Play all audios:
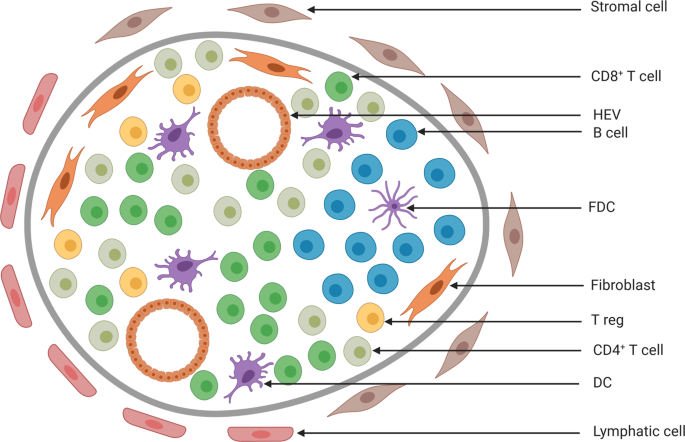
ABSTRACT Tertiary lymphoid structures (TLS) are ectopic lymphoid formations that form within nonlymphoid tissue. They share structural and functional characteristics with secondary lymphoid
structures such as lymph nodes and can contain B-cell follicles and germinal centers surrounded by a T-cell region. TLS have been described in several types of cancers and are usually
associated with positive patient outcomes. However, TLS differ vastly in cellular composition and location within tissue types. In this review, we discuss factors confounding the
interpretation of the evidence for a prognostic role for TLS in cancer and frame these factors in the context of translation to regular clinical use. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online
access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local
taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING
VIEWED BY OTHERS HETEROGENEITY OF TERTIARY LYMPHOID STRUCTURES PREDICTS THE RESPONSE TO NEOADJUVANT THERAPY AND IMMUNE MICROENVIRONMENT CHARACTERISTICS IN TRIPLE-NEGATIVE BREAST CANCER
Article 10 December 2024 IMMUNOTHERAPY RESPONSE INDUCES DIVERGENT TERTIARY LYMPHOID STRUCTURE MORPHOLOGIES IN HEPATOCELLULAR CARCINOMA Article 25 October 2024 TERTIARY LYMPHOID STRUCTURES
PREDICT SURVIVAL AND RESPONSE TO NEOADJUVANT THERAPY IN LOCALLY ADVANCED RECTAL CANCER Article Open access 02 March 2024 REFERENCES * Bergomas, F. et al. Tertiary intratumor lymphoid tissue
in colo-rectal cancer. _Cancers_ 4, 1–10 (2011). Article PubMed PubMed Central Google Scholar * Colbeck, E. J., Ager, A., Gallimore, A. & Jones, G. W. Tertiary lymphoid structures in
cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? _Front. Immunol._ 8, 1830 (2017). Article PubMed PubMed Central CAS Google Scholar *
Dieu-Nosjean, M. C., Goc, J., Giraldo, N. A., Sautes-Fridman, C. & Fridman, W. H. Tertiary lymphoid structures in cancer and beyond. _Trends Immunol._ 35, 571–580 (2014). Article CAS
PubMed Google Scholar * Trajkovski, G. et al. Tertiary lymphoid structures in colorectal cancers and their prognostic value. _Open Access Maced. J. Med. Sci._ 6, 1824–1828 (2018). Article
PubMed PubMed Central Google Scholar * Messina, J. L. et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for
immunotherapy? _Sci. Rep._ 2, 765 (2012). Article PubMed PubMed Central CAS Google Scholar * Pimenta, E. M. & Barnes, B. J. Role of tertiary lymphoid structures (TLS) in anti-tumor
immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. _Cancers_ 6, 969–997 (2014). Article CAS PubMed PubMed Central Google
Scholar * Denton, A. E., Carr, E. J., Magiera, L. P., Watts, A. J. B. & Fearon, D. T. Embryonic FAP(+) lymphoid tissue organizer cells generate the reticular network of adult lymph
nodes. _J. Exp. Med._ 216, 2242–2252 (2019). Article CAS PubMed PubMed Central Google Scholar * Carragher, D. M., Rangel-Moreno, J. & Randall, T. D. Ectopic lymphoid tissues and
local immunity. _Semin. Immunol._ 20, 26–42 (2008). Article CAS PubMed PubMed Central Google Scholar * Sautes-Fridman, C. et al. Tertiary lymphoid structures in cancers: prognostic
value, regulation, and manipulation for therapeutic intervention. _Front. Immunol._ 7, 407 (2016). Article PubMed PubMed Central CAS Google Scholar * Lin, L., Hu, X., Zhang, H. &
Hu, H. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. _Front. Immunol._ 10, 1398 (2019). Article PubMed PubMed Central CAS Google
Scholar * Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. _Nat. Rev. Cancer_ 19, 307–325 (2019).
Article CAS PubMed Google Scholar * Buisseret, L. et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. _Mod. Pathol._
30, 1204–1212 (2017). Article CAS PubMed Google Scholar * Sofopoulos, M. et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. _Cancer Immunol.
Immunother._ 68, 1733–1745 (2019). Article CAS PubMed Google Scholar * Nayar, S. et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local
pathology. _Proc. Natl Acad. Sci. USA_ 116, 13490–13497 (2019). Article CAS PubMed PubMed Central Google Scholar * Leman, J. K., Sandford, S. K., Rhodes, J. L. & Kemp, R. A.
Multiparametric analysis of colorectal cancer immune responses. _World J. Gastroenterol._ 24, 2995–3005 (2018). Article CAS PubMed PubMed Central Google Scholar * Yamaguchi, K. et al.
Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. _Oncoimmunology_ 9, 1724763 (2020). Article PubMed PubMed Central
Google Scholar * Shen, Y. C. et al. Reliability of a single-region sample to evaluate tumor immune microenvironment in hepatocellular carcinoma. _J. Hepatol._ 72, 489–497 (2020). Article
CAS PubMed Google Scholar * Pfannstiel, C. et al. BRIDGE Consortium, Germany. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes.
_Cancer Immunol. Res._ 7, 923–938 (2019). * Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. _Nature_ 577, 549–555 (2020). Article CAS PubMed
PubMed Central Google Scholar * Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. _Nature_ 577, 561–565 (2020). Article CAS PubMed Google
Scholar * Coelho, F. M. et al. Naive B-cell trafficking is shaped by local chemokine availability and LFA-1-independent stromal interactions. _Blood_ 121, 4101–4109 (2013). Article CAS
PubMed Google Scholar * Luther, S. A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis.
_J. Immunol._ 169, 424–433 (2002). Article CAS PubMed Google Scholar * de Chaisemartin, L. et al. Characterization of chemokines and adhesion molecules associated with T cell presence in
tertiary lymphoid structures in human lung cancer. _Cancer Res._ 71, 6391–6399 (2011). Article PubMed CAS Google Scholar * Engelhard, V. H. et al. Immune cell infiltration and tertiary
lymphoid structures as determinants of antitumor immunity. _J. Immunol._ 200, 432–442 (2018). Article CAS PubMed Google Scholar * Posch, F. et al. Maturation of tertiary lymphoid
structures and recurrence of stage II and III colorectal cancer. _Oncoimmunology_ 7, e1378844 (2018). Article PubMed Google Scholar * Schweiger, T. et al. Tumor-infiltrating lymphocyte
subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. _Clin. Exp. Metastasis_ 33, 727–739 (2016). Article CAS PubMed PubMed Central Google Scholar *
Kim, A. et al. The prognostic significance of tumor-infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. _PLoS ONE_ 14, e0224430
(2019). Article CAS PubMed PubMed Central Google Scholar * Seow, D. Y. B. et al. Tertiary lymphoid structures and associated plasma cells play an important role in the biology of
triple-negative breast cancers. _Breast Cancer Res. Treat._ 180, 369–377 (2020). Article CAS PubMed Google Scholar * Zhu, W. et al. A high density of tertiary lymphoid structure B cells
in lung tumors is associated with increased CD4(+) T cell receptor repertoire clonality. _Oncoimmunology_ 4, e1051922 (2015). Article PubMed PubMed Central CAS Google Scholar * Koenig,
A. & Thaunat, O. Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. _Front. Immunol._ 7, 646 (2016). Article PubMed PubMed Central CAS Google Scholar *
Schlosser, H. A. et al. B cells in esophago-gastric adenocarcinoma are highly differentiated, organize in tertiary lymphoid structures and produce tumor-specific antibodies. _Oncoimmunology_
8, e1512458 (2019). Article PubMed Google Scholar * Ikeda, A. et al. Human NKp44+ group 3 innate lymphoid cells associate with tumor-associated tertiary lymphoid structures in colorectal
cancer. _Cancer Immunol. Res._ https://doi.org/10.1158/2326-6066.CIR-19-0775 (2020). * McMullen, T. P., Lai, R., Dabbagh, L., Wallace, T. M. & de Gara, C. J. Survival in rectal cancer
is predicted by T cell infiltration of tumour-associated lymphoid nodules. _Clin. Exp. Immunol._ 161, 81–88 (2010). CAS PubMed PubMed Central Google Scholar * Stowman, A. M. et al.
Lymphoid aggregates in desmoplastic melanoma have features of tertiary lymphoid structures. _Melanoma Res._ 28, 237–245 (2018). Article PubMed PubMed Central Google Scholar * Ladanyi, A.
et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. _Cancer
Immunol. Immunother._ 56, 1459–1469 (2007). Article PubMed Google Scholar * Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral
lymphoid structures. _J. Clin. Oncol._ 26, 4410–4417 (2008). Article CAS PubMed Google Scholar * Germain, C. et al. Presence of B cells in tertiary lymphoid structures is associated with
a protective immunity in patients with lung cancer. _Am. J. Respir. Crit. Care Med._ 189, 832–844 (2014). Article CAS PubMed Google Scholar * Meshcheryakova, A. et al. B cells and
ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. _PLoS ONE_ 9, e99008 (2014). Article PubMed
PubMed Central CAS Google Scholar * Hennequin, A. et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients.
_Oncoimmunology_ 5, e1054598 (2016). Article PubMed CAS Google Scholar * Wirsing, A. M., Rikardsen, O. G., Steigen, S. E., Uhlin-Hansen, L. & Hadler-Olsen, E. Characterisation and
prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. _BMC Clin. Pathol._ 14, 38 (2014). Article PubMed PubMed Central Google Scholar * Di Caro, G. et al.
Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. _Clin. Cancer Res._ 20, 2147–2158 (2014).
Article PubMed CAS Google Scholar * Hiraoka, N. et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. _Br. J. Cancer_ 112,
1782–1790 (2015). Article CAS PubMed PubMed Central Google Scholar * Ward-Hartstonge, K. A. et al. Inclusion of BLIMP-1(+) effector regulatory T cells improves the Immunoscore in a
cohort of New Zealand colorectal cancer patients: a pilot study. _Cancer Immunol. Immunother._ 66, 515–522 (2017). Article CAS PubMed Google Scholar * Pages, F. et al. International
validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. _Lancet_ 391, 2128–2139 (2018). Article PubMed Google Scholar * Gobert, M.
et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. _Cancer
Res._ 69, 2000–2009 (2009). Article CAS PubMed Google Scholar * Calderaro, J. et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of
hepatocellular carcinoma. _J. Hepatol._ 70, 58–65 (2019). Article PubMed Google Scholar * Remark, R. et al. Characteristics and clinical impacts of the immune environments in colorectal
and renal cell carcinoma lung metastases: influence of tumor origin. _Clin. Cancer Res._ 19, 4079–4091 (2013). Article CAS PubMed Google Scholar * Cipponi, A. et al. Neogenesis of
lymphoid structures and antibody responses occur in human melanoma metastases. _Cancer Res._ 72, 3997–4007 (2012). Article CAS PubMed Google Scholar * Lee, M. et al. Presence of tertiary
lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. _Mod. Pathol._ 32, 70–80 (2019). Article CAS PubMed Google Scholar *
Basta, Y. L., Bolle, S., Fockens, P. & Tytgat, K. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. _Ann. Surg. Oncol._
24, 2669–2678 (2017). Article PubMed PubMed Central Google Scholar * Ladanyi, A. et al. Ectopic lymphoid structures in primary cutaneous melanoma. _Pathol. Oncol. Res._ 20, 981–985
(2014). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS L.M.E. was supported by the Maurice Wilkins Centre for Biodiscovery, New Zealand. J.L.R. is supported by a
Health Research Council Clinical Fellowship. We thank Hamish Angus, Ginny Niemi and Justin Tirados for critical review of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Microbiology and Immunology, University of Otago, Dunedin, New Zealand Luis Munoz-Erazo, Janet L. Rhodes & Roslyn A. Kemp * Ecole Normale Superieure de Lyon, Lyon, France
and Universite Claude Bernard Lyon 1, Lyon, France Valentine C. Marion Authors * Luis Munoz-Erazo View author publications You can also search for this author inPubMed Google Scholar * Janet
L. Rhodes View author publications You can also search for this author inPubMed Google Scholar * Valentine C. Marion View author publications You can also search for this author inPubMed
Google Scholar * Roslyn A. Kemp View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.M.E. and R.A.K. conceived the scope and content; all
authors contributed to writing the manuscript. CORRESPONDING AUTHOR Correspondence to Roslyn A. Kemp. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Munoz-Erazo, L., Rhodes, J.L., Marion, V.C. _et al._ Tertiary lymphoid structures in cancer –
considerations for patient prognosis. _Cell Mol Immunol_ 17, 570–575 (2020). https://doi.org/10.1038/s41423-020-0457-0 Download citation * Received: 05 February 2020 * Accepted: 22 April
2020 * Published: 15 May 2020 * Issue Date: June 2020 * DOI: https://doi.org/10.1038/s41423-020-0457-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * tertiary lymphoid structure * prognosis * cancer * tissue