Play all audios:
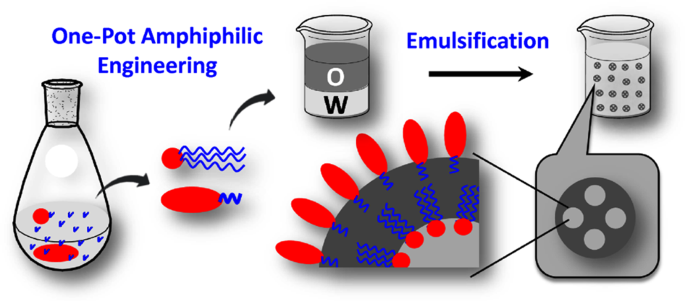
ABSTRACT In this study, we sought to develop methods for amphiphilic engineering of bioresorbable polymers, sorbitan–poly(lactic acid) (sorbitan–PLA) and poly(ethylene glycol)–polylactic
acid (PEG–PLA), by melt polycondensation of lactic acid in the presence of the binary initiators sorbitan and methoxy PEG in a single reactor. Briefly, oligo(lactic acid) (OLA) was first
prepared by distilling water out of lactic acid under vacuum; then, sorbitan and methoxy PEG were introduced in the reactor, followed by the simultaneous polycondensation of OLA onto
sorbitan and methoxy PEG, resulting in a mixture containing sorbitan–PLA/PEG–PLA copolymers. Without further purification, the recovered products were dissolved in saline buffer, mixed with
squalane oil, and then homogenized to construct a stable colloidal vesicle. Then, we conducted a mechanistic study to progressively elucidate the relationship between the dispersion
structure and the sustained release of a model protein bovine serum albumin. This one-pot approach has potential for applications and commercial use in the field of biodegradable
controlled-release delivery systems. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS DESIGN AND SYNTHESIS OF AMPHIPHILIC
ALTERNATING PEPTIDES WITH LOWER CRITICAL SOLUTION TEMPERATURE BEHAVIORS Article 08 April 2022 FACILE PREPARATION OF 2-METHYLENE-1,3-DIOXEPANE-BASED THERMORESPONSIVE POLYMERS AND HYDROGELS
Article 08 February 2021 SYNTHESIS, CHARACTERIZATION OF POLY L(+) LACTIC ACID AND ITS APPLICATION IN SUSTAINED RELEASE OF ISOSORBIDE DINITRATE Article Open access 25 March 2024 INTRODUCTION
Sorbitan fatty acid esters (Span®) and their ethoxylates (Tween®) are widely used as surfactants/emulsifying agents in biopharmaceutical formulations [1,2,3]. However, they have been
reported to trigger allergic reactions in paste bandages or anaphylactoid reactions following injection, which is a common administration route for drugs and vaccines [2, 3]. Therefore,
there is an unmet need to find alternatives to surfactants/emulsifying agents with well-defined structures to enhance the formulation efficacy without changing the tolerability. We
previously reported the use of amphiphilic bioresorbable polymers (sorbitan polyesters/PEGylated polyesters) as emulsifying agents to stabilize oil/water interfaces, introducing specialty
colloidal vesicles named polysorbasome [4]. We also investigated the potential use of polysorbasomes as sustained-release depots for proteins. The results demonstrated that polysorbasomes
may possess high stability during storage and enhanced degradability in the body compared with the conventional Span®/Tween®-based emulsion [4]. To enable a clinical study for the evaluation
of the safety and efficacy of these novel formulations, it is now necessary to prepare sufficient amounts of samples with consistent and reproducible properties. To achieve this, it is
necessary to investigate how to scale up the emulsifying process from the laboratory scale to a small pilot scale. An additional aspect of further development is to establish methods for
producing such amphiphilic polymers in a single reactor. These methods aim to simplify the separation process and reduce purification consumption while increasing the chemical yield [5].
Moreover, it is necessary to obtain comprehensive information on how the methods and compositions of new delivery systems are connected to the ability to provide sustained delivery of
bioactive molecules. In the present study, we report the one-pot amphiphilic engineering of sorbitan–poly(lactic acid) (sorbitan–PLA) and poly(ethylene glycol)–polylactic acid (PEG–PLA) by
melt polycondensation of lactic acid in the presence of the binary initiators sorbitan and methoxy PEG in a single reactor. The resulting copolymers were subjected to various
characterization techniques such as Fourier transform infrared (FTIR) spectroscopy, two-dimensional diffusion ordered spectroscopy nuclear magnetic resonance (DOSY NMR) spectroscopy,
size-exclusion chromatography (SEC), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), differential scanning calorimetry (DSC), and
thermogravimetric analysis (TGA). The amphiphilic behaviors were examined by homogenizing the polymeric aqueous solution and water-immiscible squalene oil. The stability and size
distribution were monitored to qualify the resulting colloidal vesicles. Finally, an in vitro release experiment with bovine serum albumin (BSA) as a model protein was conducted to assess
the sustained release of BSA after formulation with the squalene/aqueous colloids, either stabilized by sorbitan–PLA/PEG–PLA in a single pot or from two separated batches. MATERIALS AND
METHODS ONE-POT SYNTHESIS OF SORBITAN–PLA/PEG–PLA Lactic acid (85–90%) in aqueous solution was supplied by TEDIA (Fairfield, OH, USA). Poly(ethylene glycol) monomethyl ether flakes with an
average _M_n ~ 2000 (MePEG2000) and tin(II) 2-ethylhexanoate (SnOct2) were provided by Sigma (St. Louis, MO, USA). Sorbitan was obtained by flash distilling water out of d-sorbitol (Tokyo
Chemical Industry Co., Tokyo, Japan) at 180 °C for 3 h using phosphoric acid as the catalyst, as described in a previous report [4]. All of the solvents were of analytical grade. One-pot
sorbitan–PLA/PEG–PLA was synthesized by melt polycondensation of lactic acid in the presence of sorbitan and MePEG2000 in a single reactor. Briefly, oligo(lactic acid) (OLA) was first
obtained by flash distilling out water from lactic acid at 140 °C for 3 h in a Rotavapor® R-210 (Buchi Labortechnik AG, Switzerland) under a central suction system. Sorbitan (0.70 g),
MePEG2000 (8.82 g), and OLA (9.46 g) were introduced into the round-bottom flask, which was charged with SnOct2 (100 μL) as the catalyst. To prevent flooding, the mixture was heated at 140
°C for 6 h, followed by holding at 170 °C for 6 h. The mixture was finally cooled and solidified, and the resulting caramel-like syrup was stored in a desiccator under vacuum. MEASUREMENTS
IR spectra were recorded using a Perkin Elmer Spectrum 100 FT-IR spectrometer (PerkinElmer, Santa Clara, CA, USA). Two-dimensional DOSY NMR measurements were performed using a Varian
VNMRS-600 NMR spectrometer (Varian, Palo Alto, CA, USA). SEC was performed with size-exclusion columns (Agilent Technologies, Inc., UK) including an analytical PLgel 5-μm mixed-D column (300
× 7.5 mm) and a miniature guard column (PLgel 5 μm, 50 × 7.5 mm), and the chromatograms were recorded continuously using a refractive index detector (LabAllianceTM RI-101, Scientific System
Inc., USA). The peak molecular weight (_M_p), number-average molecular weight (_M_n), weight-average molecular weight (_M_w), and polydispersity (_M_w/_M_n) are expressed relative to
polystyrene standards (Varian, Inc., Amherst, MA, USA). MALDI-TOF MS spectra were acquired using a Micromass® MALDI micro MXTM time-of-flight mass spectrometer (Waters®, Milford, MA, USA) in
reflection mode. The thermal transition behaviors were investigated using a thermal analytical system (TA Instruments, New Castle, DE, USA) under nitrogen purging (DSC: LT-Modulate DSC 2920
calorimeter; TGA: SDT Q600 thermogravimetric analyzer). SORBITAN–PLA/PEG–PLA-BASED COLLOIDAL VESICLE In a typical procedure, the products (400 mg) recovered as described above (PEG–PLA or
one-pot sorbitan–PLA/PEG–PLA or a mixture of sorbitan–PLA and PEG–PLA with a weight ratio of 1/1) were mixed with protein medium (0.64 mL) and squalene oil (0.96 mL) and then emulsified by
using a homogenizer (Polytron® PT 2500, Kinematica AG, Swiss) at 6000 rpm for 5 min. The protein medium was prepared with BSA diluted in phosphate buffer saline (PBS). The obtained colloidal
formulation served as a stock for further characterization, namely, stability monitoring and droplet testing. The latter was performed by adding a droplet (100 μL) of colloidal stock into a
PBS-containing round-bottom tube (1 mL), followed by monitoring using an Olympus DP70 optical microscope and a Brookhaven dynamic light scattering (DLS) particle size analyzer. IN VITRO
RELEASE BSA-containing formulations (0.3 mL, 10 mg mL−1) were charged in a sample reservoir of a centrifugal device with a membrane pore size of 0.2 μm (Nanosep®, PALL Life Sciences, MI,
USA). The reservoir was capped and then inserted into a 50-mL centrifuge tube containing 2 mL PBS buffer. The tubes were placed in a thermostatic oven at 37 °C. At predetermined time points,
three samples (100 μL) were regularly taken from the filtrate receiver exterior to the reservoir and replaced with an equivalent amount of the fresh PBS buffer. The BSA content was
quantified by using a PierceTM BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). RESULTS AND DISCUSSION SYNTHESIS OF SORBITAN–PLA/PEG–PLA BY ONE-POT MELT POLYCONDENSATION Schematic
diagrams of the one-pot synthesis of sorbitan–PLA/PEG–PLA are shown in Fig. 1. To understand the effect of sorbitan and/or the PEG moiety on amphiphilic properties, we also compared their
effect with that of the individual component, sorbitan–PLA or PEG–PLA. In a typical procedure, OLA was first prepared by anhydrization of lactic acid. To prepare sorbitan–PLA, sorbitan was
then introduced into the reactor, followed by melt polycondensation of OLA on the sorbitan, resulting in an amphiphilic copolymer possessing both a hydrophilic sorbitan group and lipophilic
PLA blocks. Copolymerization initiated by methoxy PEG propagated at the hydroxyl end, and the resulting polymer had a PEG–PLA chain structure. Polymers synthesized in the absence of any
initiator can be regarded as a PLA homopolymer. When sorbitan and methoxy PEG were introduced into the reactor simultaneously, the OLA chain was extended on both sorbitan and methoxy PEG
simultaneously, resulting in a mixture containing both sorbitan–PLA and PEG–PLA copolymers. The molecular characteristics of the designed polymeric emulsifiers are summarized in Table 1.
Figure 2 shows the FTIR spectra of PLA and its derived copolymers sorbitan–PLA and PEG–PLA and the one-pot sorbitan–PLA/PEG–PLA mixture. Three characteristic absorption signals are observed
in the spectrum of PLA, with the band at 1755 cm−1 attributed to the C = O stretching vibrations and two bands at 2947 and 2997 cm−1 assigned to C–H stretching [6, 7]. In the spectrum of
PEG–PLA, another stretching vibration signal is observed at 947 cm−1, which is an absorption band feature of the PEG polymer [7]. The sorbitan–PLA copolymer absorbed in the 3100–3400 cm−1
range due to the formation of –OH end groups. DOSY NMR is often regarded as chromatographic NMR technique, and it can provide evidence on whether the recovered samples are of fusion form or
a mixture. Figure 3 shows the DOSY map of sorbitan–PLA/PEG–PLA copolymers obtained by one-pot synthesis. The NMR spectrum contained resonance signals consistent with the sorbitan molecule
(_δ_ = 3.9 ppm) [8], PEG block (_δ_ = 3.6 ppm) [7], and PLA block (_δ_ = 1.3 and 5.1 ppm for the main chain; _δ_ = 1.2 and 4.1 ppm for the end chain) [7]. These signals had two diffusion
coefficients, in agreement with the sorbitan–PLA/PEG–PLA mixture. The lighter and less viscous component was assigned to sorbitan–PLA, and the heavier and more viscous component was assigned
to PEG–PLA. SEC chromatograms (Fig. 4a) showed that sorbitan–PLA (sample S1) exhibited multiple peaks with a broad molecular weight (MW) distribution, indicating the presence of low-MW OLA
species. On the other hand, PEG–PLA (sample E1) displayed a single peak with a narrow MW distribution. It is interesting to note that the sorbitan–PLA/PEG–PLA synthesized in the same reactor
(one-pot sample O1) also showed a single peak with a narrow MW distribution, most likely due to its low sorbitan content. Using MALDI-TOF mass spectrometry, the distinct compositions of
sorbitan–PLA/PEG–PLA can be identified. As shown in Fig. 4b, the mass spectrum of a mixture sample of sorbitan–PLA (S1) and PEG–PLA (E1) was consistent with two clusters. The peaks between
1000 and 1500 _m/z_ were assigned to the MW of sorbitan–PLA and OLA oligomers, which were well resolved with the peaks separated by 72 _m/z_, corresponding to the MW of the lactyl motif. The
MW of each component was calculated according to the following equation: $${\mathrm{MW}}_{{\mathrm{sorbitan}}-{\mathrm{PLA}}} = x\left( 72.06 \right) + 164.16 + 22.99$$ where 72.06, 164.16,
and 22.99 are the MWs of lactyl units, sorbitan and Na+ ions (from Na-TFA), respectively. On the other hand, the peaks between 1500 and 2600 _m/z_ were assigned to the MW of PEG–PLA and the
homopolymers PEG and PLA, which were well resolved, with the peaks separated by 44 and 72 _m/z_. Thus, they corresponded to the MWs of the oxyethylene motif and lactyl motif, respectively.
The MW of each component was calculated according to the following equation: $${\mathrm{MW}}_{{\mathrm{PEG}}-{\mathrm{PLA}}} = x\left( 72.06 \right) + n\left( 44.03 \right) + 32.03 + 22.99$$
where 72.06, 44.03, 32.03, and 22.99 are the MWs of lactyl units, oxyethylene units, the end groups of one methyl and one hydroxyl, and Na+ ions, respectively. Similar to the mixed sample,
the one-pot sorbitan–PLA/PEG–PLA (O1) sample also presented a bimodal distribution. It is concluded that the low-MW components are due to sorbitan–PLA and that the high-MW components are due
to the PEG–PLA copolymers. THERMAL PROPERTIES OF THE COPOLYMERS SORBITAN–PLA/PEG–PLA The thermal properties of the designed copolymers were investigated using thermal analytical instruments
and compared with those of the corresponding PLA homopolymer. Prior to the analysis, the samples were preheated from room temperature to 105 °C to erase the thermal history and remove the
residue solvents. After the sample was cooled to room temperature, the endothermal information was obtained by second heating in DSC from 30 to 200 °C at a heating rate of 10 °C min−1. The
glass transition temperature (_T_g) value of each polymer sample was read from the midpoint of the transition zone of the corresponding DSC thermogram. As shown in Fig. 5a, the _T_g of the
copolymers ranged from 68 to 70 °C, showing that these materials form an organic glass at room temperature. The one-pot sorbitan–PLA/PEG–PLA showed a _T_g value very close to that of the PLA
homopolymer, indicating that the incorporation of a sorbitan molecule or PEG segment or both in the same time into the PLA chain did not significantly affect the thermal transition
intrinsic to PLA. Thermal decomposition curves of the polymers were recorded by TGA thermograms from 25 to 500 °C under nitrogen purge, as shown in Fig. 5b. PLA and sorbitan–PLA exhibited
uniform thermal decomposition profiles. On the other hand, a two-step decomposition profile was detected for the samples of PEG–PLA and one-pot sorbitan–PLA/PEG–PLA. The first decomposition
step was assigned to the PLA segments. The decomposition temperature (_T_d) was taken to be the temperature to which two straight lines with different slopes are extrapolated. The _T_d value
of the first step was determined to be 236 °C for PLA (P1), and values of 258, 240, and 226 °C were obtained for sorbitan–PLA (S1), PEG–PLA (E1), and one-pot sorbitan–PLA/PEG–PLA (O1),
respectively (Table 1). Polycondensation of lactic acid involves two reaction equilibria occurring simultaneously [9], i.e., the dehydration/hydration equilibrium for esterification and the
ring/chain equilibrium for the interconversion between PLA and lactide (Fig. 1). In the present study, OLA and initiators (sorbitan and/or MePEG2000) were heated to a temperature above _T_g
but below _T_d to improve the mobility and reaction of the end groups and to prevent thermal degradation. This process prevents undesirable side reactions in this system. It should also be
noted that a melting point of ~150 °C was detected for the PLA homopolymer (sample P1 in Fig. 5a), which can be assigned to the formation of lactides or racemization during polycondensation
[9]; however, this effect can be diminished by incorporating sorbitan and MePEG2000. FORMULATING COLLOIDAL VESICLES BASED ON SORBITAN–PLA/PEG–PLA The emulsifying abilities of the one-pot
sample and the mixture of sorbitan–PLA/PEG–PLA were tested. Without further purification, the resulting products were dissolved in PBS and then homogenized with squalene oil, constructing a
white and isotropic colloid (Fig. 6a). It appears that sorbitan–PLA is not dissolved in PBS at room temperature, and the presence of PEG–PLA in the aqueous solution helped the dispersion of
the sorbitan–PLA in the aqueous solution into polymeric micelles. It must also be noted that the PEG–PLA-emulsified squalene colloid was only stable for a few minutes before separating into
two phases, indicating that homogenization using only a single emulsifying agent failed to stabilize the squalene/water interface at this weight ratio. The sorbitan–PLA/PEG–PLA-stabilized
colloids were very stable when they were stored at 4 °C (supplementary Fig S1); on the other hand, instability occurred beyond 6 weeks upon storage at 37 °C. This result agrees with the
findings in our previous study, which showed that the physical separation of the colloids stabilized by amphiphilic bioresorbable polymers was mainly achieved by intermediate mechanisms
including coalescence, Ostwald ripening, and degradation-mediated phase separation [4]. The dispersion structure of the thus-obtained colloid was identified by its water affinity and
sustained-release ability. The water affinity of the colloid could be identified by the droplet test. Typically, a colloidal droplet in which the continuous phase was oil remained crowded on
the water surface; on the other hand, droplets in which the continuous phase was water could diffuse into the water after gentle hand stirring of the container. Using an optical microscope,
homogeneous fine particles were observed when redispersing the stock colloid in buffered saline for both the colloids obtained using the one-pot sorbitan–PLA/PEG–PLA sample (O1) and the
colloids obtained using the mixed sample (S1/E1) (Fig. 6b). The DLS pattern showed that both colloids possess a narrow size distribution with average diameters of 580 ± 340 nm (O1) and 770 ±
290 nm (S1/E1) and polydispersity index (PDI) values of 0.24 ± 0.05 (O1) and 0.24 ± 0.03 (S1/E1). This result revealed that sorbitan–PLA/PEG–PLA-stabilized colloids had high affinity for
the aqueous phase. A sustained-release investigation was conducted using hydrophilic BSA as a protein model. The release of BSA from colloidal formulation is a multifaceted process involving
diffusion from the inner core of the colloid to the surface and then into the external aqueous phase. Insofar as absorbable polymeric vesicles are concerned, a certain content of BSA
trapped within the colloid will be released by degradation-mediated phase separation. Proteins without formulation or attached to the surface of the colloid will be quickly released since no
oily barrier isolates the encapsulated molecule from the external aqueous phase. As shown in Fig. 6c, free BSA exhibited a fast release, and more than 50% of loaded BSA was released into
the aqueous solution external to the sample reservoir during the first 100-h period. The colloid made by the mixture of sorbitan–PLA/PEG–PLA showed good depot efficacy for BSA such that the
trapped BSA was released in a sustained manner during the same period. Beyond that period, the BSA release profiles appeared to reach and fluctuate around 5–10%, probably due to some BSA
molecules that were not encapsulated inside the colloid. It is interesting to note that the colloids obtained using one-pot sorbitan–PLA/PEG–PLA possess similar sustained-release
characteristics intrinsic to the individual component of the emulsifying agent mixture [4, 10]. It is known that the hydrophilic–lipophilic balance of a surfactant/emulsifying agent plays a
crucial role in the dispersion structure of water/oil-based colloids [11]. Those containing a highly hydrophilic portion in the main chain molecule have a high affinity for water and form
oil-in-water (O/W)-type dispersions, whereas those containing a poorly hydrophilic portion in the main chain molecule have a high affinity for the oily phase and form water-in-oil (W/O)
structures. In this study, a droplet test and sustained-release investigation indicated that one-pot sorbitan–PLA/PEG–PLA consisting of two emulsifying agents formed stable W/O/W
double-dispersion structures. The emulsifying systems consisting of sorbitan–PLA and PEG–PLA not only provided a potential method of stabilizing the squalene/water interfaces but also
enhanced the entrapping efficacy for hydrophilic proteins of the so-obtained colloidal formulations. Finally, all the applied excipients, including squalene core oil and polymeric
emulsifying agents, show main chain degradation into small molecules and are further absorbed in vivo, indicating high bioabsorbability of the colloidal vesicle. One-pot synthesis is desired
to simplify the separation process while maintaining the same efficacy. Further studies are underway to extend these applications to develop microencapsulation technology for prophylactic
vaccines against emerging infectious diseases and therapeutic vaccines against cancers. These applications will entail a comprehensive investigation of whether the presence of these vaccine
antigens influences the relationship between the degradation profiles of one-pot sorbitan–PLA/PEG–PLA polymers and the dispersion structure of squalene/water interfaces. CONCLUSION Here, we
conducted a rational design of a delivery system that is related to the methods of engineering amphiphilic bioresorbable polymers, namely, sorbitan–PLA and PEG–PLA, by one-pot melt
polycondensation of lactic acid in the presence of a binary initiator system of sorbitan and methoxy PEG. Without further purification, the resulting products were dissolved in PBS and then
homogenized with squalene oil to obtain an isotropic colloid. Structural features allow the vesicles to act as a depot for the sustained delivery of proteins. The work reported here
represents a significant advance to meet the requirement of a simplified separation process and will reduce the purification cost of materials while increasing chemical yield. REFERENCES *
Kalasz H, Antal I. Drug excipients. Curr Med Chem 2006;13:2535–63. Article CAS Google Scholar * Wakelin SH, Cooper S, Marren P, Shaw S. Sorbitan mono-oleate: a potential allergen in paste
bandages. Contact Dermat 1996;35:377. Article CAS Google Scholar * Sun H, Yang R, Wang J, Yang X, Tu J, Xie L, Li C, Lao Q, Sun C. Component-based biocompatibility and safety evaluation
of polysorbate 80. RSC Adv 2017;7:15127–38. Article CAS Google Scholar * Huang CY, Huang CH, Liu SJ, Chen HW, Leng CH, Chong P, Huang MH. Polysorbasome: a colloidal vesicle contoured by
polymeric bioresorbable amphiphiles as an immunogenic depot for vaccine delivery. ACS Appl Mater Interfaces 2018;10:12553–61. Article CAS Google Scholar * Ishikawa H, Suzuki T, Hayashi Y.
High-yielding synthesis of the anti-influenza neuramidase inhibitor (-)-oseltamivir by three “one-pot” preparations. Angew Chem Int Ed. 2009;48:1304–7. Article CAS Google Scholar * Li S,
Anjard S, Rashkov I, Vert M. Hydrolytic degradation of PLA/PEO/PLA triblock copolymers prepared in the presence of Zn metal or CaH2. Polymer 1998;39:5421–30. Article CAS Google Scholar *
Rashkov I, Manolova N, Li SM, Espartero JL, Vert M. Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with short poly(l-lactic acid) chains.
Macromolecules 1996;29:50–6. Article CAS Google Scholar * Wu H, Zhu H, Zhuang J, Yang S, Liu C, Cao YC. Water-soluble nanocrystals through dual-interaction ligands. Angew Chem Int Ed
2008;47:3730–4. Article CAS Google Scholar * Maharana T, Mohanty B, Negi YS. Melt–solid polycondensation of lactic acid and its biodegradability. Prog Polym Sci 2009;34:99–124. Article
CAS Google Scholar * Shum HC, Zhao YJ, Kim SH, Weitz DA. Multicompartment polymersomes from double emulsions. Angew Chem Int Ed 2011;50:1648–51. Article CAS Google Scholar * Huang MH,
Chou AH, Lien SP, Chen HW, Huang CY, Chen WW, Chong P, Liu SJ, Leng CH. Formulation and immunological evaluation of novel vaccine delivery systems based on bioresorbable poly(ethylene
glycol)-block-poly(lactide-co-ε-caprolactone). J Biomed Mater Res Part B 2009;90B:832–41. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the
National Health Research Institutes of Taiwan (grant number 108A1-IVPP19-014) and by a grant from the Ministry of Science and Technology of Taiwan (grant number MOST 106-2314-B-400-016-MY3).
AUTHOR INFORMATION Author notes * These authors contributed equally: Chiung-Yi Huang, Yu-Jhen Cheng AUTHORS AND AFFILIATIONS * National Institute of Infectious Diseases and Vaccinology,
National Health Research Institutes, Miaoli, 35053, Taiwan Chiung-Yi Huang, Yu-Jhen Cheng, Hui-Min Ho & Ming-Hsi Huang * Department of Food Science, National Taiwan Ocean University,
Keelung, 20224, Taiwan Chung-Hsiung Huang * Graduate Institute of Biomedical Sciences, China Medical University, Taichung, 40402, Taiwan Ming-Hsi Huang Authors * Chiung-Yi Huang View author
publications You can also search for this author inPubMed Google Scholar * Yu-Jhen Cheng View author publications You can also search for this author inPubMed Google Scholar * Hui-Min Ho
View author publications You can also search for this author inPubMed Google Scholar * Chung-Hsiung Huang View author publications You can also search for this author inPubMed Google Scholar
* Ming-Hsi Huang View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ming-Hsi Huang. ETHICS DECLARATIONS CONFLICT OF
INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL FOR PUBLICATION RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Huang, CY., Cheng, YJ., Ho, HM. _et al._ One-pot amphiphilic engineering of bioresorbable polymers for constructing colloidal vesicles and prolonging protein delivery. _Polym J_ 52, 237–244
(2020). https://doi.org/10.1038/s41428-019-0267-3 Download citation * Received: 27 June 2019 * Revised: 02 September 2019 * Accepted: 03 September 2019 * Published: 24 September 2019 * Issue
Date: February 2020 * DOI: https://doi.org/10.1038/s41428-019-0267-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative