Play all audios:
ABSTRACT Visual disturbance or visual failure due to toxicity of an ingested substance or a severe nutritional deficiency can present significant challenges for diagnosis and management, for
instance, where an adverse reaction to a prescribed medicine is suspected. Objective assessment of visual function is important, particularly where structural changes in the retina or optic
nerve have not yet occurred, as there may be a window of opportunity to mitigate or reverse visual loss. This paper reviews a number of clinical presentations where visual
electrophysiological assessment has an important role in early diagnosis or management alongside clinical assessment and ocular imaging modalities. We highlight the importance of vitamin A
deficiency as an easily detected marker for severe combined micronutrient deficiency. SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTRODIAGNOSTIC TESTS OF THE VISUAL PATHWAY AND APPLICATIONS IN
NEURO-OPHTHALMOLOGY Article Open access 11 June 2024 CLINICAL ELECTROPHYSIOLOGY OF THE OPTIC NERVE AND RETINAL GANGLION CELLS Article Open access 11 June 2021 VITAMIN A DEFICIENCY AND THE
RETINAL “DOUBLE CARROT” SIGN WITH OPTICAL COHERENCE TOMOGRAPHY Article 15 July 2022 METHODOLOGY Searches were conducted through Medline in January 2021 using the following search strategy:
Keywords: (generic drug name OR trade drug name) AND (adverse effects OR side effects OR toxic*) AND (eye diseases OR eye OR ocular OR ophthalmic). No date restriction was imposed. Results
were filtered for full-text and English language and were tabulated. Papers were evaluated by relevance, study type, and quality, with articles including primary evidence prioritised. Due to
the relative rarity and unpredictable incidence of many of these conditions, a large proportion of references containing primary data comprise case reports, case series, and cohorts. Review
articles were prioritised by relevance to the search criteria, comprehensiveness and date of publication. References that were deemed less relevant, of lower quality or duplicated existing
data were excluded. INTRODUCTION Most ophthalmologists will, from time to time, encounter patients who present with persistent visual disturbance or visual failure where there is clinical
suspicion either of an adverse reaction to an ingested substance or of a nutritional deficiency. Whether the onset of symptoms is acute or chronic, structural changes in the visual system
such as loss of retinal photoreceptors or optic nerve fibres will often be preceded by disturbance of visual function. At a point in the clinical course where clinical examination, ocular
coherence tomography, and magnetic resonance imaging as yet reveal no abnormality, there may be a window of opportunity for intervention to mitigate or reverse the effects of toxicity or
rectify a nutritional deficiency before irreversible structural changes in the visual system occur. In this situation, visual electrophysiological testing may be able to provide valuable
objective evidence of disordered visual function and response to treatment to supplement subjective tests of visual function such as visual acuity, perimetry, and colour vision assessment.
In some instances, retinal dysfunction may occur with no, or minimal detectable structural abnormalities, making visual electrophysiology a valuable tool to enable early detection of retinal
toxicity [1]. Electrophysiological testing may also be particularly important where the causal relationship between the suspected toxin or deficiency state and visual symptoms is unclear or
where the patient is unable to undertake subjective tests of visual function. Rather than attempting to provide an exhaustive account of ocular toxicity and deficiency states, this paper
reviews the role of visual electrophysiology testing in the diagnosis or monitoring of several important presentations which may be encountered in clinical practice. CHLOROQUINE AND
HYDROXYCHLOROQUINE Chloroquine (CQ) and hydroxychloroquine (HCQ) were introduced in 1947 and 1955, respectively for prophylaxis and treatment of malaria. Following observations that some
patients taking these medications experienced improvements in arthritis and certain skin eruptions, their use expanded to conditions such as systemic and discoid lupus erythematosus and
rheumatoid arthritis. Their immuno-modulatory, antimicrobial, anti-angiogenic, and anti-neoplastic properties have been studied extensively and there is considerable research interest in
extending their use to the treatment of a diverse range of conditions including high-risk coronary heart disease, type 2 diabetes mellitus, primary progressive multiple sclerosis, and
recurrent miscarriage [2]. CQ and HCQ are inexpensive to manufacture and are generally well tolerated. Reports of retinal toxicity in patients taking CQ appeared from the late 1950s onwards,
with a typical presentation of paracentral visual field defects, progressing to central field loss and development of a “bulls-eye” maculopathy. Some patients also developed extensive
peripheral field loss [3]. The pattern of retinal toxicity seen in patients taking HCQ is similar and although HCQ seems to be less toxic than CQ to the retina at doses that give an
equivalent therapeutic effect, the relative risk is uncertain as accurate comparison of retrospective data is difficult [4]. The risk of retinopathy increases with dose and duration of
exposure, though individual susceptibility appears to vary considerably with renal disease and concurrent use of tamoxifen identified as risk factors for toxicity [5]. Although symptomatic
retinopathy in patients taking conventional doses of HCQ is uncommon, particularly when the duration of treatment is less than 5 years, Melles and Marmor found evidence of retinopathy using
Humphrey 10-2 visual fields or spectral-domain ocular coherence tomography (SD-OCT) in 7.5% of 2361 patients taking HCQ for more than 5 years [5], raising the possibility that subclinical
toxicity is much more common than previously thought. The exact mechanism of CQ and HCQ toxicity in the retina remains uncertain, though photoreceptors appear to be the primary target, with
the retinal pigment epithelium showing damage at a later stage [6]. Early signs of CQ/HCQ toxicity include pericentral visual field defects with the Humphrey 10-2 protocol, perifoveal SD-OCT
abnormalities (particularly disruption of the outer segment ellipsoid zone and loss of the external limiting membrane) [7]. Fundus Autofluorescence (FAF) changes (perifoveal
hyper-autofluorescence with later mottled hyper- and hypo-autofluorescence) [6] and multifocal electroretinogram (mfERG) abnormalities. The mfERG is a cone-driven response recorded under
light-adapted conditions from many areas of the retina simultaneously. Quantitative comparison of responses originating from the fovea and extra-foveal areas can be made by grouping
individual focal responses into concentric rings and averaging the responses originating from each ring. Typically, the central response (usually designated Ring 1) corresponds to the
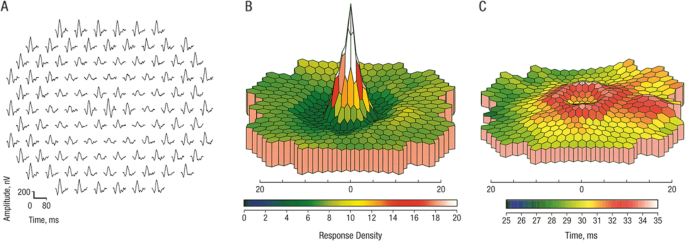
central 3 degrees of the visual field and the next concentric ring (Ring 2) corresponds to 3-10 degrees from fixation [8]. The most reliable early sign of CQ/HCQ toxicity is a depression of
the response amplitude in Ring 2 with relative preservation of the central response, expressed as the R1:R2 amplitude ratio [9] (Fig. 1). CQ/HCQ retinopathy is irreversible and once
“bulls-eye” macular changes are present, is likely to worsen for at least 3 years following cessation of the drug, though early retinopathy does not usually progress following cessation
[10]. Disruption of the external limiting membrane on SD-OCT has been identified as a specific risk factor for the progression of retinopathy after cessation of treatment [6]. The Royal
College of Ophthalmologists [11] has made recommendations for monitoring for CQ/HCQ retinopathy using SD-OCT and static perimetry with the 10-2 protocol as primary surveillance tools. A
two-year study of patients assessed in the UK using these recommendations [12] found a prevalence of retinopathy of between 0.3% and 1.6% depending on the criteria used—considerably lower
than the prevalence estimated by Melles and Marmor [5]. Although there is not currently sufficient evidence to support the use of electrophysiological testing in the routine surveillance of
patients taking CQ/HCQ, where retinopathy is suspected, or where a patient has additional risk factors for developing retinopathy, mfERG can provide additional diagnostic confirmation.
Cessation of CQ/HCQ therapy is not always a straightforward option, as therapeutic alternatives may be less effective or less well tolerated. In this situation, the patient may opt to
continue CQ/HCQ in the presence of possible early retinopathy, in which case close surveillance including serial mfERG examinations may be required. VIGABATRIN Vigabatrin (VGB) is a drug
used since the 1990s to treat epilepsy. In adults, it is used to manage refractory complex partial seizures which cannot be controlled through other medications, whereas in children it is a
first-line option for infantile spasms associated with West syndrome. Although not proven to be effective, VGB has also been proposed as an alternative treatment for cocaine dependence [13].
It selectively and irreversibly inhibits 4-aminobutanoic acid (γ‐aminobutyric acid, GABA) transaminase, resulting in increased levels of GABA in the brain and even higher concentrations in
the retina [14, 15]. First reported by Eke in 1997 [16], VGB-induced retinal toxicity has been widely documented, manifesting predominantly as bilateral irreversible peripheral visual field
defects. Visual field defects vary in severity but commonly culminate in bilateral concentric constriction with temporal field and macula preservation. Patients often have normal or
near-normal visual acuity (VA) and may be unaware of field loss unless it is severe [17]. Although the mechanism of toxicity is uncertain, animal studies have demonstrated VGB accumulation
in many cell types of the retina and have linked retinal toxicity to photopic exposure and taurine deficiency [18]. Postmortem examination of an eye of a patient with VGB-associated visual
field loss showed depletion in retinal ganglion cell and inner and outer nuclear layers [19]. There are indications from electrophysiological testing that there is dysfunction of the cone
system and particularly GABAergic amacrine cells [20]. Visual field loss appears to be dose-dependent and can occur within 6 months of exposure [21]. Visual field defects may be
non-progressive or may progress slowly up to 35 months, with longitudinal studies showing that the loss typically persists indefinitely following cessation of VGB therapy [21]. A few
isolated cases of improvement following cessation in children have been reported but may be artefactual as a result of learning effects. These findings have led to recommendations to limit
dosage to 3 g/day in adults and 50–100 mg/kg/day in children as a maintenance dose, with withdrawal encouraged if there is no clinical benefit [22]. Estimates of the prevalence of
VGB-induced retinal toxicity vary, but a systematic review by Maguire estimated a prevalence of 52% [95% confidence interval (CI) 46–59] for adults and 34% (95% CI 25–42) for children [23].
Prevalence increases with age and cumulative dose and may be higher in males [21]. Identifying early signs of VGB-induced retinal toxicity using visual field assessment remains a challenge
as perimetry is often not suitable for children and patients with cognitive impairment, learning difficulties, or fatigue, all of which are frequently reported in epilepsy [24]. Furthermore,
perimetry alone may not detect subtle peripheral field changes and apparent field constriction is not necessarily indicative of toxicity. The Royal College of Ophthalmologists has
recommended that all patients taking VGB undergo a complete eye examination including visual field assessment (preferably gradient adapted static perimetry) before starting treatment, with
perimetry repeated every 6 months for 5 years [22]. An additional threshold test extending to 30 degrees of eccentricity should be conducted if a field defect is reported [22]. Due to the
limitations of visual field assessment in paediatric patients and approximately 20% of adult epilepsy patients [25], additional tests may be utilised to detect toxicity, including
fundoscopy, optical coherence tomography (OCT), and electrophysiology. Fundoscopy is often unremarkable in patients with VGB-associated visual field loss, although macular pigment epithelial
changes and thinning of the nasal retinal nerve fibre layer (RNFL), termed ‘reverse optic atrophy’ have been reported [15]. Often these changes are subtle or only evident where there is
severe field loss. OCT allows quantitative evaluation of the RNFL, and reduced RNFL thickness has been shown to correlate with visual field defects [25]. Despite the cooperation requirements
of OCT, handheld devices can yield reliable measurements in children taking VGB [26]. Alternative VEP methods of visual field assessment for children taking VGB have been explored [27] but
have yet to be validated for routine clinical practice. As VGB toxicity appears to be predominantly an inner retinal dysfunction, the standard full-field electroretinogram (ERG) typically
shows only subtle abnormalities which may not correlate with the severity of visual field loss [28]. However, reduced amplitude of the 30 Hz flicker ERG (a cone system-driven response,
originating in the inner retina) and the photopic negative response (PhNR) (which reflects ganglion cell activity) have been reported and may provide useful supporting evidence of toxicity
in infants and children [29, 30]. Although the ERG does not usually provide unequivocal evidence of VGB toxicity, it is valuable in the initial assessment of a patient receiving VGB to
exclude other retinal causes of visual field constriction such as photoreceptor dystrophies. Electrophysiological assessment may also help to identify pre-existing ERG abnormalities,
possibly associated with other anti-epilepsy medications, which were found in 30% of patients prior to VGB treatment by Westall et al. [29]. There is, however, currently insufficient
evidence to recommend electrophysiological assessment as part of the routine ongoing monitoring of visually asymptomatic patients taking vigabatrin. ETHAMBUTOL Ethambutol was introduced in
the early 1960s for the treatment of tuberculosis and other mycobacterial infections. The first report of optic neuropathy in patients treated with ethambutol appeared whilst the drug was
still in clinical trials, with optic disc hyperaemia and swelling and the appearance of a centrocaecal scotoma, which resolved following cessation of treatment [31]. The incidence of optic
neuropathy appears to be dose and duration-dependent, with a reported incidence of 45% at doses of 60–100 mg/kg/day [31] reducing to less than 1% with a dose of 15 mg/kg/day [32]. However,
there does not appear to be a “safe” dose, and optic neuropathy has been reported with a dose of only 3.6 mg/kg/day after 6 months of treatment [33]. The toxicity of ethambutol may be
potentiated by renal impairment or co-administration of other drugs which can cause optic neuropathy in their own right, such as linezolid [34] or isoniazid [35]. In a series of 857 patients
taking ethambutol, the incidence of optic neuropathy was 1.5%, and of these, reduced visual acuity was evident in 65% and abnormal colour vision in 61% [36]. Colour vision abnormalities
ranged from mild red-green defects to complete loss of colour vision. Visual field defects attributed to ethambutol toxicity are typically central or centrocaecal, but can also be extensive
with homonymous or bitemporal patterns, raising the possibility that the optic chiasm or optic tracts may be affected in some cases [36,37,38,39]. Peripapillary RNFL thinning, particularly
in the temporal quadrant and macular nerve fibre layer thinning may be evident on OCT [33, 40]. Where the visual acuity is substantially reduced due to ethambutol optic neuropathy, the
pattern visual evoked potential (PVEP) may be reduced in amplitude or unrecordable. Where a PVEP is recordable, a delay in the P100 component is the most consistent abnormal finding [33, 38,
41]. Multifocal ERG abnormalities [42] and full-field ERG abnormalities (reduced scotopic and photopic b-wave amplitudes and loss of oscillatory potentials) [33] have also been reported,
which suggests that the toxic effect of ethambutol in the retina may not be confined to the ganglion cells but may involve other cell types such as amacrine and bipolar cells. Ethambutol
appears to render ganglion cells abnormally sensitive to glutamate ions (an excitotoxic effect), leading to a rise in intracellular calcium ions which disrupts mitochondrial function [43].
