Play all audios:
ABSTRACT Brown adipose tissue (BAT) is critical for non-shivering thermogenesis making it a promising therapeutic strategy to combat obesity and metabolic disease. However, the regulatory
mechanisms underlying brown fat formation remain incompletely understood. Here, we found SOX4 is required for BAT development and thermogenic program. Depletion of SOX4 in BAT progenitors
(_Sox4-MKO_) or brown adipocytes (_Sox4-BKO_) resulted in whitened BAT and hypothermia upon acute cold exposure. The reduced thermogenic capacity of _Sox4-MKO_ mice increases their
susceptibility to diet-induced obesity. Conversely, overexpression of SOX4 in BAT enhances thermogenesis counteracting diet-induced obesity. Mechanistically, SOX4 activates the transcription
of EBF2, which determines brown fat fate. Moreover, phosphorylation of SOX4 at S235 by PKA facilitates its nuclear translocation and EBF2 transcription. Further, SOX4 cooperates with EBF2
to activate transcriptional programs governing thermogenic gene expression. These results demonstrate that SOX4 serves as an upstream regulator of EBF2, providing valuable insights into BAT
development and thermogenic function maintenance. SIMILAR CONTENT BEING VIEWED BY OTHERS EPAC1 ENHANCES BROWN FAT GROWTH AND BEIGE ADIPOGENESIS Article Open access 09 January 2024 GRAF1
DEFICIENCY LEADS TO DEFECTIVE BROWN ADIPOSE TISSUE DIFFERENTIATION AND THERMOGENIC RESPONSE Article Open access 20 November 2024 DIET-INDUCED OBESITY AND AGING-INDUCED UPREGULATION OF TRIB3
INTERFERE WITH ENERGY HOMEOSTASIS BY DOWNREGULATING THE THERMOGENIC CAPACITY OF BAT Article Open access 02 December 2024 INTRODUCTION Obesity occurs when energy intake from food exceeds
energy expenditure. Adipose tissues are important for regulating whole-body energy homeostasis [1]. White adipose tissue (WAT) consists mainly of white adipocytes that store excess energy in
fat. Brown adipose tissue (BAT), on the other hand, utilizes lipids and glucose to generate heat, which is essential for maintaining body temperature and resisting cold-induced hypothermia
in mammals [2,3,4,5]. In brown adipocytes, activation of uncoupling protein-1 (Ucp1) which is located in the inner membrane of mitochondria causes proton leakage resulting in energy
dissipation as heat. Mice with enhanced thermogenic activity of BAT exhibited increased energy expenditure, resulting in resistance to weight gain [6]. Conversely, mice lacking BAT
thermogenic function were more susceptible to developing obesity [7]. Intensive studies have elucidated that metabolically active brown fat is present in both infants and adults [8]. And the
increased BAT activity of human is correlated with declining body weight [3, 9, 10]. Therefore, promoting BAT development and function might be a promising strategy to counteract obesity
and related metabolic disorders [6, 11, 12]. Several transcriptional regulators have been shown to participate in BAT development and function. The previous lineage studies clarified that
classic brown adipocytes derived from multipotent progenitor cells that express engrailed 1 (_En1_), paired box protein 3 (_Pax3_), _Pax_7, and myogenic factor 5 (_Myf5_) in the somatic
mesoderm during embryogenesis [13]. During BAT development, these progenitors develop to preadipocytes and subsequently differentiate to brown adipocytes with a transcriptional cascade.
Early B-cell factor 2 (EBF2) is an essential transcriptional regulator for brown fat cell fate. It could recruit peroxisome proliferator-activated receptor-γ (PPARγ), a key transcription
factor during adipocyte differentiation, to its brown-selective binding sites to maintain BAT identity [14, 15]. Removing the inhibitory effect on EBF2 enhances the thermogenic potential of
white adipocytes [16]. Recent studies have demonstrated that EBF2 enhances the transcriptional activity of an ERRα/PGC1α complex to promote expression of thermogenic genes and maintain core
temperature in BAT [17]. These findings indicate EBF2 is crucial for brown adipocyte differentiation and thermogenic function. However, the transcriptional regulator of _Ebf2_ remains
unknown. SRY-related High Mobility Group box transcription factor 4 (SOX4), a member of Sox C family, contains high-mobility group DNA-binding domain (HMG-Box domain) and C-terminal
transactivation domain (TAD domain) [18]. It plays a key role in regulating cell stemness, cell differentiation, and development of nervous system, endocrine islet, and heart [19,20,21]. We
found that SOX4 not only suppresses preadipocyte determination in WAT [22], but also acts as a coactivator of PPARγ to facilitate beige adipocyte formation during prolonged cold exposure
[23]. Although the regulation of brown and beige fat differentiation involves an overlapping set of pan-adipogenic and BAT-specific transcription factors [13], the potential involvement of
SOX4 in determining the fate of brown adipocytes remains unclear. Here, we show that SOX4 is required for BAT development and thermogenic function in mice by promoting the transcription of
_Ebf2_, which determines brown fat fate. This process could be facilitated by PKA activation. Our findings elucidate the regulatory role and mechanism of SOX4 in controlling thermogenic gene
programs, and suggest that targeting SOX4 may provide a potential strategy for enhancing energy expenditure to combat obesity. RESULTS SOX4 IS REQUIRED FOR BAT DEVELOPMENT AND MAINTENANCE
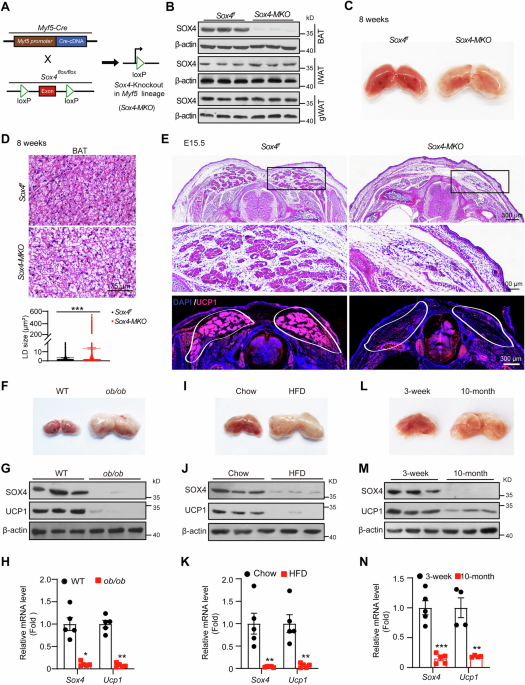
Our previous study demonstrated the requirement of SOX4 for beige adipocyte formation during prolonged cold exposure [23]. Here, we found SOX4 was mainly expressed in BAT and gonadal WAT
(gWAT) (Fig. S1A, B). Notably, the mRNA levels of Sox4 in BAT stromal vascular fraction SVF cells (SVFs) were significantly higher than those in WAT SVFs (Fig. S1C). Following a 4-hour acute
cold exposure, SOX4 mRNA levels exhibited a marked increase in both BAT tissues and BAT SVFs, while remaining unchanged in inguinal WAT (iWAT) and gWAT (Fig. S1D–F). Consequently, we sought
to investigate the role of SOX4 in BAT, which is crucial for non-shivering thermogenesis. As BAT originates from _Myf5_+ precursor cells, we generated _Myf5-Cre Sox4__f/f_ mice (referred to
as _Sox4-MKO_) by crossing _Sox4__f/f_ with _Myf5-Cre_ mice (Fig. 1A). As shown in Fig. 1B, SOX4 expression was greatly diminished in BAT of _Sox4-MKO_ mice. Additionally, the BATs from
adult or newborn _Sox4-MKO_ mice exhibited a whitened phenotype compared to that of wild type mice (Figs. 1C and S1G). H&E staining further revealed enlarged lipid droplets in BATs of
_Sox4-MKO_ mice (Figs. 1D and S1H). Since BAT develops during embryogenesis in murine models [6], we examined the development of BAT at E15.5 embryos from _Sox4-MKO_ mice. As depicted in
Fig. 1E, BAT depots in _Sox4-MKO_ mice diminished compared to that of _Sox4__f/f_ littermates and UCP1 expression was also obviously decreased. To further evaluate the role of SOX4 in BAT
development, we generated BAT-specific _Sox4_ knockout mice (_Sox4-BKO_) by crossing _Sox4__f/f_ with _Ucp1-Cre_ mice (Fig. S1I). SOX4 was specifically deleted in BAT but not in WAT (Fig.
S1J). Similar to the observations made in _Sox4-MKO_ mice, whitened BATs were observed in _Sox4-BKO_ mice (Fig. S1K) characterized by enlarged lipid droplets (Fig. S1L). Notably, whitening
of BAT is also observed in obese and older animals [24,25,26]. Accordingly, significant downregulation of SOX4 expression was detected in whitened BATs from _ob/ob_ mice (Fig. 1F–H),
high-fat diet-induced obese mice (Fig. 1I–K), and aged mice (Fig. 1L–N). Collectively, these findings suggested that SOX4 is essential for both the development and maintenance of BAT. SOX4
IS REQUIRED FOR THERMOGENESIS OF BAT Browning or whitening is highly correlated with thermogenesis of BAT which is essential for maintaining body temperature and counteracting cold exposure
[2]. To investigate the role of SOX4 in thermogenic function, we first analyzed the surface temperature of newborns of _Sox4-MKO_ and control littermates on postnatal day 1 (Fig. 2A, B).
Infrared imaging showed that the surface temperature in _Sox4-MKO_ mice (28.18 ± 0.35 °C) was remarkably lower than that of control littermates (30.15 ± 0.14 °C). Next, 8-week _Sox4__f/f_
and _Sox4-MKO_ male mice were exposed to either room temperature (25 °C) or 4 °C for 4 h, and core temperature was measured. As shown in Fig. 2C, both groups exhibited similar core
temperatures (38 °C) when maintained at room temperature. However, after 4 h cold exposure at 4 °C, core temperature of _Sox4__f/f_ mice was maintained at about 36.4 °C while that of
_Sox4-MKO_ mice declined to 34.7 °C. Since BATs primarily utilize glucose and fatty acids from plasma to generate heat in response to acute cold exposure, we measured blood glucose and free
fatty acid (FFA) levels. As shown in Fig. 2D and E, _Sox4-MKO_ mice exhibited significantly lower blood glucose levels and higher FFA levels at 4 °C, indicating a preference for glucose
consumption while exhibiting impaired fatty acid utilization under acute cold exposure. And the triglyceride (TG) levels of BATs in _Sox4-MKO_ mice were higher than those in _Sox4__f/f_ mice
at both room temperature and 4 °C, with acute cold exposure not exacerbating this difference (Fig. 2F). Additionally, the consumption of O2 and heat production of _Sox4-MKO_ mice were
significantly lower than that of _Sox4__f/f_ mice when mice were subjected into 4 °C (Fig. 2G, H). We then used CL316, 243, a β3-adrenergic receptor agonist, to mimic the effect of cold
stimulation. The increase in oxygen consumption and heat production induced by CL316, 243 treatment was significantly attenuated in _Sox4-MKO_ mice compared to _Sox4__f/f_ mice (Fig. 2I, J).
As skeletal myocytes also arise from _Myf5__+_ progenitor cells and function in shivering thermogenesis under cold stimulation [27], we examined muscle phenotype in _Sox4-MKO_ mice but no
apparent defects were observed (Fig. S2). Additionally, the role of SOX4 in BAT thermogenesis was further assessed using BAT-specific _Sox4_ knockout mice. Similar to _Sox4-MKO_ mice, both
newborns (Fig. 2K, L) and adults (Fig. 2M–R) of _Sox4-BKO_ mice exhibited decreased body temperature and impaired thermogenesis upon acute cold exposure. These results confirmed that SOX4 is
required for thermogenic function of BAT. To further investigate the role of SOX4 in facilitating thermogenesis of BAT, we characterized the phenotype of BAT from preadipocyte-specific
_Sox4_ transgenic mice (_Pref1-Sox4_). With higher expression of SOX4, BATs from _Pref1-Sox4_ mice showed a deeper brown color and smaller fat droplets (Fig. S3A–C). And the surface
temperatures of newborn WT and _Pref1-Sox4_ pups (P1) were measured using infrared imaging following a 30-minute exposure to room temperature. The surface temperature of _Pref1-Sox4_ pups
was significantly higher than that of WT pups (Fig. S3D, E). Furthermore, 8-week-old male WT and _Pref1-Sox4_ mice were subjected into a cold challenge at 4 °C for 6 hours. The body
temperature of _Pref1-Sox4_ mice declined slower than that of WT mice (Fig. S3F). After 6 hours of cold exposure, the body temperature reached 35.42 ± 0.34 °C in WT mice while it remained at
a higher level of 37.04 ± 0.42 °C in _Pref1-Sox4_ mice (Fig. S3F). Additionally, _Pref1-Sox4_ mice exhibited increased glucose consumption and triglyceride utilization compared to WT
controls (Fig. S3G, H). And metabolic cage analysis revealed a significant increase in O2 consumption and heat production in _Pref1-Sox4_ mice with acute cold stimuli (Fig. S3I, J). The
adeno-associated virus (AAV) system with _Ucp1_ mini-promoter and enhancer was used to deliver GFP (_AAV-GFP_) or SOX4 (_AAV-SOX4_) into 6-week-old male mice via tail vein injection (Fig.
S3K, L). BAT from _AAV-SOX4_ mice had deeper brown color and smaller adipocyte size (Fig. S3M, N), and their body temperatures were significantly higher than control mice by approximately 1
°C after 4 h cold exposure (Fig. S3O). Additionally, _AAV-SOX4_ mice showed elevated heat production compared to _AAV-GFP_ mice after cold stimulation (Fig. S3P). The data above suggested
that overexpression of SOX4 enhances heat production and thermogenesis of BAT. LOSS OF SOX4 IN BAT PROMOTES HFD-INDUCED OBESITY Non-shivering thermogenesis of BAT expends energy,
contributing to the maintenance of whole-body metabolic homeostasis. We investigated whether the absence of SOX4 in BAT contributes to obesity development. 6-week-old male mice with
_Sox4__f/f_ and _Sox4-MKO_ genotypes were fed either a chow diet (Fig. S4) or a high-fat diet (HFD) (Fig. 3). After 6 weeks of HFD feeding, the body weight of _Sox4-MKO_ mice significantly
exceeded that of _Sox4__f/f_ mice (Fig. 3A), while on a normal chow diet, both groups exhibited similar weight gain patterns (Fig. S4A). Furthermore, HFD-fed _Sox4-MKO_ mice displayed more
severe glucose intolerance and insulin resistance than their control counterparts (Fig. 3B, C). Body composition analysis revealed an increase in body fat, while the lean composition
remained unchanged in HFD-fed _Sox4-MKO_ mice compared to the controls (Fig. 3D). Consistently, the percentages of BAT, iWAT, gWAT, and liver were markedly higher in HFD-fed _Sox4-MKO_ mice
compared to those in controls (Fig. 3E), with larger individual fat pads and livers observed as well (Fig. 3F). Histological analysis showed significantly larger lipid droplets in BAT, iWAT,
and gWAT of HFD-fed _Sox4-MKO_ mice, along with increased lipid accumulation in liver sections (Fig. 3G, H). Moreover, the liver TG content, serum TG levels, and FFA levels were
significantly elevated in _Sox4-MKO_ mice fed a HFD (Fig. 3I–K). Consistent with an increased susceptibility to obesity, F4/80, a macrophage marker, was found to be significantly upregulated
in the gWAT of HFD-fed _Sox4-MKO_ mice compared to their _Sox4__f/f_ counterparts (Fig. 3L). Metabolic cage analysis further confirmed that HFD-fed _Sox4-MKO_ mice displayed significantly
reduced oxygen consumption and heat production, while food intake and locomotor activity remained similar to those of _Sox4__f/f_ mice (Fig. 3M–P). On normal chow diet (NCD), the mass and
the morphology of BAT, iWAT, gWAT and liver, as well as O2 consumption and heat production, were similar between _Sox4-MKO_ and _Sox4__f/f_ mice (Fig. S4B–H). These data suggest that loss of
SOX4 in BAT impairs thermogenesis and reduces energy consumption, thereby increasing susceptibility to obesity when exposed to HFD. We further investigated whether overexpression of SOX4 in
BAT could counteract HFD-induced obesity. Mice injected with _AAV-GFP_ or _AAV-SOX4_ were fed with NCD or HFD (Fig. S5A). On NCD, both groups exhibited similar weight gain (Fig. S5B). After
5 weeks on HFD, the body weight of _AAV-SOX4_ mice was significantly lower than that of _AAV-GFP_ mice (Fig. S5B). After 10 weeks of HFD treatment, _AAV-SOX4_ mice displayed improved blood
glucose tolerance and insulin sensitivity, and reduced body fat composition (Fig. S5C–E). The percentages of BAT, iWAT, gWAT and liver in _AAV-SOX4_ mice were significantly lower than those
in _AAV-GFP_ mice (Fig. S5F). Additionally, _AAV-SOX4_ mice had decreased serum TG and FFA levels (Fig. S5G, H). Consistently, compared to the control mice, _AAV-SOX4_ mice had smaller
individual fat pads and liver (Fig. S5I). H&E staining showed that _AAV-SOX4_ mice had markedly smaller lipid droplets in BAT, iWAT, gWAT and liver (Fig. S5J, K). Metabolic cage analysis
revealed increased oxygen consumption and heat production in _AAV-SOX4_ mice while food intake and locomotor activity remained similar to those of _AAV-GFP_ mice (Fig. S5L–O). Overall,
these results demonstrate that overexpressing SOX4 in BAT increases energy expenditure and attenuates HFD-induced obesity. SOX4 PROMOTES BAT-SELECTIVE GENES EXPRESSION IN VIVO We then
investigated how SOX4 regulates BAT development and thermogenesis. The brown coloration of BAT is attributed to its abundant presence of mitochondria and cytochrome, which confers a rich
metabolic capacity [28]. In S_ox4-MKO_ mice, we observed a whitening phenomenon in BAT, suggesting potential alterations in mitochondrial structure and function. Transmission electron
microscopy analysis showed that brown adipocytes from _Sox4-MKO_ mice exhibited larger lipid droplets and disorganized cristae within their mitochondria compared to control mice (Fig. 4A).
Furthermore, quantitative analysis revealed a decrease in both the number of mitochondria and mitochondrial DNA content in BAT from _Sox4-MKO_ mice (Fig. 4B, C). To further elucidate the
impact of SOX4 on BAT development, we performed RNA sequencing of BAT tissues from _Sox4__f/f_ and _Sox4-MKO_ mice. Heatmaps revealed downregulation of numerous genes involved in
mitochondria complex I-V, as well as key BAT-selective genes including _Pparα_, _Ucp1, PGC1α_, _Cidea, Cpt1b, Dio2, Ebf2_ in the BAT of _Sox4-MKO_ mice (Fig. 4D). Gene ontology (GO) analysis
revealed the downregulated genes in the _Sox4-MKO_ mice were associated with thermogenesis, mitochondrial oxidative phosphorylation, brown fat cell differentiation, TCA cycle and fatty acid
metabolism (Fig. 4E). These findings were further validated through qPCR analysis (Fig. 4F, G). PGC1α, a crucial regulator of mitochondrial biogenesis, is essential for maintaining BAT
thermogenesis [29]. The low expression of PGC1α in BAT of _Sox4-MKO_ mice suggested a potential link between SOX4 deficiency, impaired mitochondria, and compromised thermogenesis. Moreover,
levels of mitochondrial complex proteins and BAT-selective proteins were reduced in BAT from _Sox4-MKO_ mice (Fig. 4H, I). Notably, we observed a significant upregulation of WAT-selective
genes in BAT of _Sox4-MKO_ mice (Fig. 4D, and Fig. S6A), while the expression levels of common pan-adipocyte markers remained comparable to those in _Sox4__f/f_ mice (Fig. S6B). Consistent
with the whitening of BAT, the mRNA levels of lipolysis-related genes _Hsl_, _Atgl_, and _Mgll_ were significantly downregulated in _Sox4-MKO_ mice (Fig. S6C). Additionally, reduced levels
of p-HSL/HSL and phospho-PKA substrates, along with elevated PLIN1 levels in the BAT further indicated suppressed lipolysis in _Sox4-MKO_ mice (Fig. S6D, E). Moreover, the whitening of BAT
indicated increased inflammation and lipogenesis [30, 31]. Consequently, we reanalyzed the RNA-seq data from BATs of _SOX4__f/f_ and _Sox4-MKO_ mice with GO analysis, which revealed a
significant upregulation of genes associated with inflammation and lipogenesis (Fig. S6F, G). These findings were further confirmed by qPCR (Fig. S6H, I). We also performed a rescue
experiment by reintroducing SOX4 into _Sox4-MKO_ mice via adenovirus injection (Fig. S7A). 7 days after the first injection, BAT tissues were collected for analysis. qPCR analysis revealed
that the expressions of BAT-selective genes, including _Ucp1_, _Prdm16_, _Pgc1α_, and _Dio2_, were fully restored in BAT expressing SOX4 (Fig. S7B). Immunofluorescence staining further
confirmed protein levels of UCP1 and PRDM16 were restored with SOX4 expression (Fig. S7C). Additionally, histological analysis showed that SOX4 overexpression rescued the enlarged lipid
droplets observed in BAT of _Sox4-MKO_ mice (Fig. S7D, E). We also explored whether overexpression of SOX4 could promote the expression of BAT-selective genes. We observed increased
expression of BAT-specific genes and decreased expression of white-specific genes in _AAV-SOX4_ mice (Fig. S7F, G). Immunohistochemical experiments further confirmed that _AAV-SOX4_ mice
exhibited enhanced UCP1 expression compared to _AAV-GFP_ mice (Fig. S7H). Collectively, these findings demonstrate that SOX4 is essential for the expression of BAT-selective genes which are
crucial for maintain BAT characteristics. SOX4 IS REQUIRED FOR BROWN ADIPOCYTE DIFFERENTIATION IN VITRO The differentiation of brown adipocytes and the development of BAT critically rely on
genes selectively expressed in BAT [32, 33]. To investigate the essential role of SOX4 in brown adipocyte differentiation in vitro, we immortalized BAT SVF cells isolated from newborn male
WT mice for subsequent experiments. The immortalized BAT SVF cells were infected with lentivirus expressing scramble or shSox4 and then induced to differentiate into mature brown adipocytes
in vitro (Fig. 5A). Oil Red O staining revealed that the loss of SOX4 promoted lipid storage in mature brown adipocytes, resulting in significantly elevated triglyceride levels (Fig. 5B, C).
The expression of BAT-selective genes was significantly downregulated, while WAT-selective genes were upregulated (Fig. 5D, E). Western blot analysis showed that the BAT-selective protein
levels in SOX4 knockdown adipocytes were noticeably decreased, whereas the protein levels of AGT were significantly increased (Fig. 5F). Additionally, mitochondria-containing DNA was largely
depleted in SOX4 knockdown cells (Fig. 5G). Consistently, SOX4 knockdown cells displayed reduced levels of basal mitochondrial respiration and maximal mitochondrial respiratory capacity
(Fig. 5H–J). Similar results were observed in brown adipocytes differentiated from primary BAT SVFs isolated from _Sox4-MKO_ mice (Fig. S8A–J). These results demonstrate that SOX4 is
essential for brown adipocytes differentiation in vitro. SOX4 ACTIVATES TRANSCRIPTION OF EBF2 FACILITATING BAT DEVELOPMENT To elucidate the mechanism by which SOX4 regulates brown adipocyte
differentiation, we examined the expression of adipogenic regulators at different time points. Notably, expression of _Ebf2_ was remarkably decreased in Sox4-knockdown cells at the early
stage of differentiation (Fig. S9A, B). Consistently, overexpression of SOX4 promoted the expression of EBF2 (Fig. S9C). We further compared RNA-seq data from _Sox4-MKO_ BAT with that from
_Ebf2-AKO_ BAT (GSE144188) (Fig. 6A), and we found that 862 genes were down-regulated in _Sox4 MKO_ mice (KO: WT < 0.05). Among these genes, 557 overlapped with the down-regulated genes
identified in _Ebf2 AKO_ mice. GO analysis revealed that the overlapping downregulated genes were associated with thermogenesis, oxidative phosphorylation, brown fat cell differentiation,
TCA cycle and fatty acid metabolism (Fig. 6A). These indicates that SOX4 may regulate BAT development and thermogenesis via EBF2. To further confirm this hypothesis, we isolated primary BAT
SVFs from _Sox4__f/f_ and _Sox4-MKO_ mice and performed a rescue experiment in SOX4-knockout BAT SVFs. As depicted in Fig. S9D, E, overexpression of EBF2 significantly reduced TG content and
restored the expression of BAT-selective genes in SOX4-knockout cells. Furthermore, knockdown of EBF2 largely blocked enhancement of BAT-selective genes expression mediated by
overexpression of SOX4 (Fig. S9F). These results suggest that SOX4 promotes the expression of BAT-selective genes by enhancing the transcription of EBF2. To address the direct regulation of
EBF2 transcription by SOX4, we performed ChIP-seq analysis. As shown in Fig. 6B, ~4 kb region of _Ebf2_ was bound by SOX4, and ChIP-qPCR assays confirmed specific binding of SOX4 to this
region (Fig. 6C). Next, the SOX4 binding region in _Ebf2_ was cloned into a pGL4.26 vector for luciferase reporter assay. SOX4 significantly increased the luciferase activity, and this
activation could be blocked by mutation of SOX4 binding site (AACAAGT mutant to GCTCGGT) (Fig. 6D). To elucidate whether the transcriptional activation of Ebf2 relies on the HMG-box domain
(HMG-BOX DNA-binding domain) or the TAD domain (C-terminal transactivation domain) of SOX4 protein, we generated SOX4 mutants lacking either the HMG-box domain (ΔHMG) or the TAD domain
(ΔTAD) (Fig. S9G). Luciferase assay results revealed that when either the HMG-box domain or TAD domain was absent, SOX4 failed to activate Ebf2-luciferase activity (Fig. 6E). Further,
FAIRE-qPCR assay showed knockdown of SOX4 markedly reduced chromatin accessibility at _Ebf2_ enhancer (Fig. 6F). These results indicate that SOX4 directly activates the transcription of
EBF2. EBF2 determines brown adipocyte identity by recruiting PPARγ to its brown-selective binding sites [15]. We found that EBF2 protein levels in BAT were remarkably decreased in both
embryonic and adult _Sox4 MKO_ mice (Figs. 4I and 6G), while PPARγ protein levels were not affected by loss of SOX4 (Fig. S9H). Therefore, we investigated whether the loss of SOX4 reduced
PPARγ binding to BAT-selective genes. BATs from _Sox4__f/f_ and _Sox4-MKO_ mice were subjected into ChIP-qPCR using anti-PPARγ antibody. The loss of SOX4 reduced PPARγ binding at promoter
regions of _Ucp1_ and _Prdm16_, and increased PPARγ binding at promoter regions of _Agt_ (Fig. S9I). Taken together, these results suggested that SOX4 activated EBF2 expression, promoting
PPARγ binding to promoters of BAT-selective genes, which facilitates BAT development and thermogenic function. To assess the role of SOX4 at the early stages of BAT development, we examined
control and mutant embryos at E15.5. Immunostaining analysis revealed a significant decrease in EBF2 protein levels in _SOX4-MKO_ mice (Fig. 6G). We analyzed expression profiles of SOX4 and
EBF2 using scRNA-seq data from the dorsal-anterior region of mouse embryos at E10.5–E13.5 (GSE233955) (Fig. 6H) [34]. The evaluation of the cell profiles at different developmental time
points revealed a prominent presence of sclerotome at E10.5-E11.5 and the existence of _Cdh4__+_/ _Ebf2__+_ cell cluster was observed at E12.5 and E13.5 (Fig. 6I). The expression of SOX4 and
EBF2 exhibited high degree of spatial coincidence in sclerotome cells and _Ebf2__+__/Cdh4__+_ BAT preadipocytes at E10.5-E13.5 (Fig. 6J). And the peak expression of SOX4 was observed at
E12.5 which is earlier than the peak expression time of EBF2 (Fig. 6K). Furthermore, we analyzed the expression patterns of SOX4 and EBF2 using RNA-Seq data from human induced pluripotent
stem cells (iPSCs) that were differentiated to brown adipocytes (GSE131169). In this process, highest expression levels of SOX4 were observed during the early stage, followed by an increase
in EBF2 expression (Fig. 6L). Collectively, these results suggest SOX4 plays a regulatory role at early-stage of BAT development. PHOSPHORYLATION OF SOX4 BY PKA FACILITATES ITS NUCLEAR
TRANSLOCATION AND ENHANCES THE TRANSCRIPTION OF _EBF2_ Notably, induction medium could promote nuclear localization of SOX4 in brown adipocytes differentiation (Fig. 7A). We also performed
ChIP-Seq to visualize the binding of SOX4 to _Ebf2_ enhancer in the presence or absence of induction medium. As shown in Fig. 7B, SOX4 directly bound to -4 kb region of _Ebf2_, and its
binding intensity was dramatically increased by treatment of induction medium. The administration of forskolin could mimic the effect of induction medium on SOX4 nuclear translocation and
its binding to _Ebf2_ enhancer, thereby increased the mRNA levels of _Ebf2_ (Fig. 7C–E). Notably, the loss of SOX4 significantly blunted the increase in _Ebf2_ mRNA levels by forskolin
treatment (Fig. 7E). Cold stimulation can increase the population of brown precursor fat cells [35]. EBF2 specifically marks and regulates the molecular profile of brown preadipocytes [14].
Therefore, _Sox4__f/f_ and _Sox4-MKO_ mice were subjected into RT or 4 °C for 4 h, and primary BAT SVFs were isolated for qPCR analysis. As shown in Fig. 7F, the increased mRNA levels of
_Ebf2_ induced by cold stimuli were largely blunted in the BAT of _Sox4-MKO_ mice. The PKA inhibitor H89 effectively blocked the nuclear translocation of SOX4 and SOX4-mediated
transcriptional activation of EBF2 induced by forskolin (Fig. 7C, G). Subsequently, HA-SOX4 was purified from cells overexpressing either PKACA or a dominant negative variant of PKACA,
followed by comprehensive mass spectrometry analysis. The phosphorylated S235-containing peptide of SOX4 was specifically detected in PKACA expressing cells, as illustrated in Fig. 7H.
Additionally, sequence alignment analysis revealed the conservation of residue S235 in SOX4 across diverse species (Fig. 7I). To investigate the impact of S235 phosphorylation, we generated
an S235A mutant to mimic unphosphorylated SOX4 (Fig. 7I). This mutant was unable to enter the nucleus and failed to activate _Ebf2_ transcription upon forskolin stimulation (Fig. 7J, K).
Taken together, these findings suggest that forskolin induces PKA activation, leading to the phosphorylation of SOX4 at S235 and its subsequent translocation into the nucleus (Fig. 7L). SOX4
COOPERATES WITH EBF2 TO ACTIVATE THE TRANSCRIPTION OF _EBF2_ Considering the adjacent binding sites of SOX4 and EBF2 at the region of _Ebf2_ (Fig. S10A), we sought to investigate whether
these two factors could collaborate in activating EBF2 transcription. First, SOX4 and EBF2 could be coprecipitated and exhibited nuclear colocalization in BAT SVF cells (Fig. S10B–D).
Notably, luciferase experiments showed that individual expression of SOX4 or EBF2 led to about a 5-fold activation, whereas their combined effect synergistically enhanced EBF2 transcription
approximately 33-fold (Fig. S10E). We further explored the SOX4-EBF2 interaction interfaces, revealing that the TAD domain of SOX4 is required for its interaction with EBF2 (Fig. S10F, G).
Moreover, the truncation lacking either the HMG or TAD domain did not exhibit a synergistic effect with EBF2 in enhancing EBF2 transcription (Fig. S10H), thereby suggesting that the
synergistic effect between SOX4 and EBF2 relies on both the binding of the HMG domain to the promoter region and the interaction of TAD domain with EBF2. These results reveal that SOX4 could
cooperate with EBF2 to activate transcription of EBF2 (Fig. 7L). SOX4 COOPERATES WITH EBF2 TO ENHANCE EXPRESSION OF THERMOGENIC GENES Given our clear elucidation of the interaction between
SOX4 and EBF2 in promoting EBF2 transcription, we aimed to investigate their potential cooperative role in enhancing the transcription of thermogenic genes targeted by EBF2. SOX4 exhibited
colocalization with EBF2 and could precipitate with EBF2 in mature brown adipocytes (Fig. 8A, B). Next, we reanalyzed the ChIP-seq profiles of EBF2 (GSE97114) at the _Ucp1_ and _Prdm16_
enhancers, which revealed binding peaks of EBF2 in the -6k region of _Ucp1_ and -1k region of _Prdm16_ [36], which coincided with the conserved binding site for SOX4 (AACAAAG) in these
regions (Fig. S11A). Furthermore, ChIP-qPCR experiments confirmed the specific binding of SOX4 to these regions (Fig. 8C). FAIRE-qPCR experiments indicated that loss of SOX4 significantly
reduced chromatin accessibility at both _Ucp1_ and _Prdm16_ (Fig. 8D). Additionally, the luciferase assays demonstrated a significant augmentation in the transcription of _Ucp1_ and _Prdm16_
when SOX4 and EBF2 were co-overexpressed (Fig. 8E). The SOX4 truncation lacking either the HMG or TAD domain failed to cooperate with EBF2 in activating the transcription of _Ucp1_ and
_Prdm16_ (Fig. S11B, C). These findings collectively suggest that the cooperation between SOX4 and EBF2 drives the transcription of thermogenic genes in brown adipocytes (Fig. 8F).
DISCUSSION BAT is essential for non-shivering thermogenesis. It develops at the embryonic stage and is quite important for maintaining body temperature especially for newborns to counteract
cold [37]. Promoting BAT development and thermogenic function to elevate energy consumption is a potential strategy to counteract obesity and obesity-related diseases [4]. EBF2 and PRDM16
are identified as key transcription factors for activation of thermogenic gene program to determine brown adipocyte cell fate [6, 15]. In the process of brown adipocyte differentiation, EBF2
expression was stimulated at early stage while PRDM16 expressed at late stage [34, 38]. EBF2, which is highly expressed in BAT, could recruit PPARγ binding to BAT-specific genes and
activate BAT-selective genes expression [15]. This is fundamental for brown adipocytes development. However, the upstream regulator of EBF2 remains unknown. Here, we found that deletion of
SOX4 impaired EBF2 expression in BAT, thereby suppressing expression of BAT-selective genes and thermogenic genes such as UCP1 and PRDM16. In vitro, the brown adipocytes differentiated from
SOX4 knockdown BAT SVF cells exhibited reduced expression of BAT thermogenic genes, leading to the accumulation of large lipid droplets. In contrast, SOX4 overexpression enhanced BAT
thermogenic genes expression and reduced the size of lipid droplets. In vivo, similar to EBF2-promoter mice [15, 36], _Sox4 MKO_ and _Sox4 BKO_ mice exhibited deficiencies in BAT development
and thermogenic function, resulting in hypothermia with acute cold exposure. _Sox4 MKO_ mice were easy to gain weight with HFD. Elevated expression of SOX4 in mice resulted in upregulation
of thermogenic genes and increased heat production, thereby mitigating HFD-induced obesity. Besides, older or obese mice exhibited severely whitened BAT, and SOX4 expression in BAT was
significantly lower. These results indicated that SOX4 is essential for BAT development and the maintenance of thermogenic function. SOX4 is required for the early development of BAT during
mouse embryonic stages. In _Sox4-MKO_ mice, BAT depots diminished significantly compared to that in _Sox4__f/f_ littermates, and immunostaining analysis revealed a notable decrease in UCP1
and EBF2 expression at E15.5. Additionally, SOX4 directly activates the transcription of EBF2, which is a key factor determining the cell fate of brown adipocytes. The scRNA-seq data from
the dorsal-anterior region of mouse embryos at E10.5–E13.5 revealed that SOX4 and EBF2 exhibited a high degree of spatial coincidence in sclerotome cells as well as _Ebf2__+__/Cdh4__+_ BAT
preadipocytes, suggesting that SOX4 may play a crucial role in brown preadipocyte commitment. Furthermore, RNA-Seq data from human iPSCs differentiated into brown adipocytes revealed an
initial observation of elevated levels of SOX4 on day 4 of differentiation, which is expressed as early as EN1 [39]. These suggest SOX4 plays a crucial role in embryonic BAT development.
SOX4 regulates BAT development and thermogenesis via EBF2. RNA-seq analysis revealed many downregulated thermogenic genes from _Sox4-MKO_ were overlapped with down-regulated genes from _Ebf2
AKO_ mice. Overexpression of EBF2 in SOX4 knockout SVF cells significantly rescued expression of thermogenic genes. Further, ChIP-qPCR results demonstrated that SOX4 binds to promoter of
EBF2. SOX4 directly activated EBF2-luciferase in a manner dependent on its HMG and TAD domains. The HMG domain, which contains an L-shaped structure, binds to target DNA sequences and alters
chromatin conformation to facilitate transcription [40, 41]. These findings suggested SOX4 may mediate remodeling of chromatin structure in activating EBF2 transcription. FAIRE-qPCR results
revealed depletion of SOX4 significantly reduced chromatin accessibility of EBF2 promoter. EBF2, an essential transcriptional regulator of brown fat cell fate, can promote PPARγ to target
to BAT-selective genes and thermogenic genes to maintain BAT identity [15]. Consistently, in BAT of _Sox4-MKO_ mice, the binding of PPARγ to the _Prdm16_ and _Ucp1_ promoters decreased
significantly. Additionally, the cooperative interaction between SOX4 and EBF2 leads to a robust transcriptional activation of downstream thermogenic genes. Therefore, SOX4 regulates brown
fat formation and thermogenic function by mediating adipocyte remodeling via EBF2. Besides, similar to _Ebf2__-/-_ mice, SOX4-deficient mice exhibit the presence of adipocytes instead of a
total lack of BAT. Other factors, such as Ebf1 or Ebf3, may compensate for the role of Ebf2 in adipocyte differentiation in _vivo_ [15, 17]. Nevertheless, the brown characters and functions
of BATs from SOX4-deficient mice are diminished. Loss of SOX4 results in lower expression EBF2, which recruits PPARγ to its brown-selective binding sites and reduces its binding to
WAT-specific sites [15]. In the absence of SOX4/EBF2, PPARγ binding to WAT-specific sites is enhanced, resulting in a significant increase in the expression of WAT-selective genes that
ultimately promote whitening of BAT. Additionally, genes associated with lipogenesis were upregulated, whereas those associated with lipolysis exhibited downregulation in BATs from
_SOX4-MKO_ mice, thereby facilitating triacylglycerol accumulation and contributing to whitening of BAT. SOX4 plays a critical role in adaptive thermogenesis. Under acute cold exposure, SOX4
mRNA levels were increased both in BAT tissues and SVF cells. Due to defects in thermogenic gene program, SOX4-deficient mice had lower adaptive thermogenesis in response to acute cold
exposure. In adult mice, _Sox4 MKO_ and _Sox4 BKO_ mice as well as _Sox4__f/f_ mice had similar levels of heat production at room temperature. When mice were subjected to 4 °C, _Sox4__f/f_
mice exhibited higher production of heat and consumption of O2, while that of _Sox4 MKO_ and _Sox4 BKO_ mice was significantly lower. On normal chow, there was no significant difference in
the heat production of _Sox4 MKO_ and _Sox4__f/f_ mice. When mice were fed with HFD, _Sox4 MKO_ showed deficiency in thermogenesis and reduced O2 consumption. Due to decreased heat
production and energy consumption, _Sox4 MKO_ mice were prone to develop obesity and related metabolic diseases. Conversely, with SOX4 overexpressed in preadipocytes, _Pref1-Sox4_ mice could
produce more heat under acute cold exposure or HFD [23]. Overexpression of SOX4 with adenovirus or AAV promoted expression of thermogenic genes. In addition, we previously found that SOX4
is expressed in BAT as well as in WAT and SOX4 is required for beige adipocyte-mediated adaptive thermogenesis with prolonged cold exposure [23]. These findings suggest that enhancing the
expression of SOX4 in adipocytes may represent a promising strategy for promoting energy expenditure to counteract obesity. Notably, acute cold exposure treatment significantly upregulated
the expression of SOX4 and EBF2, along with an increased proportion of BAT preadipocytes in BAT SVFs [35]. Cold stimuli can activate the cAMP-PKA pathway through adrenergic receptor
signaling. Forskolin activates PKA, leading to the S235 phosphorylation of SOX4, which promotes its nuclear translocation and binding to the EBF2 promoter, thereby enhancing transcriptional
activity of EBF2. These findings suggest that cold exposure may induce brown adipocyte formation by upregulating EBF2 expression mediated by PKA-phosphorylated SOX4. There are still some
issues that need to be addressed in further experiments. Our data from experiments with differentiated cells and mouse models have demonstrated that SOX4 is required for brown adipocytes
differentiation. To further analyze the role of SOX4 in determining brown cell fate, lineage-tracing studies need to be confirmed in the future. Additionally, how the phosphorylation of SOX4
mediates its nuclear translocation and promotes its transcriptional activity remains unclear. S235A mutation of SOX4 failed to enter the nucleus and was not able to activate the
transcription of EBF2 with forskolin treatment. Through the AlphaFold protein structure website (https://alphafold.ebi.ac.uk/), we analyzed the spatial localization of serine residues at
position S235. It is located in the linker region between regular and irregular structures. Phosphorylation of S235 might alter the spatial conformation of SOX4, enabling it to be recognized
by the nuclear pore protein complex for entry into the cell nucleus. Identifying factors that enable phosphorylated SOX4 to enter the nucleus may reveal new strategies to enhance
thermogenesis and energy consumption. Promoting BAT development and function might be a promising strategy to counteract obesity and related metabolic disorders [42, 43]. Short-term cold
exposure enables brown fat remodeling in mouse and human [44, 45]. We have established that SOX4 is essential for brown fat development and maintenance. A notable decrease in SOX4 expression
was observed in the whitened BATs of genetically modified or HFD-induced obese mice, as well as in aged mice. Our findings show that AAV-mediated overexpression of SOX4 enhances thermogenic
capacity of BATs, helping mice resist HFD-induced obesity. Genetic modification of adipose tissue using AAV technology offers a promising strategy for developing innovative treatments for
obesity-related metabolic disorders [46]. In addition, recent reports suggest that the application of human brown-like adipocytes in cell-based therapies presents significant therapeutic
benefits in mouse models [47]. Elucidating the role and mechanisms by which SOX4 influences brown fat cell differentiation may facilitate the engineering of precursor cells into brown or
beige adipocytes, potentially offering new avenues for future cellular therapies. MATERIALS AND METHODS MICE Mice were housed in colony cages under specific conditions: temperature
maintained at 22–24 °C, humidity at 50–60%, and a 12-hour light/dark cycle starting at 7:00 am. All mice were age-matched male mice with a C57BL/6 genetic background, and specific ages were
detailed in the figure legends. _Sox4__f/f_ mice, generated by GemPharmatech Company (Nanjing, China), and _Pref1-Sox4_ mice, generated by Cyagen Company (Guangzhou, China), were previously
described [22, 23]. _Myf5-Cre_ mice were provided by Prof. Wei Mo at Zhejiang University, and _Ucp1-Cre_ mice were provided by Prof. Tongjin Zhao at Fudan University. _Sox4__f/f_ mice were
crossed with _Myf5-Cre_ or _Ucp1-Cre_ transgenic mice to generate _Sox4-MKO_ or _Sox4-BKO_ mice, respectively (Figs. 1A and S1I). The _ob/ob_ mice were purchased from Shanghai Model
Organisms Center. The Taq MasterMix (CWBIO, Cat#CW0690H) was used for genotyping, and the genotyping primers are listed in Supplemental Table 1. The chow diet (NCD, Xietong Organism,
Nanjing, China) consists of 67.4% carbohydrates, 20.6% protein, 12% fat. The high-fat diet (HFD, Readydietech Co., Ltd., Shenzhen, P. R. China) contains 20% calories from carbohydrate, 20%
calories from protein, and 60% calories from fat. IMMUNOHISTOCHEMISTRY AND IMMUNOFLUORESCENCE For H&E staining, mice were euthanized, and indicated tissues or embryos were fixed in 4%
paraformaldehyde (PFA) overnight at room temperature. The fixed tissues were dehydrated in ethanol, embedded in paraffin and sectioned at 4 μm. Sections were stained with hematoxylin and
eosin (Wanleibio, Cat#WLA051a) according to manufacturer’s instructions. For immunofluorescence, the slides were deparaffinized and performed heat-induced antigen retrieval with 10 mM sodium
citrate. The slides were then blocked with 3% BSA and 0.1% Triton X-100 in PBS (Pricella, Cat#PB180327) for 1 hr at room temperature, followed by incubation with the indicated primary
antibodies: SOX4 (Abcam, Cat#ab243041) (dilution 1:100), PRDM16 (Abcam, Cat#ab106410) (dilution 1:50), TOM20 (Santa Cruz, Cat#sc-17764) (dilution 1:100), EBF2 (Affinity, Cat#DF13398)
(dilution 1:100), F4/80 (Abclonal, Cat#A18637) (dilution 1:100), Flag (Sigma-Aldrich, Cat#F7425) (dilution 1:200), UCP1 (Abclonal, Cat#A5857) (dilution 1:100). Subsequently, the slides were
washed three times with PBS and incubated with indicated secondary fluorescent-conjugated antibodies: Alexa Fluor 488 (Invitrogen, Cat#A11029) (dilution 1:1000), Alexa Fluor Plus 647
(Invitrogen, A32733) (dilution 1:2000), at room temperature for 1 h in the dark. Finally, the slides were washed three times with PBS for 5 minutes and incubated with 5 μg/μl DAPI (Mei5
Biotech) for 10 minutes. For mitochondrial quantification of BAT tissue, the mitochondrial marker Tom20 was selected for mitochondrial quantity analysis. Samples were observed using the
Zeiss LSM 780 confocal microscope or Leica Aperio Versa 200 microscope [48]. For immunohistochemistry, paraffin-embedded tissues underwent deparaffinization, rehydration, and antigen
retrieval. The sections were then treated with 3% H2O2, permeabilized with 0.1% Triton X-100, and incubated with the UCP1 antibody overnight at 4 °C. Subsequently, adipocytes labeled with
the UCP1 antibody were visualized using goat anti-rabbit IgG conjugated with HRP (EpiZyme, Cat#LF101) (dilution 1:5000), following the instructions of the DAB chromogenesis kit (Elabscience,
Cat#E-IR-R217). TRANSMISSION ELECTRON MICROSCOPY Dissected BATs were cut into small pieces and fixed overnight at 4 °C in pre-cooled fixation buffer (2.5% glutaraldehyde, 0.1 M phosphate
buffer, pH 7.4). The fixed samples were rinsed three times with 0.1 M phosphate buffer and post-fixed in 1% OsO4 for 2 hr. Then the samples were rinsed and dehydrated sequentially in
concentration gradient ethanol. After embedding and slicing, thin sections were stained with uranyl acetate and lead citrate before imaging. Electron microscopy (Hitachi, HT-7800) was
utilized for image capture [49]. BODY-COMPOSITION ANALYSIS The mice were immobilized with the indicated tool and then positioned in the instrument for body composition analysis. The Echo MRI
composition analyzer (Echo Medical Systems, 100H) was employed to assess the fat mass and lean mass data of the mice. INFRARED THERMOGRAPHY AND COLD TOLERANCE TEST (CTT) Mice were genotyped
on postnatal day 1. The 1-day-old male mice were housed individually for 30 minutes at room temperature, and the surface temperature was immediately obtained with an infrared imaging device
(Junctec, Ax5). For CTT, the mice were individually housed in cages at 22 °C for one week prior to the test. On the day of experiment, mice were fasted for 4 h with water and then moved to
individual cages at 4 °C. Rectal temperature was measured with a rectal thermocouple probe (NJKEWBIO, FT3400) at indicated time. At the end of these experiments, the mice were euthanized for
subsequent experiments [50]. GLUCOSE AND INSULIN TOLERANCE TESTS For glucose tolerance test, the mice were fasted for 16 hr with assess to water only, and then they received an
intraperitoneal injection of D-glucose at a dose of 1.0 g/kg body weight. For insulin tolerance test, mice were starved for 6 hr with assess to water and then intraperitoneally injected with
human insulin at a dose of 1.5 U/kg. Blood samples were collected from the tail vein at 0, 15, 30, 60, 90 and 120 minutes after the intraperitoneal injection, and blood glucose levels were
measured using a glucometer. METABOLIC CAGE STUDY Before this experiment, the mice were individually housed at 22 °C for a week and then acclimatized to the metabolic cages for 2 days. The
Sable Promethion system was employed to meticulously record various parameters including food intake, body weight, oxygen consumption, heat production, and locomotor activities. To evaluate
metabolic data during acute cold challenge, the instrument temperature was adjusted to 4 °C after fasting the mice for 4 hr. Oxygen consumption and heat production were continuously
monitored during this period. To test metabolic data after intraperitoneal injection of CL316,243 (MCE, Cat#HY-116771A), _Sox4__f/f_ and _Sox4-MKO_ mice were injected with CL316,243 (1
mg/kg) intraperitoneally. The mice were then moved to metabolic cage, and oxygen consumption and heat production were monitored. WESTERN BLOT ANALYSIS The protein from tissues or cells were
lysed using RIPA lysis buffer (APExBIO, Cat#K1120, Houston, USA) supplemented with phosphatase inhibitor and proteinase inhibitor cocktail (TargetMol, Cat#C0001). After ultrasonication, the
lysates were centrifuged at 13,000 g for 10 min at 4 °C. The homogenized supernatants were quantified using a BCA protein assay kit (NCM biotech, Cat#WB6501). The protein samples were
separated on SDS-PAGE (CYTOCH, Cat#PW0002) and transferred onto hydrophobic PVDF membrane (Millipore, Cat#IPVH00010). The membranes were blocked with 5% defatted milk powder at room
temperature for 1 hr and then incubated with indicated primary antibodies, including SOX4 (Abcam, Cat#ab70598) (dilution 1:200), PCG1a (Millipore, Cat#AB3242) (dilution 1:1000), AGT
(Abclonal, Cat#A11689) (dilution 1:1000), OXPHOS (Abcam, Cat#ab110413) (dilution 1:1000), Flag (Sigma-Aldrich, Cat#F7425) (dilution 1:1000), HA (Bioss, Cat#BMS0966M) (dilution 1:1000), PPARγ
(Bioworld, Cat#BS79617) (dilution 1:1000), β-actin (Sino Biological, Cat#109444-T36) (dilution 1:10000), UCP1 (Diagbio, Cat#db9840) (dilution 1:1000), PRDM16 (Abcam, Cat#ab106410) (dilution
1:500), PLIN1 (Boster, Cat#AAED-16) (dilution 1:1000), HSL (ZENBIO, Cat#344379) (dilution 1:1000), p-HSL-S660 (SAB, Cat#12416) (dilution 1:1000), p-PKA Substrate (CST, Cat#9621) (dilution
1:1000). Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies: Goat Anti-Rabbit IgG Antibody (GenScript, Cat#A00098) (dilution 1:5000), Goat Anti-Mouse IgG
Antibody (GenScript, Cat#A00160) (dilution 1:5000). The membrane was visualized with enhanced chemiluminescence detection (Abbkine, Cat#BMU101). GENE EXPRESSION ANALYSIS Total RNA was
extracted from tissues or cells using TRIzol reagent (Accurate Biology, Cat#AG21101) according to standard protocols and dissolved in DEPC H2O. cDNA was synthesized by cDNA synthesis kit
(Abm, Cat#G490), and quantitative PCR (qPCR) was performed with a real-time PCR system (Bio-Rad) using SYBR Green (Swiss Affinibody LifeScience AG, Cat#Q01). _18S_ mRNA was used as the
invariant control. The primers sequences are listed in Supplementary Table 1. For RNA-Seq, BAT tissues were isolated from 15-week-old male _Sox4-MKO_ mice and control littermate mice (n =
2). The total RNA was extracted using TRIzol Reagent, and 4 µg of total RNA was used to construct biologically sequencing libraries essentially according to Illumina TruSeq RNA Sample
Preparation v2 Guide. The samples were amplified by PCR and sequenced using Illumina HiSeq2500 by AMOGENE (Xiamen, China). Genes with _p_-value < 0.05 and averaged FPKM value > 1 in at
least one genotype were defined as _Sox4_-regulated genes. Heat maps and clustering analysis were conducted by the GENE DENOVO platform available at
https://www.omicshare.com/tools/Home/Soft/heatmap. CELL CULTURE AND ADIPOCYTE DIFFERENTIATION HEK293T (ATCC, Cat#CRL-3216) and NIH3T3 (Pricella, Cat#CL-0171) cells: Cells were cultured in
growth media containing high-glucose DMEM (Viva Cell, Cat#C3103-0500), 100 U/ml penicillin, 100 mg/ml streptomycin (Biochannel, Cat#BCCE007), 1 mM sodium pyruvate (BasalMedia, Cat#S410JV),
1% NEAA (IMMOCELL, Cat#IMC-D07) and 10% FBS (EXCell Bio, Cat#FSP500) at 37 °C in an atmosphere of 5% CO2. For the immortalization of BAT preadipocytes, postnatal day 1 pups of wild-type
C57BL/6 mice were sacrificed, and BAT tissue was collected. Brown preadipocytes were isolated using 3 mg/mL collagenase II (Sigma-Aldrich, Cat#C6885) at 37 °C for 45 minutes, following a
previously described method [51]. The SVFs were cultivated in high-glucose DMEM (Sunncell) supplemented with 20% FBS (CellMax, Cat#SA111) at 37 °C in an atmosphere of 8.8% CO2 and treated
with a large T-antigen retrovirus for 2 days. Subsequently, they were cultured in fresh medium containing G418 (Aladdin, Cat#108321-42-2) (50 μg/ml) for 4 days to select immortalized cells.
For mature brown adipocytes differentiation, the immortalized cells were cultured until they reached 95% confluence and then treated with Medium A containing 1 nM T3 (Sigma-Aldrich,
Cat#T2877), 0.125 mM indomethacin (Sigma-Aldrich, Cat#I7378), 1 μM rosiglitazone (MCE, Cat#HY-17386), 5 μM dexamethasone (Sigma-Aldrich, Cat#D1756), 850 nM insulin (MCE, Cat#HY- P0035), and
0.5 mM IBMX (Sigma-Aldrich, Cat#I5879) for 2 days. After 48 hours, cells were treated with Medium B containing 1 nM T3, 1 μM rosiglitazone and 850 nM insulin. Starting from the fourth day,
the cells were cultured in medium B, and the medium was changed every two days until harvest. On day 6, mature brown adipocytes were achieved and harvested for indicated assay. OIL RED O
STAINING AND TRIGLYCERIDE MEASUREMENT ASSAY For cell staining, a 0.35% (w/v) Oil Red O stock solution (Solarbio, Cat#G1262) was prepared beforehand. The working solution was created by
mixing three parts of the stock solution with two parts of water. The cells were rinsed with PBS and then fixed with 4% formalin at room temperature for 15 minutes. After fixation, the cells
were incubated with the Oil Red O working solution for 10 minutes at room temperature in cell culture dish (Jet Biofil). Then, the samples were washed three times and subjected to
microscopic. For triglyceride levels measurement, the mature brown adipocytes were suspended in PBS and fragmented on ice using an ultrasonic processor (Cole Parmer) at 20% amplitude. The
resulting cell lysate was used for triglyceride measurement, and the data were normalized to the protein concentration in the lysate. OXYGEN CONSUMPTION RATE (OCR) MEASUREMENTS OF BROWN
ADIPOCYTES BAT SVF cells were plated into XFe96 cell culture microplate (Agilent) and differentiated into brown adipocytes, followed by OCR measurement using an XF96 Extracellular Flux
Analyzer (Seahorse Bioscience) according to the manufacturer’s instructions. Before OCR measurement, the cell culture medium was replaced with Seahorse XF DMEM (Agilent, Cat#103575-100)
supplemented with 25 mM glucose, 1 mM sodium pyruvate, and 2 mM glutamine (Keygen BioTECH). During OCR measurement, 4 µM oligomycin, 2.5 µM FCCP and 1.5 µM rotenone/antimycin were
sequentially injected into the microplate to detect the uncoupled respiration, maximal respiration and non-mitochondrial respiration, respectively. OCR was normalized to the protein content.
SERUM ANALYSIS Before the mice were sacrificed, the blood was obtained from the eyeballs and clotted at 4 °C for 4-6 hr. The samples were then centrifuged at 1500 g, 4 °C for 10 minutes and
the supernatants were collected. The commercial assay kits were utilized for TG (Nanjing Jiancheng, Cat#A110-1-1) and FFA (Bioswamp, Cat#BTK026) measurements, following the manufacturer’s
instructions. PLASMIDS, LENTIVIRUS PACKAGING AND INFECTION For the construction of lentiviral overexpression plasmids, mouse _Sox4_ and _Ebf2_ were cloned from a mouse cDNA library obtained
from mouse BAT tissue. The genes were inserted into either pLV-N-Flag/HA-XM vector (provided by Dr. Jiahuai Han at Xiamen University) or pCDH-EF1-MCS-IRES-BSD vector (provided by Dr. Tongjin
Zhao at Fudan University) with indicated tags by Seamless Assembly Kit (Shanghai Acmec Biochemical Technology Co., Ltd, Cat# AC17180). For knockdown, indicated shRNA were cloned into pLKO.1
plasmid (Addgene). The constructed plasmids were extracted according to the instructions of the plasmid extraction kit (Shandong Sparkjade Biotechnology, Cat#AD0103). For lentivirus
production, the constructed lentivirus plasmids were transfected into HEK293T cells along with pHR and pVSV-G plasmids, following previously described protocols [52]. After 48 hr, the
lentivirus were collected and concentrated (Sartorius, Cat#VS15T42). For lentivirus infections, cells were cultured to 75% confluence and then infected with lentiviruses in medium
supplemented with 10 μg/ml polybrene (HUAYUN, Cat#HYP490). After 36 hr, the infected cells were seeded into new dished and selected with 5 μg/ml puromycin (GIBCO, Cat#11138-03) for 1 week to
establish stable cell line. The primer sequences are listed in Supplementary Table 1. MITOCHONDRIA QUANTITY ANALYSIS BATs were isolated and finely minced into small pieces with scissors.
The tissue was then digested overnight at 55 °C in TNES digestion buffer (0.2 M NaCl, 0.1 M Tris, 5 mM EDTA, 0.4% SDS) supplemented with 200 μg/ml protease K (Macklin, Cat#P6321). Following
digestion, genomic DNA was extracted using 6 M NaCl, and subsequently precipitated with 100% ethanol. After drying, the DNA was resuspended in ddH2O. For mitochondria quantity analysis,
specific coding genes for NADH dehydrogenase 1, 2, or 4 (_Nd1_, _Nd2_, or _Nd4_) were selected, and the cyclophilin (_Ppib_) gene was utilized as a reference. The sample were determined by
RT-PCR, the primer sequences are listed in Supplementary Table 1. CONSTRUCTION, PURIFICATION, AND INJECTION OF ADENOVIRUS For the construction of the adenoviral vector, the CDS region of
_Sox4_ gene was cloned into a shuttle vector pAdTrack-CMV to form pAdTrack-CMV-Sox4 plasmid by T4 DNA ligase (CUSABIO, Cat#CSB-YP355583EDZ). The pAdTrack-CMV-Sox4 plasmid was then linearized
by digestion with PmeI (Novoprotein, Cat#RE106-U050) and co-transfected into Escherichia coli strain BJ5183 along with an adenoviral backbone plasmid called pAdEasy-1. The recombinant
clones were selected based on their resistance to kanamycin (YuanYe, Cat#Y69596) and verified by restriction endonuclease analysis. Subsequently, the recombinant plasmid was extracted and
linearized using Pac I restriction enzyme. The linearized recombinant plasmid was then transfected into β-5 cells cultured in 15 cm dishes (NEST Biotechnology) for packaging into adenovirus
particles. For purification of the adenovirus, cells and supernatants were collected when approximately one-third to half of the cells were detached. The collected samples were subjected to
three cycles of freeze-thawing to release the viral particles. Next, the precipitated viral particles were purified through PEG8000 (Biofroxx, Cat#1363GR500) precipitation. The resulting
precipitates were further purified using cesium chloride density gradient centrifugation at 22800 g and 20 °C for 2.5 hr. The adenovirus bands were carefully collected by puncturing the
centrifuge tubes and dialyzed overnight. The titer of the purified adenovirus was determined, and the purified adenovirus was subpackaged for future use. To achieve overexpression of SOX4 in
BAT of _Sox4-MKO_ mice, adenoviruses carrying the SOX4 were injected into BAT pads within a designed area at 1010 _pfu_ per mice. The adenoviruses were injected twice every 3 days. At day
7, mice were euthanized, and BAT tissues from the injected area were collected for indicated assays. PACKAGING AND PURIFICATION OF ADENO-ASSOCIATED VIRUS (AAV) In the study, the AAV2/9
system was utilized for overexpression of SOX4 in BAT. To construct the adeno-associated virus plasmid, the CDS region of _Sox4_ gene was cloned into pAAV-mini-_Ucp1_-GFP vector, generously
provided by Dr. Shengcai Lin at Xiamen University. Then the transgene plasmid was transfected into HEK293T cells along with packaging plasmids AAV2/9 and ΔT-6 by transfection reagent (AbBOX,
Cat#KX0110055). After 72 hr of transfection, both the cells and the medium containing the packaged viruses were collected. The cells were lysed through three rounds of freeze-thawing to
release the viral particles. The supernatants, along with the culture medium containing viruses, were collected and precipitated using 5 × polyethylene glycol (PEG) at a final concentration
of 8% PEG-8000 and 0.5 M NaCl. Subsequently, the precipitates were purified through iodixanol (Sigma-Aldrich, Cat#92339-11-2) density gradient ultracentrifugation (17%, 25%, 40%, and 60%).
qPCR with specific primers was employed to determine the copy numbers of AAV. For in vivo experiments, AAVs carrying SOX4 constructs were injected into the tail vein of 6-week-old male mice
at a dosage of 0.4×1012 copies per mouse, and littermates were injected with equal amount of AAV carrying the empty vector as the control group. After a period of 5 weeks, the mice were
euthanized, and the effectiveness of AAV-mediated gene modulation was examined. The primer sequences can be found in Supplementary Table 1. LUCIFERASE REPORTER ASSAYS To construct the
luciferase reporter, we amplified the promoter region of the specified genes from mouse genomic DNA and inserted it into the pGL3-basic or pGL4.26 plasmid (Promega). Cells were cultured
until they reached approximately 75% confluence. Subsequently, the constructed luciferase reporter plasmid, along with β-galactosidase (β-gal) and other specified plasmids, were transfected
into the cells using EZ-Trans transfection reagent (Shanghai Life-iLab Biotech, Cat#AC04L092). After a period of 36-48 hr post-transfection, the cells were harvested and subjected to
luciferase and β-galactosidase assays. The activity of luciferase serves as an indicator of gene expression, and β-galactosidase is used as an internal control. The luciferase activity was
then normalized to the corresponding β-galactosidase levels to address any variations and ensure accurate calibration of experimental errors. CHROMATIN IMMUNOPRECIPITATION (CHIP) AND
CHIP-SEQUENCING ANALYSIS The immortalized BAT SVF cells were infected with lentiviruses expressing either pLV-Flag-empty or pLV-Flag-Sox4 constructs, and the infection was allowed to proceed
for 48 hours, establishing a stable cell line. Subsequently, the cells were cultured until they reached 100% confluence and were then treated with DMSO or forskolin (MCE, Cat#HY-15371) (20
μM) for 1 hr before being harvested. For fixation, the cells were treated with 1% PFA at 37 °C for 15 minutes in 100 mm cell culture dish (SAINING). To stop the fixation process, 0.125 M
glycine was added and incubated for 5 minutes at room temperature. Following fixation, the cells were washed with cold PBS supplemented with 1 mM PMSF and lysed with ChIP SDS lysis buffer
(50 mM Tris-HCl, pH 8.1, 10 mM EDTA, 1% SDS) containing protease inhibitor and phosphatase inhibitors for 15 minutes on ice. To achieve DNA fragmentation within the desired range of 200-800
base pairs, an ultrasonic processor (SCIENTZ, Cat#SCIENTZ08-IIIA) was used on ice. Subsequent steps were performed according to the specific biotechnology protocol, and the enrichment of DNA
was measured using qPCR and normalized with the input sample. The primer sequences can be found in Supplementary Table 1. To investigate the genomic binding patterns of SOX4, we established
a stable cell line of BAT SVF overexpressing 3X HA-SOX4 for ChIP-Seq assays. ChIP-Seq assay was performed as above, except purified DNA was used for library construction and sequencing. The
library was prepared utilizing a KAPA Hyper Prep Kit as per the provided guidelines. Adaptors and primer sequences from Roche were employed for both library construction and amplification.
Subsequent to PCR amplification, fragments ranging from 250 to 450 bp were isolated by employing KAPA pure beads as per the manufacturer’s instructions. All ChIP libraries underwent pair-end
sequencing on HiSeq3000 systems at Sangon Biotech (Shanghai, China). IMMUNOPRECIPITATION The cells were lysed with IP buffer (MKbio, Cat#MP1504) supplemented with protease inhibitors and
phosphatase inhibitors. The total cell lysates were incubated with indicated antibodies or FLAG Magnetic Beads (HUABIO, Cat#HAK21011) at 4 °C for 8 hr. Then protein A/G magnetic beads (MCE,
Cat#HY-K0202) were washed with IP buffer for 3 times, and the above cell lysate were incubated with beads. The eluted fraction was analyzed by western blot using indicated antibodies.
FORMALDEHYDE-ASSISTED ISOLATION OF REGULATORY ELEMENTS (FAIRE) ASSAY The cells were cultured to 95% confluence and then fixed in 1% PFA 15 minutes at room temperature. The cross-linking
reaction was quenched by adding 0.125 M glycine (LABLEAD, Cat#GAS10-1) for 5 minutes at room temperature. After washing with cold PBS supplemented with 1 mM PMSF, the samples were collected,
the cells were lysed in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1, 1 mM PMSF and 1 mM protease inhibitor cocktail) for 10 minutes on ice. To obtain DNA fragments ranging
from 200 to 1,000 bp, the cell lysates were sonicated under appropriate conditions. After centrifugation, the supernatant from each group was collected and incubated with RNase A (YEASEN,
Cat#10405ES03) at 37 °C for 1 hr. Subsequently, the samples were divided into two equal parts. For de-crosslinking (control DNA), 10 µL of proteinase K (20 mg/mL) was added and incubated at
37 °C for 4 hr, followed by incubation at 65 °C for 6 hr. The non-de-crosslinked and de-crosslinked samples were then purified using the phenol:chloroform method. Chromatin accessibility was
assessed using qPCR and analyzed according to a previously reported calculation method. The primers used for FAIRE-PCR were listed in Supplementary Table 1. SCRNA-SEQ ANALYSIS AND SPATIAL
DECONVOLUTION ANALYSIS For the scRNA-seq analysis of mouse embryos ranging from E10.5 to E13.5, we utilized a publicly available dataset (GSE233955) and conducted the analysis using the R
package Seurat v4. Quality control involved setting cutoffs for the maximum percentage of reads mapping to mitochondrial genes for each dataset separately. Scrublet was employed to remove
potential doublets. Subsequently, we regressed out cell cycle phase, percentage of reads mapping to mitochondrial genes, and percentage of reads mapping to histone genes using the scale data
function. After performing principal component analysis (PCA), we reduced the dimensionality of each dataset using uniform manifold approximation and projection (UMAP) implemented in the
RunUMAP function. Clusters were defined using the find neighbors and find clusters functions. Fibroblasts and skeletal muscle cell clusters were identified based on the expression of
_Pdgfrα_ and _Tnnt1_, respectively, at each embryonic stage. We then used the top 3000 variable genes to create potential anchors with the find integration anchors function and integrated
the data using the integrate data function, resulting in a new matrix with 3000 features. Subsequently, we selected cell clusters predicted to localize to the dorsal mouse embryo by patial
deconvolution analysis. Next, we conducted cell type deconvolution based on spatial transcriptomic data using SpaDecon. We obtained spatial transcriptomics datasets from sagittal sections of
E10.5-E13.5 mouse embryos from the mouse organogenesis spatial transcriptomics atlas (MOSTA). The scRNA-seq dataset spanning E10.5-E13.5 was used to deconvolve the MOSTA data corresponding
to E10.5, E11.5, E12.5, and E13.5. The indicated code details are available at https://github.com/luoy-cloud/Sox4-program/tree/main. QUANTIFICATION AND STATISTICAL ANALYSIS All data in this
study were presented as mean ± SEM and analyzed using GraphPad Prism 8.0 software. Each group of data was analyzed for normal distribution using the Anderson-Darling test, D’Agostino-Pearson
test, Shapiro-Wilk test, or Kolmogorov-Smirnov test, as appropriate. An unpaired two-tailed Student’s _t_-test was used to determine significance between two groups of normally distributed
data. An unpaired two-tailed Mann–Whitney test was used to determine significance between data without a normal distribution. Multiple group comparisons were performed using the
Kruskal-Wallis test followed by Dunn’s multiple comparisons test, or one-way or two-way ANOVA followed by Tukey’s test, as indicated in the legends. Immunoblotting assay analysis and lipid
droplet size analysis were performed using ImageJ software (National Institutes of Health). The statistical details of experiments were prepared and given in the figure legends, including
exact number of mice samples and data analysis methods. Except for high-throughput sequencing experiments, at least three biological replicates were performed for mouse experiment and cell
experiment. Significant differences of all results are indicated as *_p_ < 0.05, ** _p_ < 0.01, and *** _p_ < 0.001, ns, no significance. Investigators were not blinded to group
allocation during experiments. DATA AVAILABILITY The datasets in this study are available in the following datasets: RNA-Seq data: Gene Expression Omnibus GSE263445. ChIP-Seq data: Gene
Expression Omnibus GSE263446. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may
be requested from the authors. REFERENCES * Chen J, Xu X, Li Y, Li F, Zhang J, Xu Q, et al. Kdm6a suppresses the alternative activation of macrophages and impairs energy expenditure in
obesity. Cell Death Differ. 2021;28:1688–704. Article CAS PubMed Google Scholar * Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev.
2004;84:277–359. Article CAS PubMed Google Scholar * Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult
humans. N Engl J Med. 2009;360:1509–17. Article PubMed Central CAS PubMed Google Scholar * Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat
development and energy homeostasis. Annu Rev Physiol. 2014;76:225–49. Article CAS PubMed Google Scholar * Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles
beyond heat generation. Cell Metab. 2015;22:546–59. Article PubMed Central CAS PubMed Google Scholar * Harms M, Seale P. Brown and beige fat: development, function and therapeutic
potential. Nat Med. 2013;19:1252–63. Article CAS PubMed Google Scholar * Lin SC, Li P. CIDE-A, a novel link between brown adipose tissue and obesity. Trends Mol Med. 2004;10:434–9.
Article CAS PubMed Google Scholar * Tews D, Wabitsch M. Brown adipose tissue in children and its metabolic function. Horm Res Paediatr. 2022;95:104–11. Article CAS PubMed Google
Scholar * Devlin MJ. The “Skinny” on brown fat, obesity, and bone. Am J Phys Anthropol. 2015;156:98–115. Article PubMed Google Scholar * Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki
H, Igarashi K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–9. Article PubMed Central CAS PubMed Google Scholar * Liang Q,
Zheng Q, Zuo Y, Chen Y, Ma J, Ni P, et al. SENP2 suppresses necdin expression to promote brown adipocyte differentiation. Cell Rep. 2019;28:2004–11.e2004. Article CAS PubMed Google
Scholar * Wu L, Xia M, Duan Y, Zhang L, Jiang H, Hu X, et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019;10:1–18.
Article Google Scholar * Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691–702. Article PubMed Central CAS PubMed Google Scholar * Wang
W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci USA. 2014;111:14466–71. Article
PubMed Central CAS PubMed Google Scholar * Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab.
2013;17:562–74. Article PubMed Central CAS PubMed Google Scholar * Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, et al. Zfp423 maintains white adipocyte identity
through suppression of the beige cell thermogenic gene program. Cell Metab. 2016;23:1167–84. Article PubMed Central CAS PubMed Google Scholar * Angueira AR, Shapira SN, Ishibashi J,
Sampat S, Sostre-Colón J, Emmett MJ, et al. Early B cell factor activity controls developmental and adaptive thermogenic gene programming in adipocytes. Cell Rep. 2020;30:2869–78.e2864.
Article PubMed Central CAS PubMed Google Scholar * Jang SM, Kim JW, Kim D, Kim CH, An JH, Choi KH, et al. Sox4-mediated caldesmon expression facilitates differentiation of skeletal
myoblasts. J Cell Sci. 2013;126:5178–88. CAS PubMed Google Scholar * Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ. Roles of Sox4 in central nervous system development. Brain Res Mol
Brain Res. 2000;79:180–91. Article CAS PubMed Google Scholar * Schilham MW, Oosterwegel MA, Moerer P, Ya J, de Boer PA, van de Wetering M, et al. Defects in cardiac outflow tract
formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711–4. Article CAS PubMed Google Scholar * Wilson ME, Yang KY, Kalousova A, Lau J, Kosaka Y, Lynn FC, et
al. The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes. 2005;54:3402–9. Article CAS PubMed Google Scholar * He T, Wang S, Li S, Shen
H, Hou L, Liu Y, et al. Suppression of preadipocyte determination by SOX4 limits white adipocyte hyperplasia in obesity. iScience. 2023;26:1–26. Article Google Scholar * Shen H, He T,
Wang S, Hou L, Wei Y, Liu Y, et al. SOX4 promotes beige adipocyte-mediated adaptive thermogenesis by facilitating PRDM16-PPARγ complex. Theranostics. 2022;12:7699–716. Article PubMed
Central CAS PubMed Google Scholar * Huang Z, Zhang Z, Moazzami Z, Heck R, Hu P, Nanda H, et al. Brown adipose tissue involution associated with progressive restriction in progenitor
competence. Cell Rep. 2022;39:1–16. Article Google Scholar * Tokuyama K, Himms-Hagen J. Brown adipose tissue thermogenesis, torpor, and obesity of glutamate-treated mice. Am J Physiol.
1986;251:E407–415. CAS PubMed Google Scholar * Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One.
2011;6:1–6. e17247. Article Google Scholar * Lee Y-H, Jung Y-S, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med. 2014;46:e78. Article PubMed
Central CAS PubMed Google Scholar * Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. 2014;19:25–37. * Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM.
Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–41. Article CAS PubMed Google Scholar * Lee HJ, Lee J,
Yang MJ, Kim YC, Hong SP, Kim JM, et al. Endothelial cell-derived stem cell factor promotes lipid accumulation through c-Kit-mediated increase of lipogenic enzymes in brown adipocytes. Nat
Commun. 2023;14:1–18.e2195. Google Scholar * Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, et al. Brown adipose tissue whitening leads to brown adipocyte death and
adipose tissue inflammation. J Lipid Res. 2018;59:784–94. Article PubMed Central CAS PubMed Google Scholar * Farmer SR. Molecular determinants of brown adipocyte formation and function.
Genes Dev. 2008;22:1269–75. Article PubMed Central CAS PubMed Google Scholar * Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab.
2010;11:257–62. Article PubMed Central CAS PubMed Google Scholar * Jun S, Angueira AR, Fein EC, Tan JME, Weller AH, Cheng L, et al. Control of murine brown adipocyte development by
GATA6. Dev Cell. 2023;58:2195–2205.e2195. Article PubMed Central CAS PubMed Google Scholar * Fukano K, Okamatsu-Ogura Y, Tsubota A, Nio-Kobayashi J, Kimura K. Cold exposure induces
proliferation of mature brown adipocyte in a ß3-adrenergic receptor-mediated pathway. PLoS One. 2016;11:1–16.e0166579. Article Google Scholar * Shapira SN, Lim HW, Rajakumari S, Sakers AP,
Ishibashi J, Harms MJ, et al. EBF2 transcriptionally regulates brown adipogenesis via the histone reader DPF3 and the BAF chromatin remodeling complex. Genes Dev. 2017;31:660–73. Article
PubMed Central CAS PubMed Google Scholar * Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function
in adult mice. Cell Metab. 2014;19:593–604. Article PubMed Central CAS PubMed Google Scholar * Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of
brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. Article PubMed Central CAS PubMed Google Scholar * Zhang L, Avery J, Yin A, Singh AM, Cliff TS, Yin H, et al. Generation of
functional brown adipocytes from human pluripotent stem cells via progression through a paraxial mesoderm state. Cell Stem Cell. 2020;27:784–97.e711. Article CAS PubMed Google Scholar *
Dy P, Penzo-Méndez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins-Sox4, Sox11 and Sox12-exhibit overlapping expression patterns and molecular properties. Nucleic
Acids Res. 2008;36:3101–17. Article PubMed Central CAS PubMed Google Scholar * van Houte LP, Chuprina VP, van der Wetering M, Boelens R, Kaptein R, Clevers H. Solution structure of the
sequence-specific HMG box of the lymphocyte transcriptional activator Sox-4. J Biol Chem. 1995;270:30516–24. Article PubMed Google Scholar * Suchacki KJ, Ramage LE, Kwok TnC, Kelman A,
McNeill BT, Rodney S, et al. The serotonin transporter sustains human brown adipose tissue thermogenesis. Nature Metabolism. 2023;5:1319–36. Article PubMed Central CAS PubMed Google
Scholar * Khani S, Topel H, Kardinal R, Tavanez AR, Josephrajan A, Larsen BDM, et al. Cold-induced expression of a truncated adenylyl cyclase 3 acts as rheostat to brown fat function.
Nature Metabolism. 2024;6:1053–75. Article CAS PubMed Google Scholar * Hanssen MJW, van der Lans AAJJ, Brans B, Hoeks J, Jardon KMC, Schaart G, et al. Short-term cold acclimation
recruits brown adipose tissue in obese humans. Diabetes. 2015;65:1179–89. Article PubMed Google Scholar * Inoue S-i, Emmett MJ, Lim H-W, Midha M, Richter HJ, Celwyn IJ, et al. Short-term
cold exposure induces persistent epigenomic memory in brown fat. Cell Metabolism. 2024;36:1764–78.e1769. Article CAS PubMed Google Scholar * Jimenez V, Muñoz S, Casana E, Mallol C, Elias
I, Jambrina C, et al. In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes. 2013;62:4012–22. Article PubMed Central
CAS PubMed Google Scholar * Wang CH, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD, et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate
metabolic syndrome in mice. Sci Transl Med. 2020;12:1–14. Article Google Scholar * Huang L, Pan D, Chen Q, Zhu LJ, Ou J, Wabitsch M, et al. Transcription factor Hlx controls a systematic
switch from white to brown fat through Prdm16-mediated co-activation. Nat Commun. 2017;8:1–16. Google Scholar * Shi M, Huang XY, Ren XY, Wei XY, Ma Y, Lin ZZ, et al. AIDA directly connects
sympathetic innervation to adaptive thermogenesis by UCP1. Nat Cell Biol. 2021;23:268–77. Article CAS PubMed Google Scholar * Wang J, Hao JW, Wang X, Guo H, Sun HH, Lai XY, et al. DHHC4
and DHHC5 facilitate fatty acid uptake by palmitoylating and targeting CD36 to the plasma membrane. Cell Rep. 2019;26:209–21.e205. Article PubMed Google Scholar * Lodhi IJ, Dean JM, He A,
Park H, Tan M, Feng C, et al. PexRAP inhibits PRDM16-mediated thermogenic gene expression. Cell Rep. 2017;20:2766–74. Article PubMed Central CAS PubMed Google Scholar * Xie YY, Mo CL,
Cai YH, Wang WJ, Hong XX, Zhang KK, et al. Pygo2 regulates adiposity and glucose homeostasis via β-Catenin-Axin2-GSK3β signaling pathway. Diabetes. 2018;67:2569–84. Article PubMed Google
Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Tongjin Zhao for providing _Ucp1-cre_ mouse strain and Dr. Wei Mo for providing _Myf5-cre_ mouse strain; Dr. Shuyong Lin for
providing Myc–WT-PRKACA and Myc–KD-PRKACA plasmids; Dr. Patrick Seale (University of Pennsylvania) for providing details of single-cell sequencing data and spatial transcriptomics data. We
also thank Yaying Wu, Zheni Xu, and Dr. Changchuan Xie (School of Life Sciences, Xiamen University) for mass spectrometry experiments and data analysis. FUNDING This work was supported by
grants from the National Natural Science Foundation of China (82473163 to B.-A.L., 32371223 and 32071150 to H.G., 82272944 to QL., 82170266 to Z.H), the National Key Research and Development
Program of China (2020YFA0112300 to B.-A.L.), Xiamen Municipal Bureau of Science and Technology (3502Z20224021 to W.L.), Postdoctoral Fellowship Program of CPSF under Grant Number
GZC20231412 and “Project 111” sponsored by the State Bureau of Foreign Experts and Ministry of Education (grant number B06016). AUTHOR INFORMATION Author notes * These authors contributed
equally: Shuai Wang, Ting He, Ya Luo. AUTHORS AND AFFILIATIONS * State Key Laboratory of Cellular Stress Biology, Innovation Center for Cell Signaling Network and Engineering Research Center
of Molecular Diagnostics of The Ministry of Education, School of Life Sciences, Xiamen University, 361102, Xiamen, Fujian, China Shuai Wang, Ting He, Kexin Ren, Huanming Shen, Lingfeng Hou,
Yixin Wei, Tong Fu, Wenlong Xie, Peng Wang, Jie Hu, Yu Zhu, Huiling Guo & Boan Li * Department of Cardiology, Xiamen Key Laboratory of Cardiac Electrophysiology, Xiamen Institute of
Cardiovascular Diseases, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, 361102, Xiamen, China Shuai Wang, Zhengrong Huang & Weihua Li *
National Institute for Data Science in Health and Medicine, School of Medicine, Xiamen University, 361102, Xiamen, Fujian, China Ya Luo & Qiyuan Li * Shenzhen Institute of Advanced
Technology, Chinese Academy of Science, 518055, Shenzhen, China Huanming Shen Authors * Shuai Wang View author publications You can also search for this author inPubMed Google Scholar * Ting
He View author publications You can also search for this author inPubMed Google Scholar * Ya Luo View author publications You can also search for this author inPubMed Google Scholar * Kexin
Ren View author publications You can also search for this author inPubMed Google Scholar * Huanming Shen View author publications You can also search for this author inPubMed Google Scholar
* Lingfeng Hou View author publications You can also search for this author inPubMed Google Scholar * Yixin Wei View author publications You can also search for this author inPubMed Google
Scholar * Tong Fu View author publications You can also search for this author inPubMed Google Scholar * Wenlong Xie View author publications You can also search for this author inPubMed
Google Scholar * Peng Wang View author publications You can also search for this author inPubMed Google Scholar * Jie Hu View author publications You can also search for this author inPubMed
Google Scholar * Yu Zhu View author publications You can also search for this author inPubMed Google Scholar * Zhengrong Huang View author publications You can also search for this author
inPubMed Google Scholar * Qiyuan Li View author publications You can also search for this author inPubMed Google Scholar * Weihua Li View author publications You can also search for this
author inPubMed Google Scholar * Huiling Guo View author publications You can also search for this author inPubMed Google Scholar * Boan Li View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS WS and H-LG designed the study, conducted data analysis, and drafted the manuscript. SW and TH performed the majority of experiments. YL
carried out bioinformatics analyses of scRNA-seq datasets. K-XR, TF, H-MS, L-FH, Y-XW, W-LX, PW, JH, YZ, and Z-RH contributed to mouse experiments. Q-YL, W-HL, H-LG, and B-AL supervised the
project, revised the manuscript and provided fundings. B-AL, serving as the guarantor, had full access to all study data and takes responsibility for ensuring both data integrity and the
accuracy of data analysis. CORRESPONDING AUTHORS Correspondence to Qiyuan Li, Weihua Li, Huiling Guo or Boan Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ETHICS All mouse experiments were approved and complied with the guidelines of the Institutional Animal Care and Research Advisory Committee at Xiamen University, China (Permit
Number: XMULAC20230105). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION SUPPLEMENTAL TABLE 1 UNCROPPED WESTERN BLOTS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, S., He, T., Luo, Y. _et al._ SOX4 facilitates brown fat development and
maintenance through EBF2-mediated thermogenic gene program in mice. _Cell Death Differ_ 32, 447–465 (2025). https://doi.org/10.1038/s41418-024-01397-0 Download citation * Received: 10 June
2024 * Revised: 28 September 2024 * Accepted: 01 October 2024 * Published: 15 October 2024 * Issue Date: March 2025 * DOI: https://doi.org/10.1038/s41418-024-01397-0 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative