Play all audios:
The regulation of T-cell fate is crucial for the balance between infection control and tolerance. Calcium (Ca2+) and zinc (Zn2+) signals are both induced after T-cell stimulation, but their
specific roles in the fate of activation and differentiation remain to be elucidated. Are Zn2+- and Ca2+ signals responsible for different aspects in T-cell activation and differentiation
and do they act in concert or in opposition? It is crucial to understand the interplay of the intracellular signals to influence the fate of T cells in diseases with undesirable T-cell
activities or in Zn2+-deficient patients. Human peripheral blood mononuclear cells were stimulated with the Zn2+ ionophore pyrithione and thapsigargin, an inhibitor of the
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). Intracellular Zn2+ and Ca2+ signals were monitored by flow cytometry and ELISA, quantitative PCR and western blot were used to
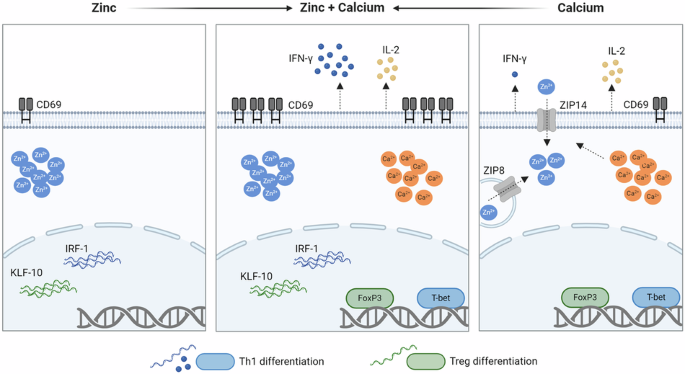
evaluate T-cell differentiation and the underlying molecular mechanism. We found that Zn2+ signals upregulated the early T-cell activation marker CD69, interferon regulatory factor 1
(IRF-1), and Krüppel-like factor 10 (KLF-10) expression, which are important for T helper cell (Th) 1 differentiation. Ca2+ signals, on the other hand, increased T-bet and Forkhead box P3
(FoxP3) expression and interleukin (IL)-2 release. Most interestingly, the combination of Zn2+ and Ca2+ signals was indispensable to induce interferon (IFN)-γ expression and increased the
surface expression of CD69 by several-fold. These results highlight the importance of the parallel occurrence of Ca2+ and Zn2+ signals. Both signals act in concert and are required for the
differentiation into Th1 cells, for the stabilization of regulatory T cells, and induces T-cell activation by several-fold. This provides further insight into the impaired immune functions
of patients with zinc deficiency.
The activation threshold of T lymphocytes and the regulation of T-cell fate are critical for the balance between infection control and tolerance. Once activated, T cells proliferate and
differentiate to mediate a highly specific immune response [1]. Stimulation of the T-cell receptor (TCR) causes the distribution of calcium (Ca2+) signals throughout the cell, depending on
the potency of the antigen [2]. An initial Ca2+ signal is induced by inositol-1,4,5-trisphosphate, causing the release of Ca2+ from the endoplasmic reticulum (ER) into the cytosol. The
depletion of Ca2+ from the ER stores is detected by sensors stromal interaction molecule 1 (STIM1) which interacts with ORAI1 in the plasma membrane and thus activating calcium
release-activated calcium (CRAC) channels. This induces a store-operated calcium entry (SOCE) from the synaptic cleft. The potentiated Ca2+ signal then causes the translocation of the
nuclear factor of activated T cells (NFAT) into the nucleus and thereby the expression of important genes for cell proliferation, such as interleukin (IL) 2 [3,4,5].
In addition to Ca2+ signals, zinc (Zn2+) signals have also become a focus of interest. Zn2+ is an essential trace element which is present in the serum with mean concentrations of 84.9 and
80.6 µg/dL in men and women, respectively [6]. Within cells, Zn2+ is mostly bound to proteins such as metallothionein [7], or to Zn2+-binding S100 proteins such as calprotectin and
calgranulin [8]. Additionally, Zn2+ is also present freely or loosely bound in the picomolar to nanomolar range. Intracellular Zn2+ signals can occur within seconds after stimulation
influencing signaling cascades (zinc flux). Therefore, Zn2+ is also regarded as a second messenger [9, 10]. Slower Zn2+ signals have also been described in mast cells, occurring a few
minutes after receptor stimulation in a Ca2+-dependent manner and are referred to as “zinc wave” [11]. Stimulation of cells may additionally cause changes in Zn2+ homeostasis after a few
days, thereby affecting gene expression [10].
In T cells especially, it has been shown that after stimulation of the TCR via dendritic cells, Zn2+ influx from the extracellular space occurs within 1 min, depending on the Zn2+
transporter Zip6. This signal occurred mostly in the subsynaptic region of the immunological synapse [9]. This is also seen in CRAC channel-mediated calcium influx where high concentrations
of calcium are localized to the subsynaptic region [12, 13]. However, calcium signals were found to rapidly diffuse throughout the cell [14]. When Zn2+ influx via Zip6 is inhibited, the
activation markers CD25 and CD69 are less expressed, indicating that T-cell activation is impaired [15]. In addition, the Zn2+ transporter Zip8 is upregulated after T-cell activation,
causing the release of Zn2+ from lysosomes into the cytoplasm. However, when Zip8 expression is inhibited, the reduced cytoplasmic Zn2+ results in reduced interferon (IFN)-γ expression [16]
and thus in T helper cell (Th) 1 differentiation.
Many studies have highlighted the importance of Zn2+ in maintaining the balance between Th1/Th2 [17, 18] in inducing regulatory T cells [19, 20]. The interplay between Zn2+ and Ca2+
signaling has also been partly investigated. Extracellular Zn2+ can sustain Ca2+ signaling after T-cell receptor stimulation [9], and in the human T-cell line Jurkat, it was seen that
different stimulants used for T-cell activation induce either a Ca2+ or a Zn2+ signal [21]. However, the mechanism by which Zn2+ and Ca2+ act in parallel has yet to be examined. Therefore,
we investigated whether Zn2+ and Ca2+ signals are responsible for different aspects in T-cell activation and differentiation. We focused on the single and combined effects of Zn2+ and Ca2+
signals independent of other activating stimulants.
To investigate the individual and the combined effects of Zn2+ and Ca2+ signals, we separately induced intracellular Zn2+ signals using the Zn2+ ionophore pyrithione (Fig. 1A) and Ca2+
signals using thapsigargin in peripheral blood mononuclear cells (PBMC) (Fig. 1B). Thapsigargin inhibits the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) and thereby reduces the
transport of Ca2+ into the endoplasmic reticulum, which results in an accumulation of Ca2+ in the cytosol [22]. PBMC were stimulated with increasing concentrations of pyrithione (0.25–2 µM)
or thapsigargin (5–150 µM). First, the cell viability was investigated by propidium iodide staining and no significant increase in dead PI+ lymphocytes was observed (Fig. S1A, B). Even with
the combined stimulation with pyrithione and thapsigargin, no significant increase in dead PI+ lymphocytes was found (Fig. S1C). Then, the induction of intracellular Zn2+ and Ca2+ signals
was measured by flow cytometry 10 min after stimulation. We confirmed that pyrithione and thapsigargin increased the intracellular Zn2+ and Ca2+ concentrations, respectively, in a
dose-dependent manner (Fig. 1A, B). 0.35 and 0.5 µM pyrithione increased the mean intracellular Zn2+ concentration from 0.048 to 0.171 and 0.248 nM, respectively. For the following
experiments, 0.35 and 0.5 µM pyrithione were used, since Zn2+ acts in the nanomolar concentration range [23].
PBMC were stained with FluoZin-3 AM for Zn2+ measurements or with Fluo-4 for Ca2+ measurements and then stimulated with pyrithione (Pyr.), thapsigargin (Thaps.) or S-nitrosocysteine (SNOC).
A 10 min after stimulation the intracellular Zn2+ or B Ca2+ concentration was measured using flow cytometry. C In addition, cells were stimulated with 2 mM SNOC and Zn2+ concentration was
measured at 15 min time intervals after stimulation. Data are presented as mean + SEM with n = 3 (A), n = 3–6 (B) and n = 4 (C) experiments. Statistical significance was determined by
one-way ANOVA with Dunnett’s multiple comparisons test (A, B) or Friedman test with Dunn’s multiple comparisons test (C) (*p