This raises the possibility that drugs that inhibit the NMDA subtype of glutamate receptor, such as memantine and amantadine may protect against ethambutol toxicity and there is evidence
that this may be the case in animal models [43, 44]. Although the prognosis for recovery from ethambutol optic neuropathy is generally good following cessation of the drug, permanent visual
loss can occur even when ethambutol is discontinued promptly [31]. Kim et al found significant delays in PVEP P100 latency at 2 months and 4 months of therapy with ethambutol even where
visual acuity and colour vision were normal, suggesting that subclinical toxicity is common [45]. Serial monitoring of asymptomatic patients taking ethambutol with PVEP and OCT retinal nerve
fibre layer thickness is a practical addition to the recording of visual acuity and colour vision during treatment and should be considered, particularly for patients who may require
prolonged treatment with ethambutol. Where symptomatic ethambutol toxicity is suspected, electrophysiological assessment may also include PERG or mfERG and full-field ERG. QUININE Quinine,
occurring naturally in the bark of the cinchona tree, has been used from antiquity to treat malaria. It is still sometimes used for the treatment of malaria caused by _Plasmodium falciparum_
and, at a lower dose, for the treatment of nocturnal leg cramps, though its use for both of these indications is declining where safer alternatives are available. It is also used at a lower
concentration in tonic water to impart a bitter flavour. It has a narrow therapeutic index and accidental or intentional overdosage with quinine may result in hypotension, life-threatening
cardiac arrhythmias, and seizures [46]. Acute overdosage (which may be accidental, or a result of attempted suicide) can also result in severe visual loss, sometimes to no perception of
light bilaterally within a few hours of ingestion. In the acute presentation, the pupils are typically fixed and semi dilated. The retina may appear normal initially, though there may be
transient retinal pallor with the appearance of a cherry-red spot at the fovea [47, 48]. Later findings typically include arteriolar attenuation, optic disc pallor, and pronounced thinning
of the inner retinal layers on SD-OCT [47, 49]. There may be spontaneous visual improvement in the days or weeks after the overdose, sometimes to a normal visual acuity, but with
night-blindness and pronounced concentric constriction of visual fields which does not improve [48]. The mechanism of damage to the retina in quinine poisoning probably involves direct
toxicity to the Müller cells, bipolar cells, amacrine cells, and ganglion cells [50], though the photoreceptors may also show evidence of damage on SD-OCT [47]. The reason why central
retinal function may improve when peripheral retinal function does not is unknown. No effective antidote to the retinal toxicity of quinine has yet been found. In the first few days
following ingestion of quinine, the bright flash dark-adapted ERG may show a delayed, supernormal a-wave and b-wave with absent oscillatory potentials [51]. Later, the bright flash
dark-adapted ERG shows a reduced b-wave with a relatively preserved a-wave, producing an “electronegative” waveform [49, 51, 52]. The single-flash photopic ERG shows a prolonged a-wave
trough and small, steeply rising b-wave. The 30 Hz flicker photopic ERG is reduced in amplitude and delayed, and the long (200 ms) flash photopic ERG has a characteristic abnormal shape with
a positive plateau following the off d-wave peak, indicative of cone ON- and OFF-pathway dysfunction [49, 50]. The pattern ERG (reflecting macular function) may be absent even in the
presence of good visual acuity [49]. The ERG changes persist indefinitely, which can help distinguish the condition from retinitis pigmentosa where a history of quinine ingestion may not be
volunteered by the patient [53]. IRON CHELATORS Iron-chelating agents are used to prevent iron accumulation in thalassaemia and other conditions which require frequent blood transfusion.
Many tissues and organs are vulnerable to iron overload, especially the heart, liver, bone and endocrine tissues. The most commonly used and studied iron chelator is desferrioxamine mesylate
(DFO); however, as it can only be administered parenterally, its use has declined with the introduction of newer oral chelators deferiprone and deferasirox. Since the 1980s, single case
reports and small case series have reported ocular toxicity associated with DFO, with reported findings of pigmentary retinopathy or optic neuropathy accompanied by reduced visual acuity and
colour vision, nyctalopia, cataract, and visual field defects [54, 55]. The primary target of DFO toxicity appears to be the retinal pigment epithelium (RPE). The mechanism is poorly
understood but may involve both direct chelation of iron stores within the RPE and secondary depletion of trace elements. Early signs of toxicity include transient opacification of the RPE
and outer retina, followed later by pigmentary change [54]. The fundoscopic appearance of DFO retinopathy is variable, typically resembling a pattern dystrophy, however vitelliform and
“bulls-eye” lesions have also been described [56, 57]. Pigmentary changes in the macula may be subtle and easily missed on fundoscopy. Historically, fluorescein angiography (FA) was widely
utilised to diagnose DFO retinopathy [54], but FAF, confocal scanning laser ophthalmoscopy, and SD-OCT are now more widely used in the detection of subtle retinal abnormalities and to
monitor progression over time [57, 58]. Reported electrophysiological abnormalities in acute DFO toxicity include markedly reduced scotopic ERG a- and b-wave amplitudes, delayed photopic
cone b-waves, and a reduced electrooculogram (EOG) light peak to dark trough ratio [54, 59]. Dettoraki [60] found that the full-field ERG and mfERG were more sensitive in detecting early
toxicity than VEP, FAF, and OCT. Where there are visible pigmentary changes involving the macula, the mfERG typically shows reduced responses in the central rings, though such changes are
not unique to DFO toxicity [57]. Some reports describe reversibility of visual defects when DFO therapy is changed from intravenous to subcutaneous administration [61] or is completely
stopped [62], but permanent visual deterioration or progression of retinopathy after DFO has been discontinued has also been documented [59]. The paucity of longitudinal studies means that
there is limited information on the incidence of ocular toxicity associated with iron chelators, although it appears to be a rare finding. A 22-year review of 88 patients receiving chelation
therapy reported only 3 patients with suspected toxicity [63]. The lack of a formal definition of DFO ocular toxicity and evidence-based guidelines for screening means that arrangements for
surveillance of patients taking DFO are typically determined by local protocols between prescribers and ophthalmology services. To minimise the risk of toxicity, it has been recommended
that dosage should not exceed 50 mg/kg of body weight in adults and 25–30 mg/kg in children [64], and regular examination using fundoscopy, FAF, and OCT is encouraged to identify early signs
of retinal and RPE damage. It is unclear if the long-term use of newer iron-chelating agents deferiprone and deferasirox will result in similar retinal toxicity. There are a few case
reports of macular changes in patients taking Deferasirox, with evidence suggesting that vision can improve after therapy is ceased [65]. Further data is required on the ocular safety of
oral iron chelators and ophthalmology services are likely to receive requests to monitor patients receiving these agents. Clinical decisions to continue, change, or discontinue therapy may
be difficult because of the need to balance the risks of irreversible organ damage from iron overload with a risk of retinal toxicity. Based on the reported electrophysiological
abnormalities in patients taking DFO, the most useful electrophysiological tests for monitoring patients receiving iron chelation therapy are likely to be the full-field ERG, with the
addition of either the PERG or mfERG to assess macular toxicity. OCULAR SIDEROSIS Iron is a component of haemoglobin, and its capacity to mediate electron transfer makes it a component or
co-factor in many enzymatic processes, including those involved in oxidative phosphorylation in mitochondria and RPE65. The ferrous (Fe2+) ion is an electron donor and readily catalyses the
conversion of hydrogen peroxide into the hydroxyl radical (OH·) which is highly reactive and causes oxidative damage to proteins, DNA, and lipids. Almost all the body’s iron is normally
bound to protein, but the mechanisms for quenching free radicals and removing iron from the eye may be overwhelmed by the presence of a retained ferrous intraocular foreign body.
Iron-containing foreign bodies are particularly liable to enter the eye during activities that involve striking metal on metal, such as using a hammer and cold-chisel [66]. A shard of metal
generated in this way may have sharp edges and sufficient momentum to penetrate the cornea or sclera. Larger foreign bodies tend to cause symptomatic intraocular haemorrhage or rapidly
evolving cataract, but smaller fragments may lodge in the ciliary body, iris, lens, or anterior chamber drainage angle with few, if any, short-term symptoms. If the injury has gone unnoticed
by the patient or if clinical assessment has failed to identify an entry wound, the undetected ferrous foreign body will release iron as it slowly degrades. The features of established
ocular siderosis include cataract with brown deposits on the lens near the pupil margin, brown or greenish discolouration of the iris, a dilated tonic pupil with light-near dissociation,
brown discolouration of the corneal stroma, and increased pigmentation of the trabecular meshwork, diffuse retinal pigmentary changes, retinal arteriolar attenuation and cystoid macular
oedema. Not all of these features will necessarily be present, and the changes can be subtle. Signs of siderosis can become evident anywhere from a few weeks to several years after the
injury [67]. The bright flash dark-adapted ERG b-wave amplitude has been reported to be transiently supernormal (125% or more of that of the fellow eye) with a normal a-wave in some cases of
early siderosis [66]. The reason for this is uncertain, but as the condition advances, the rod and cone b-waves, the oscillatory potentials, and the 30 Hz flicker response become
progressively attenuated with relative preservation of the a-wave, suggesting that the inner retina is affected earlier than the photoreceptors. Ultimately the scotopic and photopic ERG may
become undetectable. The time between the earliest detectable ERG changes and extinction of the ERG varies but can be less than 18 months [68, 69]. Following surgical removal of the foreign
body, ERG changes did not recover in 6 cases reported by Hope-Ross [69] even though the final visual acuity was 6/12 in all cases. There is evidence that delayed removal of an intraocular
foreign body may be followed by continuing deterioration of the ERG [70]. However, substantial recovery of the ERG was reported following surgery at about a month post-injury by Imaizumi et
al. [71] in a young adult, although the oscillatory potentials and 30 Hz flicker amplitudes did not normalise. These cases suggest that identification and early removal of an iron-containing
intraocular foreign body before clinical or electroretinographic signs of siderosis appear may be important for the prevention of ocular siderosis. However, this goal is not always
achievable as patients may decline surgery or the foreign body may be difficult to locate or inaccessible. Small foreign bodies buried in the pars plicata of the ciliary body can be
particularly difficult to locate, even with the assistance of modern imaging equipment. Hwang records a case of a ferrous foreign body embedded in the anterior part of the optic nerve with
good visual acuity. A decision was made to leave the foreign body in situ and there was no evidence of siderosis after 2 years [72]. In a situation where surgery to remove a foreign body
carries a high short-term risk to vision, it may be reasonable to monitor the patient with serial ERGs and re-evaluate the risks and benefits of surgery should ERG changes suggestive of
early siderosis appear. Electroretinography may also provide useful prognostic information in delayed presentations of established siderosis, including patients who present with a dense
cataract and an uncertain history of previous trauma. Mild or moderate ERG abnormalities may be compatible with good vision [69] though an extinguished ERG suggests a poor prognosis for
recovery of vision. METHANOL Methanol is found in some brands of antifreeze and is used as an ingredient of methylated spirits to denature ethanol, rendering it unfit for human consumption,
whilst still allowing it to be used as a solvent or a fuel. Methanol may also be a contaminant in illegally distilled spirits, which has resulted in mass-poisonings [73]. The effects of
accidental or intentional ingestion of methanol are dose-related and include blindness, acute encephalopathy (which may be fatal), and metabolic acidosis. Methanol is less intoxicating than
ethanol, so victims may report early symptoms at a point when intervention may be effective. Methanol is oxidised to methanal in the liver by alcohol dehydrogenase and undergoes further
enzymatic oxidation to methanoic (formic) acid, which inhibits cytochrome-c oxidase, thereby reducing ATP production in mitochondria in metabolically active sites such as ganglion cell
axons. Hayreh et al. demonstrated in rhesus monkeys that, following ingestion of methanol, a combination of arrest of axonal transport and swelling of oligodendrocytes in the retrolaminar
optic nerve produces a clinical picture resembling severe papilloedema which develops gradually over 1–2 days [74]. Accounts of acute methanol poisoning in humans show similar findings and
have a similar time course, with gradual resolution of optic disc swelling and development of optic atrophy over 1–2 months [75]. The optic discs may later develop pronounced cupping,
resembling advanced normal-tension glaucoma, which suggests that there is also loss of glia in the prelaminar optic nerve [76, 77]. Presenting visual symptoms range from blurring or greying
of vision to complete loss of vision, and a central scotoma may be evident, but visual improvement has been documented as disc swelling subsides [75]. The severity of metabolic acidosis at
presentation appears to correlate with the severity of visual loss and electrophysiological abnormalities [74, 78]. Urban et al. found pattern-reversal visual evoked potentials (PVEP)
abnormalities in 43% of a cohort of 47 victims of a mass-poisoning event, with a mild or moderate delay of the P100 component in subjects (typically 118–130 ms) in subjects where the P100
amplitude was preserved, though the PVEP was unrecordable in those with severe visual loss, as expected [73]. Five subjects had a delayed PVEP despite normal visual acuities. There was a
correlation between the biochemical severity of poisoning and VEP abnormalities. Reported full-field ERG abnormalities following methanol poisoning include selective reduction of the b-wave
[79], combined scotopic a-wave and b-wave reduction and reduced 30 Hz flicker amplitude [80] which suggests that methanol is also toxic to the retina. The treatment of methanol poisoning
includes resuscitation, correction of metabolic acidosis, and administration of fomepizole (4-methylpyrazole), an inhibitor of alcohol dehydrogenase, which delays the conversion of methanol
to its more toxic metabolites. Electrophysiological assessment of patients with known or suspected methanol poisoning should include PVEP (and flash VEP where the PVEP is unrecordable), PERG
and full-field ERG. TOXIC-NUTRITIONAL OPTIC NEUROPATHY This term is used to describe a bilateral optic neuropathy of gradual onset in adults characterised by reduced contrast sensitivity,
dyschromatopsia, and the appearance of central or centrocaecal scotomas. Descriptions of this clinical presentation appeared from the mid-18th Century onwards, but the first systematic
description is attributed to de Schweinitz in 1896 [81] and anecdotal observations that heavy alcohol or tobacco consumption seemed to be associated with the condition led to the older term
‘tobacco-alcohol amblyopia’. Several epidemics with a similar pattern of optic neuropathy have occurred, notably in Cuba in 1992–93 when more than 50,000 cases of optic neuropathy associated
with peripheral and auditory neuropathy were linked to deficiencies in B vitamins and folate during an economic embargo [82], raising the possibility that the condition is caused primarily
by dietary deficiency of B vitamins or other micronutrients, given that these are common in people who are heavily dependent on alcohol. Although the onset of toxic-nutritional optic
neuropathy is usually insidious with few fundoscopic signs, transient optic disc swelling and telangiectases, and peripapillary haemorrhages sometimes occur [83], prompting comparisons with
methanol and ethambutol optic neuropathy and Leber hereditary optic neuropathy (LHON). The most likely mechanism which links possible toxic, nutritional, and genetic aetiologies of this
clinical entity is decompensation of aerobic respiration in the mitochondria of ganglion cells which serve the central visual field. Carbon monoxide, cyanide ions (both components of
cigarette smoke), and methanoic acid (a metabolite of methanol) exert a direct effect on the cytochrome enzymes. The antibiotic linezolid probably interferes with oxidative phosphorylation
indirectly via inhibition of mitochondrial ribosomes [35]. Ethambutol probably exerts an indirect excitotoxic effect on oxidative phosphorylation by increasing intracellular calcium via the
NMDA glutamate receptor [43]. Thiamine, riboflavin, niacin, pyridoxine, vitamin B12, folate, and copper are all required for mitochondrial metabolism [84]. The G3460A, G11778A and T14484C
mutations of mitochondrial DNA responsible for >90% of cases of LHON all disrupt the function of Complex 1 of the electron transport chain [85]. The OPA1 gene encodes a GTPase which is
involved in the control of mitochondrial fusion and fission and mutations in this gene have been documented to cause autosomal dominant optic atrophy [86]. In addition to measurement of
visual acuity, colour vision, and visual fields, imaging and visual electrophysiology can be helpful. SD-OCT may show RNFL thinning which is not necessarily limited to the temporal quadrant
[87]. The a-wave and b-wave of the dark adapted and light-adapted full-field ERG are likely to be normal, but the amplitude of the photopic negative response (PhNR) of the full-field ERG
which reflects ganglion cell function is likely to be reduced, as is also the case in LHON [88]. The pattern ERG (PERG) is a macular-dominated response and usually shows preservation of the
P50 component with selective reduction of the N95 component, reflecting abnormal ganglion cell function [89]. The PVEP to a reversing checkerboard stimulus typically shows predominantly a
reduction in amplitude of the P100 component, although delays in latency may also be seen [89]. When the visual acuity deteriorates to 6/60 or worse, the PVEP even to large (60’) checks may
be unrecordable. An individual presenting with toxic-nutritional optic neuropathy may have more than one risk factor for ganglion cell mitochondrial decompensation, for example, a diet
deficient in B-complex vitamins and heavy tobacco consumption. These can potentially be addressed to prevent further deterioration of vision. However, there may also be underlying genetic
factors that influence the level of individual vulnerability to optic neuropathy. Electrophysiological assessment of patient with suspected toxic-nutritional optic neuropathy should include
PVEP (and flash VEP where the PVEP is unrecordable), PERG and full-field ERG, ideally including measurement of the photopic negative response. MICRONUTRIENT DEFICIENCIES Many micronutrients
are essential to maintain the functioning of the visual system and prolonged deficiencies may cause vision loss and affect the structural integrity of the eye. The prevalence of
micronutrient deficiencies may be increasing, due to the rise in malabsorption syndromes secondary to inflammatory bowel diseases and gastric bariatric surgery, the popularity of strict
vegan and vegetarian diets, high rates of alcohol dependency, and avoidant restrictive food intake disorder (ARFID). Although ARFID is a relatively new diagnosis, emerging evidence suggests
that ARFID in Autistic Spectrum Disorder (ASD) is a significant and rising cause of micronutrient deficiency in paediatric populations [90, 91]. Patients with ARFID may avoid particular
foods due to associations with a specific sensory aspect such as colour or texture, fear of contamination, or past traumatic events related to food [92]. Individuals with restricted diets
often have multiple vitamin and trace element deficiencies, including vitamin A, B9, B12, C, D, E, zinc, iron, and copper, of which the mechanisms of interaction are not fully understood.
Although there are no population studies of ARFID, Hamilton et al. [93] reported 3 cases of vision loss secondary to highly restrictive dietary intake in patients with ASD. The causes of
reduced vision were retinal dysfunction, optic neuropathy, dysfunction, or a combination of these, and visual loss was severe in 2 cases. All patients were deficient in vitamin A. A review
of a larger series of cases is in progress and vitamin A deficiency was prominent feature of most of these (Ruth Hamilton, personal communication). Vitamin A deficiency (VAD) retinopathy is
the most extensively reported nutritional retinopathy. Vitamin A is essential for rhodopsin and cone opsin formation and the maintenance of corneal and conjunctival epithelial cell function.
Patients with VAD can present with nyctalopia, xerophthalmia, ocular surface keratinisation (Bitot spots), and retinal white spots, although ocular examination may show no abnormality [94,
95]. The ERG in VAD shows characteristic abnormalities. The response to a rod-specific stimulus is absent or markedly reduced and the bright-flash dark- adapted ERG shows a contribution from
dark-adapted cones, with loss of the rod system component. The light-adapted transient and 30 Hz flicker ERG are relatively preserved but reduced in amplitude [96]. The ERG to a red flash
under dark-adapted conditions is particularly useful to identify selective rod dysfunction in these patients and can be used to verify the cone system origin of residual scotopic bright
flash ERG response [97] (Fig. 2). The dark-adapted ERG responses typically show recovery following prolonged dark adaptation. Dark adaptometry confirms delayed recovery from bleach,
particularly, though not exclusively for the rod component of the dark adaptation curve [98]. Additional changes in s-cone function, mfERG, microperimetry, and pERG have been described in
VAD patients [95, 96]. Identifying characteristic rod dysfunction through visual electrophysiology enables early detection and treatment for VAD retinopathy and associated micronutrient
deficiencies, which is important as vision loss is often reversible through early and adequate supplementation. ERG and dark adaptometry studies have demonstrated the recovery of rod
function and are valuable as a monitoring tool once treatment is initiated [95, 98]. The full impact of nutritional deficiencies on the visual system is still unknown. Severe combined
micronutrient deficiency as reported in children with ARFID can have a major and lasting impact on visual function. The high prevalence of VAD in these children suggests that behavioural or
electroretinographic evidence of vitamin A deficiency should be regarded as a ‘red flag’ for specialist referral, even if the vision is otherwise good. Electrophysiological assessment of
patients with suspected VAD or severe combined micronutrient deficiency, even where there is no clinical evidence of impaired night vision, should include a full-field ERG, including
dark-adapted responses to a red flash and, where possible, a full-field ERG following prolonged dark adaptation. Where visual acuity is reduced, the assessment should also include PERG, PVEP
(and, where the PVEP is unrecordable, a flash VEP). SUMMARY The referral of a patient with visual loss associated with possible exposure to a toxic substance or a severe deficiency can be
challenging for clinicians, especially when symptoms are acute, associated with a prescribed medicine, or are related to self-harm. If symptoms have arisen more gradually, the nature and
extent of exposure to a suspected toxin may be difficult to ascertain. Where the suspected toxin is a prescribed medicine, a decision to reduce the dose or discontinue therapy may not be
straightforward because it may precipitate a relapse of the condition for which it was prescribed, and alternative therapies may be less well tolerated. Under these circumstances, it is
desirable to establish a link between the suspected toxin or deficiency state and the visual symptoms with as high a degree of certainty as possible. However, this process may be complicated
by interactions between potential toxins, deficiency states, and individual genetic variation, or by a lack of detailed information about the circumstances of exposure (for instance, where
illicit substances may have been involved). Visual electrophysiology testing can play an important role in providing evidence to establish a causal connection, particularly where structural
changes have not yet evolved, where subjective tests of visual function are unreliable, or when investigating the effects of prenatal exposure to potential toxins on postnatal visual
development. It may also have an important role in monitoring a response to intervention or supporting clinical decision-making where withdrawal of a prescribed medication is undesirable or
has been declined by the patient. Selection of an appropriate range of electrophysiological tests allows the function of the visual pathway from the photoreceptors and RPE to the primary
visual cortex to be probed, and regional disturbances of retinal function to be elicited (Table 1). This paper has reviewed a number of clinically important toxicity or deficiency states
involving the visual system where there is evidence that visual electrophysiological testing can assist the clinician. New hazards and side-effects of new treatments which affect the visual
system are likely to emerge in the future and visual electrophysiology will continue to play a part in establishing causal connections with presenting symptoms, identifying the mechanism of
damage to the visual system, and monitoring the response to intervention. REFERENCES * Dettoraki M, Moschos MM. 2016. The role of multifocal electroretinography in the assessment of
drug-induced retinopathy: a review of the literature. Ophthalmic Res. 2016;56:169–77. Article CAS PubMed Google Scholar * Plantone D, Koudriavtseva T. Current and future use of
chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig. 2018;38:653–71. Article CAS PubMed Google Scholar *
Lloyd LA, Hilz JW. Ocular complications of chloroquine therapy. Canad Med Ass J. 1965;92:508–13. CAS PubMed PubMed Central Google Scholar * Costedoat-Chalumeau N, Dunogué B, Leroux G,
Morel N, Jallouli M, Le Guern V, et al. A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol. 2015;49:317–26. Article CAS PubMed
Google Scholar * Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453–60. Article PubMed Google Scholar
* Reichel C, Berlin A, Radun V, Tarau IS, Hillenkamp J, Kleefeldt N, et al. Quantitative fundus autofluorescence in systemic chloroquine/hydroxychloroquine therapy. Transl Vis Sci Technol.
2020;28:42. Article Google Scholar * Scarinci F, Shaarawy A, Narala R, Jampol LM, Fawzi AA. Loss of external limiting membrane integrity predicts progression of hydroxychloroquine retinal
toxicity after drug discontinuation. Retina. 2016;36:1951–7. Article CAS PubMed Google Scholar * Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, et al. ISCEV standard for
clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol. 2012;124:1–13. Article PubMed Google Scholar * Lyons JS, Severns ML. Detection of early hydroxychloroquine
retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143:801–9. Article CAS PubMed Google Scholar * Marmor MF, Hu J. Effect of
disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132:1105–12. Article PubMed Google Scholar * Yusuf IH, Foot B, Lotery AJ. The Royal College of
Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary. Eye(Lond). 2021,
https://doi.org/10.1038/s41433-020-01380-2. * Gobbett A, Kotagiri A, Bracewell C, Smith J. Two years’ experience of screening for hydroxychloroquine retinopathy. Eye. 2021;35:1171–7. Article
CAS PubMed Google Scholar * Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the
safety and efficacy of vigabatrin for treating cocaine dependence. JAMA psychiatry. 2013;70:630–7. Article CAS PubMed Google Scholar * Schechter PJ, Tranier Y, Jung MJ, Böhlen P.
Audiogenic seizure protection by elevated brain GABA concentration in mice: Effects of γ-acetylenic GABA and γ-vinyl GABA, two irreversible GABA-T inhibitors. Eur J Pharm. 1977;45:319–28.
Article CAS Google Scholar * Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ. Characteristic retinal atrophy with secondary “inverse” optic atrophy identifies vigabatrin
toxicity in children. Ophthalmology. 2004;111:1935–42. Article PubMed Google Scholar * Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin.
BMJ. 1997;314:180. Article CAS PubMed PubMed Central Google Scholar * Besch D, Kurtenbach A, Apfelstedt-Sylla E, Sadowski B, Dennig D, Asenbauer C, et al. Visual field constriction and
electrophysiological changes associated with vigabatrin. Doc Ophthalmol. 2002;104:151–70. Article PubMed Google Scholar * Jammoul F, Wang Q, Nabbout R, Coriat C, Duboc A, Simonutti M, et
al. Taurine deficiency is a cause of vigabatrin‐induced retinal phototoxicity. Ann Neurol. 2009;65:98–107. Article CAS PubMed PubMed Central Google Scholar * Ravindran J, Blumbergs P,
Crompton J, Pietris G, Waddy H. Visual field loss associated with vigabatrin: pathological correlations. J Neurol Neurosurg Psychiatry. 2001;70:787–9. Article CAS PubMed PubMed Central
Google Scholar * Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–8. Article
CAS PubMed Google Scholar * Hardus PL, Verduin WM, Postma G, Stilma JS, Berendschot TT, Van Veelen CW. Long term changes in the visual fields of patients with temporal lobe epilepsy using
vigabatrin. Br J Ophthalmol. 2000;84:788–90. Article CAS PubMed PubMed Central Google Scholar * Hawker MJ, Astbury NJ. The ocular side effects of vigabatrin (Sabril): information and
guidance for screening. Eye(Lond). 2008;22:1097–8. CAS Google Scholar * Maguire MJ, Hemming K, Wild JM, Hutton JL, Marson AG. Prevalence of visual field loss following exposure to
vigabatrin therapy: a systematic review. Epilepsia. 2010;51:2423–31. Article PubMed Google Scholar * Lagogianni C, Gatzonis S, Patrikelis P. Fatigue and cognitive functions in epilepsy: a
review of the literature. Epilepsy Behav. 2020;114:107541. Article PubMed Google Scholar * Harding GF, Wild JM, Robertson KA, Rietbrock S, Martinez C. Separating the retinal
electrophysiologic effects of vigabatrin: treatment versus field loss. Neurology. 2000;55:347–52. Article CAS PubMed Google Scholar * Ji X, Wright T, Vanden, Hoven C, MacKeen L,
McFarlane M, et al. Reliability of handheld optical coherence tomography in children younger than three years of age undergoing vigabatrin treatment for childhood epilepsy. Transl Vis Sci
Technol. 2020;9:9. Article PubMed PubMed Central Google Scholar * Harding GF, Spencer EL, Wild JM, Conway M, Bohn RL. Field-specific visual-evoked potentials: identifying field defects
in vigabatrin-treated children. Neurology. 2002;58:261–5. Article Google Scholar * Harding GF, Robertson K, Spencer EL, Holliday I. Vigabatrin; its effect on the electrophysiology of
vision. Doc Ophthalmol. 2002;104:213–29. Article CAS PubMed Google Scholar * Westall CA, Logan WJ, Smith K, Buncic JR, Panton CM, Abdolell M. The hospital for sick children, Toronto,
longitudinal ERG study of children on vigabatrin. Doc Ophthalmol. 2002;104:133–49. Article PubMed PubMed Central Google Scholar * Dracopoulos A, Westall C. Reduction of the photopic
negative response (PhNR) in children with childhood epilepsy on vigabatrin therapy. Abstracts of the 7th Annual OSA Fall Vision Meeting. J Vis. 2007;7:58. Article Google Scholar * Carr RE,
Henkind P. Ocular manifestations of ethambutol, Toxic amblyopia after administration of an experimental antituberculous drug. Arch Ophthalmol. 1962;67:566–71. Article CAS PubMed Google
Scholar * Chan RY, Kwok AK. Ocular toxicity of ethambutol. Hong Kong Med J. 2006;12:56–60. CAS PubMed Google Scholar * Addy LK, Harrison WW. Case report: long-term structural and
functional effects of ethambutol optic neuropathy. Optom Vis Sci. 2020;97:555–60. Article PubMed Google Scholar * Libershteyn Y. Ethambutol/linezolid toxic optic neuropathy. Optom Vis
Sci. 2016;93:211–7. Article PubMed Google Scholar * Kulkarni HS, Keskar VS, Bavdekar SB, Gabhale Y. Bilateral optic neuritis due to isoniazid (INH). Indian Pediatr. 2010;47:33–5. Article
Google Scholar * Lee EJ, Kim SJ, Choung HK, Kim JH, Yu YS. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. J Neuroophthalmol. 2008;28:269–77. Article
PubMed Google Scholar * Melamud A, Kosmorsky GS, Lee MS. Ocular ethambutol toxicity. Mayo Clin Proc. 2003;78:1409–11. Article PubMed Google Scholar * Lim SA. Ethambutol-associated optic
neuropathy. Ann Acad Med Singap. 2006;35:274–8. PubMed Google Scholar * Mehta S. Patterns of ethambutol ocular toxicity in extended use therapy. Cureus. 2019;11:e4408. PubMed PubMed
Central Google Scholar * Pavan Taffner BM, Mattos FB, Cunha MCd, Saraiva FP. The use of optical coherence tomography for the detection of ocular toxicity by ethambutol. PLoS ONE. 2018,
https://doi.org/10.1371/journal.pone.0204655. * Menon V, Jain D, Saxena R, Sood R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol.
2009;93:1251–4. Article CAS PubMed Google Scholar * Behbehani RS, Affel EL, Sergott RC, Savino PJ. Multifocal ERG in ethambutol associated visual loss. Br J Ophthalmol. 2005;89:976–82.
Article CAS PubMed PubMed Central Google Scholar * Heng JE, Vorwerk CK, Lessell E, Zurakowski D, Levin LA, Dreyer EB. Ethambutol is toxic to retinal ganglion cells via an excitotoxic
pathway. Invest Ophthalmol Vis Sci. 1999;40:190–6. CAS PubMed Google Scholar * Zubair M, Tahir M, Sheikh TH, Lone KP, Samee W, Munir B. Prevention of ethambutol induced changes by
memantine in optic nerve of rabbit. Biomedica. 2009;25:19–23. Google Scholar * Kim KL, Park SP. Visual function test for early detection of ethambutol induced ocular toxicity at the
subclinical level. Cutan Ocul Toxicol. 2016;35:228–32. Article CAS PubMed Google Scholar * AlKadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53:385–91. Article CAS
PubMed Google Scholar * Meshi A, Belkin A, Koval T, Kornhouser T, Assia EI, Rotenstreich Y. An experimental treatment of ocular quinine toxicity with high-dose 9-cis Beta-carotene. Retin
Cases Brief Rep. 2015;9:157–61. Article PubMed Google Scholar * Bacon P, Spalton DJ, Smith SE. Blindness from quinine toxicity. Br J Ophthalmol. 1988;72:219–24. Article CAS PubMed
PubMed Central Google Scholar * Su D, Robson AG, Xu D, Lightman S, Sarraf D. Quinine toxicity: multimodal retinal imaging and electroretinography findings. Retin Cases Brief Rep.
2017;11(Suppl):S102–S106. Article PubMed Google Scholar * Georgiou A, Neveu M, Calcagni A, Robson A. Characterisation of retinal dysfunction associated with quinine toxicity in 20
patients. Abstracts of the 58th Annual Symposium of the International Society for Clinical Electrophysiology of Vision (ISCEV). Doc Ophthalmol. 2020;141:1–37. S24. Google Scholar * Zahn JR,
Brinton GF, Norton E. Ocular quinine toxicity followed by electroretinogram, electro-oculogram, and pattern visually evoked potential. Am J Optom Physiol Opt. 1981;58:492–8. Article CAS
PubMed Google Scholar * Verdon W. Clinical electrophysiology in quinine induced retinal toxicity. Optom Vis Sci. 2008;85:17–26. Article PubMed Google Scholar * Freund PR, Wright T,
Margolin EA. Toxic optic neuropathy from quinine overdose. J Neuroophthalmol. 2020;40:258–61. Article PubMed Google Scholar * Haimovici R, D’Amico D, Gragoudas E, Sokol S. The expanded
clinical spectrum of deferoxamine retinopathy. Ophthalmology. 2002;109:164–71. Article PubMed Google Scholar * Lakhanpal V, Schocket S, Jiji R. Deferoxamine (Desferal®)-induced toxic
retinal pigmentary degeneration and presumed optic neuropathy. Ophthalmology. 1984;91:443–51. Article CAS PubMed Google Scholar * Georgakopoulos C, Tsapardoni F, Kostopoulou E, Makri O.
Pattern dystrophies in patients treated with deferoxamine: report of two cases and review of the literature. BMC Ophthal. 2018;18:246. * Di Nicola M, Barteselli G, Dell’Arti L, Ratiglia R,
Viola F Functional and Structural Abnormalities in Deferoxamine Retinopathy: A Review of the Literature. BioMed Res Int. 2015, https://doi.org/10.1155/2015/249617. * Viola F, Barteselli G,
Dell’Arti L, Vezzola D, Mapelli C, Villani E, et al. Multimodal imaging in deferoxamine retinopathy. Retina. 2014;34:1428–38. Article PubMed Google Scholar * Simon S, Athanasiov P,
Gilhotra J, Jain R, Raymond G. Desferrioxamine-related ocular toxicity: a case report. Indian J Ophthalmol. 2012;60:315. Article PubMed PubMed Central Google Scholar * Dettoraki M,
Kattamis A, Ladas I, Maragkos K, Koutsandrea C, Chatzistefanou K, et al. Electrophysiological assessment for early detection of retinal dysfunction in β-thalassemia major patients. Graefes
Arch Clin Exp Ophthalmol. 2017;255:1349–58. Article CAS PubMed Google Scholar * Baath J, Lam W, Kirby M, Chun A. Deferoxamine-related ocular toxicity: incidence and outcome in a
pediatric population. Retina. 2008;28:894–9. Article PubMed Google Scholar * Rahi A, Hungerford J, Ahmed A. Ocular toxicity of desferrioxamine: light microscopic histochemical and
ultrastructural findings. Br J Ophthalmol. 1986;70:373–81. Article CAS PubMed PubMed Central Google Scholar * Thuangtong A. Incidence of ocular toxicity from iron chelating agents at
Siriraj Hospital. Siriraj Med J. 2020;72:209–13. Article Google Scholar * Olivieri N, Brittenham G. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–61. Article
CAS PubMed Google Scholar * Pan Y, Keane P, Sadun A, Fawzi A. Optical coherence tomography findings in deferasirox-related maculopathy. Retin Cases Brief Rep. 2010;4:229–32. Article
PubMed Google Scholar * Casini G, Sartini F, Loiudice P, Benini G, Menchini M Ocular siderosis: a misdiagnosed cause of visual loss due to ferrous intraocular foreign bodies-epidemiology,
pathogenesis, clinical signs, imaging and available treatment options. Doc Ophthalmol. 2020, https://doi.org/10.1007/s10633-020-09792-x. * O’Duffy D, Salmon JF. Siderosis bulbi resulting
from an intralenticular foreign body. Am J Ophthalmol. 1999;127:218–9. Article PubMed Google Scholar * Eagling EM, Roper-Hall MJ. Eye injuries, an illustrated guide. (Gower Medical
Publishing Ltd, London, UK, 1986) 9–10. * Hope-Ross M, Mahon GJ, Johnston PB. Ocular siderosis. Eye (Lond). 1993;7:419–25. Article Google Scholar * Schechner R, Miller B, Merksamer E,
Perlman I. A long term follow up of ocular siderosis: quantitative assessment of the electroretinogram. Doc Ophthalmol. 1990;76:231–40. Article PubMed Google Scholar * Imaizumi M,
Matsumoto CS, Yamada K, Nanba Y, Takaki Y, Nakatsuka K. Electroretinographic assessment of early changes in ocular siderosis. Ophthalmologica. 2000;214:354–9. Article CAS PubMed Google
Scholar * Hwang YS, Lin KK, Chen KJ, Lai CC. Iron foreign body in the optic nerve without ocular siderosis. J Neuroimaging. 2010;20:201–3. Article PubMed Google Scholar * Urban P,
Zakharov S, Diblík P, Pelclová D, Ridzoň P. Visual evoked potentials in patients after methanol poisoning. Int J Occup Med Environ Health. 2016;29:471–8. Article PubMed Google Scholar *
Hayreh MS, Hayreh SS, Baumbach GL, Cancilla P, Martin-Amat G, Tephly TR, et al. Methyl alcohol poisoning III. Ocular toxicity. Arch Ophthalmol. 1977;95:1851–8. Article CAS PubMed Google
Scholar * Scrimgeour EM, Dethlefs RF, Kevau I. Delayed recovery of vision after blindness caused by methanol poisoning. Med J Aust. 1982;13:481–3. Article Google Scholar * Sharma M, Volpe
NJ, Dreyer EB. Methanol-induced optic nerve cupping. Arch Ophthalmol. 1999;117:286. Article CAS PubMed Google Scholar * Galvez-Ruiz A, Elkhamary SM, Asghar N, Bosley TM. Cupping of the
optic disk after methanol poisoning. Br J Ophthalmol. 2015;99:1220–3. Article PubMed Google Scholar * Hantson P, de Tourtchaninoff M, Simoens G, Mahieu P, Boschi A, Beguin C, et al.
Evoked potentials investigation of visual dysfunction after methanol poisoning. Crit Care Med. 1999;27:2707–15. Article CAS PubMed Google Scholar * Potts AM, Praglin J, Farkas I, Orbison
L, Chickering D. Studies on the visual toxicity of methanol. VIII. Additional observations on methanol poisoning in the primate test object. Am J Ophthalmol. 1955;40:76–83. Article CAS
PubMed Google Scholar * McKellar MJ, Hidajat RR, Elder MJ. Acute ocular methanol toxicity: clinical and electrophysiological features. Aust N Z J Ophthalmol. 1997;25:225–30. Article CAS
PubMed Google Scholar * Ruedemann AD. Toxic amblyopia. Clevel Clin J Med. 1939;6:241–7. Article Google Scholar * Roman GC. An epidemic in Cuba of optic neuropathy, sensorineural
deafness, peripheral sensory neuropathy and dorsolateral myeloneuropathy. J Neurol Sci. 1994;127:11–28. Article CAS PubMed Google Scholar * Monferrer-Adsuara C, García-Villanueva C,
Mata-Moret L, Ortiz-Salvador M, Remolí-Sargues L, Cervera-Taulet E. Case report: nutritional and toxic optic neuropathy: a diagnostic dilemma. Optom Vis Sci. 2020;97:477–81. Article PubMed
Google Scholar * Atan D. Challenges and opportunities in the diagnosis of nutritional optic neuropathy. Expert Rev Ophthalmol. 2020;15:67–70. Article CAS Google Scholar * Kirches E.
LHON: mitochondrial mutations and more. Curr Genomics. 2011;12:44–54. Article CAS PubMed PubMed Central Google Scholar * Maeda-Katahira A, Nakamura N, Hayashi T, et al. Autosomal
dominant optic atrophy with OPA1 gene mutations accompanied by auditory neuropathy and other systemic complications in a Japanese cohort. Mol Vis. 2019;25:559–73. PubMed PubMed Central
Google Scholar * Vieira LM, Silva NF, Dias dos Santos AM, dos Anjos RS, Pinto LA, Vicente AR, et al. Retinal ganglion cell layer analysis by optical coherence Tomography in toxic and
nutritional optic neuropathy. J Neuroophthalmol. 2015;35:242–5. Article PubMed Google Scholar * Karanjia R, Berezovsky A, Sacai PY, Cavascan NN, Liu HY, Nazarali S, et al. The photopic
negative response: an objective measure of retinal Ganglion Cell function in patients with leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2017;58:BIO300–BIO306. Article
PubMed Google Scholar * Holder GE. Electrophysiological assessment of optic nerve disease. Eye (Lond). 2004;18:1133–43. Article CAS Google Scholar * American Psychiatric Association
(APA). Diagnostic and statistical manual of mental disorders (DSM-5®). 5th edn. (American Psychiatric Publishing, Arlington VA, 2013). * Spicer L, Strudwick K, Kelly V. GP82 Prevalance rates
for avoidant restrictive food intake disorder (ARFID) in tertitary feeding clinic in UK. Arch Dis Child. 2019;104(Suppl):3. Google Scholar * Coglan L, Otasowie J. Avoidant/restrictive food
intake disorder: what do we know so far? BJPsych Adv. 2019;25:90–98. Article Google Scholar * Hamilton R, Mansfield D, Ali S, Millar E, Mulla U. Three cases of ‘fussy-eating’ induced
vision loss. Abstr 13th Annu Meet Br Soc Clin Electrophysiol Vis (BRISCEV). 2015;13:11. https://briscev.org.uk/index.php/notice-board/meetings. Google Scholar * World Health Organization
(WHO). Xerophthalmia and night blindness for the assessment of clinical vitamin A deficiency in individuals and populations. WHO/NMH/NHD/EPG/14.4 (2014),
https://www.who.int/nutrition/publications/micronutrients/indicators_xerophthalmia_vad/en/. * McBain VA, Egan CA, Pieris SJ, Supramaniam G, Webster AR, Bird AC, et al. Functional
observations in vitamin A deficiency: diagnosis and time course of recovery. Eye (Lond). 2007;21:367–76. Article CAS Google Scholar * Saker S, Morales M, Jhittay H, Wen Y, Amoaku W.
Electrophysiological and microperimetry changes in vitamin A deficiency retinopathy. Doc Ophthalmol. 2015;130:231–40. Article PubMed Google Scholar * Thompson DA, Fujinami K, Perlman I,
Hamilton R, Robson AG. ISCEV extended protocol for the dark-adapted red flash ERG. Doc Ophthalmol. 2018;136:191–7. Article PubMed PubMed Central Google Scholar * Kemp CM, Jacobson SG,
Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988;46:185–97. Article CAS PubMed Google Scholar Download references AUTHOR
INFORMATION Author notes * These authors contributed equally: Emily K. O’Neill, Richard Smith. AUTHORS AND AFFILIATIONS * Clinical and Academic Department of Ophthalmology, Great Ormond
Street Hospital for Children, London, UK Emily K. O’Neill * Eye Department, Stoke Mandeville Hospital, Aylesbury, Buckinghamshire, UK Richard Smith Authors * Emily K. O’Neill View author
publications You can also search for this author inPubMed Google Scholar * Richard Smith View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
The authors contributed equally to this work. EO was responsible for designing and conducting literature searches, screening potential eligible studies, creating a summary table, selecting
figures, and writing the report. RS contributed to designing the review, adapting the summary table and writing the report, including the introduction and summary sections. CORRESPONDING
AUTHORS Correspondence to Emily K. O’Neill or Richard Smith. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE O’Neill, E.K., Smith, R. Visual electrophysiology in the assessment
of toxicity and deficiency states affecting the visual system. _Eye_ 35, 2344–2353 (2021). https://doi.org/10.1038/s41433-021-01663-2 Download citation * Received: 07 March 2021 * Revised:
17 June 2021 * Accepted: 21 June 2021 * Published: 21 July 2021 * Issue Date: September 2021 * DOI: https://doi.org/10.1038/s41433-021-01663-2 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative