Play all audios:
ABSTRACT In many human cancers the control of apoptosis is dysregulated, for instance as a result of the overexpression of pro-survival BCL-2 proteins. This promotes tumorigenesis by
protecting nascent neoplastic cells from stress and renders malignant cells resistant to anti-cancer agents. Therefore, several BH3 mimetic drugs targeting distinct pro-survival proteins
have been developed. The BCL-2 inhibitor Venetoclax/ABT-199, has been approved for treatment of certain blood cancers and tens of thousands of patients have already been treated effectively
with this drug. To advance the clinical development of MCL-1 and BCL-XL inhibitors, a more detailed understanding of their distinct and overlapping roles in the survival of malignant as well
as non-transformed cells in healthy tissues is required. Here, we discuss similarities and differences in pro-survival BCL-2 protein structure, subcellular localisation and binding
affinities to the pro-apoptotic BCL-2 family members. We summarise the findings from gene-targeting studies in mice to discuss the specific roles of distinct pro-survival BCL-2 family
members during embryogenesis and the survival of non-transformed cells in healthy tissues in adults. Finally, we elaborate how these findings align with or differ from the observations from
the clinical development and use of BH3 mimetic drugs targeting different pro-survival BCL-2 proteins. You have full access to this article via your institution. Download PDF SIMILAR CONTENT
BEING VIEWED BY OTHERS THE BCL-2 PROTEIN FAMILY: FROM DISCOVERY TO DRUG DEVELOPMENT Article Open access 09 April 2025 LYMPHOMA CELLS LACKING PRO-APOPTOTIC BAX ARE HIGHLY RESISTANT TO
BH3-MIMETICS TARGETING PRO-SURVIVAL MCL-1 BUT RETAIN SENSITIVITY TO CONVENTIONAL DNA-DAMAGING DRUGS Article Open access 08 February 2023 BREAST CANCER DEPENDENCE ON MCL-1 IS DUE TO ITS
CANONICAL ANTI-APOPTOTIC FUNCTION Article Open access 31 March 2021 FACTS * Pro-survival BCL-2 proteins have overlapping roles in securing the survival of certain cell types, whereas other
cell populations rely on the expression of a specific pro-survival BCL-2 family member. * Many cell types during embryogenesis as well as in the adult cannot tolerate the loss of the
pro-survival protein MCL-1. * On-target toxic effects of BH3 mimetic drugs targeting specific pro-survival BCL-2 proteins on healthy cells are generally less severe than the defects seen in
gene-targeted mice lacking these proteins. OPEN QUESTIONS * Why can the survival dependence of distinct cell types not be explained exclusively by the expression profile of the different
pro-survival BCL-2 proteins? * Why is MCL-1 critical for early embryogenesis and the survival of so many cell types? * Are there apoptosis-unrelated functions of pro-survival BCL-2 proteins,
in particular MCL-1, that are critical for the survival of distinct cell types? * Do the reported apoptosis unrelated functions of pro-survival proteins play a role in the efficacy and/or
on-target toxic effects of BH3 mimetic drugs? PRO-SURVIVAL BCL-2 PROTEINS—SAME, SAME BUT DIFFERENT Mammals have five pro-survival BCL-2 family members: BCL-2, BCL-XL, MCL-1, BCL-W and
A1(murine)/BFL-1(human). They regulate mitochondrial apoptosis through interactions with two sub-groups of pro-apoptotic BCL-2 family members [1,2,3]; the BH3-only proteins (PUMA, BIM, tBID,
NOXA, BMF, BIK, HRK, BAD) that are critical for apoptosis initiation as well as the multi-BH (BCL-2 homology) domain effectors of apoptosis, BAX and BAK [2]. The BAX/BAK-related
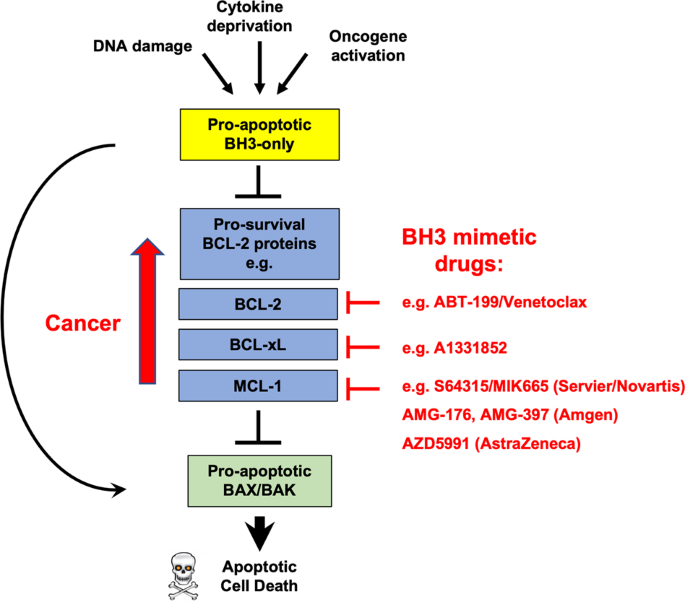
multi-BH-domain protein BOK is not regulated by pro-survival BCL-2 proteins [4, 5]. Apoptosis is initiated in response to diverse stresses, such as cytokine deprivation or oncogene
activation, resulting in the transcriptional and/or post-transcriptional upregulation of pro-apoptotic BH3-only proteins [6]. BH3-only proteins bind the pro-survival BCL-2 proteins with high
affinity and thereby neutralise them. This unleashes BAX and BAK from their restraint by the pro-survival BCL-2 proteins, allowing them to cause mitochondrial outer membrane
permeabilisation (MOMP) [2, 3]. Some BH3-only proteins can reportedly also directly activate BAX and BAK [7,8,9]. MOMP results in the release of apoptogenic factors (e.g., Cytochrome c) from
the mitochondrial inter-membrane space leading to the activation of the caspase cascade that causes the ordered dismantling of dying cells (Graphical Abstract) [3]. All pro-survival
proteins contain four BH domains and interact with both sub-groups of pro-apoptotic BCL-2 family members through their hydrophobic BH3 binding groove (Fig. 1A, B). Even though highly related
to each other, different pro-survival BCL-2 proteins have some distinct features. BCL-2, BCL-XL, MCL-1 and BCL-W have a C-terminal transmembrane (TM) domain that allows them to anchor to
different intracellular membranes (Fig. 1A). BCL-2 is found at the endoplasmic reticulum (ER), the mitochondrial outer membrane (MOM) and the nuclear envelope (NE) [10,11,12]. MCL-1 is found
at the ER, the MOM as well as the mitochondrial inter-membrane space, the NE and in the cytosol [13,14,15,16,17]. BCL-XL is located in the cytosol and at the MOM but has also been detected
at the NE and the ER [18,19,20]. BCL-W is cytosolic but can insert into the MOM in response to intracellular stress [21]. A1 lacks a TM domain and is therefore mainly found in the cytosol
but it is also detected at the MOM, the ER and the NE [22,23,24] (Fig. 2). MCL-1 has a long N-terminal stretch harbouring 4 putative PEST sequences, and this is in part responsible for its
short (20–30 min) half-life [25, 26]. In contrast, BCL-2, BCL-XL and BCL-W have half-lives of >20 h [27, 28]. A1/BFL-1 protein has a half-life of only 10 min [29] (Fig. 1A). The
pro-survival BCL-2 proteins differ in their binding to the apoptosis effectors BAX and BAK. All pro-survival BCL-2 proteins can restrain BAX, but only BCL-XL, MCL-1 and A1 also bind BAK [30,
31]. Furthermore, whereas all pro-survival BCL-2 proteins can bind to the BH3-only proteins BIM, PUMA and tBID, NOXA interacts only with MCL-1 and A1, while BAD selectively binds BCL-2,
BCL-XL and BCL-W [9, 30, 32] (Fig. 1B). THE ROLES OF THE DIFFERENT PRO-SURVIVAL BCL-2 PROTEINS IN EMBRYOGENESIS During the initial stages of embryogenesis (days 0–2) the expression of
proteins in the zygote is driven from maternal mRNA. Only after several cell divisions (6–8 cell state) around embryonic day 3 (E3) the embryonic genome is activated to allow mRNA and
protein synthesis [33]. Among the pro-survival BCL-2 proteins, the poorly studied BCL-B(human)/DIVA(murine) is the most prominently expressed at the mRNA level in oocytes and early embryos
[34]. However, loss of BCL-B/DIVA has no impact on oocytes or early embryos [35]. Of note, some reports indicate that BCL-B/DIVA lacks a BH3 domain and its function in regulating cell death
is therefore unclear [36,37,38]. Neither A1 nor BCL-W are expressed during early embryogenesis. MCL-1 and BCL-XL are highly expressed from both maternally inherited mRNA as well as embryonic
transcripts [39]. BCL-2 expression levels are low during early embryogenesis but increase after the late blastocyst stage (~E4.5). Here, we focus on the most relevant pro-survival BCL-2
family members during embryogenesis, MCL-1, BCL-XL and BCL-2. BCL-2 (Table 1): BCL-2-deficient mice are born but die soon after weaning due to polycystic kidney disease, a disorder that
commences during embryogenesis [40]. This demonstrates an essential role of BCL-2 for renal epithelial cell survival during embryogenesis. BCL-2 levels are high in the central nervous system
during embryogenesis but decline after neuronal tube formation [39]. In contrast, high levels of BCL-2 are maintained in the peripheral neuronal system [10, 41,42,43]. BCL-2-deficient mice
show normal neuronal development during embryogenesis but exhibit a significant loss of sympathetic, motor and sensory neurons during the early postnatal period [44]. BCL-XL (Table 2):
Constitutive absence of BCL-XL (encoded by the _Bcl2l1_ gene) results in embryonic lethality ~E13.5 due to erythroid and neuronal defects [45, 46]. To further study the role of BCL-XL during
development and in adulthood, chimaeric mice were generated through injection of _Bcl2l1__-/-_ (in the following referred to as _Bcl-x__-/-_) or control embryonic stem (ES) cells into
wild-type blastocysts. No significant differences in the contribution of _Bcl-x__-/-_ _vs_ control cells were observed in the heart, kidney or muscle [46], but BCL-XL was indispensable for
the survival of embryonic erythroid progenitors (EryP) and definitive erythrocytes (EryD) in the adult [45]. Furthermore, _Bcl-x__-/-_ ES cells contributed less to the spleen and thymus
compared to control ES cells [45]. _Bcl-x__-/-_ embryos present with massive apoptotic death of post-mitotic immature neurons in the developing brain, spinal cord, and dorsal root ganglia
[46]. Accordingly, extensive cell death was detected in cultures of telencephalic neurons derived from _Bcl-x__-/-_ embryos, and this was rescued by loss of BAX [47, 48]. BCL-XL deficiency
specifically in catecholaminergic neurons (tyrosine-hydroxylaseCre-_Bcl-x__fl/fl_ mice) resulted in viable offspring, although this neuronal population was reduced by one-third [49].
However, some catecholaminergic neurons without BCL-XL were present [49], indicating that their survival can be safeguarded by additional pro-survival BCL-2 proteins. MCL-1 (Table 3):
Amongst the pro-survival BCL-2 proteins, only MCL-1 is essential for early embryogenesis. Constitutive absence of MCL-1 in mice results in peri-implantation lethality (~E3.5) [50]. The
authors of this study failed to detect an increase in apoptotic cells in the _Mcl-1__-/-_ blastocysts and therefore hypothesised that the essential role of MCL-1 during implantation is
unrelated to its role in inhibiting apoptosis. Further investigations are needed to clarify whether the peri-implantation lethality caused by the absence of MCL-1 is triggered by excess cell
death or defects in apoptosis-unrelated functions of MCL-1. Tissue-restricted gene deletion revealed an essential role for MCL-1 in neuronal and cardiac tissues in embryos.
_NestinCre-Mcl-1__fl/fl_ mice, with neuronal-restricted loss of MCL-1, developed only until ~E12.5 [42, 51]. A wave of apoptosis was detected at E9.5 in the brainstem and cervical spinal
cord and at E10.5 in the forebrain. Interestingly, while loss of BAX was sufficient to protect the majority of neuronal precursor cells from apoptosis, this rescued only ~50% of cells in the
dorso-medial brainstem and ventral-thoracic spinal cord [51]. Thus, MCL-1 ensures NPC survival mainly by restraining BAX in the brain stem, while the survival of other brain cells must also
rely on the inhibition of BAK. The critical role of MCL-1 in neurogenesis in the forebrain was further demonstrated in _Foxg1Cre-Mcl-1__fl/fl_ mice, which all died before E15 [42].
_CkmmCre-Mcl-1__fl/fl_ mice, in which MCL-1 is removed from cardiomyocytes and skeletal muscle cells die around post-natal day 10 due to myocardial degeneration, demonstrating that MCL-1 is
essential for cardiomyocyte survival [52]. The loss of MCL-1 had only minor impact on skeletal muscle cells, at least within the short lifetime of the pups [52], suggesting that their
survival is safeguarded by additional pro-survival BCL-2 proteins. THE ROLES OF PRO-SURVIVAL BCL-2 PROTEINS IN ADULT MICE Some healthy cells in the adult are protected from apoptosis by
mostly one pro-survival BCL-2 family member, whereas others are safeguarded by two or more (Fig. 3). Moderate to high expression of MCL-1 is found in most adult tissues, including the brain,
digestive system, liver, kidney, reproductive organs (male and female), smooth and skeletal muscle and cardiomyocytes [53,54,55]. BCL-2 expression is reported as low in the adult heart,
liver, skeletal muscle but moderate to high in the colon, male and female reproductive organs, skin, colon and kidneys [53,54,55]. Moderate to high levels of BCL-XL have been observed in
many adult tissues, however, others such as cardiac and smooth muscles as well as the skin lack BCL-XL [53,54,55]. BCL-W is expressed in the brain, spinal cord, colon, testes, pancreas,
liver, heart, spleen and mammary glands of pregnant mice [21]. A1 is only found in haematopoietic cells [53,54,55] (Fig. 3). BCL-2 (Table 1): BCL-2-deficient mice die ~30 days after birth
(C57BL/6 background) due to polycystic kidney disease, a disorder that starts during embryogenesis [40, 56]. BCL-2-deficient mice also present with substantial reductions in mature B and T
lymphocytes and melanocytes, the latter causing premature greying [40, 56,57,58]. All defects caused by the absence of BCL-2 could be prevented by concomitant loss of the pro-apoptotic
BH3-only protein BIM [59, 60]. This demonstrates that BCL-2 safeguards the survival of several cell types by preventing BIM-induced apoptosis. BCL-2-deficient mice also exhibit an abnormal
reduction of sympathetic, motor and sensory neurons during the early postnatal period [44]. _NestinCreERT2-Bcl-2__fl/fl_ mice were generated to facilitate inducible deletion of _Bcl-2_
specifically in NPCs in adult mice. This revealed that BCL-2 is essential for the survival of doublecortin-expressing immature neurons in the central and peripheral nervous systems [61].
BCL-2 is also critical for endothelial cell survival. Abnormally decreased numbers of endothelial cells and pericytes were observed in retinas from BCL-2-deficient mice, resulting in
decreased retinal vascular density [62]. BCL-XL (Table 2): The loss of only one allele of _Bcl2l1_ (encoding _Bcl-x_) impairs male fertility, although with incomplete penetrance [63]. A
critical role of BCL-XL in male and female reproductive organs was confirmed in a _Bcl-x_ hypomorphic mouse strain which exhibited reduced germ cell survival during gonadogenesis. All
_Bcl-x_ hypomorphic males were sterile and had abnormally small testes. Interestingly, 25% of the _Bcl-x_ hypomorphic females were fertile but had abnormally small litters [63].
CreERT2-induced whole-body deletion of _Bcl-x_ in _Bcl-x__fl/fl__;RosaCreERT2_ mice was fatal at ~25 days due to severe anaemia and thrombocytopenia caused by the loss of erythroid
progenitors and platelets [64]. Unexpectedly, deletion of BCL-XL in all cells other than haematopoietic ones also caused fatal anaemia and thrombocytopenia, in this case due to severe kidney
damage resulting in the loss of the red blood cell-stimulating and megakaryocyte-stimulating hormones erythropoietin (EPO) and thrombopoietin (TPO), respectively [64]. This identifies an
essential role of BCL-XL for the survival of mitochondria-rich renal tubular cells in the kidneys [64]. Hepatocyte-specific loss of BCL-XL in _AlbCre-Bcl-x__fl/fl_ mice caused hepatocyte
apoptosis and liver fibrosis [65]. _RIPCre-Bcl-x__fl/fl_ mice, with specific loss of BCL-XL in pancreatic β-cells, are viable and show normal development of the pancreatic islets but these
cells are hypersensitive to apoptosis-inducing agents [66]. Approximately 50% of mice in which BCL-XL is absent in lung epithelial cells (_SftpcCre-Bcl-x__fl/fl_) die soon after birth.
Interestingly, the analysis of the viable offspring revealed that BCL-XL expression is not essential for lung development, but the pulmonary epithelial cells are hypersensitive to apoptotic
stimuli [67]. MCL-1 (Table 3): Loss of MCL-1 in cardiomyocytes is fatal in adults [19, 52, 68]. _MyhCreER-Mcl-1__fl/fl_ as well as _MerCreMer-Mcl-1__fl/fl_ mice died around 3–4 weeks after
induced deletion of _Mcl-1_ [52, 68, 69]. Interestingly, while _CkmmCre-Mcl-1__fl/fl_ as well as tamoxifen-treated (to activate CreERT2) _MyhCreERT2-Mcl-1__fl/fl_ mice developed until late
adulthood in the absence of BAX and BAK (_CkmmCre-Mcl-1__fl/fl__;Bax__fl/fl__;Bak__-/-_ and _MyhCreERT2-Mcl-1__fl/fl__;Bax__fl/fl__;Bak__-/-_ mice), defects in mitochondrial dynamics and
oxygen consumption were observed in these triple knockout mice [52]. It was therefore proposed that loss of an apoptosis-unrelated function of MCL-1 in mitochondrial dynamics and energy
production is at least partially responsible for the cardiac defects observed [52, 68]. Specifically, it was reported that a proteolytically cleaved form of MCL-1 is imported into the
mitochondrial intra-membrane space where it regulates mitochondrial fusion and mitochondrial respiratory complex assembly [70]. However, the CRE-mediated deletion of the floxed _Mcl-1_ and
_Bax_ alleles in _CkmmCre-Mcl-1__fl/fl__;Bax__fl/fl__;Bak__-/-_ mice as well as tamoxifen-treated _MyhCreERT2-Mcl-1__fl/fl__;Bax__fl/fl__;Bak__-/-_ mice was achieved simultaneously [52].
Given the large difference in the half-life of MCL-1 (~20 min) [25, 26] _vs_ BAX (24 h) [71], it cannot be excluded that the observed defects in mitochondrial ultrastructure and oxygen
consumption were a consequence of BAX-mediated induction of MOMP, because these cells would experience several hours when MCL-1 is no longer present but BAX is still there. In order to avoid
this complication and be able to better investigate the importance of the postulated apoptosis-unrelated function of MCL-1, a mouse model that allows the deletion of _Mcl-1_ and _Bax_
independently is needed (e.g., first deletion of _Bax_ using the FRT/_flp_ system and then deletion of _Mcl-1_ using the CreERT2/_loxP_ system). The deletion of MCL-1 in neuronal cells of
adult mice was achieved by stereotaxic injection of _NestinCre_-expressing plasmids into the lateral ventricles of adult _Mcl-1__fl/fl_ mice. This demonstrated a critical role of MCL-1 in
adult NPCs of the subventricular zone [19]. MCL-1 is also needed for hepatocyte survival [72]. _AlbCre-Mcl-1__fl/fl_ mice are born with abnormally small livers due to high rates of
hepatocyte apoptosis, resulting in chronic liver damage and hepatic pericellular fibrosis [72]. Other studies revealed that MCL-1 and BCL-XL cooperatively maintain the integrity of
hepatocytes in developing and adult mice [73]. Accordingly, _AlbCre-Bcl-x__fl/fl__Mcl-1__fl/fl_ mice display a massive reduction in hepatocytes and abnormally small livers and die within 1
day after birth [73]. A critical role for MCL-1 was reported for epithelial cells, including thymic epithelial (TECs) as well as intestinal epithelial cells (IECs) [74, 75]. In contrast,
loss of BCL-2 or BCL-XL had no impact on the survival of TECs or IECs. _Foxn1Cre-Mcl-1__fl/fl_ mice, in which MCL-1 is absent in TECs produce viable offspring. However, these mice are
severely immunocompromised due to early thymic atrophy and T-cell lymphopenia, with near complete loss of thymic tissue by 2 months of age [75]. The loss of MCL-1 in IECs caused increased
apoptosis leading to severe entero-colopathy. The increased IEC apoptosis was associated with hyperproliferative crypts, driven by compensatory proliferation of cells that had not recombined
_Mcl-1__fl_, and this led to epithelial barrier dysfunction, chronic inflammation and accumulation of DNA damage in hyperproliferating IECs. This caused the development of intestinal
tumours with morphological and genetic features of human adenomas and carcinomas [74]. MCL-1 is also essential for mammary epithelial cell survival and lactation using _K5Cre-Mcl-1__fl/fl__,
MMTVCre-Mcl-1__fl/fl_ and _WAPiCre-Mcl-1__fl/fl_ mice [76] as well as the survival of endothelial cells. Even though some endothelial cell specific _Mcl-1_-deleted mice develop until
adulthood, most _Tie2Cre-Mcl-1__fl/fl_ pups are lost before weaning. They exhibit abnormally high rates of apoptosis in the angiogenic vasculature and a decline in vessel density [77].
BCL-W: BCL-W _(_encoded by the _Bcl2l2_ gene_)-_deficient mice develop normally and most tissues from BCL-W_-_deficient mice are indistinguishable from those of wild-type controls [21, 78].
However, BCL-W_-_deficient males are infertile, demonstrating a critical role for BCL-W in spermatogenesis [78, 79]. A1: BFL-1 is expressed from a single gene in humans (_BCL2A1_) but there
are four _A1_ genes in the mouse genome [80]. _Bcl2a1a_, _Bcl2a1b_ and _Bcl2a1d_ are expressed and almost identical in sequence, whereas _Bcl2a1c_ is a pseudogene. Mice lacking all three
functional _A1_ genes develop and age normally and only exhibit minor abnormalities in certain haematopoietic cell subsets [80, 81]. This is not surprising, given that A1 expression is
largely restricted to haematopoietic cell lineages (Fig. 4) [80]. THE ROLES OF THE DIFFERENT PRO-SURVIVAL BCL-2 PROTEINS IN HAEMATOPOIESIS Haematopoiesis describes the production and
differentiation of all mature blood cell subsets from haematopoietic stem and progenitor cells (HSPCs). HSPCs have self-renewal capability and give rise to multipotent progenitor cells
(MPPs) which in turn give rise to the lineage-committed progenitor cells, including the common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). The latter further
differentiate into megakaryocyte-erythroid progenitor (MEPs) or granulocyte-macrophage progenitors (GMPs). CLPs are the origin of all lymphoid cells, including B as well as T lymphocytes and
natural killer (NK) cells. MEPs give rise to erythroid cells and megakaryocytes, which shed platelets, while granulocytes (e.g., neutrophils, eosinophils, monocytes) originate from GMPs.
The survival of immature and mature haematopoietic cells is safeguarded by pro-survival BCL-2 proteins with distinct family members being critical in different cell subsets (Fig. 4). MCL-1
is highly expressed in HSPCs and many mature haematopoietic cell subsets in both mice and humans [82,83,84,85]. In contrast, the expression of BCL-XL is moderate throughout haematopoiesis,
but with relatively high levels found in erythroid cells and megakaryocytes [82,83,84,85]. BCL-2 is most prominently expressed in certain B and T lymphoid populations as well as NK cells and
moderate expression is also found in stem and progenitor populations, including HSCs, MPPs, CMPs and CLPs, but only low expression is seen in erythroid cells [82,83,84,85]. A1 is found in
antigen receptor stimulated T and B lymphocytes, dendritic cells, neutrophils, eosinophils and monocytes [82,83,84,85]. BCL-W is expressed at comparatively low levels in the haematopoietic
compartment (Fig. 4) [82,83,84,85]. Conditional gene deletion studies in mice have identified the essential roles of distinct pro-survival BCL-2 proteins in the survival of different
haematopoietic cell types. BCL-2 (Table 1): Examination of chimaeric mice with a BCL-2-deficient haematopoietic compartment revealed that BCL-2-deficient mature T cells developed normally in
vivo but had abnormally short lifespan in vitro and increased sensitivity to glucocorticoids and γ-irradiation compared to control T cells [86]. Antigen receptor stimulation enhanced the in
vitro survival of T cells lacking BCL-2 [86], presumably because this causes NF-kB-driven up-regulation of BCL-XL [87]. In these chimaeric mice BCL-2-deficient T and B cells disappeared
from the bone marrow, thymus, and peripheral lymphoid organs by 4 weeks of age [86, 88]. Characterisation of a _Bcl-2_ YFP reporter mouse strain revealed that BCL-2 is critical for the
survival of effector and memory T lymphocytes [89, 90]. Notably, the survival defects of all lymphoid subsets caused by the absence of BCL-2 could be rescued by the concomitant loss of only
a single allele of pro-apoptotic _Bim_ [60]. This demonstrates that the function of BCL-2 in mature B and T lymphocytes is mainly to oppose BIM-induced apoptosis. The absence of BCL-2 had no
impact on the numbers of myeloid as well as erythroid cells and platelets. Notably, even cells that express moderate to high levels of BCL-2 (e.g., pro-myelocytes, normoblasts,
megakaryocytes) are present at normal numbers in BCL-2-deficient mice [88]. Tissue-restricted deletion of floxed _Bcl-2_ revealed that BCL-2 contributes to NK cell survival
(_Ncr1Cre-Bcl-2__fl/fl_ mice) [91] but is dispensable for the survival of megakaryocytes and platelets (_Pf4-Cre-Bcl-2__fl/fl_ mice) [92]. BCL-XL (Table 2): Even though moderate to high
expression of BCL-XL has been reported for most haematopoietic cell populations (Fig. 4), the absence of BCL-XL only impacts the survival of some. The role of BCL-XL in haematopoiesis was
studied in chimaeric mice that were generated by injection of _Bcl-x__-/-_ ES cells into wild-type or _Rag1__-/-_ blastocysts, the latter unable to give rise to mature T or B cells [46].
This revealed impaired survival of immature lymphocytes, such as CD4+CD8+ thymocytes, but not mature lymphocytes [46, 93]. Conditional deletion of _Bcl-x_ specifically in T lymphoid cells
from an early stage of differentiation (_LckCre-Bcl-x__fl/fl_ mice) revealed that the development of effector and memory T lymphocytes was not impacted by the loss of BCL-XL [94]. Analysis
of the aforementioned chimaeric mice revealed that _Bcl-x__-/-_ ES cells did not contribute to circulating EryD cells in the peripheral blood, demonstrating that BCL-XL plays an important
role in erythroid progenitor survival. In vitro differentiation analysis confirmed that BCL-XL is critical for the survival of both primitive (EryP) and definite erythroid (EryD) progenitor
cells [45]. Interestingly, the differentiation of _Bcl-x__-/-_ and wild-type ES cells in vitro yielded similar numbers of EryP and EryD cells, however, prominent apoptosis of _Bcl-x__-/-_
EryP and EryD occurred upon further maturation. This demonstrates that BCL-XL is critical at later stages of erythropoiesis. Accordingly, _MMTVCre-Bcl-x__fl/fl_ transgenic mice that express
the CRE recombinase in various secretory tissues but also the haematopoietic system [95,96,97,98] develop fatal anaemia [99]. Notably, the loss of only a single allele of _Bcl-x_ or point
mutations that reduce BCL-XL protein half-life (_Bcl-x__Plt16/Plt16_ and _Bcl-x__Plt20/Plt20_ mice) cause a significant reduction in platelets [100]. Platelets specifically depend on BCL-XL
to inhibit BAK-mediated apoptosis. When platelets are shed from megakaryocytes, they no longer produce much protein. Therefore, the relative levels of BCL-XL _vs_ BAK set up a timer of
platelet lifespan and consequently a reduction in BCL-XL (e.g., in platelets from _Bcl-x__+/-_ mice) reduces platelet survival [100]. Conditional deletion of _Bcl-x_ in megakaryocytes
(_Pf4-Cre-Bcl-x__fl/fl_ mice) demonstrated that BCL-XL is dispensable for the development and survival of these cells [101]. In fact, megakaryocyte survival is safeguarded by the combination
of BCL-XL and MCL-1, with only the absence of both causing their depletion [102, 103]. MCL-1 (Table 3): A reduction in lymphocytes, red blood cells (RBCs) but not platelets was observed in
_Mcl-1__+/-_ mice in which MCL-1 protein levels are reduced by ~40% compared to wild-type cells [104]. Conditional _Mcl-1_ gene-targeted mice were generated to identify the role of MCL-1 in
the survival of different haematopoietic cell populations. _LckCre-Mcl-1__fl/fl_ mice with MCL-1 loss from early stages of T lymphocyte development lack all immature and mature T cell
populations, with their development arrested at the progenitor (CD3-4-8- triple-negative (TN)) stage [32]. _CD19Cre-Mcl-1__fl/fl_ mice with loss of MCL-1 in B cells present with an arrest of
B lymphocyte differentiation at the pro-B cell stage [32]. Although these studies identify a critical role for MCL-1 in the survival of early T and B cell progenitors, they do not provide
insight into possible roles of MCL-1 at later stages of T and B cell differentiation. Inducible deletion of MCL-1 using _MxCre-Mcl-1__fl/fl_ mice was able to reveal a critical role of MCL-1
for the survival of mature B and T cells in culture [105]. Moreover, MCL-1 is required for the survival of activated B cells and the formation of the germinal centre [106].
_Ncr1Cre-Mcl-1__fl/fl_ mice that lack MCL-1 in NK cells are completely deficient in this cell population [107], identifying an essential role of MCL-1 for NK cell survival. The
characterisation of _CD11c-Cre-Mcl-1__fl/fl_ revealed a critical role of MCL-1 in the survival of conventional as well as plasmacytoid dendritic cells [108]. A prominent role for MCL-1 was
also reported for erythroid progenitors. _EpoR-Cre-Mcl-1__fl/fl_ embryos die ~E13.5 due to severe anaemia [109]. Interestingly, MCL-1 is only required during early stages of definitive
erythropoiesis but is dispensable for the survival of later stage erythroid progenitor cells that instead depend on BCL-XL [45, 99, 109]. MCL-1 has a less prominent role in myeloid cells and
megakaryocytes. _LysMCre-Mcl-1__fl/fl_ mice have normal numbers of monocytes and macrophages but display an abnormal reduction in neutrophils [110, 111]. It is, however, noteworthy that the
_LysMCre_ transgene is not as effective at recombining floxed genes as other _Cre_ transgenes; therefore the importance of MCL-1 in myeloid cell survival may have been underestimated.
Examination of _Pf4-Cre-Mcl-1__fl/fl_ mice revealed that MCL-1 is dispensable for megakaryocyte development and survival as well as platelet lifespan [102, 103] with megakaryocyte survival
safeguarded by both BCL-XL and MCL-1. BCL-W: Moderate BCL-W expression was detected in most haematopoietic cell populations [21, 112] (Fig. 4). Nevertheless, BCL-W_-_deficient mice had
normal distributions of all immature as well as mature haematopoietic cell types [78]. Enforced expression of BCL-W renders lymphoid and myeloid cells refractory to diverse cytotoxic
conditions [112]. Therefore, it was proposed that BCL-W may play a role in the development of haematological cancers. However, initial reports [113, 114] that BCL-W is a driver of
lymphomagenesis in various B cell lymphomas could not be reproduced [115]. A1: A1 is expressed in certain haematopoietic cell types but only minor defects are observed in A1_-_deficient
mice, including a small reduction in TCRγ/δ T cells, antigen-experienced conventional as well as regulatory CD4+ T cells in vivo and impaired survival of conventional dendritic cells (cDCs)
in culture [80]. The absence of A1 did not impair T cell responses during viral infection in mice [81]. A1 expression is induced by inflammatory cytokines, suggesting a role in the survival
of inflammatory cells [116,117,118,119]. However, no defects in neutrophil survival during infection were observed in A1-deficient mice [120]. TARGETING PRO-SURVIVAL BCL-2 FAMILY PROTEINS
WITH BH3 MIMETIC DRUGS FOR CANCER THERAPY In many human cancers apoptosis is dysregulated, often as a result of the overexpression of BCL-2, BCL-XL or MCL-1, and many cancers rely on their
aberrant expression for sustained growth [121]. Accordingly, BH3-mimetic drugs that target distinct pro-survival proteins have been developed and the BCL-2 inhibitor Venetoclax is approved
for CLL and AML. Given the essential roles of pro-survival BCL-2 proteins for the survival of many non-malignant cells, these compounds may cause undesirable on-target side effects to
healthy tissues that may be predicted from the analysis of gene knock-out studies in mice (Table 4). All on-target toxicities observed in patients and mice treated with BH3 mimetic drugs
targeting BCL-2, BCL-XL (e.g., thrombocytopenia) or MCL-1 (cardiac, intestinal and haematopoietic toxicity) were also seen in mice deficient for these proteins. Obversely, some of the
damages to tissues caused by constitutive or cell type-restricted knockout of pro-survival BCL-2 proteins were not seen in patients or mice treated with BH3 mimetic drugs targeting these
proteins. This is not surprising since in contrast to genetic deletion, drug-mediated inhibition is transient and likely does not result in a complete loss of function of a pro-survival
BCL-2 protein. Therefore, it is expected that the on-target drug-induced toxicities are milder than the defects caused by constitutive loss of a pro-survival BCL-2 protein upon its genetic
deletion. Here, we compare and discuss the consequences of genetic deletion _vs_ drug-mediated inhibition of pro-survival BCL-2 proteins in major tissues. ABT-737 is the first BH3-mimetic
compound described, followed by its orally available derivative ABT-263/Navitoclax that was tested in clinical trials [122, 123]. Both compounds are potent inhibitors of BCL-2, BCL-XL and
BCL-W [124,125,126,127]. Despite high potency in killing several types of malignant cells in pre-clinical tests, clinical trials of Navitoclax in chronic lymphocytic leukaemia were halted
because of platelet toxicity [123]. The thrombocytopenia and anaemia observed in Navitoclax-treated patients [122, 123] aligns with the essential role of BCL-XL in platelets and erythroid
progenitor cells identified in gene-targeted mice [99, 100]. Accordingly, toxic effects on platelets were also observed in mice treated with BCL-XL-specific inhibitors [128,129,130,131].
This will likely complicate clinical development of such agents unless they can be appropriately scheduled to minimise dose limiting toxicity or modified to preferentially target cancerous
cells, for example by coupling to an antibody that binds to malignant cells. Another promising approach is the PROTAC-based degradation of BCL-XL, targeting E3-ubiquitin ligases that are not
present in platelets but highly expressed in cancerous cells [132,133,134]. Prolonged treatment with Navitoclax did not cause liver or kidney toxicities that are caused by genetic deletion
of BCL-XL [135]. Of note, Navitoclax and ABT-737 were both shown to preferentially inhibit BCL-2 rather than BCL-XL in cells in vivo [136]. This may limit conclusions regarding the spectrum
of toxicities related to specific pro-survival BCL-2 proteins from clinical studies of Navitoclax. As platelets express only low levels of BCL-2 and BCL-W, BCL-XL can be inferred to be the
primary target of Navitoclax in these cells. In contrast, cells in the kidney express significant levels of BCL-2, BCL-XL and MCL-1 (Fig. 3), and thus BCL-XL might not be efficiently
targeted by Navitoclax in this tissue. MCL-1 was shown to safeguard hepatocyte survival in the absence of BCL-XL in genetic knock-out studies [73], perhaps explaining why Navitoclax did not
cause liver toxicity. Since no BCL-XL-specific inhibitor has entered clinical trials, it cannot be excluded that consequences of genetic loss of BCL-XL might actually occur in patients
treated with such a drug. To prevent dose-limiting thrombocytopenia in cancer patients, the BCL-2-specific inhibitor Venetoclax/ABT-199 was developed [137]. Treatment with Venetoclax is
well-tolerated in patients, with no toxicity in major organs, such as the kidney or liver [138]. This agrees with observations from BCL-2-deficient mice, showing that even though BCL-2 is
essential for kidney development in the embryo, it is dispensable for the function of major adult organs [40, 60]. In line with what was seen in BCL-2-deficient mice, many patients develop
transient neutropenia (~40%) [139, 140]. While investigators have attributed thrombocytopenia and anaemia as adverse events to single agent Venetoclax, this occurred with a large proportion
of enroled patients with relapsed/refractory CLL having cytopenia at baseline [139, 140]. As most haematologic adverse events occurred early in treatment and decreased or resolved over time,
these observations are most likely secondary to the high burden of disease rather than a direct effect of Venetoclax. Importantly, the neutropenia in Venetoclax-treated patients could be
managed with growth factor support [139, 140]. In contrast to the genetic deletion of BCL-2, treatment with Venetoclax does not cause neuronal toxicity in patients [138]. This may be
explained by the poor blood-brain barrier penetration of Venetoclax, with drug concentration in the central nervous system only reaching 0.1% of that observed in the plasma [141]. Despite
the essential role of MCL-1 for the survival of many critical cell types, pre-clinical studies in mice have identified a therapeutic window for MCL-1-specific inhibitors [142,143,144,145].
This is in line with observations that loss of even a single allele of _Mcl-1_ obliterates the expansion of MYC-driven lymphomas in mice, unless they carry a mutation in p53 [146], while
loss of one allele of _Mcl-1_ is well-tolerated in mice with only minor reductions in mature B lymphocytes and erythroid cells [104]. Conclusions from pre-clinical observations prompted the
initiation of phase 1 clinical trials with several MCL-1 inhibitors. Trials of AMG-397 (Amgen) and AZD5991 (AstraZeneca) were paused due to cardiac toxicity signals [147,148,149,150]. In
contrast, phase 1 clinical dose escalation studies for S64315/MIK665 (Servier/Novartis) have been completed without major adverse events being reported. The cardiotoxicity of AMG-397 and
AZD5991 is in line with the findings from genetic studies that MCL-1 is essential for cardiomyocyte survival in mice [52, 68]. MCL-1 is also the major survival factor for HSPCs, and
accordingly, AMG-176 induced neutropenia and severe anaemia in patients, which may be caused by a reduction in HSPCs [151]. Consistent with this idea, human HSPCs were depleted during in
vitro treatment with S64315/MIK665 [151]. MCL-1 loss results in intestinal epithelial cell apoptosis [74] and, accordingly, treatment with AMG-176 caused gastro-intestinal toxicity in
patients. Pre-clinical data demonstrate that the combined inhibition of MCL-1 and BCL-XL causes liver toxicity [152], a scenario that could be predicted from gene-targeting studies, showing
that MCL-1 and BCL-XL collectively ensure hepatocellular survival [65, 72, 73]. In conclusion, mouse models have been invaluable to identify the critical roles of the different pro-survival
BCL-2 proteins for the survival of a broad range of non-transformed cell types during embryogenesis and in the adult. This highlights the importance of using genetic mouse models to form the
foundation for developing effective and safe new treatments for cancer patients. DATA AVAILABILITY There are no primary data presented in this review article. REFERENCES * Adams JM, Cory S.
The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. Article CAS PubMed PubMed Central Google Scholar * Czabotar PE, Lessene G, Strasser A, Adams
JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. Article CAS PubMed Google Scholar * Green DR. The
coming decade of cell death research: five riddles. Cell. 2019;177:1094–107. Article CAS PubMed PubMed Central Google Scholar * Ke FFS, Vanyai HK, Cowan AD, Delbridge ARD, Whitehead L,
Grabow S, et al. Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX, BAK, and BOK. Cell. 2018;173:1217–30 e1217. Article CAS PubMed Google Scholar * Llambi
F, Wang YM, Victor B, Yang M, Schneider DM, Gingras S, et al. BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell. 2016;165:421–33. Article
CAS PubMed PubMed Central Google Scholar * Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of
BH3-only proteins. Cell Death Differ. 2002;9:505–12. Article CAS PubMed Google Scholar * Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of
Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–18. Article CAS PubMed Google Scholar * Marani M, Tenev T,
Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–89. Article
CAS PubMed PubMed Central Google Scholar * Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2
homologs, not Bax or Bak. Science. 2007;315:856–9. Article CAS PubMed Google Scholar * Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular
distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–14. CAS PubMed Google Scholar
* Lithgow T, van Driel R, Bertram JF, Strasser A. The protein product of the oncogene Bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial
membrane. Cell Growth Differ. 1994;5:411–7. CAS PubMed Google Scholar * Janiak F, Leber B, Andrews DW. Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem.
1994;269:9842–9. Article CAS PubMed Google Scholar * Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF. Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol
Cell Biol. 2005;25:3117–26. Article CAS PubMed PubMed Central Google Scholar * Nakajima W, Hicks MA, Tanaka N, Krystal GW, Harada H. Noxa determines localization and stability of MCL-1
and consequently ABT-737 sensitivity in small cell lung cancer. Cell Death Dis. 2014;5:e1052. Article CAS PubMed PubMed Central Google Scholar * Weng SY, Yang CY, Li CC, Sun TP, Tung
SY, Yen JJ, et al. Synergism between p53 and Mcl-1 in protecting from hepatic injury, fibrosis and cancer. J Hepatol. 2011;54:685–94. Article CAS PubMed Google Scholar * Yang T, Kozopas
KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–84. Article CAS PubMed
Google Scholar * Jamil S, Sobouti R, Hojabrpour P, Raj M, Kast J, Duronio V. A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with
Cdk1. Biochem J. 2005;387:659–67. Article CAS PubMed PubMed Central Google Scholar * Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol
Chem. 1997;272:13829–34. Article CAS PubMed Google Scholar * Malone CD, Hasan SM, Roome RB, Xiong J, Furlong M, Opferman JT, et al. Mcl-1 regulates the survival of adult neural precursor
cells. Mol Cell Neurosci. 2012;49:439–47. Article CAS PubMed Google Scholar * Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of Mcl-1 is required for the
initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–86. Article CAS PubMed PubMed Central Google Scholar * O’Reilly LA, Print C, Hausmann G, Moriishi K,
Cory S, Huang DC, et al. Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ. 2001;8:486–94. Article PubMed CAS Google Scholar * Werner
AB, de Vries E, Tait SW, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J Biol Chem. 2002;277:22781–8.
Article CAS PubMed Google Scholar * Guedes RP, Rocha E, Mahiou J, Moll HP, Arvelo MB, Taube JM, et al. The C-terminal domain of A1/Bfl-1 regulates its anti-inflammatory function in human
endothelial cells. Biochim Biophys Acta. 2013;1833:1553–61. Article CAS PubMed PubMed Central Google Scholar * Kucharczak JF, Simmons MJ, Duckett CS, Gelinas C. Constitutive
proteasome-mediated turnover of Bfl-1/A1 and its processing in response to TNF receptor activation in FL5.12 pro-B cells convert it into a prodeath factor. Cell Death Differ.
2005;12:1225–39. Article CAS PubMed Google Scholar * Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–200.
Article CAS PubMed Google Scholar * Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis
by destabilization of MCL-1. Mol Cell. 2006;21:749–60. Article CAS PubMed Google Scholar * Blagosklonny MV, Alvarez M, Fojo A, Neckers LM. bcl-2 protein downregulation is not required
for differentiation of multidrug resistant HL60 leukemia cells. Leuk Res. 1996;20:101–7. Article CAS PubMed Google Scholar * Rooswinkel RW, van de Kooij B, de Vries E, Paauwe M, Braster
R, Verheij M, et al. Antiapoptotic potency of Bcl-2 proteins primarily relies on their stability, not binding selectivity. Blood. 2014;123:2806–15. Article CAS PubMed Google Scholar *
Herold MJ, Zeitz J, Pelzer C, Kraus C, Peters A, Wohlleben G, et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281:13663–71. Article
CAS PubMed Google Scholar * Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows
complementary apoptotic function. Mol Cell. 2005;17:393–403. Article CAS PubMed Google Scholar * Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3
domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. Article CAS PubMed Google
Scholar * Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6.
Article CAS PubMed Google Scholar * Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J.
1982;1:681–6. Article CAS PubMed PubMed Central Google Scholar * Boumela I, Assou S, Haouzi D, Dechaud H, Ait-Ahmed O, Hamamah S. Developmental regulated expression of anti- and
pro-apoptotic BCL-2 family genes during human early embryonic development. Curr Med Chem. 2014;21:1361–9. Article CAS PubMed Google Scholar * Russell HR, Lee Y, Miller HL, Zhao J,
McKinnon PJ. Murine ovarian development is not affected by inactivation of the bcl-2 family member diva. Mol Cell Biol. 2002;22:6866–70. Article CAS PubMed PubMed Central Google Scholar
* Ke N, Godzik A, Reed JC. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J Biol Chem. 2001;276:12481–4. Article CAS PubMed Google Scholar *
Lee R, Chen J, Matthews CP, McDougall JK, Neiman PE. Characterization of NR13-related human cell death regulator, Boo/Diva, in normal and cancer tissues. Biochim Biophys Acta.
2001;1520:187–94. Article CAS PubMed Google Scholar * Song Q, Kuang Y, Dixit VM. Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J. 1999;18:167–78.
Article CAS PubMed PubMed Central Google Scholar * Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25:37–45. Article CAS PubMed
Google Scholar * Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell.
1993;75:229–40. Article CAS PubMed Google Scholar * Abe-Dohmae S, Harada N, Yamada K, Tanaka R. Bcl-2 gene is highly expressed during neurogenesis in the central nervous system. Biochem
Biophys Res Commun. 1993;191:915–21. Article CAS PubMed Google Scholar * Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, et al. Mcl-1 is a key regulator of
apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–78. Article CAS PubMed PubMed Central Google Scholar * Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ. bcl-2
protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994;120:301–11. Article CAS PubMed Google Scholar * Michaelidis TM,
Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, et al. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early
postnatal development. Neuron. 1996;17:75–89. Article CAS PubMed Google Scholar * Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. bcl-x prevents apoptotic cell death of both
primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999;189:1691–8. Article CAS PubMed PubMed Central Google Scholar * Motoyama N, Wang F, Roth KA, Sawa H,
Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–10. Article CAS PubMed Google Scholar * Roth
KA, Motoyama N, Loh DY. Apoptosis of bcl-x-deficient telencephalic cells in vitro. J Neurosci. 1996;16:1753–8. Article CAS PubMed PubMed Central Google Scholar * Shindler KS, Latham
CB, Roth KA. Bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 1997;17:3112–9. Article CAS PubMed PubMed Central Google Scholar *
Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25:6721–8. Article CAS PubMed PubMed Central
Google Scholar * Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–7. Article CAS PubMed
PubMed Central Google Scholar * Flemmer RT, Connolly SP, Geizer BA, Opferman JT, Vanderluit JL. The role of Mcl-1 in embryonic neural precursor cell apoptosis. Front Cell Dev Biol.
2021;9:659531. Article PubMed PubMed Central Google Scholar * Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S, et al. Deletion of MCL-1 causes lethal cardiac failure and
mitochondrial dysfunction. Genes Dev. 2013;27:1351–64. Article CAS PubMed PubMed Central Google Scholar * Sjostedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, et al. An
atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367. * Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for
normal and cancer tissues based on antibody proteomics. Mol Cell Proteom. 2005;4:1920–32. Article CAS Google Scholar * Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P,
Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. Article PubMed CAS Google Scholar * Kamada S, Shimono A, Shinto Y, Tsujimura T,
Takahashi T, Noda T, et al. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and
distorted small intestine. Cancer Res. 1995;55:354–9. CAS PubMed Google Scholar * Mak S-S, Moriyama M, Nishioka E, Osawa M, Nishikawa S-I. Indispensable role of Bcl2 in the development of
the melanocyte stem cell. Dev Biol. 2006;291:144–53. Article CAS PubMed Google Scholar * Yamamura K, Kamada S, Ito S, Nakagawa K, Ichihashi M, Tsujimoto Y. Accelerated disappearance of
melanocytes in bcl-2-deficient mice. Cancer Res. 1996;56:3546–50. CAS PubMed Google Scholar * Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2
balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–75. Article CAS PubMed PubMed Central Google Scholar * Bouillet P, Cory S, Zhang LC,
Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–53. Article CAS PubMed Google Scholar *
Ceizar M, Dhaliwal J, Xi Y, Smallwood M, Kumar KL, Lagace DC. Bcl-2 is required for the survival of doublecortin-expressing immature neurons. Hippocampus. 2016;26:211–9. Article CAS PubMed
Google Scholar * Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2-/- mice. Dev Biol.
2005;279:205–19. Article CAS PubMed Google Scholar * Rucker EB 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, et al. Bcl-x and Bax regulate mouse primordial germ
cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–52. Article CAS PubMed Google Scholar * Brinkmann K, Waring P, Glaser SP, Wimmer V, Cottle DL, Tham MS, et
al. BCL-XL exerts a protective role against anemia caused by radiation-induced kidney damage. EMBO J. 2020;39:e105561. Article CAS PubMed PubMed Central Google Scholar * Takehara T,
Tatsumi T, Suzuki T, Rucker EB 3rd, Hennighausen L, Jinushi M, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses.
Gastroenterology. 2004;127:1189–97. Article CAS PubMed Google Scholar * Carrington EM, McKenzie MD, Jansen E, Myers M, Fynch S, Kos C, et al. Islet beta-cells deficient in Bcl-xL develop
but are abnormally sensitive to apoptotic stimuli. Diabetes. 2009;58:2316–23. Article CAS PubMed PubMed Central Google Scholar * Staversky RJ, Vitiello PF, Yee M, Callahan LM, Dean DA,
O’Reilly MA. Epithelial ablation of Bcl-XL increases sensitivity to oxygen without disrupting lung development. Am J Respir Cell Mol Biol. 2010;43:376–85. Article CAS PubMed Google
Scholar * Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27:1365–77.
Article CAS PubMed PubMed Central Google Scholar * Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, Hennek T, et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res.
2003;31:e12. Article PubMed PubMed Central CAS Google Scholar * Perciavalle RM, Opferman JT. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol.
2013;23:22–9. Article CAS PubMed Google Scholar * Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxbeta: a constitutively active human Bax isoform that is under tight regulatory control by the
proteasomal degradation mechanism. Mol Cell. 2009;33:15–29. Article CAS PubMed Google Scholar * Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, et al. Knockout of myeloid cell
leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–36. Article CAS PubMed Google Scholar * Hikita H, Takehara T,
Shimizu S, Kodama T, Li W, Miyagi T, et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–26. Article CAS
PubMed Google Scholar * Healy ME, Boege Y, Hodder MC, Bohm F, Malehmir M, Scherr AL, et al. MCL1 is Required for Maintenance of Intestinal Homeostasis and Prevention of Carcinogenesis in
Mice. Gastroenterology. 2020;159:183–99. Article CAS PubMed Google Scholar * Jain R, Sheridan JM, Policheni A, Heinlein M, Gandolfo LC, Dewson G, et al. A critical epithelial survival
axis regulated by MCL-1 maintains thymic function in mice. Blood. 2017;130:2504–15. Article CAS PubMed Google Scholar * Fu NY, Rios AC, Pal B, Soetanto R, Lun AT, Liu K, et al.
EGF-mediated induction of Mcl-1 at the switch to lactation is essential for alveolar cell survival. Nat Cell Biol. 2015;17:365–75. Article CAS PubMed Google Scholar * Watson EC,
Whitehead L, Adams RH, Dewson G, Coultas L. Endothelial cell survival during angiogenesis requires the pro-survival protein MCL1. Cell Death Differ. 2016;23:1371–9. Article CAS PubMed
PubMed Central Google Scholar * Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise
redundant. Proc Natl Acad Sci USA. 1998;95:12424–31. Article CAS PubMed PubMed Central Google Scholar * Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, et al.
Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–6. Article CAS PubMed Google Scholar * Schenk RL, Tuzlak S, Carrington EM, Zhan Y, Heinzel S, Teh CE, et al.
Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ.
2017;24:534–45. Article CAS PubMed PubMed Central Google Scholar * Tuzlak S, Schenk RL, Vasanthakumar A, Preston SP, Haschka MD, Zotos D, et al. The BCL-2 pro-survival protein A1 is
dispensable for T cell homeostasis on viral infection. Cell Death Differ. 2017;24:523–33. Article CAS PubMed PubMed Central Google Scholar * de Graaf CA, Choi J, Baldwin TM, Bolden JE,
Fairfax KA, Robinson AJ, et al. Haemopedia: an expression atlas of murine hematopoietic cells. Stem Cell Rep. 2016;7:571–82. Article Google Scholar * Uhlen M, Karlsson MJ, Zhong W, Tebani
A, Pou C, Mikes J, et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science. 2019;366:6472. Article CAS Google Scholar * Choi J, Baldwin TM, Wong
M, Bolden JE, Fairfax KA, Lucas EC, et al. Haemopedia RNA-seq: a database of gene expression during haematopoiesis in mice and humans. Nucleic Acids Res. 2019;47:D780–5. Article CAS
PubMed Google Scholar * Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, et al. Densely interconnected transcriptional circuits control cell states in human
hematopoiesis. Cell. 2011;144:296–309. Article CAS PubMed PubMed Central Google Scholar * Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, et al. Disappearance of the
lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–8. Article CAS PubMed Google Scholar * Grossmann M, O’Reilly LA, Gugasyan R, Strasser A, Adams JM,
Gerondakis S. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 2000;19:6351–60. Article CAS PubMed
PubMed Central Google Scholar * Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney
disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–4. Article CAS PubMed PubMed Central Google Scholar * Dunkle A, Dzhagalov I, Gordy C, He YW. Transfer of CD8+ T cell
memory using Bcl-2 as a marker. J Immunol. 2013;190:940–7. Article CAS PubMed Google Scholar * Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, et al. Bcl-2
allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol. 2011;186:5729–37. Article CAS PubMed Google Scholar * Viant C, Guia S, Hennessy RJ, Rautela J,
Pham K, Bernat C, et al. Cell cycle progression dictates the requirement for BCL2 in natural killer cell survival. J Exp Med. 2017;214:491–510. Article CAS PubMed PubMed Central Google
Scholar * Debrincat MA, Pleines I, Lebois M, Lane RM, Holmes ML, Corbin J, et al. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis. 2015;6:e1721. Article CAS
PubMed PubMed Central Google Scholar * Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA.
1995;92:4763–7. Article CAS PubMed PubMed Central Google Scholar * Zhang N, He YW. The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T
lymphocytes. J Immunol. 2005;174:6967–73. Article CAS PubMed Google Scholar * Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the
mammary gland. Nucleic Acids Res. 1997;25:4323–30. Article CAS PubMed PubMed Central Google Scholar * Choi YW, Henrard D, Lee I, Ross SR. The mouse mammary tumor virus long terminal
repeat directs expression in epithelial and lymphoid cells of different tissues in transgenic mice. J Virol. 1987;61:3013–9. Article CAS PubMed PubMed Central Google Scholar * Ross SR,
Hsu CL, Choi Y, Mok E, Dudley JP. Negative regulation in correct tissue-specific expression of mouse mammary tumor virus in transgenic mice. Mol Cell Biol. 1990;10:5822–9. CAS PubMed
PubMed Central Google Scholar * Stewart TA, Hollingshead PG, Pitts SL. Multiple regulatory domains in the mouse mammary tumor virus long terminal repeat revealed by analysis of fusion
genes in transgenic mice. Mol Cell Biol. 1988;8:473–9. CAS PubMed PubMed Central Google Scholar * Wagner KU, Claudio E, Rucker EB 3rd, Riedlinger G, Broussard C, Schwartzberg PL, et al.
Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–58. Article CAS PubMed Google Scholar *
Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. Article CAS PubMed Google
Scholar * Josefsson EC, James C, Henley KJ, Debrincat MA, Rogers KL, Dowling MR, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive
and produce platelets. J Exp Med. 2011;208:2017–31. Article CAS PubMed PubMed Central Google Scholar * Debrincat MA, Josefsson EC, James C, Henley KJ, Ellis S, Lebois M, et al. Mcl-1
and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood. 2012;119:5850–8. Article CAS PubMed Google Scholar * Kodama T, Hikita H, Kawaguchi T, Shigekawa M, Shimizu S, Hayashi Y,
et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ. 2012;19:1856–69. Article CAS PubMed PubMed Central Google
Scholar * Brinkmann K, Grabow S, Hyland CD, Teh CE, Alexander WS, Herold MJ, et al. The combination of reduced MCL-1 and standard chemotherapeutics is tolerable in mice. Cell Death Differ.
2017;24:2032–43. Article CAS PubMed PubMed Central Google Scholar * Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the
survival of hematopoietic stem cells. Science. 2005;307:1101–4. Article CAS PubMed Google Scholar * Vikstrom I, Carotta S, Lüthje K, Peperzak V, Jost PJ, Glaser S, et al. Mcl-1 is
essential for germinal center formation and B cell memory. Science. 2010;330:1095–9. Article CAS PubMed PubMed Central Google Scholar * Sathe P, Delconte RB, Souza-Fonseca-Guimaraes F,
Seillet C, Chopin M, Vandenberg CJ, et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun. 2014;5:4539. Article CAS PubMed Google Scholar
* Carrington EM, Zhang JG, Sutherland RM, Vikstrom IB, Brady JL, Soo P, et al. Prosurvival Bcl-2 family members reveal a distinct apoptotic identity between conventional and plasmacytoid
dendritic cells. Proc Natl Acad Sci USA. 2015;112:4044–9. Article CAS PubMed PubMed Central Google Scholar * Turnis ME, Kaminska E, Smith KH, Kartchner BJ, Vogel P, Laxton JD, et al.
Requirement for antiapoptotic MCL-1 during early erythropoiesis. Blood. 2021;137:1945–58. Article CAS PubMed PubMed Central Google Scholar * Dzhagalov I, St John A, He YW. The
antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–6. Article CAS PubMed PubMed Central Google Scholar * Steimer DA, Boyd
K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–15. Article
CAS PubMed PubMed Central Google Scholar * Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, et al. bcl-w, a novel member of the bcl-2 family, promotes cell
survival. Oncogene. 1996;13:665–75. CAS PubMed Google Scholar * Adams CM, Kim AS, Mitra R, Choi JK, Gong JZ, Eischen CM. BCL-W has a fundamental role in B cell survival and
lymphomagenesis. J Clin Invest. 2017;127:635–50. Article PubMed PubMed Central Google Scholar * Adams CM, Mitra R, Gong JZ, Eischen CM. Non-Hodgkin and Hodgkin lymphomas select for
overexpression of BCLW. Clin Cancer Res. 2017;23:7119–29. Article CAS PubMed PubMed Central Google Scholar * Diepstraten ST, Chang C, Tai L, Gong JN, Lan P, Dowell AC, et al. BCL-W is
dispensable for the sustained survival of select Burkitt lymphoma and diffuse large B-cell lymphoma cell lines. Blood Adv. 2020;4:356–66. Article CAS PubMed PubMed Central Google Scholar
* Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–4. Article CAS PubMed
Google Scholar * Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–96.
Article CAS PubMed Google Scholar * Lin EY, Orlofsky A, Berger MS, Prystowsky MB. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to
bcl-2. J Immunol. 1993;151:1979–88. CAS PubMed Google Scholar * Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target
of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–7. Article CAS PubMed PubMed Central Google Scholar * Gangoda L, Schenk RL, Best SA, Nedeva C, Louis C,
D’Silva DB, et al. Absence of pro-survival A1 has no impact on inflammatory cell survival in vivo during acute lung inflammation and peritonitis. Cell Death Differ. 2022;29:96–104. Article
CAS PubMed Google Scholar * Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. Article CAS PubMed Google
Scholar * Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of
navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. Article CAS PubMed Google Scholar * Wilson WH, O'Connor OA, Czuczman MS, LaCasce LS,
Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics,
pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. Article CAS PubMed PubMed Central Google Scholar * Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir
SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharm. 2010;66:869–80. Article CAS Google
Scholar * Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature.
2005;435:677–81. Article CAS PubMed Google Scholar * Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel
of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. Article CAS PubMed Google Scholar * Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al.
ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. Article CAS PubMed Google Scholar * Koehler MF, Bergeron P, Choo EF, Lau K, Ndubaku C,
Dudley D, et al. Structure-guided rescaffolding of selective antagonists of BCL-XL. ACS Med Chem Lett. 2014;5:662–7. Article CAS PubMed PubMed Central Google Scholar * Lessene G,
Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9:390–7. Article CAS PubMed Google Scholar *
Sleebs BE, Kersten WJ, Kulasegaram S, Nikolakopoulos G, Hatzis E, Moss RM, et al. Discovery of potent and selective benzothiazole hydrazone inhibitors of Bcl-XL. J Med Chem. 2013;56:5514–40.
Article CAS PubMed Google Scholar * Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CH, et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med
Chem Lett. 2014;5:1088–93. Article CAS PubMed PubMed Central Google Scholar * Khan S, Zhang X, Lv D, Zhang Q, He Y, Zhang P, et al. A selective BCL-XL PROTAC degrader achieves safe and
potent antitumor activity. Nat Med. 2019;25:1938–47. Article CAS PubMed PubMed Central Google Scholar * Zhang X, He Y, Zhang P, Budamagunta V, Lv D, Thummuri D, et al. Discovery of
IAP-recruiting BCL-XL PROTACs as potent degraders across multiple cancer cell lines. Eur J Med Chem. 2020;199:112397. Article CAS PubMed PubMed Central Google Scholar * Zhang X,
Thummuri D, Liu X, Hu W, Zhang P, Khan S, et al. Discovery of PROTAC BCL-XL degraders as potent anticancer agents with low on-target platelet toxicity. Eur J Med Chem. 2020;192:112186.
Article CAS PubMed PubMed Central Google Scholar * Zhao X, Ogunwobi OO, Liu C. Survivin inhibition is critical for Bcl-2 inhibitor-induced apoptosis in hepatocellular carcinoma cells.
PLoS One. 2011;6:e21980. Article CAS PubMed PubMed Central Google Scholar * Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not
equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–16. Article CAS PubMed PubMed Central Google Scholar * Souers AJ, Leverson
JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. Article
CAS PubMed Google Scholar * Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J
Med. 2016;374:311–22. Article CAS PubMed Google Scholar * Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax
monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24:4371–9. Article CAS PubMed Google Scholar * Davids MS, von Keudell G, Portell CA,
Cohen JB, Fisher DC, Foss F, et al. Revised dose ramp-up to mitigate the risk of tumor lysis syndrome when initiating venetoclax in patients with mantle cell lymphoma. J Clin Oncol.
2018;36:3525–7. Article CAS Google Scholar * Reda G, Cassin R, Dovrtelova G, Matteo C, Giannotta J, D’Incalci M, et al. Venetoclax penetrates in cerebrospinal fluid and may be effective
in chronic lymphocytic leukemia with central nervous system involvement. Haematologica. 2019;104:e222–3. Article CAS PubMed PubMed Central Google Scholar * Strasser GLKAA. Toward
targeting antiapoptotic MCL-1 for cancer therapy. Annu Rev Cancer Biol. 2020;4:299–313. Article Google Scholar * Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D, et al. AMG
176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Disco. 2018;8:1582–97. Article CAS Google Scholar *
Szlavik Z, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, et al. Discovery of 04315, a potent and selective Mcl-1 inhibitor. J Med Chem. 2020;63:13762–95. Article CAS PubMed Google
Scholar * Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute
myeloid leukemia. Nat Commun. 2018;9:5341. Article CAS PubMed PubMed Central Google Scholar * Kelly GL, Grabow S, Glaser SP, Fitzsimmons L, Aubrey BJ, Okamoto T, et al. Targeting of
MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28:58–70. Article CAS PubMed PubMed Central Google Scholar * Safety, tolerability,
pharmacokinetics and efficacy of AMG 397 in subjects with selected relapsed or refractory hematological malignancies. _Clinicaltrials.gov.au_ 2018–2019. * FDA places trials of MCL-1
inhibitor on clinical hold. Ash Clinical News; 2019. * FDA places clinical hold on trials of AZD5991 following cardiac complications. Ash Clinical News; 2021. * Study of AZD5991 alone or in
combination with venetoclax in relapsed or refractory haematologic malignancies. _Clinicaltrialsgov_ 2022. * Spencer A, Rosenberg AS, Jakubowiak A, Raje N, Chatterjee M, Trudel S, et al. A
Phase 1, first-in-human study of AMG 176, a selective MCL-1 inhibitor, in patients with relapsed or refractory multiple myeloma. Clin Lymphoma, Myeloma Leuk. 2019;19:E53–4. Article Google
Scholar * Bohler S, Afreen S, Fernandez-Orth J, Demmerath EM, Molnar C, Wu Y, et al. Inhibition of the anti-apoptotic protein MCL-1 severely suppresses human hematopoiesis. Haematologica.
2021;106:3136–48. CAS PubMed Google Scholar * Weeden CE, Ah-Cann C, Holik AZ, Pasquet J, Garnier JM, Merino D, et al. Dual inhibition of BCL-XL and MCL-1 is required to induce tumour
regression in lung squamous cell carcinomas sensitive to FGFR inhibition. Oncogene. 2018;37:4475–88. Article CAS PubMed Google Scholar * Yamashita J, Datta NS, Chun YH, Yang D-Y, Carey
AA, Kreider JM, et al. Role of Bcl2 in osteoclastogenesis and PTH anabolic actions in bone. J Bone Miner Res. 2008;23:621–32. Article CAS PubMed Google Scholar * Walton KD, Wagner KU,
Rucker EB 3rd, Shillingford JM, Miyoshi K, Hennighausen L. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does
not compromise cell function during lactation. Mech Dev. 2001;109:281–93. Article CAS PubMed Google Scholar * Hon H, Rucker EB 3rd, Hennighausen L, Jacob J. bcl-xL is critical for
dendritic cell survival in vivo. J Immunol. 2004;173:4425–32. Article CAS PubMed Google Scholar * Peperzak V, Vikström I, Walker J, Glaser SP, LePage M, Coquery CM, et al. Mcl-1 is
essential for the survival of plasma cells [published correction appears in Nat Immunol. 2013 Aug;14(8):877]. Nat Immunol. 2013;14:290–7. Article CAS PubMed PubMed Central Google Scholar
* Jakubowska MA, Kerkhofs M, Martines C, Efremov DG, Gerasimenko JV, Gerasimenko OV, et al. ABT-199 (Venetoclax), a BH3-mimetic Bcl-2 inhibitor, does not cause Ca2+ -signalling
dysregulation or toxicity in pancreatic acinar cells. Br J Pharmacol. 2019;176:4402–15. Article CAS PubMed Google Scholar * Robinson EJ, Aguiar SP, Kouwenhoven WM, Starmans DS, von
Oerthel L, Smidt MP, van der Heide LP. Survival of midbrain dopamine neurons depends on the Bcl2 factor Mcl1. Cell Death Discov. 2018;4:107. Download references ACKNOWLEDGEMENTS We thank Dr
Marco Herold for discussion of the data that are reviewed here. We thank Dr Andrew Roberts for sharing his experience from clinical treatment of patients with Venetoclax and Dr David Huang
for sharing his insights into the stability of BAX protein. FUNDING The work by the authors was supported by grants and fellowships from the Deutsche Krebshilfe (Dr Mildred Scheel
post-doctoral fellowship to KB), the National Health and Medical Research Council (Program Grant #1113133, Fellowship 1116937, Investigator Grant #2007887, Synergy Grant #2010275; all to AS)
and the Leukaemia and Lymphoma Society (SCOR Grant #7015-18 to AS). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The Walter and Eliza Hall Institute of Medical Research, Melbourne, Vic,
Australia Kerstin Brinkmann, Ashley P. Ng, Carolyn A. de Graaf & Andreas Strasser * The Department of Medical Biology, University of Melbourne, Melbourne, Vic, Australia Kerstin
Brinkmann, Ashley P. Ng, Carolyn A. de Graaf & Andreas Strasser Authors * Kerstin Brinkmann View author publications You can also search for this author inPubMed Google Scholar * Ashley
P. Ng View author publications You can also search for this author inPubMed Google Scholar * Carolyn A. de Graaf View author publications You can also search for this author inPubMed Google
Scholar * Andreas Strasser View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS KB and AS discussed and interpreted all primary research papers
mentioned in this review. KB wrote the manuscript with the help of AS. KB designed Figs. 1 and 2. CdG analysed publicly available gene expression data and designed Figs. 3 and 4. AN and KB
produced Table 4 and AN described the findings about on-target toxicities of BH3 mimetic drugs in patients and pre-clinical tests in mice. CORRESPONDING AUTHORS Correspondence to Kerstin
Brinkmann or Andreas Strasser. ETHICS DECLARATIONS COMPETING INTERESTS All authors are employees of The Walter and Eliza Hall Institute (WEHI). WEHI receives royalties and milestone payments
from the sale of Venetoclax. AS has received funding for his research from Servier and has served on a strategic advisory board from Servier. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by G. Melino SUPPLEMENTARY INFORMATION SIGNED AUTHORSHIP CHANGE
FORM RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Brinkmann, K., Ng, A.P., de Graaf, C.A. _et al._ What can we learn from mice lacking pro-survival
BCL-2 proteins to advance BH3 mimetic drugs for cancer therapy?. _Cell Death Differ_ 29, 1079–1093 (2022). https://doi.org/10.1038/s41418-022-00987-0 Download citation * Received: 28
September 2021 * Revised: 04 March 2022 * Accepted: 15 March 2022 * Published: 06 April 2022 * Issue Date: June 2022 * DOI: https://doi.org/10.1038/s41418-022-00987-0 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative