Play all audios:
ABSTRACT Vascular endothelial cells are the predominant cell type in the cardiovascular system, and their dysfunction and death following hypoxic injury contribute to vascular lesions,
playing an essential role in cardiovascular disease. Despite its importance, the mechanisms underlying vascular endothelial cell injury under hypoxia and potential therapeutic interventions
remain poorly understood. Here, we constructed both an in vivo hypoxia model in C57BL/6 mice and an in vitro hypoxia model in HUVEC cells. Our findings demonstrated that hypoxia induces
necroptosis in vascular endothelial cells and exacerbates inflammatory injury in vivo and in vitro, as evidenced by immunofluorescence and western blot. We identified FADD as a critical
regulator of hypoxia-mediated necroptosis, with FADD knockdown significantly reversing hypoxia-induced necroptosis. Mechanistically, hypoxia affected protein conformation through SUMOylation
of FADD and competitively inhibited its ubiquitination, leading to an increase in protein half-life and protein level of FADD. Furthermore, SUMOylation increased the interaction between
FADD and RIPK1 and induced the formation of the FADD-RIPK1-RIPK3 complex, thereby promoting necroptosis in vascular endothelial cells. The SUMOylation inhibitor ginkgolic acid (GA) notably
reduced hypoxia-induced vascular endothelial injury and inflammatory responses in male mice. Taken together, our research has uncovered a new process by which SUMOylation of FADD regulates
hypoxia-induced necroptosis in endothelial cells, providing potential therapeutic targets for hypoxia-related cardiovascular diseases. SIMILAR CONTENT BEING VIEWED BY OTHERS SUPPRESSION OF
PFKFB3-DRIVEN GLYCOLYSIS RESTRAINS ENDOTHELIAL-TO-MESENCHYMAL TRANSITION AND FIBROTIC RESPONSE Article Open access 01 September 2022 HDAC11 PROMOTES BOTH NLRP3/CASPASE-1/GSDMD AND
CASPASE-3/GSDME PATHWAYS CAUSING PYROPTOSIS VIA ERG IN VASCULAR ENDOTHELIAL CELLS Article Open access 12 March 2022 HO-1 NUCLEAR ACCUMULATION AND INTERACTION WITH NPM1 PROTECT AGAINST
STRESS-INDUCED ENDOTHELIAL SENESCENCE INDEPENDENT OF ITS ENZYMATIC ACTIVITY Article Open access 26 July 2021 INTRODUCTION Hypoxia is a disorder caused by a reduction in the availability of
oxygen to tissues or organs. It is associated with a variety of human diseases, including ischemic damage to the brain, heart, liver, and kidney [1,2,3]. Under hypoxia, HIF-1α accumulates in
cells to promote cell adaptation and survival [4]. However, under extensive severe hypoxic stress, cells activate death mechanisms leading to cell death. Vascular endothelial cell (ECs) is
the most common cell type in the cardiovascular system, being the first layer to be exposed to changes in oxygen levels [1]. Hypoxia and HIF signaling stimulate endothelial cell activation,
leading to endothelial dysfunction [5]. Dysfunction of ECs ultimately leads directly not only to altered vasoconstrictor activity, intimal thickening, and remodeling but also to the
recruitment of monocytes, which induces an increase in inflammatory cytokines, leading to further vascular lesions [6,7,8]. However, the molecular regulatory mechanism of how hypoxia
mediates vascular endothelial cell injury remains elucidated. Necroptosis, a novel type of programmed cell death, involves cell swelling, plasma membrane rupture, loss of cytoplasmic
contents, cell permeabilization, and release of damage-associated molecular patterns (DAMP) [9, 10]. Hypoxia is closely associated with necroptosis. By inhibiting necroptosis, it is possible
to improve hypoxia-mediated retinal neoangiogenesis [11] and facilitate hypoxic brain injury as well as ischemic brain injury caused by middle cerebral artery obstruction [12, 13]. However,
how necroptosis is involved in the pathogenesis of ischemic-hypoxic vascular endothelial injury has yet to be studied. FADD (Fas-associated via death domain) is a crucial junction protein
in death receptor-mediated apoptosis [14]. In addition to its role in apoptosis, FADD is involved in other non-apoptotic processes, such as necroptosis. Caspase 8, dependent on FADD
activation, inhibits necroptosis by shearing RIPK1/RIPK3 [15]. Interestingly, FADD can promote necroptosis in some studies, and the FADD-RIPK1-RIPH3-NEMO complex can induce BAX/BAK-dependent
mitochondrial bioenergetic catabolism to promote TNFα-driven necroptosis [16, 17]. The precise role of FADD remains elusive and opposite. Thus, the role and molecular mechanism of FADD in
hypoxia-induced necroptosis need to be further investigated. SUMOylation is one of the post-transcriptional modifications (PTMs) in eukaryotic protein. SUMOylation and ubiquitination are
similar but functionally distinct. Ubiquitin-modified proteins mainly make them recognized and degraded by the proteasome. In contrast, SUMO-modified proteins are more stable, while
SUMOylation can modulate protein-protein interactions to mediate target protein localization and functional regulation [18, 19]. Studies have shown that FADD has three SUMOylation sites:
K120, K125, and K149 [20, 21]. Here, we identified a novel mechanism of SUMOylation of FADD in necroptosis and its impact on hypoxic endothelial injury. Under hypoxia, SUMOylation of FADD
competitively inhibited its ubiquitination by affecting the protein conformation, leading to an increase in the protein half-life and protein level of FADD while inducing the formation of
the FADD-RIPK1-RIPK3 complex, which promotes necroptosis in vascular ECs. Our findings provide new insights into hypoxia-mediated necroptosis, suggesting that targeting SUMOylation and FADD
has the potential to prevent and treat vascular ischemic injury and related cardiovascular diseases. RESULT HYPOXIA MEDIATES VASCULAR ENDOTHELIAL DAMAGE WITH CONCOMITANT INFLAMMATORY
RESPONSES To investigate the effects of hypoxia on vascular function, we constructed an in vivo hypoxia model in C57BL/6 mice, which were placed in an animal hypoxia chamber (10% O2) for 1,
2, 3, and 4 weeks. We measured dead cells in the aortic vasculature of C57BL/6 mice (8–10 weeks old). The number of TUNEL+ cells in the aorta increased progressively starting at 3 weeks
(Fig. 1A, Supplementary S1A). Additionally, TUNEL/CD31 fluorescence staining revealed that these TUNEL+ cells were primarily localized within the vascular endothelium (Supplementary S1C). An
inflammatory response often accompanies vascular injury. We observed that hypoxia for 4 weeks could significantly increase vascular inflammation by IL-1β immunofluorescence (Fig. 1B). The
serum levels of IL-1β and TNFα were significantly increased (Fig. 1C, D). Similarly, in the HUVEC with hypoxia intervention, the expression of TNFα reached a peak at hypoxia 12 h, the
protein expressions of other inflammatory factors (IL-1β, ICAM, VCAM) reached their peak after 6 h of hypoxia (Fig. 1E, F). These results suggest that hypoxia induces vascular EC dysfunction
and cell death. HYPOXIA INDUCES NECROPTOSIS IN VASCULAR ENDOTHELIAL CELLS Since hypoxia increased TUNEL+ levels in vascular ECs, we further determined the cell viability of HUVEC by CCK8
assay. The results showed that hypoxia significantly inhibited the cell viability in a time-dependent manner (Fig. 2A). To confirm the cytotoxic effect of hypoxia on HUVEC, the cell
viability was examined under various inhibitors, including caspase inhibitor z-VAD-fmk, RIPK1 kinase inhibitor Nec-1, lipid ROS scavenger Fer-1, and antioxidant NAC. The results showed that
Nec-1 and NAC partially prevented hypoxia (48 h)-induced cell death (Fig. 2B), and Nec-1 significantly prevented necrotic cells after hypoxia (48 h) treatment (Fig. 2C). There were typical
morphological structures of necroptotic cells, such as cytoplasmic hyalinization, loss of cytoplasmic contents, and incomplete cell membranes (indicated by red arrows) in HUVEC after hypoxia
(12 h) as observed by TEM (Fig. 2D). Meanwhile, the necroptosis activation markers (p-RIPK3/RIPK3 and p-MLKL/MLKL) gradually increased from hypoxia 6 h (Supplementary S1F, G), and the above
results were effectively reversed by Nec-1 pretreatment (Fig. 2E). p-RIPK3 immunofluorescence showed similar results to Western Blot (Fig. 2F). Since necrosomes formed by RIPK1, RIPK3, and
MLKL are central to necroptosis [22], we observed an increase in RIPK1-MLKL-RIPK3 interactions after hypoxia (12 h) treatment by co-IP assay (Fig. 2G), suggesting the formation of
necrosomes. In in vivo experiments, immunofluorescence of aortic vessel sections for p-MLKL/CD31 also showed that p-MLKL accumulated more in vascular endothelial cells (Fig. 2H). The
expression of p-MLKL/MLKL and inflammatory factors (TNFα, IL-1β) in mouse aortic vessels was significantly increased after 4 weeks of hypoxia (Fig. 2I). These results further confirm that
hypoxia promotes the formation of necrosomes and activates the necroptotic pathway, which is responsible for endothelial damage. FADD IS INVOLVED IN NECROPTOSIS OF ENDOTHELIAL CELLS UNDER
HYPOXIA In previous studies, FADD was reported to regulate necroptosis, with different effects observed in different models (Fig. 3A) [16]. We found that hypoxia (12 h) led to an increase in
the interaction of FADD with RIPK1/RIPK3 by co-IP, which suggested that FADD was involved in necroptosis (Fig. 3B). To investigate the specific role of FADD in hypoxia-mediated necroptosis
in endothelial cells, we co-incubated siRNA targeting FADD with HUVEC before hypoxia for 48 h. We observed that FADD knockdown significantly restored hypoxia-induced HUVEC cell viability and
reduced necrotic cells (Fig. 3C, D). Further, FADD knockdown significantly inhibited the levels of p-RIPK3 and p-MLKL (Fig. 3E, F). On the other hand, we co-incubated the plasmid targeting
FADD with HUVEC for 48 h before hypoxia. Western Blot results showed that FADD overexpression significantly increased the levels of p-MLKL and p-RIPK3 (Fig. 3G). These data suggest that FADD
plays a vital role in regulating necroptosis in hypoxic ECs. HYPOXIA PROMOTES FADD SUMOYLATION, THEREBY INCREASING ITS PROTEIN STABILITY To elucidate the mechanism underlying FADD-mediated
necroptosis, we focused on the changes in the FADD expression. We found that FADD protein levels significantly increased after 12 h of hypoxia in HUVECs (Fig. 4A). Combined with the in vivo
experiments, hypoxia for 4 weeks also considerably increased FADD protein levels (Supplementary S2). Interestingly, the mRNA levels of FADD remained unchanged following hypoxia (12 h) (Fig.
4B), suggesting that hypoxia may regulate FADD expression by affecting protein stability rather than transcriptional activity. To explore this hypothesis, we analyzed the rate of protein
degradation by using the protein synthesis inhibitor cycloheximide (CHX). As anticipated, hypoxia (12 h) prolonged the half-life of FADD protein (Fig. 4C). Additionally, treatment with the
autophagy activator rapamycin did not affect FADD protein levels (Supplementary S3A), further indicating that hypoxia-induced increases in FADD are not mediated by autophagy-related
pathways. Considering the importance of PTMs in regulating protein stability [23], we focused on PTMs of FADD. FADD is known to undergo various PTMs, including ubiquitination,
phosphorylation, SUMOylation, etc. Notably, SUMOylation can compete with ubiquitination, potentially influencing protein stability [19, 20]. To this end, we found that hypoxia (12 h)
enhanced the interaction between FADD and SUMO2/3 in HUVECs (Fig. 4D, E). To determine whether SUMOylation affects its protein stability, we constructed SUMOylation site mutant plasmids of
FADD (three sites mutated to arginine) by targeted mutagenesis (Fig. 4F). We then transfected HUVEC cells with either wild-type FADD (FADD-OE WT) or mutant FADD (FADD-OE MUT) plasmids,
confirming successful transfection by co-immunoprecipitation (co-IP) and Western blot analysis (Supplementary S4C-E). The results showed that the FADD-OE (WT) plasmids significantly
prolonged the half-life of the FADD protein under hypoxia (12 h). In contrast, the FADD-OE (MUT) plasmids prevented the hypoxia-induced prolongation of the FADD protein half-life (Fig. 4G).
Additionally, treatment with the SUMOylation inhibitor ginkgolic acid (GA) reduced FADD protein levels (Supplementary S3B) and shortened the half-life of FADD protein under hypoxia (12 h)
(Fig. 4H). Notably, FADD has multiple ubiquitination binding sites (K149, K153) [18, 20, 24]. We hypothesized that the SUMOylation of FADD might alter its conformation, thereby competitively
inhibiting its ubiquitination. As expected, molecular docking results showed that SUMOylated FADD masked its ubiquitination site (K149, K153), effectively inhibiting its ubiquitination
(Fig. 4I). Moreover, the ubiquitination levels of FADD were significantly reduced under hypoxia (12 h), whereas the FADD-OE (MUT) plasmids restored the ubiquitination level (Fig. 4J). The
above results suggest that hypoxia (12 h) promotes the SUMOylation of FADD in HUVEC, which in turn enhances its protein stability by inhibiting ubiquitination. SUMOYLATED FADD INCREASES ITS
BINDING TO RIPK1 AND RIPK3, THEREBY PROMOTING NECROPTOSIS Previous studies have shown that FADD can participate in necrosome formation to promote necroptosis [25,26,27]. We speculate that
SUMOylation of FADD influences its interaction with RIPK1 and regulates necroptosis. To test this hypothesis, we first elucidate the effect of SUMOylation of FADD on ECs necroptosis. In
HUVECs, treatment with FADD-OE (MUT) plasmids significantly suppressed the expression of p-MLKL and p-RIPK3 under hypoxia (12 h) (Fig. 5A, Supplementary S5A). Co-IP results further showed
that mutating the SUMOylation site of FADD significantly reduced the formation of the necrosomes (Fig. 5B). Similarly, treatment with the SUMOylation inhibitor GA effectively inhibited
hypoxia-induced expression of p-RIPK3/RIPK3 and p-MLKL/MLKL (Supplementary S5B). TEM images showed that GA treatment markedly reverses the number of necroptotic cells (Fig. 5C). FADD-OE(WT)
plasmids treatment significantly decreased cell viability and increased cytotoxicity under both hypoxia (48 h) and normoxia. In contrast, FADD-OE (MUT) plasmids did not result in an increase
in dead or necrotic cells to the same extent as FADD-WT overexpression (Fig. 5D, E). Meanwhile, GA pretreatment effectively restored cell viability and significantly reduced necrotic cells.
(Supplementary S5C, D). These data suggest that inhibiting FADD SUMOylation can suppress necroptosis in ECs. Next, we explored whether the interaction of FADD with RIPK1/3 depended on its
SUMOylation. Co-IP results showed that FADD-OE (WT) plasmids induced the interaction of FADD with RIPK1/3 under hypoxia (12 h), which were markedly reduced by mutating the SUMOylation site
of FADD (Fig. 5F). The results suggest that SUMOylation of FADD is essential for the FADD-RIPK1-RIPK3 complex. Molecular docking analyses of the protein crystal structures of FADD and RIPK1
revealed that the complex formed by SUMOylated FADD (FADD-SUMO) and the death domain (DD) of RIPK1 (RIPK1-DD) was more geometrically stable (Fig. 5G, Supplementary S5G). In contrast, the
complex formed by docking of FADD mutants and RIPK1 showed more instability (Fig. 5H), with reduced interaction contact area and elevated free energy of binding (Fig. 5I, J), as well as
reduction in hydrogen bonds (Fig. 5K). FADD interacted with RIPK1 through the amino acid network of SER122-ASP660, ASP123-SER657, THR151-LLE659, THR151-SER663, ASN150-TYR667, and
ARG146-TYR667 (Fig. 5K). Moreover, SUMO facilitated the interaction between FADD and RIPK1 by forming hydrogen bonds with RIPK1 through residues such as GLY64-TYR667, GLN65-SER664,
TNR70-ASN593, HIS37-ASP588, etc., which facilitates the interaction between FADD and RIPK1 and stabilizes FADD-SUMO-RIPK1 complexes (Supplementary S5H). In summary, these results suggest
that SUMOylated FADD increases its binding to RIPK1/3, thereby mediating necroptosis. SUMOYLATION INHIBITOR GA AMELIORATES HYPOXIA-INDUCED VASCULAR ENDOTHELIAL INJURY AND INFLAMMATORY
RESPONSE Having demonstrated that SUMOylation can contribute to hypoxia-induced necroptosis via FADD, we explored whether inhibiting SUMOylation could serve as an effective strategy to
mitigate endothelial injury. To assess this, we investigated the protective effects of GA on hypoxia-induced vascular endothelial injury and inflammatory responses in mice. After one week of
hypoxia treatment, C57BL/6 mice were administered GA at a concentration of 10 mg/ml by gavage every two days for a duration of three weeks (Fig. 6A) [28]. Consistent with expectations,
aortic vascular sections from the GA-treated hypoxic group of mice exhibited less accumulation of TUNEL+ cells and IL-1β (Fig. 6B–D). The serum of GA-treated mice showed lower levels of TNFα
and IL-1β (Fig. 6E, F). GA significantly reduced hypoxia-induced upregulation of inflammatory factor expression and phosphorylation of MLKL in aortic vascular tissues (Fig. 6G, H,
Supplementary S2). Meanwhile, the level of ICAM-1 in the aortic vessels of mice in the GA group was significantly reduced (Supplementary S6). GA reduced organ damage in the liver and spleen
(Supplementary S7). These results suggest that GA protects hypoxia-induced vascular endothelial injury by inhibiting necroptosis and inflammatory responses. DISCUSSION Hypoxia can be defined
as a physiological or pathological state in which there is a lack of sufficient oxygen supply at the tissue level to meet the needs of the cells or tissues [1, 29]. It is present in
numerous diseases, such as atherosclerosis, where an imbalance between oxygen supply and demand results in high-fat arterial wall lesions and plaque formation, increased lipid accumulation,
and inflammatory responses [6,7,8]. In contrast, the specific mechanisms by which hypoxia induces vascular lesions and necroptosis activation are not known. Here, we found that necroptosis
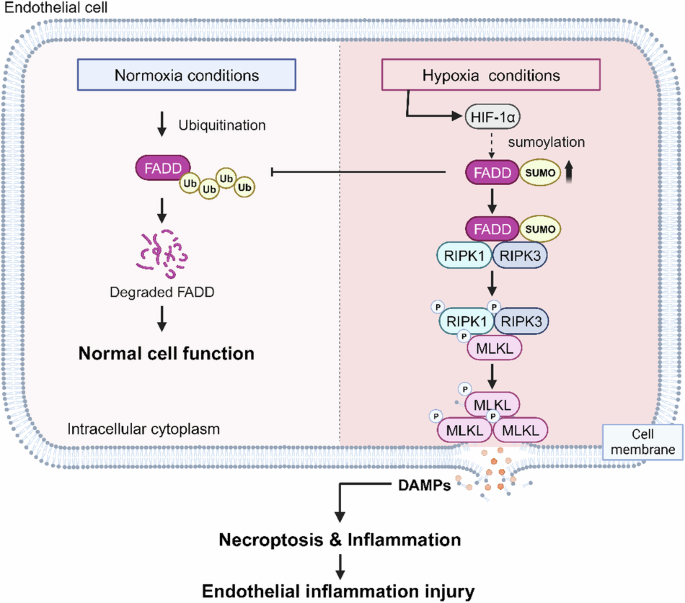
may be an essential cause of hypoxic endothelial injury, while SUMOylated FADD plays a vital role (Fig. 7), which contributes to provide new and promising targets of hypoxic diseases
treatment. Necroptosis, an emerging programmed cell death pathway, is involved in and regulates multiple diseases, such as cancer, neurological diseases, and retinal diseases [30,31,32].
Nevertheless, there is a paucity of studies that have investigated the role of necroptosis in hypoxia-mediated vascular endothelial injury. Our in vivo and in vitro experiments demonstrated
that hypoxia induces necroptosis in vascular endothelial cells (ECs) by promoting the formation of the necrosome, which ultimately leads to endothelial dysfunction. Studies have shown that
necroptosis is an inflammatory cell death, leading to the production of inflammatory cytokines [9, 10]. Here, we demonstrated that inflammatory factors were produced in vascular tissues as
well as the serum of mice after hypoxia. Meanwhile, inflammatory factors were highly expressed in the ECs and smooth muscle cells, but p-MLKL was only expressed in vascular ECs (Figs. 1B,
2H, I). This may be due to the release of inflammatory cytokines after necroptosis of ECs, which induces the activation of inflammatory signals in smooth muscle cells. Previous studies have
demonstrated that pro-inflammatory factors in the inflammatory microenvironment, such as TNFα, are the classical promoters of necroptosis [9, 10]. However, the present study did not delve
deeper into the predominant factors of hypoxia-induced necroptosis in the endothelium, which requires further exploration. As a crucial bridging protein, FADD has been identified as a master
regulator of apoptosis, necroptosis, and inflammation, depending on cell type and context [16]. Most studies have reported the inhibitory effect of FADD on necroptosis [16]. Interestingly,
the present study demonstrates that FADD plays a pro-necroptotic role in hypoxia-mediated necroptosis of endothelial cells [14,15,16]. The conflicting results observed in these studies may
be attributed to the activity of caspase 8 within the cells. We observed a decrease in caspase 8 activity under hypoxic conditions (Supplementary S1B), which resulted in a shift in FADD to
form a complex with RIPK1/RIPK3, thereby promoting necroptosis. SUMOylation plays an important role in hypoxic diseases, being an essential PTM of proteins that can regulate protein-protein
interactions, protein stability, and cellular localization [33]. In a model of pulmonary arterial hypertension constructed in mice with chronic hypoxia, the FIS1 deSUMOylation-SUMOylation
transition in pulmonary endothelium is an intrinsic pathogenesis of hypoxic PH [33]. Similarly, FADD can undergo SUMOylation after hypoxia treatment, but the effect of SUMOylation of FADD on
its protein function and its effect on hypoxia-induced endothelial damage was unknown. In the present study, we demonstrate that SUMOylation of FADD increases protein stability while
recruiting RIPK1/RIPK3 to promote necrotic apoptosis under hypoxia. The effects of ubiquitination and SUMOylation on protein function are controversial due to the shared nature of lysine
protein residues between ubiquitinated and SUMOylated proteins [34]. A recent study has indicated that the SUMOylation of HIF-1α induced by hypoxia targets HIF-1α for degradation through the
von Hippel-Lindau protein-mediated UPS [35]. In this case, there is a competition between SUMOylation and ubiquitination in FADD, rather than a collaborative process, resulting in a notable
increase in its protein stability. Our molecular docking results provide an explanation for this observation, as the SUMOylated FADD has masked the two ubiquitination sites. Both
SUMOylation and ubiquitination require E3 ligases to function [18, 19], and a shortcoming of this study is that it did not investigate which specific E3 ligase functions in SUMOylation
processes. We herein identified a crucial role for SUMOylated FADD in hypoxia-induced necroptosis. So far, the importance of SUMOylation has been established in multiple cellular death
patterns, including apoptosis, autophagy, senescence, and pyroptosis [36,37,38,39,40]. In pyroptosis, SUMOylation of NLRP3 restrains inflammasome activation [37]. We now report an additional
regulation of necroptosis by SUMOylation, and SUMOylation of FADD promotes its binding to RIPK1/RIPK3, thereby facilitating necroptosis. We further show that inflammatory injury as well as
necroptosis of aortic vascular endothelium in hypoxic mice can be alleviated by inhibiting SUMOylation. Therefore, we suggest the possibility of inhibiting FADD SUMOylation as a therapeutic
strategy for reducing hypoxic disease pathogenesis by inhibiting vascular inflammation and ECs necroptosis. To our knowledge, our finding is the first demonstration of the critical role of
SUMOylation in necroptosis in the vascular system. One major limitation of the present study is that we omitted investigations into the relationship between SUMOylation and hypoxia, which
will be focused on in our future studies. Nec-1 is a well-known small-molecule inhibitor of necroptosis and can attenuate a wide range of injuries caused by necroptosis in animal experiments
[41, 42]. However, a safe and effective drug to block necroptosis is still lacking in the clinic. Ginkgolic acid is an alkyl phenolic constituent extracted from the Ginkgo biloba [28].
Previous studies have shown that GA possesses numerous biological activities, including anti-inflammatory, anti-tumor, and anti-bacterial [43, 44]. Here, we demonstrated that GA inhibited
necroptosis, inflammatory responses, and endothelial damage in vascular tissues. Since GA can also inhibit SUMOylation of proteins, we did not investigate in depth whether the effect of GA
in inhibiting necroptosis originated from its anti-inflammatory or SUMOylation inhibition effect. In conclusion, our study reveals the role of SUMOylation of FADD in hypoxia-mediated
necroptosis in ECs. This study on necroptosis also provides new insights into therapeutic targets for endothelial injury. GA could play an essential role as a necroptosis inhibitor in the
clinical treatment of hypoxic diseases. MATERIALS AND METHODS ANIMAL STUDIES Healthy male C57BL/6 mice (8–10 weeks old, weighing 15–20 g) were provided by the Animal Centre of Chongqing
Medical University. All mice were housed in regulated experimental animal facilities with no more than 5 mice per cage, equipped with light (12 h light and 12 h dark environmental cycle),
suitable temperature (24 ± 2 °C), humidity (50 ± 10%), and adequate food and water. Block pseudo-randomization was used for experimental group allocation. The investigators were blinded to
grouping assignments. For the hypoxia intervention, mouse cages were placed in a hypoxic laboratory mouse incubator (PH-AM Wuxi Bodho). The hypoxic chamber was filled with nitrogen to
replace oxygen to reduce the oxygen concentration, and the oxygen concentration of the hypoxic chamber was set at 10%. The mice were rapidly euthanized by intraperitoneal injection of 150
mg/kg sodium pentobarbital at the indicated time points, and tissues were quickly collected for histological studies. CELL CULTURE AND HYPOXIA INTERVENTION Human Umbilical Vein Endothelial
Cells (HUVEC) were acquired from ScienCell Research Laboratories, Inc. (USA) and cultured in DMEM medium with 10% fetal bovine serum (FBS) (Adamas Life, China). To establish hypoxic
conditions for cell culture, the Whitely H35 HEPA Hypoxia Workstation (DonWhitley Scientific Limited, UK) was used, with gas levels set to 1% O2, 5% CO2, and 94% N2. HUVECs were incubated
under either normoxic or hypoxic conditions, following standard culture protocols, and experimental reagents were added at designated time points for each experimental setup. WESTERN BLOT
Protein samples were collected by lysing cells or vascular tissues with RIPA lysis solution (Beyotime, China). Aliquots of denatured protein samples were separated by SDS-PAGE gel and
transferred to the PVDF membrane (Millipore, United States). After being blocked with TBST solution containing 5% BSA, the membranes were incubated with primary antibodies at 4 °C overnight.
Subsequently, the membranes were incubated with secondary antibodies and exposed to chemiluminescence imaging system (UVP) development using an ECL kit (Biosharp, China). Protein levels
were analyzed by ImageJ software. The following primary antibodies were used: Anti-β-Actin (HRP-66009, Proteintech Group, United States), anti-RIPK3 (17563-1-AP, Proteintech Group),
anti-RIPK1 (17519-1-AP, Proteintech Group), anti-MLKL (66675-1-Ig, Proteintech Group), anti-FADD (14906-1-AP, Proteintech Group), anti-SUMO2/3 (11251-1-AP, Proteintech Group), anti-p-RIPK3
(ab209384, Abcam, United Kingdom), anti-TNF-α (SC-1351, Santa Cruz Biotech, United States), anti-IL-1β (A17361, Santa Cruz Biotech), anti-HIF-1α (AF1009, Affinity Biosciences), anti-p-MLKL
(AF7420, Affinity Biosciences, United States), VCAM (A0279, ABclonal Technology), ICAM (A5597, ABclonal Technology). IMMUNOFLUORESCENCE ASSAY For immunofluorescence of cells, HUVEC cells
were seeded into 24-well plates containing 10 mm × 10 mm slides. The cells were fixed with 4% PFA and blocked with 5% BSA. Subsequently, the cells were incubated with the primary antibody at
4 °C overnight, the secondary antibody at room temperature for 1 h, and DAPI staining for 10 min. For immunofluorescence of tissue sections, tissues collected from mice were fixed with 4%
PFA, and paraffin-embedded sections were performed. After dewaxing and hydration, the paraffin sections were antigenically repaired with 10 mM citrate buffer, blocked with 5% BSA, and
incubated with primary antibody overnight at 4 °C. After being washed by PBST, it was incubated with a secondary antibody at room temperature and stained with DAPI for 5 min. Fluorescence
images were collected using a confocal microscope system (Leica, Japan) using 20× or 40× objective lenses. The following primary antibodies were used: Anti-p-RIPK3 (ab209384, Abcam),
anti-HIF-1α (A22041, ABclonal Technology), anti-p-MLKL (AF7420, Affinity Bioscience), anti-HIF-1α (AF1009, Affinity Biosciences), anti-IL-1β (A17361, Santa Cruz Biotech), anti-CD31
(ab182981, Abcam). RT-QPCR Total RNA was extracted using the Trizol method, and RNA concentration was measured using an Implen Ultra-Micro Spectrophotometer. cDNA was prepared using Evo
M-MLV Mix Kit with gDNA Clean for qPCR (Accurate Biology, China). cDNA quantification for each sample was performed using SYBR® Green Realtime Master qPCR Mix (Accurate Biology, China) for
Real-time PCR. Quantitative cDNA was subjected to Real-time PCR using SYBR® Green Realtime Master qPCR Mix (Accurate Biology, China). Data were collected using Bio-Rad CFX Maestro (Bio-Rad,
United States) software and analyzed using the 2^-ΔΔCt method. The gene-specific primer sequences used for PCR are shown in Table 1. SIRNA INTERFERENCE FADD siRNA was purchased from Tsingke
Biotech (China) and Lipofectamine™ 2000 from Invitrogen (United States). According to the instruction manual, siRNA was transfected into HUVEC cells using Lipofectamine™ 2000. The siRNA
sequences used for interference are shown in Table 2. PLASMID TRANSFECTION The K120, K125, and K149 mutant plasmids of FADD (lysine mutated to arginine) were purchased from Shanghai
Biotechnology, and the Neofect® DNA transfection reagent was purchased from Genomtech (China). According to the instruction manual, HUVEC cells were transfected with Neofect® DNA
transfection reagent for the plasmid transfection. IMMUNOPRECIPITATION Protein samples were collected by lysing cells with RIPA lysis solution. Protein A+G magnetic beads (MCE) were
incubated with the indicated antibodies for 4 h at 4 °C, and then cell lysates were incubated with magnetic beads overnight at 4 °C to capture the immune complexes. After three washes with
PBST, the magnetic beads were boiled in SDS-PAGE uploading buffer at 99 °C for 10 min, and the samples were separated by SDS-PAGE and transferred to PVDF membranes for immunoblotting
analysis with the indicated antibodies. ANALYSIS OF CYTOTOXICITY AND VIABILITY HUVEC cells were inoculated in 96-well plates at 2000 cells per well. Necrotic cells were determined by
detecting cellular LDH release using the LDH Cytotoxicity Assay Kit (Beyotime, China) according to the instruction manual. Cell viability was detected using Enhanced Cell Counting Kit 8
(WST-8/CCK8) (Elabscience, China) according to the instruction manual. TUNEL TISSUE STAINING TUNEL staining was performed using the One Step TUNEL Apoptosis Assay Kit (Beyotime, China)
according to the instruction manual. TRANSMISSION ELECTRON MICROSCOPY (TEM) HUVEC cells were fixed with 3% glutaraldehyde, dehydrated, and embedded in Epon 812 to make ultrathin sections
(60–90 nm). After staining using uranyl acetate and lead citrate, they were observed by transmission electron microscopy (JEM-1400FLASH, Japan). ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA)
Serum levels of cytokines IL-1β and TNFα were measured using Mouse IL-1 beta ELISA Kit (KE10003, Proteintech Group) and Mouse TNF-alpha ELISA Kit (KE10002, Proteintech Group) according to
the instruction manual. DATA AND STATISTICAL ANALYSIS Sample size calculation was not conducted, while sample sizes were based on previous studies using similar analysis [45]. Data in this
study are expressed as mean ± SEM, and all experiments were repeated at least three times to ensure experimental reproducibility. Data were statistically analyzed using Prism 8.0 (GraphPad
Software, USA) using the Two-tailed unpaired t-test or one-way ANOVA. Differences were considered statistically significant if _p_ < 0.05. DATA AVAILABILITY Data will be made available on
request. REFERENCES * Zhang T, Xu D, Liu J, Wang M, Duan L-J, Liu M, et al. Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and
inflammation. Nat Cell Biol. 2023;25:950–62. Article CAS PubMed PubMed Central Google Scholar * Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of
ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–69. Article CAS PubMed PubMed Central Google Scholar * Eltzschig HK, Eckle T. Ischemia and reperfusion-from
mechanism to translation. Nat Med. 2011;17:1391–401. Article CAS PubMed Google Scholar * Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress.
Mol Cell. 2010;40:294–309. Article CAS PubMed PubMed Central Google Scholar * Janaszak-Jasiecka A, Siekierzycka A, Ploska A, Dobrucki IT, Kalinowski L. Endothelial dysfunction driven by
hypoxia-the influence of oxygen deficiency on NO bioavailability. Biomolecules. 2021;11:982. Article CAS PubMed PubMed Central Google Scholar * Yu B, Wang X, Song Y, Xie G, Jiao S, Shi
L, et al. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol Ther. 2022;238:108186. Article CAS PubMed Google Scholar * Abe H, Semba H, Takeda N. The roles of
hypoxia signaling in the pathogenesis of cardiovascular diseases. J Atheroscler Thromb. 2017;24:884–94. Article CAS PubMed PubMed Central Google Scholar * Marsch E, Sluimer JC, Daemen
MJAP. Hypoxia in atherosclerosis and inflammation. Curr Opin Lipido. 2013;24:393–400. Article CAS Google Scholar * Kearney CJ, Martin SJ. An inflammatory perspective on necroptosis. Mol
Cell. 2017;65:965–73. Article CAS PubMed Google Scholar * Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. Article CAS PubMed Google
Scholar * He C, Liu Y, Huang Z, Yang Z, Zhou T, Liu S, et al. A specific RIP3+ subpopulation of microglia promotes retinopathy through a hypoxia-triggered necroptotic mechanism. Proc Natl
Acad Sci USA. 2021;118:e2023290118. Article CAS PubMed PubMed Central Google Scholar * Yang X-S, Yi T-L, Zhang S, Xu Z-W, Yu Z-Q, Sun H-T, et al. Hypoxia-inducible factor-1 alpha is
involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep. 2017;7:5818. Article PubMed PubMed Central Google Scholar * Li J, Zhang J, Zhang Y,
Wang Z, Song Y, Wei S, et al. TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis. 2019;10:328. Article PubMed PubMed Central Google
Scholar * Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–9. Article CAS PubMed Google Scholar * Oberst A, Dillon CP, Weinlich R, McCormick LL,
Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. Article CAS PubMed PubMed Central Google
Scholar * Lee E-W, Seo J, Jeong M, Lee S, Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012;45:496–508. Article CAS PubMed Google Scholar * Irrinki KM,
Mallilankaraman K, Thapa RJ, Chandramoorthy HC, Smith FJ, Jog NR, et al. Requirement of FADD, NEMO, and BAX/BAK for aberrant mitochondrial function in tumor necrosis factor alpha-induced
necrosis. Mol Cell Biol. 2011;31:3745–58. Article CAS PubMed PubMed Central Google Scholar * Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–82. Article CAS
PubMed Google Scholar * Vertegaal ACO. Signalling mechanisms and cellular functions of SUMO. Nat Rev Mol Cell Biol. 2022;23:715–31. Article CAS PubMed Google Scholar * Seyrek K,
Ivanisenko NV, Richter M, Hillert LK, König C, Lavrik IN. Controlling cell death through post-translational modifications of DED proteins. Trends Cell Biol. 2020;30:354–69. Article CAS
PubMed Google Scholar * Choi S-G, Kim H, Jeong EI, Lee H-J, Park S, Lee S-Y. et al. SUMO-modified FADD recruits cytosolic Drp1 and caspase-10 to mitochondria for regulated necrosis. Mol
Cell Biol. 2017;37:e00254–16. Article CAS PubMed PubMed Central Google Scholar * Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates
necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. Article CAS PubMed Google Scholar * Lee JM, Hammarén HM, Savitski MM, Baek SH. Control of protein stability by
post-translational modifications. Nat Commun. 2023;14:201. Article CAS PubMed PubMed Central Google Scholar * Seo J, Lee E-W, Shin J, Seong D, Nam YW, Jeong M, et al. K6 linked
polyubiquitylation of FADD by CHIP prevents death inducing signaling complex formation suppressing cell death. Oncogene. 2018;37:4994–5006. Article CAS PubMed Google Scholar * Cho YS,
Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell.
2009;137:1112–23. Article CAS PubMed PubMed Central Google Scholar * Zhang D-W, Shao J, Lin J, Zhang N, Lu B-J, Lin S-C, et al. RIP3, an energy metabolism regulator that switches
TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. Article CAS PubMed Google Scholar * Meng H, Liu Z, Li X, Wang H, Jin T, Wu G, et al. Death-domain
dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc Natl Acad Sci USA. 2018;115:E2001–E2009. Article CAS PubMed PubMed Central Google
Scholar * Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52:727–49. Article CAS
PubMed Google Scholar * Fan Y, Li J, Fang B. A tale of two: when neural stem cells encounter hypoxia. Cell Mol Neurobiol. 2023;43:1799–816. Article CAS PubMed Google Scholar * Wang H,
Sun L, Su L, Rizo J, Liu L, Wang L-F, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. Article
CAS PubMed Google Scholar * Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196.
Article PubMed PubMed Central Google Scholar * Zhang Q, Hu X-M, Zhao W-J, Ban X-X, Li Y, Huang Y-X, et al. Targeting necroptosis: a novel therapeutic option for retinal degenerative
diseases. Int J Biol Sci. 2023;19:658–74. Article CAS PubMed PubMed Central Google Scholar * Zhou X, Jiang Y, Wang Y, Fan L, Zhu Y, Chen Y, et al. Endothelial FIS1 DeSUMOylation
protects against hypoxic pulmonary hypertension. Circ Res. 2023;133:508–31. Article CAS PubMed Google Scholar * Li K, Xia Y, He J, Wang J, Li J, Ye M, et al. The SUMOylation and
ubiquitination crosstalk in cancer. J Cancer Res Clin Oncol. 2023;149:16123–46. Article CAS PubMed Google Scholar * Cheng J, Kang X, Zhang S, Yeh ETH. SUMO-specific protease 1 is
essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–95. Article CAS PubMed PubMed Central Google Scholar * Yan L, Zhang T, Wang K, Chen Z, Yang Y, Shan B, et al.
SENP1 prevents steatohepatitis by suppressing RIPK1-driven apoptosis and inflammation. Nat Commun. 2022;13:7153. Article CAS PubMed PubMed Central Google Scholar * Barry R, John SW,
Liccardi G, Tenev T, Jaco I, Chen C-H, et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat Commun. 2018;9:3001. Article PubMed PubMed Central Google Scholar *
Zhang H, Wang Y, Zhu A, Huang D, Deng S, Cheng J, et al. SUMO-specific protease 1 protects neurons from apoptotic death during transient brain ischemia/reperfusion. Cell Death Dis.
2016;7:e2484. Article CAS PubMed PubMed Central Google Scholar * Zhang L, Xie F, Zhang J, Dijke PT, Zhou F. SUMO-triggered ubiquitination of NR4A1 controls macrophage cell death. Cell
Death Differ. 2017;24:1530–9. Article CAS PubMed PubMed Central Google Scholar * Sheng Z, Zhu J, Deng Y-N, Gao S, Liang S. SUMOylation modification-mediated cell death. Open Biol.
2021;11:210050. Article CAS PubMed PubMed Central Google Scholar * Shen B, Mei M, Pu Y, Zhang H, Liu H, Tang M, et al. Necrostatin-1 attenuates renal ischemia and reperfusion injury via
meditation of HIF-1α/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids. 2019;17:701–13. Article CAS PubMed PubMed Central Google Scholar * Vandenabeele P, Galluzzi L, Vanden Berghe
T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. Article CAS PubMed Google Scholar * Chen X, Zhu M, Zou X, Mao Y,
Niu J, Jiang J, et al. CCL2-targeted ginkgolic acid exerts anti-glioblastoma effects by inhibiting the JAK3-STAT1/PI3K-AKT signaling pathway. Life Sci. 2022;311:121174. Article CAS PubMed
Google Scholar * Zhang J, Yan J. Protective effect of ginkgolic acid in attenuating LDL induced inflammation human peripheral blood mononuclear cells via altering the NF-κB signaling
pathway. Front Pharm. 2019;10:1241. Article CAS Google Scholar * Salyha N, Oliynyk I. Hypoxia modeling techniques: a review. Heliyon. 2023;9:e13238. Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS This research was funded by the Chongqing Talent Plan Project (grant number: cstc2021ycjh-bgzxm0004). The authors would like to thank the
support of Chongqing Key Laboratory for Pharmaceutical Metabolism Research on this project. Additionally, the authors would like to thank Yifan Bao (Department of Physiological Chemistry,
University of Vienna) for BioRender mapping. AUTHOR INFORMATION Author notes * These authors contributed equally: Liming Yang, Yilin Wen. AUTHORS AND AFFILIATIONS * College of Pharmacy,
Chongqing Medical University, Chongqing, China Liming Yang, Yilin Wen, Zhiyi Yuan, Dezhang Zhao, Ping Weng, Yueyue Li, Qingyang Chen, Wanping Zhang, Hui Hu & Chao Yu * Chongqing Key
Laboratory for Pharmaceutical Metabolism Research, Chongqing, China Liming Yang, Yilin Wen, Zhiyi Yuan, Ping Weng, Yueyue Li, Qingyang Chen, Wanping Zhang & Chao Yu Authors * Liming Yang
View author publications You can also search for this author inPubMed Google Scholar * Yilin Wen View author publications You can also search for this author inPubMed Google Scholar * Zhiyi
Yuan View author publications You can also search for this author inPubMed Google Scholar * Dezhang Zhao View author publications You can also search for this author inPubMed Google Scholar
* Ping Weng View author publications You can also search for this author inPubMed Google Scholar * Yueyue Li View author publications You can also search for this author inPubMed Google
Scholar * Qingyang Chen View author publications You can also search for this author inPubMed Google Scholar * Wanping Zhang View author publications You can also search for this author
inPubMed Google Scholar * Hui Hu View author publications You can also search for this author inPubMed Google Scholar * Chao Yu View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS Liming Yang: Conceptualization, Validation, Formal analysis, Investigation, Writing - Original Draft, Data curation, Software. Yilin Wen:
Conceptualization, Methodology, Writing - Review & Editing, Data curation, Software. Zhiyi Yuan and Dezhang Zhao: Methodology, Software, Data curation, Resources. Ping Weng and Yueyue
Li: Methodology, Formal analysis, Investigation. Qingyang Chen: Data Curation. Wanping Zhang and Hui Hu: Methodology, Software. Chao Yu: Conceptualization, Funding acquisition, Writing -
Review & Editing, Supervision, Resources. CORRESPONDING AUTHOR Correspondence to Chao Yu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare competing interest. ETHICS APPROVAL
AND CONSENT TO PARTICIPATE All animal experiments were conducted in strict accordance with the guidelines provided by the National Research Council’s Guide for the Care and Use of Laboratory
Animals. All procedures involving animals were approved by the Institutional Animal Care and Use of Chongqing Medical University (IACUC-CQMU). Approval number: IACUC-CQMU-2023-0375.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by Sergio Lavandero
SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIALS SUPPLEMENTAL MATERIALS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, L., Wen, Y., Yuan, Z. _et al._ Hypoxia-mediated SUMOylation of FADD
exacerbates endothelial cell injury via the RIPK1-RIPK3-MLKL signaling axis. _Cell Death Dis_ 16, 121 (2025). https://doi.org/10.1038/s41419-025-07441-2 Download citation * Received: 10
February 2024 * Revised: 13 January 2025 * Accepted: 11 February 2025 * Published: 21 February 2025 * DOI: https://doi.org/10.1038/s41419-025-07441-2 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative