest but following treatment, neither
of these manoeuvrers was needed. In terms of patients with infrahepatic (IVC clamping with no liver mobilisation) planned at baseline, two (N0201 and N0904) actually had thrombus milked back
into the renal vein and side clamping performed. A further three patients had improvement in surgery but no change in Mayo level (N0103, N0904 and N0901). One patient with a Mayo response
did not have surgery as described above (N0801). INTRA- AND POST-OPERATIVE DETAILS AND COMPLICATIONS Median operation time was 240 min (range 120–720 min). Median estimated blood loss was
1000 ml (range 50–7000 ml). Six patients had an intraoperative complication, five related to bleeding with two patients requiring a transfusion and one patient had an intraoperative
cerebrovascular accident (CVA; identified post-operatively). Six patients had a post-operative complication of any grade (35.3%). Four complications were Clavien–Dindo 1/2 (expected CPAP
post operation, persistent wound pain, one chest and one wound infection) and two were Grade 3 or above (11.8%). Poor wound healing is a concern during VEGFR TKI use, but all patients had
discontinued axitinib prior to surgery and no issues of wound healing were reported. One patient had a cardiorespiratory arrest requiring one round of CPR to resuscitate (IVa), another had a
CVA intraoperatively and died (V) (1/17 = 5.9% mortality rate). None of these events was considered to have been caused by axitinib. Seven patients had planned or unplanned ITU admissions
post-operatively (41.2%). No patients had a delayed surgical complication at 6 or 12-weeks post-surgery follow-up. AXITINIB DOSE DELIVERED AND DURATION OF THERAPY Supplementary Fig. S3a
illustrates the axitinib dose received per patient. Axitinib dose was escalated in 12 of 21 patients (57%), two patients (9.5%) required dose reduction from the 5 mg b.d. starting dose. The
median daily dose received (excluding breaks) was 5.8 mg b.d. (range 3.1–8.0 mg b.d.). The total dose of axitinib was not significantly different between patients with or without a
Mayo-level response (_P_ = 0.405). However, patients who did not have an improvement in Mayo level or a RECIST response received a significantly lower total dose of axitinib (_P_ = 0.030)
(Supplementary Fig. S3b) and had a shorter duration of axitinib treatment (excluding breaks) compared to patients who had a Mayo-level improvement (_P_ = 0.026) (Supplementary Fig. S3c) or
had either a Mayo or a RECIST response (_P_ = 0.007) (Supplementary Fig. S3d). There was no correlation between the total dose of axitinib and VTT reduction at week 9 (Pearson’s _r_(16) =
0.07, _P_ = 0.78). ADVERSE EVENTS (AES) Serious AEs whilst on axitinib were myasthenia gravis (recovered following nephrectomy, not after stopping axitinib), pathological fracture,
hyperglycaemia, left cerebellar mass development, wound pain, confusion and hyperkalaemia. None were judged by local investigators to be related to axitinib. Table 2 and Supplementary Fig.
S4 detail AEs related to axitinib by CTCAEv4 grade. AEs were consistent with previous data and did not delay surgery. No grade 4 or 5 AEs were observed. Correlations with clinical features
are detailed in supplementary results. Patients with either a Mayo-level response (_P_ = 0.0034) and/or those with a RECIST response (_P_ = 0.0003) had significantly lower maximum levels of
proteinuria during treatment than non-responders (range 0–1 in responders vs 0–3 in non-responders). Baseline proteinuria was not significantly different between responders and
non-responders (_P_ = >0.05). Neither mean baseline systolic or diastolic blood pressure (BP), change in systolic or diastolic BP during treatment, nor maximum systolic or diastolic BP
reached during treatment correlated with Mayo response (_P_ = >0.05). TRANSLATIONAL ANALYSES Baseline biopsies, available from 17 patients, were assessed for the presence of markers
associated with treatment outcome in ccRCC [21,22,23]. There was a trend for higher CD31 microvessel density in responders (Fig. 4a, c) and higher Ki67 index in non-responders (Fig. 4b, d).
Non-responders exhibited trends toward higher T-cell infiltration but populations shifted towards exhausted (PD-1 + ) or regulatory (FOXP3 + ) phenotypes compared to an activated (PD-1-
granzyme B + ) phenotype in responders (CD8 + cells: Fig. 4e–h; CD4 + cells: Supplementary Fig. S5a–c). No differences were observed in other stromal markers (Supplementary Fig. S5d, e).
Consistent with previous studies showing low detection of ctDNA in RCC, only 25% (5/20) of patients (two in plasma, three in urine) had detectable ctDNA at baseline. There was no concordance
in the levels or composition of ctDNA between the plasma and urine. Only 20% (1/5) of patients with detectable ctDNA at baseline showed an improvement in Mayo level or RECIST response.
DISCUSSION NAXIVA is the first prospective study to evaluate drug treatment in managing RCC VTT, a frequently discussed question in clinical practice. The trial met its primary and secondary
endpoints demonstrating that it is feasible to use systemic therapy to downstage VTT of all Mayo levels and reduce the extent of surgery in patients with resectable M0 and M1 ccRCC.
Importantly, axitinib and surgical toxicity, morbidity and mortality were as expected [2] and no patient had clinically relevant VTT progression. Ordinarily, surgery for patients with VTT
would be expedited because of concern about disease progression and metastasis. In NAXIVA no participants progressed from non-metastatic to metastatic disease. Two patients did not proceed
to surgery due to the progression of their known metastatic disease, suggesting that, consistent with results from the SURTIME trial [24], pre-surgical systemic therapy in M1 ccRCC may allow
time for very aggressive disease to declare itself and ultimately enable patients to avoid inappropriate surgery. Reassuringly, the patterns of eventual VTT response at week 9 were mirrored
on the 3-week safety MRI scan (originally included to ensure that any patient with clinically relevant progression could undergo surgery immediately); indeed, two patients had surgery
expedited following a 3-week scan showing extension of VTT. If confirmed in future studies, this suggests that scans performed early during treatment could be a useful strategy as both a
response prediction and reassuring safety feature for neoadjuvant systemic therapy [25, 26]. A shorter duration of neoadjuvant treatment may also be possible for an adequate response.
Patients with M0 and M1 disease and all levels of VTT, from those within the RV only to those with VTT extending to the right atrium were included in NAXIVA because all were hypothesised to
benefit from a reduction in VTT extent if axitinib treatment reduced the extent of surgery and the associated surgical morbidity. The broad inclusion criteria in a small feasibility study
limits firm conclusions on each subgroup, but conversely allowed signal seeking from each stage of the disease which informs future trials. The positive results showing significant
reductions in VTT length (regardless of M0 or M1 status) are clinically relevant as they are linked to subsequent changes in surgical approach in 7/17 patients (41.1%). Importantly, axitinib
treatment resulted in less extensive surgery such as avoidance of open nephrectomy in favour of laparoscopic/robotic procedures, and reduced requirement for intrathoracic approaches,
cardiac bypass or Pringle manoeuvre which are associated with significant morbidity [2]. Conversely, reduction from Level 2 to Level 1 VTT appear less significant in changing the surgery
undertaken, while the patient is still exposed to drug toxicity. The Mayo levels at which downstaging of VTT make most clinical difference are levels 0, 1, 3 and 4, although further
investigation would be prudent given the relatively small numbers of such patients investigated within NAXIVA. Although no unexpected perioperative complications were reported, future
studies should specifically measure this using the EAU Intraoperative Adverse Incident Classification (EAUiaiC) [27]. In NAXIVA, axitinib was used, a potent TKI, with an established
aggressive dose escalation regime which has previously been demonstrated to have proven effect in non-metastatic and metastatic ccRCC [15]. After 8 weeks of axitinib, 16.7% patients had a
partial response (10% in M0 patients). This compares with 45.8% in the Phase 2 trial of Karam et al. where axitinib treatment was given for 12 weeks. This suggests that a longer period of
treatment is needed for deeper response, although by 9 weeks 41.1% of patients had >30% response in the VTT, downstaging of which was the aim of NAXIVA, suggesting this was an adequate
treatment duration to assess the endpoints of this trial. Interestingly, results from NAXIVA are superior to previous retrospective studies, 37.5% vs 14.9–32.9% reduction in Mayo Levels 1–4
[5,6,7,8,9,10,11,12]. Despite permissive product labels in advanced disease, VEGFR TKIs do appear less active in non-ccRCC [28], and we caution against extrapolation of the findings of
NAXIVA to patients in whom there is not pre-treatment histological proof of ccRCC. An important question is whether baseline information or that obtained early during treatment can be used
to select patients that may benefit, or not, from a period of neoadjuvant treatment. Previous studies have identified a number of molecular, genetic and other factors correlating with
response to TKI [29]. We saw similar trends in predictive markers of angiogenesis, immune infiltrate and proliferation to those seen in large scale published datasets [21, 23]. We
reconfirmed ctDNA is challenging to detect in RCC [19] and our finding that detectable ctDNA at baseline generally predicts poor response to axitinib may be clinically relevant and warrants
investigation in larger cohorts. Additionally, although previous studies have shown that TKI-related AEs may correlate with response [30], we showed that non-responders received a
significantly lower total dose of axitinib and had a shorter duration of treatment, with responders having significantly lower maximum levels of proteinuria during treatment than
non-responders. This highlights the importance of active management of TKI-related AEs during neoadjuvant treatment to ensure patients remain on drug to enable effective tumour control. A
limitation of NAXIVA is that axitinib is now used in combination with immunotherapy in the first-line metastatic setting, and only used as a single agent in subsequent lines of treatment.
Coupled with our finding that the immune profile in non-responders is consistent with an exhausted and regulatory T-cell phenotype suggests future trials should evaluate combinations such as
IO-TKI where there is potential to improve the response rate in patients unlikely to respond to TKI alone, and enable both rapid downstaging with the TKI component and immune priming which
could have longer-term survival implications [31,32,33]. However, we hypothesise that the downstaging effect may not be significantly greater with an IO-TKI combination compared with TKI
alone. In the Neoavax neoadjuvant study of 12 weeks of axitinib/avelumab, there was a 30% PR, compared with 43% in the 12-week axitinib neoadjuvant protocol of Karam et al. [15, 34]. In
addition, none of 17 patients treated with three every-2-week doses of neoadjuvant nivolumab had a PR [35]. Future randomised studies should explore the impact on overall survival,
differences in the extent of surgery and optimisation of treatment schedule and duration. In conclusion, the results from NAXIVA showed the feasibility that systemic therapy, such as
axitinib, can be used to downstage RCC VTT in 35% of patients and reduce the extent of surgery to a less morbid option in 41%. As newer combination therapies are associated with higher
response rates in advanced ccRCC, the study of these combinations in patients with operable locally advanced disease should now be prioritised. DATA AVAILABILITY The datasets generated and
analysed during NAXIVA are available from the corresponding author following assessment of a brief research proposal which will form the basis of a data-sharing agreement. All reasonable
requests will be granted. REFERENCES * Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol. 2007;52:658–62. Article PubMed Google
Scholar * Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and
venous tumour thrombus. BJU Int. 2004;94:33–41. Article PubMed Google Scholar * Parra J, Drouin SJ, Hupertan V, Comperat E, Bitker MO, Rouprêt M. Oncological outcomes in patients
undergoing radical nephrectomy and vena cava thrombectomy for renal cell carcinoma with venous extension: a single-centre experience. Eur J Surg Oncol. 2011;37:422–8. Article CAS PubMed
Google Scholar * Lambert EH, Pierorazio PM, Shabsigh A, Olsson CA, Benson MC, McKiernan JM. Prognostic risk stratification and clinical outcomes in patients undergoing surgical treatment
for renal cell carcinoma with vascular tumor thrombus. Urology. 2007;69:1054–8. Article PubMed Google Scholar * Cost NG, Delacroix SE, Sleeper JP, Smith PJ, Youssef RF, Chapin BF, et al.
The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59:912–8. Article CAS PubMed Google Scholar * Bigot P, Fardoun
T, Bernhard JC, Xylinas E, Berger J, Rouprêt M, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J
Urol. 2014;32:109–14. Article CAS PubMed Google Scholar * Peng C, Gu L, Wang L, Huang Q, Wang B, Guo G, et al. Role of presurgical targeted molecular therapy in renal cell carcinoma with
an inferior vena cava tumor thrombus. Onco Targets Ther. 2018;11:1997–2005. Article PubMed PubMed Central Google Scholar * Fukuda H, Kondo T, Takagi T, Iizuka J, Nagashima Y, Tanabe K.
Limited benefit of targeted molecular therapy for inferior vena cava thrombus associated with renal cell carcinoma. Int J Clin Oncol. 2017;22:767–73. Article CAS PubMed Google Scholar *
Ujike T, Uemura M, Kawashima A, Nagahara A, Fujita K, Miyagawa Y, et al. Clinical and histopathological effects of presurgical treatment with sunitinib for renal cell carcinoma with inferior
vena cava tumor thrombus at a single institution. Anticancer Drugs. 2016;27:1038–43. Article CAS PubMed PubMed Central Google Scholar * Horn T, Thalgott MK, Maurer T, Hauner K, Schulz
S, Fingerle A, et al. Presurgical treatment with sunitinib for renal cell carcinoma with a level III/IV vena cava tumour thrombus. Anticancer Res. 2012;32:1729–35. CAS PubMed Google
Scholar * Tanaka Y, Hatakeyama S, Hosogoe S, Tanaka T, Hamano I, Kusaka A, et al. Presurgical axitinib therapy increases fibrotic reactions within tumor thrombus in renal cell carcinoma
with thrombus extending to the inferior vena cava. Int J Clin Oncol. 2018;23:134–41. Article CAS PubMed Google Scholar * Terakawa T, Hussein AA, Bando Y, Guru KA, Furukawa J, Shigemura
K, et al. Presurgical pazopanib for renal cell carcinoma with inferior vena caval thrombus: a single-institution study. Anticancer Drugs. 2018;29:565–71. Article CAS PubMed Google Scholar
* Wood CG, Ferguson JE, Parker JS, Moore DT, Whisenant JG, Maygarden SJ, et al. Neoadjuvant pazopanib and molecular analysis of tissue response in renal cell carcinoma. JCI Insight.
2020;5:132852. Article PubMed Google Scholar * Rini BI, Plimack ER, Takagi T, Elson P, Wood LS, Dreicer R, et al. A Phase II study of pazopanib in patients with localized renal cell
carcinoma to optimize preservation of renal parenchyma. J Urol. 2015;194:297–303. Article CAS PubMed Google Scholar * Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al.
Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol. 2014;66:874–80. * Dindo D, Demartines N, Clavien P-A.
Classification of surgical complications. Ann Surg. 2004;240:205–13. Article PubMed PubMed Central Google Scholar * Simon R. Optimal two-stage designs for phase II clinical trials.
Control Clin Trials. 1989;10:1–10. Article CAS PubMed Google Scholar * Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–54. Article PubMed
PubMed Central Google Scholar * Smith CG, Moser T, Mouliere F, Field-Rayner J, Eldridge M, Riediger AL, et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of
patients with renal tumors. Genome Med. 2020;12:23. Article CAS PubMed PubMed Central Google Scholar * Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, et al.
Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8:1324. Article PubMed PubMed Central Google Scholar * Motzer RJ,
Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38:803–17.e4.
Article CAS PubMed PubMed Central Google Scholar * McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to
atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–57. Article CAS PubMed PubMed Central Google Scholar * Motzer RJ,
Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101
trial. Nat Med 2020;26:1733–41. Article CAS PubMed PubMed Central Google Scholar * Bex A, Mulders P, Jewett M, Wagstaff J, van Thienen JV, Blank CU, et al. Comparison of immediate vs
deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5:164–70. Article
PubMed Google Scholar * Welsh SJ, Thompson N, Warren A, Priest AN, Barrett T, Ursprung S, et al. Dynamic biomarker and imaging changes from a phase II study of pre- and post-surgical
sunitinib. BJU Int. 2021. https://doi.org/10.1111/bju.15600. Online ahead of print. * Ursprung S, Priest AN, Zaccagna F, Qian W, Machin A, Stewart GD, et al. Multiparametric MRI for
assessment of early response to neoadjuvant sunitinib in renal cell carcinoma. PLoS ONE. 2021;16:e0258988. Article CAS PubMed PubMed Central Google Scholar * Biyani CS, Pecanka J,
Rouprêt M, Jensen JB, Mitropoulos D. Intraoperative adverse incident classification (EAUiaiC) by the European association of urology ad hoc complications guidelines panel. Eur Urol.
2020;77:601–10. Article PubMed Google Scholar * Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Final results from the large sunitinib global expanded-access
trial in metastatic renal cell carcinoma. Br J Cancer. 2015;113:12–9. Article CAS PubMed PubMed Central Google Scholar * Sharma R, Kadife E, Myers M, Kannourakis G, Prithviraj P, Ahmed
N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res. 2021;40:186. Article PubMed PubMed Central Google
Scholar * Gadd M, Pranavan G, Malik L. Association between tyrosine-kinase inhibitor induced hypertension and treatment outcomes in metastatic renal cancer. Cancer Rep. 2020;3:e1275. *
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl J Med. 2021;384:829–41
Article CAS PubMed PubMed Central Google Scholar * Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell
carcinoma. N Engl J Med. 2021;384:1289–300. Article CAS PubMed Google Scholar * Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus
sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. Article CAS PubMed Google Scholar * Dynamic changes of the immune infiltrate after neoadjuvant
avelumab/axitinib in patients (pts) with localized renal cell carcinoma (RCC) who are at high risk of relapse after nephrectomy (NeoAvAx). J Clin Oncol. 2022.
https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.4573. * Gorin MA, Patel HD, Rowe SP, Hahn NM, Hammers HJ, Pons A, et al. Neoadjuvant nivolumab in patients with high-risk
nonmetastatic renal cell carcinoma. Eur Urol Oncol. 2021. https://www.sciencedirect.com/science/article/pii/S2588931121000766. * Stewart GD, Welsh SJ, Ursprung S, Gallagher F, Mendichovszky
I, Riddick A, et al. NAXIVA: a phase II neoadjuvant study of axitinib for reducing extent of venous tumor thrombus in clear cell renal cell cancer (RCC) with venous invasion. JCO.
2021;39:275–275. Article Google Scholar * Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:390–5. Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to The Renal Cross Channel Group and especially Jean-Jacque Patard and Bernard Escudier for the initial inspiration for developing
NAXIVA. Thanks to James Paul CRUK Glasgow Clinical Trials Unit for advice on trial design. We are grateful to the excellent assistance from the NAXIVA TSC and in particular the IDMC (Jose
Karam, Paul Nathan, Steve Bromage and Elaine McCarthy). GDS and AYW are supported by The Mark Foundation for Cancer Research, the Cancer Research UK Cambridge Centre [C9685/A25177] and NIHR
Cambridge Biomedical Research Centre (BRC-1215-20014). The Cambridge Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre. We acknowledge the support of
the National Institute for Health Research Clinical Research Network (NIHR CRN). Plasma and urine ctDNA and tumour DNA extractions, sequencing and bioinformatics analysis were performed by
the Cancer Molecular Diagnostics Laboratory and Blood Processing Laboratory, which is supported by Cambridge NIHR Biomedical Research Centre, Cambridge Cancer Centre and the Mark Foundation
of Cancer Research. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Funding and Medicine for this
Investigator Sponsored Research study were provided by Pfizer Ltd. NAXIVA was presented at ASCO GU, and the abstract was published [36]. FUNDING NAXIVA was endorsed by Cancer Research UK
(A23471). We acknowledge the support of the National Institute for Health Research Clinical Research Network (NIHR CRN). Funding and Medicine for this Investigator Sponsored Research study
was provided by Pfizer Ltd. GDS and AYW are supported by The Mark Foundation for Cancer Research, the Cancer Research UK Cambridge Centre (C9685/A25177) and NIHR Cambridge Biomedical
Research Centre (BRC-1215-20014). The Cambridge Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre. AUTHOR INFORMATION Author notes * Jacqui Shields
Present address: School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK * Lisa E. M. Hopcroft Present address: Nuffield Department of Primary Care Health Sciences,
University of Oxford, Oxford, UK * Unaffiliated: Alison Fielding. AUTHORS AND AFFILIATIONS * University of Cambridge, Cambridge, UK Grant D. Stewart, Sarah J. Welsh, Stephan Ursprung, Ferdia
A. Gallagher, James O. Jones, Thomas J. Mitchell, Anne Y. Warren, Tim Eisen, Iosif A. Mendichovszky, Andrew N. Priest, Lauren Wallis, Ruby Cross, Sarah W. Burge, Anne George, Tobias Klatte,
Sabrina Rossi, Charlie Massie, Shubha Anand, Tiffany Haddow, Marc Dodd, Wenhan Deng, Ezequiel Martin, Philip Howden, Stephanie Wenlock & Evis Sala * Cambridge University Hospitals NHS
Foundation Trust, Cambridge, UK Grant D. Stewart, Sarah J. Welsh, Ferdia A. Gallagher, James O. Jones, Thomas J. Mitchell, Anne Y. Warren, Tim Eisen, Kate Fife, Iosif A. Mendichovszky,
Andrew N. Priest, Antony C. P. Riddick, Athena Matakidou, Cara Caasi, James Watson, Tevita F. Aho, James N. Armitage, Sabrina Rossi & Evis Sala * MRC Cancer Unit, University of
Cambridge, Cambridge, UK James O. Jones & Jacqui Shields * CRUK Cambridge Institute, Cambridge, UK Christopher G. Smith * Wellcome Sanger Institute, Cambridge, UK Thomas J. Mitchell *
Royal Free London NHS Foundation Trust, London, UK Axel Bex, Ekaterini Boleti, Faiz Mumtaz, Thomas Powles, Anna Pejnovic, Sophia Tincey & Lee Grant * Scottish Clinical Trials Research
Unit, Public Health Scotland, Edinburgh, UK Jade Carruthers, Michelle Welsh, Kathleen Riddle, Lisa E. M. Hopcroft, Niki Couper, Lisa E. M. Hopcroft & Robert Hill * Broomfield Hospital,
Chelmsford, UK Abdel Hamid, Martin Nuttall, Lucy Willsher & Christian Barnett * Western General Hospital, Edinburgh, UK Alexander Laird, Steve Leung, Jahangeer Malik, Stefan Symeonides,
Lynn Ho, Jennifer Baxter, Stuart Leslie, Duncan McLaren, John Brush & Marie O’Donnell * Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK Alexander Laird, Stefan
Symeonides & Duncan McLaren * NHS Greater Glasgow and Clyde, Glasgow, UK Grenville Oades, Balaji Venugopal, Alisa Griffin, Ruth Orr, Catriona Cowan & Robert J. Jones * University of
Glasgow, Glasgow, UK Balaji Venugopal & Robert J. Jones * Royal Marsden Hospital, London, UK David Nicol & James Larkin Authors * Grant D. Stewart View author publications You can
also search for this author inPubMed Google Scholar * Sarah J. Welsh View author publications You can also search for this author inPubMed Google Scholar * Stephan Ursprung View author
publications You can also search for this author inPubMed Google Scholar * Ferdia A. Gallagher View author publications You can also search for this author inPubMed Google Scholar * James O.
Jones View author publications You can also search for this author inPubMed Google Scholar * Jacqui Shields View author publications You can also search for this author inPubMed Google
Scholar * Christopher G. Smith View author publications You can also search for this author inPubMed Google Scholar * Thomas J. Mitchell View author publications You can also search for this
author inPubMed Google Scholar * Anne Y. Warren View author publications You can also search for this author inPubMed Google Scholar * Axel Bex View author publications You can also search
for this author inPubMed Google Scholar * Ekaterini Boleti View author publications You can also search for this author inPubMed Google Scholar * Jade Carruthers View author publications You
can also search for this author inPubMed Google Scholar * Tim Eisen View author publications You can also search for this author inPubMed Google Scholar * Kate Fife View author publications
You can also search for this author inPubMed Google Scholar * Abdel Hamid View author publications You can also search for this author inPubMed Google Scholar * Alexander Laird View author
publications You can also search for this author inPubMed Google Scholar * Steve Leung View author publications You can also search for this author inPubMed Google Scholar * Jahangeer Malik
View author publications You can also search for this author inPubMed Google Scholar * Iosif A. Mendichovszky View author publications You can also search for this author inPubMed Google
Scholar * Faiz Mumtaz View author publications You can also search for this author inPubMed Google Scholar * Grenville Oades View author publications You can also search for this author
inPubMed Google Scholar * Andrew N. Priest View author publications You can also search for this author inPubMed Google Scholar * Antony C. P. Riddick View author publications You can also
search for this author inPubMed Google Scholar * Balaji Venugopal View author publications You can also search for this author inPubMed Google Scholar * Michelle Welsh View author
publications You can also search for this author inPubMed Google Scholar * Kathleen Riddle View author publications You can also search for this author inPubMed Google Scholar * Lisa E. M.
Hopcroft View author publications You can also search for this author inPubMed Google Scholar * Robert J. Jones View author publications You can also search for this author inPubMed Google
Scholar CONSORTIA NAXIVA TRIAL GROUP * Grant D. Stewart * , Niki Couper * , Kathleen Riddle * , Michelle Welsh * , Jade Carruthers * , Lisa E. M. Hopcroft * , Robert Hill * , Sarah J. Welsh
* , Kate Fife * , Athena Matakidou * , Tim Eisen * , Cara Caasi * , James Watson * , Lauren Wallis * , Ruby Cross * , Sarah W. Burge * , Anne George * , Iosif A. Mendichovszky * , Ferdia A.
Gallagher * , Tobias Klatte * , Stephan Ursprung * , Anne Y. Warren * , Thomas J. Mitchell * , Tevita F. Aho * , Antony C. P. Riddick * , James N. Armitage * , Sabrina Rossi * , Charlie
Massie * , Shubha Anand * , Tiffany Haddow * , Marc Dodd * , Wenhan Deng * , Ezequiel Martin * , Philip Howden * , Stephanie Wenlock * , Evis Sala * , Steve Leung * , Alexander Laird * ,
Jahangeer Malik * , Stefan Symeonides * , Lynn Ho * , Jennifer Baxter * , Stuart Leslie * , Duncan McLaren * , John Brush * , Marie O’Donnell * , Grenville Oades * , Balaji Venugopal * ,
Robert J. Jones * , Alisa Griffin * , Ruth Orr * , Catriona Cowan * , Faiz Mumtaz * , Axel Bex * , Ekaterini Boleti * , Thomas Powles * , Anna Pejnovic * , Sophia Tincey * , Lee Grant * ,
Abdel Hamid * , Martin Nuttall * , Lucy Willsher * , Christian Barnett * , David Nicol * , James Larkin * & Alison Fielding CONTRIBUTIONS Conception and design: GDS and RJJ. Acquisition
of the data: GDS, SJW, SU, FAG, RJJ, JS, CGS, TJM, AYW, AB, EB, TE, KF, AH, AL, SL, JM, IAM, FM, GO, ACPR, BV and RRJ. Analysis and interpretation of the data: GDS, SJW, SU, FAG, JOJ, JS,
CGS, TJM, AYW, AB, EB, TE, KF, AH, AL, SL, JM, IAM, FM, GO, ACPR, BV, MW, KR, LEMH and JOJ. Drafting of the manuscript: GDS, SJW and RJJ. Review and revision of the manuscript: GDS, SJW, SU,
FAG, RJJ, JS, CGS, TJM, AYW, AB, EB, TE, KF, AH, AL, SL, JM, IAM, FM, GO, ACPR, BV, MW, KR, LEMH and RJJ. Statistical analysis: LEMH and JC. Funding: GDS. Administrative support: GDS.
Supervision: GDS. CORRESPONDING AUTHOR Correspondence to Grant D. Stewart. ETHICS DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE Ethics approval was granted by the East of
England—Cambridgeshire and Hertfordshire Research Ethics Committee (REC reference: 17/EE/0240). All patients were consented to following GCP principles. The study was performed in accordance
with the Declaration of Helsinki. CONSENT TO PUBLISH Consent for publication of imaging (Supplementary Fig. S1) is provided via a trial consent form. COMPETING INTERESTS GDS—educational
grants from Pfizer, AstraZeneca and Intuitive Surgical; consultancy fees from Pfizer, Merck, EUSA Pharma and CMR Surgical; Travel expenses from Pfizer and Speaker fees from Pfizer.
TE—employment: Roche (current), AstraZeneca (to March 2020); stock: AstraZeneca, Roche; research support: AstraZeneca, Bayer, Pfizer. FAG—research support from GE Healthcare; Grants from
GSK; Consulting for AZ on behalf of the University of Cambridge. KF has received advisory, consultancy or speaker fees from ESAI, Ipsen, Roche, Novartis, Merck, Pfizer, EUSA Pharma, BMS, MSD
and Sanofi and conference support from Novartis, Ipsen, MSD and EUSA Pharma and Institutional research funding from Roche, Merck and Exelixis. AB received honoraria for participation in
advisory boards for Pfizer, Novartis, and Ipsen, and an educational grant from Pfizer. RJ has received educational grants from Astellas, Bayer, Clovis and Exelixis; consultancy fees from
Roche, AstraZeneca, Bristol Myers Squibb, Bayer, Novartis/AAA, Astellas, Janssen, MSD, Pfizer, Merck Serono; honoraria from Roche, AstraZeneca, Bristol Myers Squibb, Bayer, Astellas,
Janssen, MSD, Pfizer, Merck Serono; conference support from MSD and Bayer; advisory board payment from Roche. The remaining authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY METHODS
SUPPLEMENTARY FIGURES STUDY PROTOCOL SAMPLING HANDLING MANUAL SUBMISSION CHECKLIST RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Stewart, G.D., Welsh, S.J., Ursprung, S. _et al._ A Phase II study of neoadjuvant
axitinib for reducing the extent of venous tumour thrombus in clear cell renal cell cancer with venous invasion (NAXIVA). _Br J Cancer_ 127, 1051–1060 (2022).
https://doi.org/10.1038/s41416-022-01883-7 Download citation * Received: 14 March 2022 * Revised: 25 May 2022 * Accepted: 01 June 2022 * Published: 23 June 2022 * Issue Date: 05 October 2022
* DOI: https://doi.org/10.1038/s41416-022-01883-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative