Play all audios:
The developmental origins of mesenchymal progenitor cells (MPCs) and molecular machineries regulating their fate and differentiation are far from defined owing to their complexity.
Osteoblasts and adipocytes are descended from common MPCs. Their fates are collectively determined by an orchestra of pathways in response to physiological and external cues. The canonical
Wnt pathway signals MPCs to commit to osteogenic differentiation at the expense of adipogenic fate. In contrast to ß-catenin, p53’s anti-osteogenic function is much less understood. Both
activities are thought to be achieved through targeting Runx2 and/or Osterix (Osx, Sp7) transcription. Precisely, how Osx activity is dictated by ß-catenin or p53 is not clarified and
represents a knowledge gap that, until now, has largely been taken for granted. Using conditional lineage-tracing mice, we demonstrated that chondrocytes gave rise to a sizable fraction of
MPCs, which served as progenitors of chondrocyte-derived osteoblasts (Chon-ob). Wnt/ß-catenin activity was only required at the stage of chondrocyte-derived mesenchymal progenitor (C-MPC) to
Chon-ob differentiation. ß-catenin– C-MPCs lost osteogenic ability and favored adipogenesis. Mechanistically, we discovered that p53 activity was elevated in ß-catenin– MPCs including
ß-catenin– C-MPCs and deleting p53 from the ß-catenin– MPCs fully restored osteogenesis. While high levels of p53 were present in the nuclei of ß-catenin– MPCs, Osx was confined to the
cytoplasm, implying a mechanism that did not involve direct p53-Osx interaction. Furthermore, we found that p53’s anti-osteogenic activity was dependent on its DNA-binding ability. Our
findings identify chondrocytes as an additional source for MPCs and indicate that Wnt/ß-catenin discretely regulates chondrocyte to C-MPC and the subsequent C-MPC to osteoblast developments.
Most of all we unveil a previously unrecognized functional link between ß-catenin and p53, placing p53’s negative role in the context of Wnt/ß-catenin signaling-induced MPC osteogenic
differentiation.
Endochondral bone formation occurs through a cartilage to bone conversion process, during which cartilaginous tissue serves both as a template for ossification and as an innate source of
osteoblasts1,2,3,4,5,6. The cellular means by which a fully differentiated chondrocyte gains the plasticity to evolve into a mature osteoblast, as well as what signaling pathways govern this
event, remains elusive.
Canonical Wnt signaling plays diverse roles at different stages of bone development and growth5,6,7,8,9,10. In Osx-expressing MPCS, Wnt/ß-catenin plays a switch role between osteogenic and
adipogenic fates. Despite the lack of convincing evidence7, it is currently accepted that ß-catenin promotes osteogenesis through activating Runx2 and/or Osx transcription11,12,13.
p53 is a well-established tumor suppressor. It is also a vital regulator of cell fate and differentiation14. The precise functions and regulatory mechanisms of p53’s physiological roles
remain much less understood and appreciated. In limited reports, crosstalk between p53 and Wnt/ß-catenin signaling has been shown to play various roles in a context-dependent manner, such as
in smooth muscle cells15 and in embryonic stem cells16.
p53 exhibits osteo-inhibitory activity in various mouse models17,18 Nonetheless, p53 downstream molecular events leading to osteogenic inhibition are not yet defined. p53 null marrow
mesenchymal stem cells are more osteogenic and display no apparent difference in their adipogenic and chondrogenic capacities19,20. One study shows that p53 inhibits osteoblastic
differentiation through microRNA-34-mediated Runx2 suppression21,22. To date, the physiological context of this inhibitory function remains entirely elusive.
Here we used Collagen X (Col10a1) and Aggrecan (Agc1)-driven ß-catenin conditional lineage-tracing mice to delineate how Wnt/ß-catenin regulates chondrocyte to osteoblast reprogramming. We
showed that chondrocytes evolved into osteoblasts through at least two steps, which were differentially regulated by Wnt/ß-catenin. Mechanistically, we discovered that the
ß-catenin-deficient MPCS acquired elevated p53 activity and their defect in osteogenic capacity was fully reinstated by merely deleting p53 from them, indicating that Wnt/ß-catenin promotes
osteogenesis via a p53 suppression-dependent mechanism.
To acquire a mechanistic understanding of the Wnt/β-catenin action initiated from chondrocytes, we generated chondrocyte-lineage-tracing mouse models containing either deleted or stabilized
ß-catenin alleles, and systematically quantified and compared the reporter-expressing cells categorized by location and association with bone matrix.
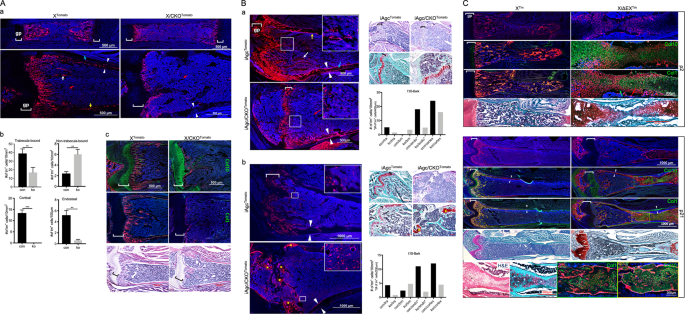
In the femurs of postnatal animals, an abundant number of Tomato-expressing (Tm+) cells was observed within the marrow cavity of both Col10a1-Cre;ROSA26R-Tomato (XTomato) control and
Col10a1-Cre;Ctnnb1fl/fl;ROSA26R-Tomato (X/CKOTomato) mutant animals, each of which presented a distinct pattern of distribution. At postnatal day 2 (p2) of XTomato control animal, the
majority of Col10a1-Cre-induced Tm+ (X/Tm+) cells were localized at the primary spongiosa physically in contact with trabeculae (trabecula-bound) and showed mature osteoblast-like morphology
(Fig. 1Aa). In contrast, most of the Tm+ (XCKO/Tm+) cells in the p2 X/CKOTomato mutant animal were scattered rather evenly throughout the marrow cavity. Most of them were not connected with
trabeculae (non-trabecula-bound) and were morphologically distinct from mature osteoblasts (Fig. 1Aa). Similarly, at p16, there were more non-trabecula-bound Tm+ cells in the X/CKOTomato
mutant than in the XTomato control mice (6/ko vs 2.4/con cells/10 mm2), whereas fewer XCKO/Tm+ cells were found on the endostea (0.63/ko vs 6/con cells/100 µm), embedded within cortices
(0/ko vs 13.4/con cells/10 mm2) or trabecular matrixes (16.6/ko vs 38.9/con cells/10 mm2) compared to the X/Tm+ cells (Fig. 1Ab). This phenotype persisted and became progressively pronounced
with age. In the marrow of 4- and 8-month-old X/CKOTomato mice, there were visibly more non-trabecula-bound XCKO/Tm+ cells than in that of p16, whose non-trabecula-bound XCKO/Tm+ cells
remained scattered among the stromal cells and were not embedded within the cortexes (Supplementary Fig. S1a, b).
A: a Confocal images of the p2 (upper) and p16 (lower) femurs. b Quantitative comparisons of Cre-induced Tm+ cells categorized by distribution between p16 XTomato (con) and X/CKOTomato (ko)
mice, n = 3, **p