Play all audios:
ABSTRACT The response of endothelial cells to signaling stimulation is critical for vascular morphogenesis, homeostasis and function. Vascular endothelial growth factor-a (VEGFA) has been
commonly recognized as a pro-angiogenic factor in vertebrate developmental, physiological and pathological conditions for decades. Here we report a novel finding that genetic ablation of
CDP-diacylglycerol synthetase-2 (CDS2), a metabolic enzyme that controls phosphoinositide recycling, switches the output of VEGFA signaling from promoting angiogenesis to unexpectedly
inducing vessel regression. Live imaging analysis uncovered the presence of reverse migration of the angiogenic endothelium in _cds2_ mutant zebrafish upon VEGFA stimulation, and endothelium
regression also occurred in postnatal retina and implanted tumor models in mice. In tumor models, CDS2 deficiency enhanced the level of tumor-secreted VEGFA, which in-turn trapped tumors
into a VEGFA-induced vessel regression situation, leading to suppression of tumor growth. Mechanistically, VEGFA stimulation reduced phosphatidylinositol (4,5)-bisphosphate (PIP2)
availability in the absence of CDS2-controlled-phosphoinositide metabolism, subsequently causing phosphatidylinositol (3,4,5)-triphosphate (PIP3) deficiency and FOXO1 activation to trigger
regression of CDS2-null endothelium. Thus, our data indicate that the effect of VEGFA on vasculature is context-dependent and can be converted from angiogenesis to vascular regression.
SIMILAR CONTENT BEING VIEWED BY OTHERS SLUG REGULATES THE DLL4-NOTCH-VEGFR2 AXIS TO CONTROL ENDOTHELIAL CELL ACTIVATION AND ANGIOGENESIS Article Open access 26 October 2020 THE LOSS OF
_DHX15_ IMPAIRS ENDOTHELIAL ENERGY METABOLISM, LYMPHATIC DRAINAGE AND TUMOR METASTASIS IN MICE Article Open access 15 October 2021 THE HIPPO PATHWAY COMPONENT _WWC2_ IS A KEY REGULATOR OF
EMBRYONIC DEVELOPMENT AND ANGIOGENESIS IN MICE Article Open access 22 January 2021 INTRODUCTION The blood vessel system, consisting of arteries, veins and interconnecting capillaries, can
deliver oxygen, nutrients, metabolites and carry circulating blood cells to support human life. Vessels interweaved within tissues also coordinate developmental organogenesis, regulate
injury repair and regeneration, and form niches for adult stem cells1,2,3,4,5. Vascular homeostasis is maintained mainly through its remodeling, including growth and pruning/regression.
Dysregulation or dysfunction of the vascular system is associated with numerous types of human diseases6. Vascular growth is mainly governed by angiogenesis, a well-studied process of new
blood vessel formation from the existing vessels, and vasculogenesis, the de novo formation of blood vessels using stem cells, involving extracellular matrix remodeling, tip or stalk cell
fate determination, endothelial cell migration, proliferation and the subsequent stabilization by mural cells7. This process is managed by numerous angiogenic signaling factors such as
VEGFs, FGF2, PDGFs, etc.8,9. VEGFA, a well-known pro-angiogenic factor, governs vasculogenesis and angiogenesis and maintains vascular stability in embryonic development, physiological and
pathological conditions. Activation of VEGFA signaling leads to activation of downstream signal arms, including PLCγ and PI3K, both of which utilize membrane
lipid-phosphatidylinositol-4,5-bisphosphate (PIP2) to mediate signal transduction and exert their angiogenic effects10. As important as vasculogenesis and angiogenesis, vessel regression
also plays important roles in embryonic development and tissue homeostasis. It governs the elimination of hyaloid vessels11 and the maturation of retinal and brain vasculature12,13,14.
Vascular regression also occurs in the adult luteolysis and couples with breast endothelium degeneration after lactation15,16. Mechanistically, vessel regression is regulated by hemodynamics
and numerous signaling pathways13. Generally, lack of VEGFA in tissues is an important reason for the cease of blood flow, endothelial apoptosis and subsequent regression of
endothelium17,18,19,20. Besides, DLL4/Notch signaling activation promotes retinal vessel regression by inducing vasoconstriction and interrupting blood flow21, and loss of Wnt signaling
transducers (β-catenin, Lef1, Evi/Wls or Rspo3) results in reduction of retinal endothelial cell proliferation and blood vessel regression22,23,24. CDP-diacylglycerol (CDP-DAG) synthase-2
(CDS2), an enzyme utilizing phosphatidic acid (PA) to produce CDP-DAG, is responsible for the recycling of phospholipids, including phosphatidylglycerol (PG), phosphatidylinositol (PI) and
their derivates, cardiolipin (CL), PIP2 and phosphatidylinositol (3,4,5)-triphosphate (PIP3)25,26. Disruption of CDS in photoreceptor cells of _Drosophila_ limits PIP2 availability and
PLC-mediated signaling, leading to a decreased amplitude of light response26. Loss of CDS also reduces PI and PI-derived PIP3 levels, resulting in accumulation of neutral lipids along with
reduced cell and organ size in the _Drosophila_25. Vertebrates have two CDS genes, _CDS1_ and _CDS2_, both of which regulate the growth of lipid droplets and adipocyte development in
cultured human cells27. Our previous study in zebrafish model shows that _cds2_, with enriched mRNA expression in endothelium, plays an important role in vascular morphogenesis. _cds2_
mutants show reduced VEGFA signaling activity and inefficient angiogenesis phenotype, which can be rescued by delivery of PIP2-containg liposome, suggesting that the recycling of
phosphoinositides is essential for angiogenesis28. In this study, we report an unexpected observation that VEGFA stimulation promotes vessel regression in CDS2-deficient endothelia. Without
the CDS2-controlled phosphoinositide metabolic circuits, the VEGFA-PLCγ signaling axis hydrolyzes PIP2, leading to depletion of PIP3 and FOXO1 nucleus accumulation/activation to trigger
reverse migration of angiogenic endothelium. Importantly, these observations and mechanisms are conserved in both zebrafish and mice. Thus, the outcome of VEGFA stimulation may switch from
promoting neovascularization to inducing vascular regression, which depends on the specific endothelial metabolic status and signaling crosstalk. RESULTS VEGFA TRIGGERS VESSEL REGRESSION IN
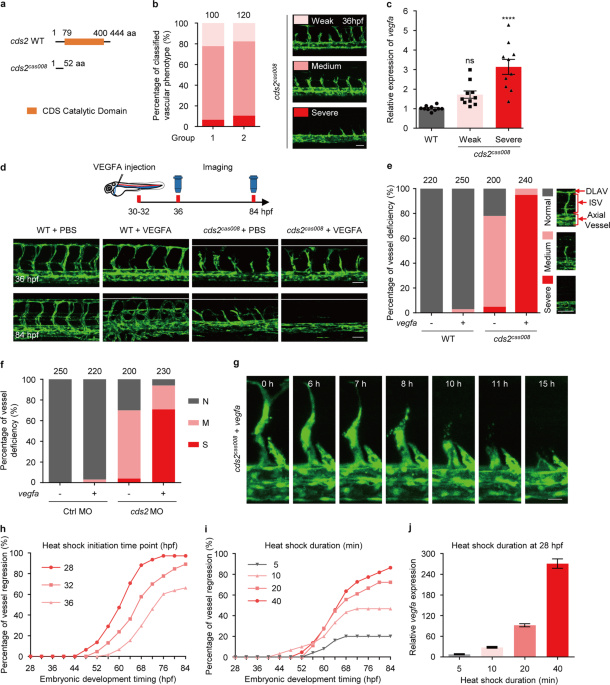
_CDS2_-DEFICIENT ZEBRAFISH EMBRYOS We previously reported that zebrafish _cds2_ mutants carried endothelium-specific morphogenic defects with impaired angiogenesis of intersegmental vessel
(ISV) and reduced VEGFA signal activity28. During the phenotype scoring on zebrafish _cds2_ mutants (Fig. 1a, b), we found there were always about 5–10% embryos showing more severe, while
about 15–20% showing less severe vascular defects, compared to the majority of _cds2_ mutants (Fig. 1b). After checking the expression level of _vegfaa_ (the major _vegfa_ isoform in
zebrafish embryos that governs vasculogenesis and angiogenesis)29,30,31, we found these _cds2_ mutants with severe vascular defects expressed a higher level of _vegfaa_, compared to those
with a weaker phenotype (Fig. 1c), which is opposite to our expectation. We further analyzed Hif1a pathway and found that Hif1a protein was accumulated and its downstream signaling was
activated in _cds2_ mutants with severe defective vessel phenotype (Supplementary information, Fig. S1a, b), indicating hypoxia might cause upregulation of _vegfaa_. To understand whether
increased VEGFA level correlates with the severity of vascular defects and explore the role of VEGFA in the vascular morphogenesis in _cds2_ mutants, we injected purified recombinant VEGFA
protein into the circulation of _cds2_ mutants (with normal or weaker phenotype) (Fig. 1d). Interestingly, almost all ISVs in VEGFA-injected _cds2_ mutants disappeared in 50 h (Fig. 1d). To
further confirm this phenomenon with statistical analysis, _cds2_ mutants were crossed into _Tg(hsp:vegfaa)_ transgenic background32. After heat shock induction, _vegfaa_ expression reached
a peak level in 4 h and then remained at least 5 folds higher compared to that in siblings without VEGFA induction (Supplementary information, Fig. S1c). _vegfa_ over-expression (OE) caused
hyper-branching in wildtype siblings (Supplementary information, Fig. S1d, e), however, over 90% of the _cds2_ mutants displayed complete loss of ISVs in their trunk regions at 84 h post
fertilization (hpf), which was over 50 h post heat shock induction of _vegfa_ (Fig. 1e). This was about the same time at which VEGFA protein injection caused the phenotype (Fig. 1d and
Supplementary information, Fig. S1e). We also tested _cds2_ morphants, which phenocopied _cds2_ mutants showing VEGFA-induced loss of angiogenic vasculature (Supplementary information, Fig.
S1f, g, Fig. 1f). Live imaging analysis revealed that ISVs in _cds2_ mutants initiated reverse migration at about 16 h post heat shock induction of _vegfaa_ and about 65% endothelial cells
underwent apoptosis before merging into axial vessels (Fig. 1g, Supplementary information, Fig. S1h, Video S1, 2 and 4), while EC proliferation barely happened in this process compared with
that in normal angiogenesis (Supplementary information, Video S3 and S4). Time-lapse scoring of vascular phenotype also confirmed that vessel regression initiated at 16–20 h post heat shock
induction and took about 40 h in-total to reach over 80% regression (Fig. 1h and Supplementary information, Fig. S1i). The duration of vessel regression remained unchanged when we
sequentially delayed the heat shock induction time points and the percentage of ISV regression depended on _vegfa_ expression level (Fig. 1h–j). The morphology of axial vessels, including
dorsal aorta and cardinal vein, remained intact during ISV regression. In addition, we found that regression of ISV also occurred in zebrafish _cds2_ mutants without _vegfa_ OE
(Supplementary information, Video S2). Such regression occurred slowly and stopped around 52 hpf, probably due to moderate stimulation of ventral (lower level) to dorsal (higher level)
gradient of transiently expressed endogenous _vegfa_33. VESSEL REGRESSION IN ENDOTHELIUM-SPECIFIC _CDS2_ KNOCKOUT MICE The observed VEGFA-induced vessel regression has never been reported
before. We therefore set to verify whether it is conserved in vertebrates, especially in mammals. Endothelial-specific tamoxifen-inducible _Cds2_ knockout mice (_Cds2__iΔEC_) were generated
by putting _loxP_ sites on both side of _exon2_ in _Cds2_ locus and crossing the floxed _Cds2_ mice into _Cdh5:Cre-ERT2_ transgenic background34 (Supplementary information, Fig. S2a, b).
Significant downregulation of _Cds2_ was validated in the endothelium of _Cds2__iΔEC_ mice. We also found that the expression of _Cds2_ was highly enriched in endothelium in wildtype mice
(Supplementary information, Fig. S2c, d). First of all, we performed phenotypic analysis on retinal vasculature (Fig. 2a–d), a system that has been well established for studying vascular
morphogenesis35. _Cds2__iΔEC_ caused significant reduction in endothelium coverage and the number of branch points, compared to those of retinal vasculature in the control mice (Fig. 2b, d).
Furthermore, there were significantly reduced endothelial sprouts, but increased Collagen 4 (COL4)-positive and isolectin B4 (IB4)-negative sleeves (an indication of regressed vessel) in
the angiogenic front of retinal vasculature in _Cds2__iΔEC_ mice (Fig. 2c, d). Importantly, as observed in zebrafish _cds2_ mutants, this defective vascular morphogenesis in _Cds2__iΔEC_
mice could be further enhanced by injection of recombinant VEGFA at postnatal day (P) 4 and 5, which resulted in blunted angiogenic front with further reduced endothelial sprouts (Fig.
2a–d). We next assessed whether cell apoptosis and proliferation were altered in _Cds2__iΔEC_ mice. In line with zebrafish study, endothelial apoptosis was also observed in the retinal
vasculature of _Cds2__iΔEC_ mice (Supplementary information, Fig. S2e, f), and a reduction of EC proliferation in _Cds2__iΔEC_ mice was revealed by EdU incorporation, which was further
declined by VEGFA stimulation (Supplementary information, Fig. S2g, h). VEGFA has been reported to govern vasculogenesis and angiogenesis in most of developmental, physiological and
pathological processes, including tumor angiogenesis9,36,37. The B16 melanoma or TC-1 lung carcinoma cells have been reported to progress in an angiogenesis-dependent manner38,39,40.
Interestingly, we found that either B16 or TC-1 cells could barely form tumors after implantation into the _Cds2__iΔEC_ mice, whereas they formed tumors readily in the control mice
(Supplementary information, Fig. S3a–j). Further analysis showed that there were dramatically reduced vascular densities inside those tiny tumors harvested from the _Cds2__iΔEC_ mice,
compared to those from the control mice (Supplementary information, Fig. S3a–j). To further examine the vascular dynamics in the anti-tumor role of CDS2 deficiency, endothelium-specific
_Cds2_ knockout was induced by tamoxifen injection after tumor cell implantation (Fig. 2e and Supplementary information, Fig. S4a). These tumors could grow as rapidly in the initial 7 days
as those in the control mice, but then grew slower and even regressed in _Cds2__iΔEC_ mice (Fig. 2e and Supplementary information, Fig. S4a). We tracked B16 tumor growth retardation in
_Cds2__iΔEC_ mice up to 35 days without finding resistance or reoccurrence, while most of B16 tumors in control mice reached the size limitation (1000 mm3, allowed by Institute Animal
Research Protocol) within 18 days (Fig. 2e). More interestingly, all TC-1 tumors were dramatically regressed in _Cds2__iΔEC_ KO mice and finally disappeared in 21–25 days, without
reoccurrence up to 35 days (Supplementary information, Fig. S4a, b), and we did observe high frequent tumor necrosis in both B16 and TC-1 tumors (Fig. 2f and Supplementary information, Fig.
S4c). Immunostaining analysis showed severely reduced vascular density inside the tumors, which highly correlated with reduced tumor weight (Fig. 2f–h and Supplementary information, Fig.
S4c, d). In addition, more endothelium regression (COL4-positive/CD31-negative sleeves) was observed in retarded B16 tumors in _Cds2__iΔEC_ mice (Fig. 2i, j). Because TC-1 tumor cells
themselves expressed high level of COL4, ultrasound imaging assay showed gradually reduced blood flow inside TC-1 tumors after endothelium-specific _Cds2_ KO in the recipient mice
(Supplementary information, Fig. S4e). After checking the expression level of _Vegfa_ in these tumors, like we did in zebrafish _cds2_ mutants, we found that both B16 and TC-1 tumors,
harvested from _Cds2__iΔEC_ mice at 9 days post-implantation, expressed much higher _Vegfa_ than tumors harvested from the control mice, although the vasculature inside these _Cds2__iΔEC_
tumors had dramatically regressed (Fig. 2k and Supplementary information, Fig. S4f). Thus, increased VEGFA seems not to promote angiogenesis inside _Cds2__iΔEC_ tumors, but correlates with
vessel regression as observed in zebrafish _cds2_ mutants (Fig. 1 and Supplementary information, Fig. S1). As expected, tumor cells in _Cds2__iΔEC_ mice were more hypoxic than those in
control mice and expressed much higher VEGFA compared to non-tumor cells (Supplementary information, Fig. S4g–i). Therefore, we hypothesized that, once tumors reached certain size, hypoxia
would drive VEGFA expression which would normally trigger angiogenesis, but caused vessel regression in the absence of _Cds2_. Reduced or reversed angiogenesis would further enhance the
hypoxia condition, which subsequently caused more VEGFA expression and triggered the greater vessel regression, eventually leading to retarded growth or regression of the tumors (Fig. 2l).
Intriguingly, _Cds2__iΔEC_ mice were viable during all experiments and vasculature in organs, including heart, kidney and liver, was intact (Supplementary information, Fig. S5a, b), which
might be due to the highly expressed VEGFA in _Cds2__iΔEC_ tumors compared to these organs (Supplementary information, Fig. S5c). Beyond VEGFA, the expression levels of other angiogenic
ligands, including FGF2, PIGF and PDGFB were also upregulated (Supplementary information, Fig. S5d), which was commonly seen in tumors with anti-VEGFA antibody or anti-VEGFR2 inhibitor
administration for rapid development of resistance. However, we did not observe any resistance or reoccurrence of tumor in _Cds2__iΔEC_ mice. Microinjection of ectopic FGF2 also failed to
rescue ISV defects in _cds2_ mutant zebrafish with _vegfa_ OE, although it is sufficient to cause hyper-branching ISV phenotype in control zebrafish embryos (Supplementary information, Fig.
S5e, f). PIP3 METABOLIC EXHAUSTION GOVERNS VEGFA-INDUCED VESSEL REGRESSION To explore the mechanism by which CDS2 deficiency converts VEGFA signaling from pro-angiogenic effect to promoting
vessel regression, we carried out liposome rescue experiments on _cds2_ zebrafish mutants with _vegfa_ OE. CDP-DAG, the product of CDS225,26, could be further converted to either PG or PI to
supply PIPs and CL, both of which have been reported to play important roles as cell signaling messengers or regulators41,42,43,44,45 (Fig. 3a). We found that microinjection of liposomes,
carrying synthetic PI, its derivates PI(4,5)P2 (PIP2) or PI(3,4,5)P3 (PIP3), but not the PG-containing liposome, could significantly rescue the formation of ISV sprouts in _vegfa_-OE _cds2_
mutants (Fig. 3b–d). To distinguish the role of PIP2 and PIP3 in VEGFA-induced vessel regression in _cds2_ mutants, we knocked down PTEN to block the conversion of PIP3 back to PIP246,47,
and found PTEN inhibition could significantly rescue the vascular defects in _cds2_ mutants with or without _vegfa_ OE, without affecting _vegfa_ OE level (Fig. 4a–c). Lipid quantitation
showed that VEGFA stimulation indeed caused dramatic reduction of PIP2 and PIP3 in CDS2-deficient endothelium, and PTEN knockdown rescued the level of PIP3, but not PIP2, in CDS2-deficient
endothelium under VEGFA stimulation (Fig. 4d and Supplementary information, Fig. S5g). In addition, we found inhibition of PTEN through small molecular compound, bpV48,49, could also rescue
vascular defects in retinal vasculature of _Cds2__iΔEC_ mice even with VEGFA stimulation, by increasing the number of angiogenic sprouts and reducing vessel regression (Fig. 4e–g). These
results indicate that the availability of PIP3 plays a key role in VEGFA-induced regression of CDS2-deficient endothelium. Vessel regression or inefficient angiogenesis has been linked to
hyper-activation of Notch signaling21,50,51, inactivation of Wnt/β-catenin or silencing of Ca2+ signaling22,23,24,52. To test whether these signal pathways are involved in VEGFA-induced
vessel regression, we firstly treated _vegfa_-OE _cds2_ mutants with Notch inhibitor, DAPT. _vegfa_ OE indeed activated Notch signaling, which could be completely abolished by DAPT treatment
(Supplementary information, Fig. S6a). However, vessel regression persisted in _cds2_ mutants with _vegfa_ OE (Supplementary information, Fig. S6b). Furthermore, neither activation of
Wnt/β-catenin signaling (endothelium-specific expression of constitutively activated form of β-catenin)53 nor activation of Ca2+ signaling (Inomycin treatment)54,55 could rescue vessel
regression phenotype in _cds2_ mutants with _vegfa_ OE (Supplementary information, Fig. S6c–f). In addition, blood flow in axial vessels remained normal during ISV reverse migration of
_cds2_-deficient embryos with _vegfa_ OE and pericyte or smooth muscle cell coverage of the vessels occurred later than vessel regression in zebrafish embryogenesis56, so the possibilities
that ISV regression caused by blood flow blockage or abnormal coverage of mural cells12,14,57 are very unlikely. FOXO1 ACTIVATION IN VEGFA-INDUCED CDS2-DEFICIENT ENDOTHELIUM REGRESSION FOXO1
has been reported to govern angiogenesis by regulating endothelium metabolism and apoptosis48,58,59, and it is negatively regulated by PIP3/Akt signaling through limiting its nuclear
translocation and hence transcription activity60,61,62. Endothelium-specific knockout of _Foxo1_ causes hyper-branching of retinal vasculature, while overexpression of constitutively
activated FOXO1 (AKT phosphorylation sites are mutated, constitutive nuclear localization) causes deficient vascular morphogenesis58, which is similar to that observed in the retinal
vasculature of _Cds2__iΔEC_ mice (Fig. 2b, d). Immunostaining analysis on retinal vasculature showed that, nuclear FOXO1 mainly appeared in the remodeling plexus region, but not the
angiogenic front in the retinal vasculature in the control mice, which was consistent to previous reports58 (Fig. 5a–d and Supplementary information, Fig. S7a). We found, different from that
in the control mice, FOXO1 protein predominantly located in endothelial nuclei in the front area of retinal vasculature in _Cds2__iΔEC_ mice (3.4 folds increase, compared to Control), which
could be further enhanced by additional VEGFA stimulation (9.1 folds increase, compared to control with recombinant VEGFA stimulation) that resulted in dramatically increased nucleus FOXO1
in blunted angiogenic front with significantly reduced sprouts (Fig. 5a–c). This FOXO1 activation was further validated by transcript analysis of FOXO1 target genes in retinal endothelial
cells (Supplementary information, Fig. S7b)58. To examine whether activated FOXO1 signaling governs vessel regression in _Cds2__iΔEC_ mice, we applied the treatment of FOXO1-specific small
molecular inhibitor, AS1842856 (AS), in the same experiments48. We found that AS treatment could significantly increase the endothelium coverage, the number of branch points and the
angiogenic sprouts in the retinal vasculature of _Cds2__iΔEC_ mice (Fig. 5e–g). Specifically, COL4-positive/IB4 negative sleeves, remained by vessel regression, was remarkably reduced in the
frontier retinal _Cds2__iΔEC_ vasculature even with ectopic VEGFA injection (Fig. 5f, g), indicative of the essential role of FOXO1 activation in VEGFA-induced vessel regression of
CDS2-deficient endothelium. Furthermore, consistent with that in mice model, FOXO1 nuclear translocation also occurred in the endothelium of zebrafish _cds2_ mutants, but not in wildtype
siblings even with VEGFA stimulation (Fig. 6a), which is further supported by reduced phospho-FOXO1 level in CDS2-deficient HUVECs (Supplementary information, Fig. S7c). The level of
endothelial nuclear FOXO1 in _cds2_ mutants could be enhanced by VEGFA stimulation (Fig. 6a), but rescued by PTEN knockdown (Fig. 6b and Supplementary information, Fig. S7d, e), which
further indicated that the FOXO1 nuclear translocation and activation was under the control of PIP3 availability upon VEGFA stimulation (Fig. 4). In addition, AS treatment could also
successfully rescue ISV angiogenic sprouts in _cds2_ mutants even with _vegfa_ OE, although angiogenic front was disorganized, most likely due to the loss of PIP3 (Fig. 6c–e). Thus, we
conclude that VEGFA can trigger the regression of _cds2-null_ vessels in a FOXO1-dependent manner, which should be blocked by the PI3K-PIP3-Akt axis of VEGFA signaling in normal
angiogenesis. PIP3 EXHAUSTION AS THE RESULT OF PLCΓ-MEDIATED PIP2 HYDROLYSIS IN VEGFA-STIMULATED CDS2-DEFICIENT ENDOTHELIUM To understand how VEGFA stimulation triggers PIP3 reduction in
endothelium of CDS2-deficient zebrafish embryos, we tested another signaling arm of VEGFA transduced by PLCγ. PLCγ hydrolyzes PIP2 to produce DAG and IP3, both of which are second messengers
for signal transduction to stimulate angiogenesis and will be recycled in a CDS2-mediated PI metabolic circuit to supply the pool of phosphatidylinositides26. PIP2 level is relative
abundant, compared to that of PIP363, thus PLCγ might serve as a major pathway to consume PIP2. We found partial knockdown of PLCγ (0.5 ng morpholino is not sufficient to block
vasculogenesis or angiogenesis) could block vessel regression in _cds2_ mutants with _vegfa_ OE, without affecting the level of _vegfa_ OE induction (Fig. 7a–c). And lipid quantitation also
showed that VEGFA-triggered PIP3 and PIP2 reduction in _cds2_-deficient endothelial cells could indeed be rescued by PLCγ knockdown (Fig. 7d). These results indicate that, without
CDS2-mediated phosphoinositide recycling, activation of PLCγ signaling by VEGFA causes PIP2 hydrolysis and its eventual reduction, which subsequently causes the reduction of the PIP3 level,
leading to FOXO1 activation and vessel regression, as summarized in Fig. 7e. DISCUSSION Homeostasis and remodeling of blood vessels interweaved within tissues and organs are tightly
controlled by intercellular metabolism and intracellular signaling network7,64, the crosstalking among which determines the specificity and flexibility of final outputs. It has been
documented that VEGFB, a close family member to VEGFA, is a context-dependent cytokine. It works as a pro-angiogenic factor in inefficient angiogenesis, but attenuates excessive vascular
branches to stabilize vasculature65. Here, we reported that the outcome of VEGFA could be altered from pro-angiogenesis to vessel regression when CDS2-dependent phosphoinositide recycling
was blocked. Furthermore, we found that VEGFA-induced vessel regression occurred mainly in the angiogenic front of retina and in growing tumors, but not in other vasculature-enriched normal
organs, including heart, liver or kidney in _Cds2__iΔEC_ mice (Supplementary information, Fig. S5a, b). We hypothesized that the endothelium under angiogenic status might require more PIP3
availability to balance VEGFA downstream signaling and be more sensitive to FOXO1 activation than those vessels that had been connected to each other or well-covered by pericytes/smooth
muscle cells, and might be in quiescent or different metabolic status58. Although the phenotype and causal mechanism of VEGFA-induced vessel regression reported in this study are novel, a
number of questions still need to be addressed in future studies. First of all, vertebrate CDS1 and CDS2 share very similar catalytic domains and play similar roles in lipid metabolism
regarding energy storage27. However, ectopic expression of CDS1 could not rescue vascular phenotype in CDS2-deficient zebrafish embryos either with or without _vegfa_ OE. On the other hand,
FGF2 also utilizes PLCγ and PI3K to transduce signals, ectopic FGF2 protein injection is sufficient to induce ISV hyper-branching phenotype in WT embryos, but failed to trigger vessel
regression in CDS2-deficient zebrafish embryos. Thus, mechanistic interaction between CDS2-controlled phosphoinositide recycling and VEGFA signaling remains elusive. Our new data argue that
PIP3, but not PIP228,66, equilibrates the angiogenic status of endothelial cells upon VEGFA stimulation. However, treatment of PI3K inhibitors on zebrafish embryos (28–76 hpf) did not cause
severe ISV regression as CDS2 deficiency did (Supplementary information, Fig. S8a, b), although they were sufficient to block angiogenesis if administration was applied (20–32 hpf) as
previously reported67 (Supplementary information, Fig. S8c, d). In B16 tumor model, loss of CDS2 showed much more potency to cause tumor inhibition with vascular defects, especially vessel
regression, compared to that of PI3K inhibitor (BEZ235 and BKM120) administration (Supplementary information, Fig. S8e–h). In other word, CDS2 inhibition, as a novel multi-targeting
approach, could work more efficient than PI3K inhibitors on vessel regression under microenvironmental VEGFA stimulation. This might be due to multiple effects caused by the disruption of
phospholipid recycling upon CDS2 deficiency. Actually, deprivation of CDS2 was also reported to lead to phosphatidic acid (PA) accumulation27, which served as an important second messenger
for signaling transduction68,69,70. Thus, combined effect of upregulated PA and inhibited PIP3 on vascular morphogenesis should be tested under VEGFA stimulation in future studies.
Phosphoinositide recycling is generally considered as a housekeeping metabolic circuit, and currently, there is no commercially available inhibitor to modulate CDS2 activity. In implanted
tumor models, the levels of tumor-secreted VEGFA are very high, compared to those in normal tissue or organs. Such expression could be further increased by 4–10 folds in _Cds2__iΔEC_ mice
(Fig. 2k and Supplementary information, Fig. S4f). After retro-orbital or intravenous injection of cell-permeable CDS2-specfiic vivo-morpholinos (chemical-modified morpholino with cell
penetrating capability) into mice with implanted B16 tumors, they worked efficiently to trigger vessel regression and block tumor progression as that occurred in _Cds2_ endothelium-specific
knockout mice (Supplementary information, Fig. S8i–k). However, these reagents could only be used as CDS2 inhibitors for research purpose, a CDS2-specific inhibitory small molecular compound
will be helpful in future mechanistic characterization and translational applications. A variety of studies demonstrate that an increased VEGF level usually accompanies with impaired
angiogenic activity in diabetes71,72,73,74,75. Although a chronic and sustained stimulation of upregulated VEGFA was proposed to be a causal effect, we think that this hypothesis remains
controversial since upregulated VEGFA can also result in diabetic retinopathy by excessive angiogenesis76. In addition, impaired PI3K-Akt signaling was reported in the myocardium of type 2
diabetic patients with chronic coronary heart disease, coupling with upregulated VEGFA71. Elevated VEGFA165 was also reported to exacerbate human type-2 diabetic nephropathy77, and
administration of VEGFA neutralizing antibody resulted in amelioration of long-term renal changes in obese type-2 diabetic mice78. Thus, it will be interesting to know whether upregulated
VEGFA associates with deficient phosphoinositide recycling in diabetic condition in heart or kidney, and tissue/organ specific signaling crosstalking and metabolic status must be considered
to elucidate the underlying mechanism. Given that numerous types of cells can secrete VEGFA to induce or regulate vascular niche in various physiological and pathological conditions,
including adult stem cells, progenitors, immune cells and others79,80,81,82,83,84, the vascular defects, previously described as impaired angiogenesis, may need to be revisited from the
angle of vessel regression by checking microenvironmental VEGFA, phosphoinositides availability, PIP3-Akt activation and FOXO1 nucleus accumulation. MATERIAL AND METHODS ZEBRAFISH HUSBANDRY
AND TRANSGENIC LINES All zebrafish studies in this work were performed according to the guidelines of the Animal Ethics Committee and Institutional Animal Care and Use Committee of Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Zebrafish maintenance and breeding were performed by standard methods85. The transgenic line
_Tg(fli1a:eGFP)_, _Tg(hsp:vegfaa)_, _Tg(fli1a:gal4)_, _Tg(tp1:dsGFP)_, _Tg(HuC:gal4)_, _Tg(fli1a:ngfp)_ and _Tg(uas:GCaMP5)_ were described previously14,32,86,87,88. Most experiments in this
study were carried out in the embryos generated by outcross of _Tg(hsp:vegfaa)_ with other lines if possible. The _cds2_ mutant was generated by CRISPR/Cas9 mutagenesis (Supplementary
information, Table S1). The heterozygotes or homozygotes were identified by genomic PCR, followed by sequencing. All PCR Oligos was listed in Table S1. MICROINJECTION Morpholino oligos (MOs)
were purchased from Gene Tools Company and dissolved in nuclease free water. Embryos were injected at one-cell stage with 1–6 ng control MO, 1 ng _cds2_ MO28, 0.5 ng _plcg1_ MO89, 6 ng
_ptena_ MO90, 6 ng _ptenb_ MO90 or a mixture of MOs if required. The MO sequences was listed in Table S1. For _pTol2:flk1-ΔN β-catenin-2a-mcherry_ rescue experiments, embryos were injected
with 50 pg of _pTol2:flk1-ΔN β-catenin-2a-mcherry_ construct53 together with 10 pg _transposase_ mRNA. _transposase_ mRNA was synthesized in vitro by SP6 mMessage mMachine Transcription Kit
(Ambion). For protein delivery into circulation, 1–2 nl VEGF-A (Sino Biological, 11066-HNAH-5; 250 μg ml−1) or FGF2 (Novoprotein, C044; 200 μg ml−1) dissolved in water was injected into the
common cardinal vein (CCV) of the embryos at 28–32 hpf. For phospholipid delivery, phosphatidylinositol (PI) (Echelon Biosciences, P-0004; 1 mg ml−1), phosphatidylglycerol (PG) (Sigma,
P8318; 2.5 mg ml−1), phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) (Echelon Biosciences, P-3904; 1 mg ml−1) and phosphatidylinositol 4, 5-bisphosphate (PIP2) (Echelon Biosciences,
P-9045; 1 mg ml−1) were dissolved in water and incubated with equal molar concentration of histone H1 carrier (Echelon Biosciences, P-9C2) for 10 min at room temperature before injection.
Then, 1–2 nl lipid–carrier mixture were delivered into the CCV of _cds2_ mutants or control embryos at 28–30 hpf. Histone H1 carrier alone-injected embryos were used as controls. Vessel
regression assessments were done at 76–84 hpf. HEATSHOCK INDUCTION AND COMPOUND TREATMENT ON ZEBRAFISH MODEL For heat-shock induction, zebrafish embryos in _Tg(hsp:vegfaa)_ background were
heat-shocked at 28–36 hpf at 37 °C for 1 h and then analyzed for ISV phenotype at the indicated stages. For _vegfa_ expression level determination, total RNA from the heat-shocked embryos
(10–20 embryos pooled for each sample) at 30–32 hpf was extracted and reverse transcribed to cDNA for qPCR analysis. For small molecular compound treatments, all chemicals were dissolved in
DMSO and diluted in egg water containing 0.045% 1-phenyl-2-thiourea (PTU, Sigma) at the indicated concentrations, including DAPT67 (Selleck; 30 μM), AS1842856 (Selleck; 20 nM), Ionomycin
(Sigma; 500 nM), BKM120 (TargetMol; 5 μM) and BEZ235 (MCE; 10 μM). Embryos were manually dechorionated and incubated with chemicals at the indicated stage until analysis. To validate the
function of ionomycin on zebrafish, embryos were treated at 30 hpf for 10 min and then mounted for confocal imaging analysis. ZEBRAFISH TUNEL ASSAY Zebrafish embryos were collected at 54–60
hpf and fixed with 4% PFA. After methanol dehydration, rehydration, proteinase K digestion and acetone treatment, the embryos were permeabilized in incubating buffer (0.1% Triton X-100, 0.1%
sodium citrate in PBS) for 30 min at RT. Then TUNEL staining was performed using the In Situ Cell Death Detection Kit TMR red (Roche) as the manufacturer’s instruction. After that, the
embryos were incubated with mouse anti-GFP antibody (Abmart, M20004, 1:500) followed by incubation of Alexa-Fluor 488-conjugated secondary antibody. IMAGING ANALYSIS ON LIVE ZEBRAFISH
EMBRYOS Confocal images of zebrafish live transgenic embryos were obtained by a Zeiss LSM 710 inverted confocal microscope (Carl Zeiss, Germany) or Olympus FV1000 scanning confocal
microscope. Live embryos were anesthetized with 0.03% Tricaine (Sigma) and then mounted in 1% low-melt agarose. Images were taken using a Plan Apochromat 10× objective or UPLSAPO 20×
objective with 488 nm and/or 559 nm lasers. For time-lapse imaging, embryos were anesthetized with 0.03% Tricaine (Sigma-Aldrich), mounted with 1% low-melt agarose in custom-built chamber
and imaged using a Zeiss LSM 880 upright confocal microscope (Carl Zeiss, Germany) equipped with a 20× or 40× objective. Z-stacks were acquired at 2–3 μm increments every 10–14 min. Images
were processed by ImageJ (NIH) and Imaris (Bitplane) software. LIPID QUANTIFICATION Endothelial cells were sorted by FACS from _Tg(fli1a:eGFP)_ background embryos (2000 per sample) at 48 hpf
(before vessel regression), followed by lipid extraction as previously described91. In brief, cells were lysed in 3.75 volumes methanol:chloroform:HCl (40:20:1) mixture on ice, followed
with 1 volume chloroform and 2.25 volumes water. After vortexing for 1 min, samples were centrifuged at 3000 rpm for 2 min at 4 °C, and the lower organic phase was collected and dried under
nitrogen stream. Quantitative analysis on PIP2 or PIP3 was performed by commercial available kits (Echelon Biosciences, K-4500 and K-2500s), with synthetic PIP2 or PIP3 (Echelon Biosciences)
as standard control. GENETIC MANIPULATION AND HUSBANDRY OF MICE MODEL All mice were maintained under specific pathogen-free conditions and handled according to the Animal Ethics Committee
and Institutional Animal Care and Use Committee of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Project license of mice study used in this work
was SIBS-2017-PWJ-1. Floxed Cds2 mice were commercially generated (Shanghai Model Organisms Center). _Cds2__fl_/_fl_ mice were bred into _Cdh5-CreERT2_ mice34 to generate inducible knockout
line in the endothelium. All genotypings were determined by PCR analyses with oligos listed in Table S1. Cre activation in newborn mice was induced by three consecutive intraperitoneal
injections of 50 μl tamoxifen (Sigma, T5648; 2 mg ml−1) on postnatal day (P) 3-P5. Injections of vehicle or 1 μg mVEGF-A (Sino Biological, 50159-MNAB; 100 μg ml−1) dissolved in PBS were
administrated subcutaneously in the region surrounding the eye at P4 and P5. Retina vasculature was analyzed at P7. Tamoxifen-injected _Cre_− littermate animals were used as control. To
characterize the efficiency of mouse endothelium-specific _Cds2_ knockout, endothelial cell isolation was performed as previously described92. In brief, organs were harvested, cut into small
pieces and dissociated into single cell suspension by a digestion with 1 mg ml−1 collagenase/dispase (Roche, 10269638001). Then cell suspension was incubated with anti-CD31 antibody (BD
Biosciences, 553370) pre-incubated microbeads (Miltenyi Biotec) for endothelial cell separation by manufacturer’s protocol. Total RNA of endothelial cells was extracted and reversely
transcribed into cDNA. _Cds2_ expression relative to the housekeeping gene _Actb_ was determined by qPCR. COMPOUND TREATMENT ON MICE RETINA MODEL For pharmacological inhibition experiments
in mouse pups, pilot experiments were performed to estimate the optimum concentration of each small molecular compound. To inhibit FOXO1 or PTEN, AS1842856 (Calbiochem, 344355; 2.5 mg kg−1)
or bpV (EMD Millipore, 203701; 2 mg kg−1) were injected subcutaneously in the region surrounding the eye at P2, P4 and P6 before dissection at P7. DMSO (5%) or saline was used as vehicle and
experimental control. CELL CULTURE AND MANIPULATION Mouse lung carcinoma cell line TC-1 and melanoma cell line B16 were purchased from the cell bank of Shanghai Institutes for Biological
Sciences. These lines are not listed in the International Cell Line Authentication Committee database. GFP-labeled B16 cells were generated by retroviral transduction with GFP-expressing
construct as previously reported93. Blasticidin was used for stable cell line selection. TC-1 and B16 cells were cultured in RPMI-1640 and DMEM respectively, supplemented with 10% FBS, 1%
Penicillin/Streptomycin. HUVECs (PromoCell) were cultured in endothelial growth medium-2 (PromoCell, C-22011) on plates pre-coated with 1% collagen (Corning, 354236). HUVECs less than
passage 5 were used in experiments and tested negative for mycoplasma. CDS2 knockdown was performed as previously described28. All cells were tested negative for mycoplasma contamination
before experiments by PCR. Primer sequences for this test can be found in Table S1. TUMOR XENOGRAFTS For tumor angiogenesis study, mice aged between 8–12 weeks were injected with 80 mg per
kg (weight) tamoxifen intraperitoneally, once daily for 5 days to induce _Cds2_ deletion before tumor cell inoculations. Cells were harvested in 100 μl RPMI-1640 or DMEM medium and gently
mixed 2:1 (volume ratio) with matrigel (Corning, 356237). Then, mice were inoculated subcutaneously on the lateral flanks with 106 TC-1 cells or 0.5 million B16 cells (150 μl volume per
injection). Vernier caliper was used to measure the approximate tumor volume calculated as length × width2/2, and final tumor weight measurements were taken at the termination of the
experiments. Subsequently, tumors as well as other organs were harvested for histology analysis. For vessel regression determination, mice were injected with 3 × 106 TC-1 cells or 106 B16
cells (150 μl volume per injection) two days prior to a five-day consecutive injection of tamoxifen, allowing for a robust angiogenic response. Tumors were measured once two days for 25 days
or until reaching an average diameter of 1.5 cm, and tumor necrosis was monitored daily during this process. For TC-1 tumors, ultrasound imaging and analysis were performed at day 7, 11 and
15 post-implantation to assess the blood perfusion. Meanwhile, a portion of tumors without necrosis were excised at day 9 or 13 for tumor weight measurements, histology and qRT-PCR
analysis. To determine the _Vegfa_ expression in tumor cells, GFP-labeled B16 cells were inoculated as described above. Then day 9 tumors were excised, cut into pieces and digested with
collagenase/dispase (Roche, 1 mg ml−1) in DMEM at 37 °C for 30 min. GFP-positive and -negative cells were sorted by FACS to perform qPCR analysis. To pharmacologically inhibit PI3K
signaling, BKM120 or BEZ235 dissolved in 10% N-methyl-2-pyrrolidone (NMP) with 90% PEG400 was orally administrated at 40 mg kg−1 daily from day 5 to day 9 after B16 cell inoculation. Then,
tumors were excised at day 10 for weight measurement and immunohistochemistry analysis. For vivo morpholino (vMO) administration, _Cds2_ vMOs (Gene Tools) were administrated at 1 mg kg−1
daily through intravenous injection from day 1 post B16 cell implantation until the experimental termination. Tumor size was measured once two days for 15 days or until reaching size
limitation guided by Animal Protocol. ULTRASOUND IMAGING AND ANALYSIS Ultrasound imaging was performed using the Vevo 2100 high-frequency ultrasound system (VisualSonics, Toronto, ON,
Canada). Tumor-bearing mice were anesthetized with isoflurane at 1.5% concentration delivered with medical air through a vaporizer. Mice were positioned on a heated platform (THM 150; Indus
Instruments, Webster, TX). A solid state transducer (MS-250) was placed on the tumor and held in position by a clamp mounted on the Vevo Rail System. 21-MHz B-mode imaging was used to
identify lesions suspicious for cancer (hypoechoic foci), and once a lesion was identified, the 21-MHz transducer was fixed into position on the imaging platform and switched with the 18-MHz
transducer for nonlinear contrast-enhanced imaging. Nonlinear Contrast Mode imaging was employed to detect the presence of Vevo MicroMarker® Non-Targeted Contrast Agent (VisualSonics,
Toronto, ON, Canada). A bolus injection of 100 μl contrast agent (concentration of 2 × 107 microbubbles ml−1) was delivered through the tail vein catheter. Acquisition parameters were kept
constant at 4% power, 9 dB contrast gain, gate size of 6, high line density and wide beam width. Tumor sectional area was determined by manually outlining the borders of the tumor.
Motion-compensated data analysis on stored cine loops of perfusion and imaging was performed by commercially available software (VevoCQ; VisualSonics). IMMUNOFLUORESCENCE ANALYSIS OF MOUSE
TISSUES To analyze vascular morphogenesis in the neonatal retina, eyes were dissected and fixed in 4% paraformaldehyde (Sigma) for 1 h on ice, washed in PBS before dissection of retinas.
After blocking in TNB buffer (0.5% blocking reagent (PerkinElmer, FP1012), 150 mM NaCl, 100 mM Tris-HCl (pH 7.4), 0.4% Triton X-100) for 2 h at room temperature, the retinas were incubated
with Alexa-Fluor-conjugated isolectin B4 (Invitrogen, I21411, 20 μg ml−1 in TNB) and primary antibody (if required) overnight at 4 °C. Anti-collagen IV (AbD Serotec, 2150–1470, 1:400),
anti-ERG (Abcam, Ab92513, 1:200) or FOXO1 (Cell Signaling Technology, 2880, 1:100) and then Alexa-Fluor 546-conjugated secondary antibody (Invitrogen, 1:400) were applied in sequence.
Afterwards, retinas were flat-mounted with Fluromount-G (Invitrogen) and examined by Olympus FV1000 or Zeiss LSM 710 confocal microscope. To label the proliferative endothelial cells with
EdU, pups were injected i.p. with 60 μl EdU (Invitrogen, C10338, 0.5 mg ml−1 in PBS) 3 h before experiments. EdU detection was performed by Click-iT EdU Cell Proliferation Kit for Imaging
(Invitrogen, C10338). Retinas were stained by In Situ Cell Death Detection Kit TMR red (Roche) as the manufacturer’s instruction for determination of apoptotic ECs. In implanted tumor
assays, mouse tissues were fixed in 4% paraformaldehyde overnight at 4 °C, washed in PBS, equilibrated in 30% sucrose overnight, embedded in OCT (Sakura), and sectioned at 5 μm. Slides were
treated with 0.2% Triton X-100 in PBS and incubated in blocking solution (1% BSA, 5% goat serum in PBS) for 1 h at room temperature. Primary antibodies anti-CD31 (BD Biosciences, 553370,
1:200) and anti-collagen IV (AbD Serotec, 2150–1470, 1:200) were incubated overnight at 4 °C, followed with Alexa-Fluor 488- and Alexa-Fluor 546-conjugated secondary antibody (Invitrogen,
1:400) stainings for 2 h at room temperature. Samples were stained by DAPI solution for nuclei and then mounted by Fluromount-G. Slides were examined by Olympus BX-53 upright or Zeiss LSM
710 confocal microscope. Image analysis was accomplished with ImageJ. To assess tumor vessel density, CD31- positive area was measured and normalized with the tumor area. For analysis of
vessel regression, the collagen IV-positive area was measured and divided by the CD31-positive area as previously reported24. QUANTITATIVE ANALYSIS OF THE RETINAL VESSELS All quantifications
were performed with Volocity software on high-resolution confocal images representing a thin _z_ section of the retina. The sprouts, EC area, branch points and collagen IV+/isolectin B4-
sleeves were measured according to the published protocols24,35,94. In brief, the sprouts were defined as protrusive endothelial cells above the angiogenic front line and were quantified in
a minimum of 8 fields (sized 1270 μm × 1270 μm). For each vascularized field, the proportion of EC coverage was calculated by measuring the isolectin B4-positive area normalized to the total
area in a minimum of 8 fields per group. Regions of quantification were selected in 1270 μm × 1270 μm fields for EC coverage or in the vascular plexus (sized 635 μm × 635 μm) between an
artery and vein excluding the angiogenic front for branching points. For the analysis of vessel stability, regions at the angiogenic front (around 400 μm × 200 μm) were selected and a
minimum of 8 fields per group were taken for measurements. Vascular regression analysis was accomplished by counting collagen IV-positive and isolectin B4-negative structures and correlating
them to the IB-4-positive area. For analysis of FOXO1 activation, IB4+/FOXO1+ cell nuclei were counted and correlated to the IB-4-positive area. For analysis of proliferation and apoptosis,
EdU+/ERG+ nuclei or TUNEL+/IB4+ signals were counted and normalized to the vessel area. All the images shown are representative vascular phenotype observed in samples from at least two
distinct litters per group. RETINAL ENDOTHELIAL CELL SORTING P7 retinas were dissected, minced into fragments and then digested with collagenase/dispase (Roche, 1 mg ml−1) in DMEM at RT for
2 min and filtered through 40 μm nylon mesh. Cell suspensions were incubated with eFluor 660-conjugated VE-cadherin antibody (eBioscience, 50-1441-82, 1:200) in 1% BSA/PBS for 1 h on ice.
eFluor 660-positive endothelial cells were sorted through the instrument (BD SORP FACS Aria) and authenticated by CD31 expression. QUANTITATIVE REAL-TIME PCR ANALYSIS Total RNA in sorted
endothelial cells, embryos, normal or tumor tissues (grinded in liquid nitrogen) was extracted by Trizol and reverse transcribed into cDNA by RT Master Mix (TOYOBO) according to the
manufacturer’s instructions. Subsequent quantitative PCR was performed with SYBR Green Real-time PCR Master Mix (TOYOBO). Gene expression was normalized to the endogenous control zebrafish
_actb_ or mouse _Actb_. All qPCR reactions were run on ABI VIIA7 real-time PCR instrument and data were calculated using the ΔΔ_C__t_ method. Primers used for qPCR analysis were listed in
Table S1. WESTERN BLOTTING ANALYSIS Zebrafish embryos were collected at the indicated stage and directly lysed in SDS sample buffer. For nuclear FOXO1 detection, cytoplasmic and nuclear
protein samples were obtained from 48 hpf zebrafish embryos using Nuclear and Cytoplasmic Protein Extraction Kit (Thermo fisher) according to the manufacturer’s instruction. Tumor tissue
lysates were prepared in RIPA buffer for 30 min on ice. HUVEC samples were directly harvested into SDS sample buffer. Proteins were separated by SDS-PAGE and blotted onto polyvinylidene
fluoride membranes. The following antibodies were used: anti-MYC (Genomics Technology, SG4110-18, 1:1000), anti-a-Tubulin (Sigma, T9026, 1:1000), anti-GAPDH (Absci, 21612-2, 1:2000),
anti-HIF1a (Ruiying Biological, RLT2133, 1:500), anti-phospho-FOXO1 (Cell Signaling Technology, 9464, 1:500), anti-FOXO1 (Cell Signaling Technology, 2880, 1:500) and anti-Histone-3 (Cell
Signaling Technology, 9715, 1:2000). STATISTICAL ANALYSIS No statistical methods were used to predetermine sample size. The experiments were not randomized and investigators were not blinded
to allocation during experiments and outcome assessment. Statistical analysis was performed by Graphpad Prism 7 software. Data were analyzed by unpaired two-tailed Student’s _t_-test for
two group comparison. When variances were significantly different in two groups, Welch’s correction was applied. For multiple group comparison, one-way ANOVA or two-way ANOVA (analysis of
variance) followed by Bonferroni’s multiple comparison test was performed. Results were represented as mean ± SEM. _P_ values <0.05 were considered as significant, *_P_ _<_ 0.05, **_P_
< 0.01, ***_P_ < 0.001, ****_P_ < 0.0001 or ns (_P_ ≥ 0.05). Each experiment was independently performed at least three times. DATA AVAILABILITY The data that support the findings
of this study are available from the corresponding author upon reasonable request. REFERENCES * Ramasamy, S. K., Kusumbe, A. P. & Adams, R. H. Regulation of tissue morphogenesis by
endothelial cell-derived signals. _Trends Cell Biol._ 25, 148–157 (2015). Article PubMed Google Scholar * Ramasamy, S. K., Kusumbe, A. P., Wang, L. & Adams, R. H. Endothelial Notch
activity promotes angiogenesis and osteogenesis in bone. _Nature_ 507, 376–380 (2014). Article CAS PubMed PubMed Central Google Scholar * Ding, B. S. et al. Inductive angiocrine signals
from sinusoidal endothelium are required for liver regeneration. _Nature_ 468, 310–315 (2010). Article CAS PubMed PubMed Central Google Scholar * Rafii, S., Butler, J. M. & Ding,
B. S. Angiocrine functions of organ-specific endothelial cells. _Nature_ 529, 316–325 (2016). Article CAS PubMed PubMed Central Google Scholar * Li, D. et al. VCAM-1(+) macrophages
guide the homing of HSPCs to a vascular niche. _Nature_ 564, 119–124 (2018). Article CAS PubMed PubMed Central Google Scholar * Carmeliet, P. Angiogenesis in health and disease. _Nat.
Med._ 9, 653–660 (2003). Article CAS PubMed Google Scholar * Herbert, S. P. & Stainier, D. Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. _Nat.
Rev. Mol. Cell Biol._ 12, 551–564 (2011). Article CAS PubMed PubMed Central Google Scholar * De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour
angiogenesis. _Nat. Rev. Cancer_ 17, 457–474 (2017). Article PubMed CAS Google Scholar * Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. _Nature_ 407, 249–257
(2000). Article CAS PubMed Google Scholar * Fiedler, L. VEGF signaling: methods and protocols. Preface. _Methods Mol. Biol._ 1332, v–vi (2015). PubMed Google Scholar * Ito, M. &
Yoshioka, M. Regression of the hyaloid vessels and pupillary membrane of the mouse. _Anat. Embryol._ 200, 403–411 (1999). Article CAS Google Scholar * Franco, C. A. et al. Dynamic
endothelial cell rearrangements drive developmental vessel regression. _PLoS Biol._ 13, e1002125 (2015). Article PubMed PubMed Central CAS Google Scholar * Korn, C. & Augustin, H.
G. Mechanisms of vessel pruning and regression. _Dev. Cell_ 34, 5–17 (2015). Article CAS PubMed Google Scholar * Chen, Q. et al. Haemodynamics-driven developmental pruning of brain
vasculature in zebrafish. _PLoS Biol._ 10, e1001374 (2012). Article CAS PubMed PubMed Central Google Scholar * Modlich, U., Kaup, F. J. & Augustin, H. G. Cyclic angiogenesis and
blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. _Lab. Investig._ 74, 771–780 (1996). CAS PubMed
Google Scholar * Andres, A. C. & Djonov, V. The mammary gland vasculature revisited. _J. Mammary Gland Biol. Neoplasia_ 15, 319–328 (2010). Article PubMed Google Scholar * Scott, A.
et al. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. _PloS ONE_ 5, e11863 (2010). Article PubMed PubMed Central CAS
Google Scholar * Baffert, F. et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. _Am. J. Physiol. Heart Circ. Physiol._ 290,
H547–H559 (2006). Article CAS PubMed Google Scholar * Benjamin, L. E., Golijanin, D., Itin, A., Pode, D. & Keshet, E. Selective ablation of immature blood vessels in established
human tumors follows vascular endothelial growth factor withdrawal. _J. Clin. Investig._ 103, 159–165 (1999). Article CAS PubMed PubMed Central Google Scholar * Meeson, A. P., Argilla,
M., Ko, K., Witte, L. & Lang, R. A. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. _Development_ 126, 1407–1415 (1999). Article CAS PubMed
Google Scholar * Lobov, I. B. et al. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. _Blood_ 117,
6728–6737 (2011). Article CAS PubMed Google Scholar * Phng, L. K. et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. _Dev. Cell_ 16,
70–82 (2009). Article CAS PubMed PubMed Central Google Scholar * Korn, C. et al. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis.
_Development_ 141, 1757–1766 (2014). Article CAS PubMed Google Scholar * Scholz, B. et al. Endothelial RSPO3 controls vascular stability and pruning through non-canonical WNT/Ca(2+)/NFAT
signaling. _Dev. Cell_ 36, 79–93 (2016). Article CAS PubMed Google Scholar * Liu, Y., Wang, W., Shui, G. & Huang, X. CDP-diacylglycerol synthetase coordinates cell growth and fat
storage through phosphatidylinositol metabolism and the insulin pathway. _PLoS Genet._ 10, e1004172 (2014). Article PubMed PubMed Central CAS Google Scholar * Wu, L., Niemeyer, B.,
Colley, N., Socolich, M. & Zuker, C. S. Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. _Nature_ 373, 216–222 (1995). Article CAS PubMed Google Scholar
* Jenny Zhou, H. et al. Endothelial exocytosis of angiopoietin-2 resulting from CCM3 deficiency contributes to cerebral cavernous malformation. _Nat. Med._ 22, 1033–1042 (2016). Article
PubMed PubMed Central CAS Google Scholar * Pan, W. et al. CDP-diacylglycerol synthetase-controlled phosphoinositide availability limits VEGFA signaling and vascular morphogenesis.
_Blood_ 120, 489–498 (2012). Article CAS PubMed PubMed Central Google Scholar * Liang, D. et al. Cloning and characterization of vascular endothelial growth factor (VEGF) from
zebrafish, Danio rerio. _Biochim. Biophys. Acta_ 1397, 14–20 (1998). Article CAS PubMed Google Scholar * Bahary, N. et al. Duplicate VegfA genes and orthologues of the KDR receptor
tyrosine kinase family mediate vascular development in the zebrafish. _Blood_ 110, 3627–3636 (2007). Article CAS PubMed PubMed Central Google Scholar * Nasevicius, A., Larson, J. &
Ekker, S. C. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. _Yeast_ 17, 294–301 (2000). Article CAS PubMed PubMed Central Google Scholar * Jin, D. et
al. Vegfa signaling regulates diverse artery/vein formation in vertebrate vasculatures. _J. Genet. Genom. = Yi chuan xue bao_ 44, 483–492 (2017). Article Google Scholar * Liang, D. et al.
The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. _Mech. Dev._ 108, 29–43 (2001). Article CAS PubMed
Google Scholar * Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. _Nature_ 465, 483–486 (2010). Article CAS PubMed Google Scholar * Pitulescu, M. E.,
Schmidt, I., Benedito, R. & Adams, R. H. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. _Nat. Protoc._ 5, 1518–1534 (2010). Article
CAS PubMed Google Scholar * Olsson, A. K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling—in control of vascular function. _Nat. Rev. Mol. Cell Biol._ 7,
359–371 (2006). Article CAS PubMed Google Scholar * Carmeliet, P. Angiogenesis in life, disease and medicine. _Nature_ 438, 932–936 (2005). Article CAS PubMed Google Scholar * Croci,
D. O. et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. _Cell_ 156, 744–758 (2014). Article CAS PubMed Google Scholar *
Yuan, T. L. et al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. _Proc. Natl Acad. Sci. USA_ 105, 9739–9744 (2008). Article CAS PubMed PubMed Central
Google Scholar * Liu, Q. et al. Genetic targeting of sprouting angiogenesis using Apln-CreER. _Nat. Commun._ 6, 6020 (2015). Article CAS PubMed Google Scholar * Insall, R. H. &
Weiner, O. D. PIP3, PIP2, and cell movement-similar messages, different meanings? _Dev. Cell_ 1, 743–747 (2001). Article CAS PubMed PubMed Central Google Scholar * Cremona, O. et al.
Essential role of phosphoinositide metabolism in synaptic vesicle recycling. _Cell_ 99, 179–188 (1999). Article CAS PubMed Google Scholar * Maguire, J. J. et al. Known unknowns of
cardiolipin signaling: the best is yet to come. _Biochim. Biophys. Acta_ 1862, 8–24 (2017). Article CAS Google Scholar * Kagan, V. E. et al. Cardiolipin signaling mechanisms: collapse of
asymmetry and oxidation. _Antioxid. Redox Signal._ 22, 1667–1680 (2015). Article CAS PubMed PubMed Central Google Scholar * Kagan, V. E., Chu, C. T., Tyurina, Y. Y., Cheikhi, A. &
Bayir, H. Cardiolipin asymmetry, oxidation and signaling. _Chem. Phys. Lipids_ 179, 64–69 (2014). Article CAS PubMed Google Scholar * Funamoto, S., Meili, R., Lee, S., Parry, L. &
Firtel, R. A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. _Cell_ 109, 611–623 (2002). Article CAS PubMed Google Scholar * Cantley,
L. C. The phosphoinositide 3-kinase pathway. _Science_ 296, 1655–1657 (2002). Article CAS PubMed Google Scholar * Dang, L. T. H. et al. Hyperactive FOXO1 results in lack of tip stalk
identity and deficient microvascular regeneration during kidney injury. _Biomaterials_ 141, 314–329 (2017). Article CAS PubMed PubMed Central Google Scholar * Schmid, A. C., Byrne, R.
D., Vilar, R. & Woscholski, R. Bisperoxovanadium compounds are potent PTEN inhibitors. _FEBS Lett._ 566, 35–38 (2004). Article CAS PubMed Google Scholar * Siekmann, A. F. &
Lawson, N. D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. _Nature_ 445, 781–784 (2007). Article CAS PubMed Google Scholar * Hellstrom, M. et al.
Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. _Nature_ 445, 776–780 (2007). Article PubMed CAS Google Scholar * Lobov, I. B. et al. WNT7b mediates
macrophage-induced programmed cell death in patterning of the vasculature. _Nature_ 437, 417–421 (2005). Article CAS PubMed PubMed Central Google Scholar * He, X. et al. Chemical
biology reveals CARF as a positive regulator of canonical Wnt signaling by promoting TCF/beta-catenin transcriptional activity. _Cell Discov._ 3, 17003 (2017). Article CAS PubMed PubMed
Central Google Scholar * Fan, X. et al. New statistical methods enhance imaging of cameleon fluorescence resonance energy transfer in cultured zebrafish spinal neurons. _J. Biomed. Opt._
12, 034017 (2007). Article PubMed CAS Google Scholar * Helassa, N. et al. Fast-response calmodulin-based fluorescent indicators reveal rapid intracellular calcium dynamics. _Sci. Rep._
5, 15978 (2015). Article CAS PubMed PubMed Central Google Scholar * Wiens, K. M. et al. Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel
formation. _PloS ONE_ 5, e11324 (2010). Article PubMed PubMed Central CAS Google Scholar * Simonavicius, N. et al. Pericytes promote selective vessel regression to regulate vascular
patterning. _Blood_ 120, 1516–1527 (2012). Article CAS PubMed Google Scholar * Wilhelm, K. et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. _Nature_
529, 216–220 (2016). Article CAS PubMed PubMed Central Google Scholar * Daly, C. et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription
factor FKHR (FOXO1) _Dev_. _Genes Dev._ 18, 1060–1071 (2004). Article CAS PubMed PubMed Central Google Scholar * Van Der Heide, L. P., Hoekman, M. F. & Smidt, M. P. The ins and outs
of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. _Biochem. J._ 380, 297–309 (2004). Article Google Scholar * Brunet, A. et al. Akt promotes cell
survival by phosphorylating and inhibiting a Forkhead transcription factor. _Cell_ 96, 857–868 (1999). Article CAS PubMed Google Scholar * Guo, S. et al. Phosphorylation of serine 256 by
protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response
sequence. _J. Biol. Chem._ 274, 17184–17192 (1999). Article CAS PubMed Google Scholar * Guillou, H., Stephens, L. R. & Hawkins, P. T. Quantitative measurement of phosphatidylinositol
3,4,5-trisphosphate. _Methods Enzymol._ 434, 117–130 (2007). Article CAS PubMed Google Scholar * Li, X., Kumar, A. & Carmeliet, P. Metabolic pathways fueling the endothelial cell
drive. _Annu. Rev. Physiol._ 81, 483–503 (2019). Article CAS PubMed Google Scholar * Li, X., Kumar, A., Zhang, F., Lee, C. & Tang, Z. Complicated life, complicated VEGF-B. _Trends
Mol. Med._ 18, 119–127 (2012). Article PubMed CAS Google Scholar * Im, E. & Kazlauskas, A. Regulating angiogenesis at the level of PtdIns-4,5-P2. _EMBO J._ 25, 2075–2082 (2006).
Article CAS PubMed PubMed Central Google Scholar * Herbert, S. P. et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation.
_Science_ 326, 294–298 (2009). Article CAS PubMed PubMed Central Google Scholar * Fang, Y., Vilella-Bach, M., Bachmann, R., Flanigan, A. & Chen, J. Phosphatidic acid-mediated
mitogenic activation of mTOR signaling. _Science_ 294, 1942–1945 (2001). Article CAS PubMed Google Scholar * Wang, X., Devaiah, S. P., Zhang, W. & Welti, R. Signaling functions of
phosphatidic acid. _Prog. Lipid Res._ 45, 250–278 (2006). Article CAS PubMed Google Scholar * Andresen, B. T., Rizzo, M. A., Shome, K. & Romero, G. The role of phosphatidic acid in
the regulation of the Ras/MEK/Erk signaling cascade. _FEBS Lett._ 531, 65–68 (2002). Article CAS PubMed Google Scholar * Sasso, F. C. et al. Increased vascular endothelial growth factor
expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. _J. Am. Coll. Cardiol._ 46,
827–834 (2005). Article CAS PubMed Google Scholar * Cooper, M. E. et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental
diabetes. _Diabetes_ 48, 2229–2239 (1999). Article CAS PubMed Google Scholar * Waltenberger, J. VEGF resistance as a molecular basis to explain the angiogenesis paradox in diabetes
mellitus. _Biochem. Soc. Trans._ 37, 1167–1170 (2009). Article CAS PubMed Google Scholar * Ray, D. et al. Association of the VEGF gene with proliferative diabetic retinopathy but not
proteinuria in diabetes. _Diabetes_ 53, 861–864 (2004). Article CAS PubMed Google Scholar * Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy.
_Nature_ 565, 505–510 (2019). Article CAS PubMed PubMed Central Google Scholar * Bahrami, B., Hong, T., Gilles, M. C. & Chang, A. Anti-VEGF therapy for diabetic eye. _Dis. Asia-Pac.
J. Ophthalmol._ 6, 535–545 (2017). CAS Google Scholar * Li, X., Wu, T. T., Chen, J. & Qiu, W. Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis
factor-alpha and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy. _J. Diabetes Investig._ 8, 108–114 (2017). Article CAS PubMed Google Scholar *
Flyvbjerg, A. et al. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. _Diabetes_ 51, 3090–3094 (2002).
Article CAS PubMed Google Scholar * Avramis, I. A., Kwock, R. & Avramis, V. I. Taxotere and vincristine inhibit the secretion of the angiogenesis inducing vascular endothelial growth
factor (VEGF) by wild-type and drug-resistant human leukemia T-cell lines. _Anticancer Res._ 21, 2281–2286 (2001). CAS PubMed Google Scholar * Ziogas, A. C. et al. VEGF directly
suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. _Int. J. Cancer_ 130, 857–864 (2012). Article CAS PubMed Google Scholar *
Medyouf, H. et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. _Cell Stem Cell_ 14, 824–837 (2014).
Article CAS PubMed Google Scholar * Jiang, Y. et al. A PPARgamma transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. _Nat. Commun._ 8, 15926
(2017). Article CAS PubMed PubMed Central Google Scholar * Rehn, M. et al. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in
the low-oxygenic niche. _Blood_ 118, 1534–1543 (2011). Article CAS PubMed Google Scholar * Verma, M. et al. Muscle satellite cell cross-talk with a vascular niche maintains quiescence
via VEGF and notch signaling. _Cell Stem Cell_ 23, 530–543 e539 (2018). Article CAS PubMed PubMed Central Google Scholar * Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B.
& Schilling, T. F. Stages of embryonic development of the zebrafish. _Dev. Dyn._ 203, 253–310 (1995). Article CAS PubMed Google Scholar * Lawson, N. D. & Weinstein, B. M. In vivo
imaging of embryonic vascular development using transgenic zebrafish. _Dev. Biol._ 248, 307–318 (2002). Article CAS PubMed Google Scholar * Nicenboim, J. et al. Lymphatic vessels arise
from specialized angioblasts within a venous niche. _Nature_ 522, 56–61 (2015). Article CAS PubMed Google Scholar * Yao, Y. et al. Visual cue-discriminative dopaminergic control of
visuomotor transformation and behavior selection. _Neuron_ 89, 598–612 (2016). Article CAS PubMed Google Scholar * Jing, C. B. et al. Phospholipase C gamma-1 is required for granulocyte
maturation in zebrafish. _Dev. Biol._ 374, 24–31 (2013). Article CAS PubMed Google Scholar * Liu, D., Yu, Y. & Schachner, M. Ptena, but not Ptenb, reduces regeneration after spinal
cord injury in adult zebrafish. _Exp. Neurol._ 261, 196–205 (2014). Article CAS PubMed Google Scholar * Irie, F., Okuno, M., Pasquale, E. B. & Yamaguchi, Y. EphrinB-EphB signalling
regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. _Nat. Cell Biol._ 7, 501–509 (2005). Article CAS PubMed PubMed Central Google Scholar *
Vitorino, P. et al. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. _Nature_ 519, 425–430 (2015). Article CAS PubMed Google Scholar * Tang, Y. C. et al.
Aneuploid cell survival relies upon sphingolipid homeostasis. _Cancer Res._ 77, 5272–5286 (2017). Article CAS PubMed PubMed Central Google Scholar * Nowak-Sliwinska, P. et al. Consensus
guidelines for the use and interpretation of angiogenesis assays. _Angiogenesis_ 21, 425–532 (2018). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We
thank Ralf Adams for sharing _Cdh5:CreERT2_ transgenic mice, Ruilin Zhang and Jiu-lin Du for sharing zebrafish transgenic line, _Tg(fli1a:gal4), Tg(tp1:dsGFP), and Tg(HuC:gal4)_,
_Tg(uas:GCaMP5)_ respectively. We are also grateful to members of Weijun Pan laboratory for technical support. This work was supported by Ministry of Science and Technology of China
(2018YFA0800200 and 2017YF0503600), National Natural Science Foundation of China (31571505 and 31371461), CAS Strategic Priority Research Program (XDB19030000) and Scientific Research
Equipment Development Project (YZ201646) to WJP. AUTHOR INFORMATION Author notes * These authors contributed equally: Wencao Zhao, Le Cao, Hanru Ying AUTHORS AND AFFILIATIONS * Key
Laboratory of Tissue Microenvironment and Tumor, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences,
Chinese Academy of Sciences (CAS), Shanghai, China Wencao Zhao, Le Cao, Wenjuan Zhang, Dantong Li, Wenzhi Xue, Shuang Wu, Mengye Cao, Cong Fu, Haonan Qi, Yimei Hao, Yun-Chi Tang, Jun Qin
& Weijun Pan * Department of Plastic and Reconstructive Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, China Hanru Ying &
Xiaoxi Lin * Shanghai Key Laboratory of Regulatory Biology, Institute of Molecular Medicine, East China Normal University School of Life Sciences, Shanghai, China Xiaolong Zhu & Tao P.
Zhong * Innovative Research Team of High-level Local University in Shanghai, Shanghai, China Xiaoxi Lin & Weijun Pan * Institute of Genetics, College of Life Sciences, Zhejiang
University, Hangzhou, China Luyang Yu * State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China Xuri Li * State Key Laboratory of
Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, CAS, Shanghai, China Lin Li *
Department of Pharmacology, Vascular Biology and Therapeutic Program, School of Medicine, Yale University, New Haven, CT, USA Dianqing Wu Authors * Wencao Zhao View author publications You
can also search for this author inPubMed Google Scholar * Le Cao View author publications You can also search for this author inPubMed Google Scholar * Hanru Ying View author publications
You can also search for this author inPubMed Google Scholar * Wenjuan Zhang View author publications You can also search for this author inPubMed Google Scholar * Dantong Li View author
publications You can also search for this author inPubMed Google Scholar * Xiaolong Zhu View author publications You can also search for this author inPubMed Google Scholar * Wenzhi Xue View
author publications You can also search for this author inPubMed Google Scholar * Shuang Wu View author publications You can also search for this author inPubMed Google Scholar * Mengye Cao
View author publications You can also search for this author inPubMed Google Scholar * Cong Fu View author publications You can also search for this author inPubMed Google Scholar * Haonan
Qi View author publications You can also search for this author inPubMed Google Scholar * Yimei Hao View author publications You can also search for this author inPubMed Google Scholar *
Yun-Chi Tang View author publications You can also search for this author inPubMed Google Scholar * Jun Qin View author publications You can also search for this author inPubMed Google
Scholar * Tao P. Zhong View author publications You can also search for this author inPubMed Google Scholar * Xiaoxi Lin View author publications You can also search for this author inPubMed
Google Scholar * Luyang Yu View author publications You can also search for this author inPubMed Google Scholar * Xuri Li View author publications You can also search for this author
inPubMed Google Scholar * Lin Li View author publications You can also search for this author inPubMed Google Scholar * Dianqing Wu View author publications You can also search for this
author inPubMed Google Scholar * Weijun Pan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS WCZ and WJP developed the concepts and designed
the experiments. WCZ, LC, HRY, WJZ, SW, MYC, CF, YMH and WJP performed the experiments. WCZ analyzed data and made figures and models. DTL assisted making figure and models. XLZ assisted
retinal vessel immunostaining and analysis. WZX assisted imaging with Zeiss 880. HNQ assisted the schematic illustration of working model. XXL, YCT, JQ, ZT, YMH, LYY, XR.L., LL and DQW were
involved in result interpretation and manuscript writing. WCZ and WJP wrote the paper. WJP supervised the project. CORRESPONDING AUTHOR Correspondence to Weijun Pan. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION, FIGURE S1 SUPPLEMENTARY INFORMATION, FIGURE S2 SUPPLEMENTARY INFORMATION,
FIGURE S3 SUPPLEMENTARY INFORMATION, FIGURE S4 SUPPLEMENTARY INFORMATION, FIGURE S5 SUPPLEMENTARY INFORMATION, FIGURE S6 SUPPLEMENTARY INFORMATION, FIGURE S7 SUPPLEMENTARY INFORMATION,
FIGURE S8 SUPPLEMENTARY INFORMATION, TABLE S1 SUPPLEMENTARY INFORMATION, SUPPLEMENTARY VIDEO LEGENDS SUPPLEMENTARY INFORMATION, SUPPLEMENTARY VIDEO S1 SUPPLEMENTARY INFORMATION,
SUPPLEMENTARY VIDEO S2 SUPPLEMENTARY INFORMATION, SUPPLEMENTARY VIDEO S3 SUPPLEMENTARY INFORMATION, SUPPLEMENTARY VIDEO S4 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, W., Cao, L., Ying, H. _et al._ Endothelial CDS2 deficiency
causes VEGFA-mediated vascular regression and tumor inhibition. _Cell Res_ 29, 895–910 (2019). https://doi.org/10.1038/s41422-019-0229-5 Download citation * Received: 08 April 2019 *
Accepted: 23 August 2019 * Published: 09 September 2019 * Issue Date: November 2019 * DOI: https://doi.org/10.1038/s41422-019-0229-5 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative