Play all audios:
ABSTRACT BACKGROUND/OBJECTIVES To measure the endothelial cell density (ECD) of the in toto pre-stripped endothelial Descemet membrane lamellae (EDML) and to describe the impact of pre- and
intraoperative endothelial cell loss (ECL) on postoperative midterm clinical outcome. SUBJECTS/METHODS The ECD of 56 Corneoscleral Donor Discs (CDD) was first measured with an inverted
specular microscope (t0pre). The measurement was then repeated non-invasively after the preparation of the EDML (t0post). DMEK was performed the next day using these grafts. Follow-up
examinations took place 6 weeks, 6 months and 1 year postoperatively where the ECD was assessed. In addition, the impact of ECL 1 (during preparation) and ECL 2 (during surgery) on the ECD,
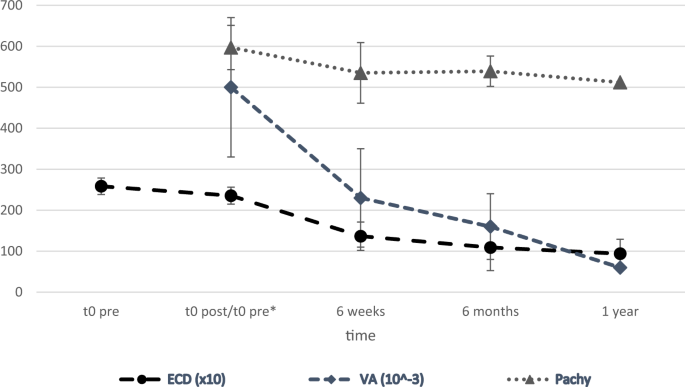
visual acuity (VA) and pachymetry at 6 months and 1 year was investigated. RESULTS The average ECD (in cells/mm²) at time points t0pre, t0post, 6 weeks, 6 months & 1 year was 2584 ± 200,
2355 ± 207, 1366 ± 345, 1091 ± 564 and 939 ± 352. The average logMAR VA and pachymetry (in µm) was 0.50 ± 0.27 and 597 ± 63, 0.23 ± 0.17 and 535 ± 54, 0.16 ± 0.12 and 535 ± 54, 0.06 ± 0.08
and 512 ± 37, respectively The ECL 1 (9% on average) had no significant impact on the main outcome measures after 6 months and 1 year (p > 0.11). The ECL 2 correlated significantly with
the ECD and the pachymetry at 1 year postop (_p_ < 0.02). CONCLUSION Our results indicate that the non-invasive ECD measurement of the prestripped EDML roll before its transplantation is
feasible. Despite significantly decreasing ECD up to 6 months postoperatively, visual acuity further improved and thickness further decreased up to 1 year postoperatively. You have full
access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS THINNING RATE OVER 24 MONTHS IN ULTRATHIN DSAEK Article 15 March 2022 THE PREDICTABILITY OF
GRAFT THICKNESS FOR DESCEMET’S STRIPPING AUTOMATED ENDOTHELIAL KERATOPLASTY USING A MECHANICAL MICROKERATOME SYSTEM Article Open access 23 December 2022 OPTIMIZED LABORATORY TECHNIQUES FOR
ASSESSING THE QUALITY OF PRE-STRIPPED DMEK GRAFTS Article Open access 04 March 2025 INTRODUCTION From its introduction in 2006 [1] up to now, Descemet-Membrane Endothelial Keratoplasty
(DMEK) has become the gold standard therapy for corneal endothelial diseases [2]. This is due to its good visual outcomes and low post-operative complication rates in comparison to
penetrating keratoplasty (PKP) and is since reflected in its increasing frequency in Germany [3]. In the literature the reported Endothelial Cell Loss (ECL) after DMEK varied a lot between
different studies [4,5,6]. In order to reduce the ECL to a minimum, an accomplished, minimally invasive preparation method of the endothelial Descemet membrane lamellae (EDML) is necessary
[7]. The measurement of the ECL caused by the preparation of the EDML could be an additional screening method to monitor the quality of the grafts preoperatively. Unfortunately, already
existing measurement methods could not provide precise results due to two potential problems: (1) The measurement of the ECD after preparation was invasive because of unrolling and fixating
the EDML in order to count endothelial cells. This may lead to an overestimation of the ECL [7,8,9,10,11]. (2) The measurement protocol was not applied after a simulation of the entire
preparation protocol and, therefore, led to an underestimation of ECL [12,13,14]. A reproducible, non-invasive measurement of ECL was introduced in 2020 [15]. Safi et al. analysed 30 rolled
endothelial Descemet membrane lamellae (EDML) directly after their preparation and counted their endothelial cell density (ECD) by means of inverted light microscopy. In contrast to former
methods, the performed measurements were non-invasive and took place after the complete preparation procedure with no further manipulation of the EDML. For reproducibility and accuracy
purposes, each measurement was repeated 5 times, each time a minimum of 3 pictures were taken (two in the periphery, one in the centre of the EDML). This new, non-invasive measurement
protocol showed a high reproducibility (standardised Cronbach’s alpha of 0,924) and satisfying results in terms of ECL [15]. In order to provide a better understanding of the impact of the
ECL caused by the preparation and due to the transplantation itself on the midterm clinical outcome, the purpose of this study was to correlate this pre- and intraoperative ECL with ECD, VA
and pachymetry at 6 months and 1 year after DMEK. MATERIALS & METHODS STUDY SET-UP Since 2013, more than 1100 DMEKs have been performed by 5 microsurgeons in the Department of
Ophthalmology, University Saarland Medical Center in Homburg/Saar (UKS) [16]. For this retrospective study, 56 Corneoscleral Donor Discs (CDD) were dissected from 43 donors with a mean age
of 75 ± 10 (range 54–95) years and transplanted into 56 eyes of 55 DMEK recipients from July 2019 to February 2021. Since, we are not performing non-invasive specular microscopy of the donor
roll on a routine basis, we originally planned on having 70 consecutive grafts for the present study, what seemed to be enough for a clinically significant statement. However, 14 patients
were lost to follow-up for unknown reasons. The indication for all the DMEK operations performed in this study was Fuchs endothelial corneal dystrophy. For the presented study, donor corneas
were provided in house by the _Klaus Faber Center for Corneal Diseases, incl. LIONS-Eye Bank Saar-Lor-Lux, Trier/Westpfalz_. The study followed the tenets of the 1964 Declaration of
Helsinki and was approved by the Ethics Commission of the German Medical Association (Identification-Number: BU217/20).N All the transplanted grafts had passed a strict quality control in
the eye bank including negative serology, negative swap for bacteria and a sufficient ECD > 2200 cells/mm2. Therefore, they fulfilled the criteria of a typical DMEK operation. The CDD
were originally stored in organ culture medium I (Biochrom AG, Berlin, Germany) without dextran. ECD measurements were performed on the CDD before the preparation of the EDML (t0pre) and
then after the preparation on the rolled EDML (t0post). DMEK follow-up examinations at 6-weeks, 6-months and 1-year included: ECD, visual acuity (VA), intraocular pressure (IOP) and
pachymetry. TISSUE PREPARATION Following the inhouse guidelines of Seitz et al. [16, 17], an experienced surgeon performed the preparation of the EDML from the CDD in the operating theatre
one day before DMEK surgery. Briefly, the first step included the fixation of the CDD epithelium-down on a suction block (‘Hanna trephination system’, Moria Surgical, Antony, France) and
then staining it for 30 s with Blue Color Caps (BCC) (Croma GmbH, Leobendorf, Austria) to optimise visualisation. After carefully rinsing the BCC with organ culture medium I, a circular mark
of 7.5 mm diameter was applied on the endothelium using the ‘Moria descemet’s stripping automated endothelial keratoplasty (DSAEK) trephination system’. Then, using a razor blade, 1–1.5 mm
incisions were made in a hexagonal shape outside the existing 7.5 mm mark. Afterwards, a slow detachment of the entire EDML from the periphery to the centre was attempted via a small, blunt
forceps. 120-degree rotations of the underlying suction block with repetition of the previous step helped to detach the EDML step by step until leaving only a small central part of the EDML
attached to the remaining stroma. After that, complete trephination of the EDML with the 7.5-mm trephine was applied resulting in a curvilinear edge. Then 3 asymmetric half-circle marks at
the edge of EDML according to Bachmann et al. [18] to avoid upside-down attachment of the graft in the patients’ eye later. The remaining small central part of EDML attached to the stroma
could now be completely and safely detached from the stroma and by means of another larger forceps carefully put into a well plate containing organ culture medium I without dextran, where
the EDML took a rolled configuration. One day after the EDML preparation, DMEK took place, in which the EDML was inserted into the anterior chamber by means of a Geuder glass cartridge. The
graft was then attached to the recipient’s stroma by sulphur hexafluoride (SF6 20%) gas injection [16]. MEASUREMENT OF ENDOTHELIAL CELL DENSITY (ECD) Before the preparation of the EDML, ECD
of the CDD was measured by an inverted light microscope (model 090-135.001; Leica Microsystems, Wetzlar, Germany). To ensure precise and comparable ECD measurements before and after the EDML
preparation, preoperatively, one image was taken in the centre of the CDD, and two were taken in the middle peripheral region (5–7 mm away from the centre). Around 5 h after preparation,
the rolled EDML with a diameter of 7.5 mm was placed under the same microscope to measure the ECD again. Without any further manipulation, three pictures were again taken, two in the
periphery (again 5–7 mm away from centre) and one in the centre of the EDML. A specialised software first automatically calculated ECD by using a 5.5 cm sided square (=116.599 micrometre of
the EDML) within which endothelial cells were counted. The automated cell counting was then manually corrected by an experienced medical technician to achieve the most accurate ECD. The
total ECD was achieved by taking the average of the three taken pictures. Around 20–40 cells are usually counted in one region of interest of an endothelial image. The reliability,
reproducibility and precision of this non-invasive counting method has been verified earlier [15]. This method to detect the ECD was used before as well as around 5 h after the preparation,
to allow for a certain degree of stabilisation of the endothelial cell layer after migration and redistribution of EC, in order to achieve a more valid ECD. Until their transplantation on
the next day, the EDML were stored in an incubator at 36 °C. The postoperative ECD measurements in all patients were performed using a clinical specular microscope _(Tomey specular
microscope EM-3000__©__; Tomey GmbH, Erlangen, Germany)_ at 6-weeks, 6-months and 1-year intervals. All measurements were performed by a well-trained, medical technician using the automatic
cell count modality but without manual correction. ADDITIONAL MAIN OUTCOME MEASURES Corneal pachymetry was performed preoperatively, 6-weeks, 6-months, and 1-year after DMEK with an anterior
segment optical coherence tomograph (AS-OCT) _(CASIA2; Tomey GmbH, Erlangen, Germany)_ and was measured in μm. Additionally, VA and IOP measurements took place preoperatively, 6-weeks,
6-months, and 1-year after DMEK and were retraced by means of digital patients’ records. VA results are shown in logMAR and IOP was measured by Goldmann applanation tonometry in mmHg.
STATISTICAL ANALYSIS Excel (Microsoft, Redmond, Washington) and SPSS software (IBM, Armonk, New York) were used for analysing all statistical data. All descriptive statistics were shown as
mean ± standard deviation (SD) and median. The evolution of ECD, pachymetry, VA and IOP over time were presented in a ‘Related-samples Friedman’s Two-Way Analysis of Variance by Ranks’ with
an alpha set to be 0.05. For each multivariate test, Pillai’s trace was significant. All significant values (_p_ < 0.05) have been adjusted by the Bonferroni correction for multiple
tests. The Spearman Rank correlation test was used for correlation of pre- and intraoperative ECL with clinical main outcome measures. RESULTS QUANTIFICATION OF ENDOTHELIAL CELL LOSS (ECL)
Preoperatively, the mean ECD at t0pre was 2584 ± 200 c/mm2. The ECD was decreasing significantly during follow-up (Table 1, Fig. 1). Using ECD values, the ECL at each step was calculated
(ECL 1: Difference in ECD between t0pre and t0post; ECL 2: Difference in ECD between t0post and 6-weeks after DMEK; ECL 3: Difference in ECD between 6-weeks and 6-months after DMEK; ECL 4:
Difference in ECD between 6-months and 1-year after DMEK). All results were statistically significant except for ECL 4 (Table 2). PACHYMETRY Pachymetry was assessed from t0pre to 1-year
after DMEK during follow-up. Preoperatively at t0pre the mean pachymetry was 597 ± 63 μm. Corneal thickness was decreasing significantly during follow-up (Table 1, Fig. 1). There was a
significant decrease in the corneal thickness (CT) at all time points (_p_ < 0.001) except between 6-weeks and 6-months after DMEK (_p_ = 0.74) (Fig. 1). VISUAL ACUITY (LOGMAR) Visual
acuity increased significantly during follow-up (Table 1, Fig. 1). The course of the VA over time showed a significant improvement with a _p_ < 0.001 when comparing the results of t0preop
and 6-weeks after DMEK, and _p_ = 0.003 when comparing 6-months and 1-year after DMEK. There was only a small insignificant improvement of the VA between 6-weeks and 6-months with _p_ =
0.23. INTRAOCULAR PRESSURE (IOP) A mean of 13 (±3) mmHg was measured at t0preop. IOP remained stable during each follow-up appointment. CORRELATIONS OF PRE- AND INTRAOPERATIVE ENDOTHELIAL
CELL LOSS WITH ECD, PACHYMETRY AND VA AT 6 MONTHS AND 1 YEAR POSTOPERATIVELY The ECL 1 due to preparation was 9% on average. This did not correlate with any of the postoperative clinical
main outcome measures (_p_ > 0.11). Intraoperative ECL 2 (42% on average) correlated significantly with ECD at 1 year postoperatively (_p_ = 0.027, Fig. 2) and with pachymetry at 1 year
postoperatively (_p_ = 0.019, Fig. 2). DISCUSSION The non-invasive method of counting the ECD on the EDML, introduced in 2020 by Safi et al. [15], can be used directly before DMEK in order
to exactly know the ECL caused by the EDML preparation itself. Our results demonstrated that measuring the ECD on the rolled EDML is feasible and revealed on average a 9% ECL attributed to
the preparation procedure itself. Despite a rather high ECL rate during the transplantation, pachymetry, VA and intraocular pressure showed favourable results. The intraoperative ECL was
correlated with ECD and pachymetry after 1 year of follow-up. Even though DMEK has become the gold standard therapy for treating corneal endothelial dysfunction in Germany [2, 3], a high ECL
postoperatively may finally result in graft failure. In a large literature review, it was shown that ECL varies between different studies from 19% to 44% 6 months postoperatively [19]. In
2020, Basak et al. [20] for instance presented 27.2% and 33.5% ECL after 6 and 12 months postoperatively. In addition, Birbal et al. (2020) [5] stated an ECL of 37% at 6 months and 40% at 1
year postoperatively. All these different studies have one common ground, which is a steep loss of endothelial cells (EC) early after the operation and a subsequent flattening of the ECL
curve. Overestimation of the ECD by the eye banks might be a potential explanation for this initial, large loss of EC. However, a high ECL during the preparation could also explain this
problem [21,22,23,24]. We assume that there are at least 2 causes of this rapid ECL. First, the manipulation and stripping of the tissue itself during the preparation may cause immediate
ECL. The second cause of this rapid ECL shortly (around 5 h) after the preparation might be a migration and redistribution of the endothelial cells. It is believed that corneal endothelial
cells close the wound gap mainly via migration and increased cell spreading (Inoda et al. 2020). In vitro, endothelial cell migration phenomenon from Quarter-DMEK grafts was proven and seem
to occur along the radial cut edges of the graft (Miron et al. 2018). Although we do not have enough information, this could be one of the reasons for the measured ECL after preparation. But
since the time period between stripping and repeat measurement is very short (around 5 h), this concept of explanation seems to be of minor impact. Another source of this high ECL might be
the 1 day storage after the preparation and before surgery took place. As mentioned in Safi et al. 2020, an average of 8% additional loss was detected on day 1 after preparation of the
tissue. Therefore, it might be advisable to plan tissue preparation and surgery on the same day when it is logistically doable. Again, further scientific research concerning this issue seems
to be necessary. Since there were only either invasive and overestimating methods for ECD measurement after the preparation [7,8,9,10,11], or methods which only involved a partial
preparation and therefore an underestimation of the ECL [12,13,14], it was necessary to find a non-invasive and reproducible method to count the ECD preoperatively on the rolled DMEK graft
[15]. Mayko et al. [12] for instance had a different and incomplete preparation technique, whereby after stripping the Descemet membrane, a peripheral hinge was maintained and not stripped,
and was used as an anchor for the EDML. The EDML was then spread again in its placed on the CDD (which was used as a support system), in contrast to our preparation method in which the EDML
was totally stripped from the CDD and placed in the culture medium as a roll simulating the exact preparation method used in a typical DMEK operation. On the other hand, in our study we used
an inverse light microscope to measure the ECD, whereas in the study performed by Mayko et al. [12], a clinical specular microscope was used. Due to the differences between the preparation
and measurement methods, different results may have been obtained. Giving another example of a study which contradicts our ECL findings due to tissue preparation, Böhm et al. [25] reported a
similar lack of change in ECD for preloaded DMEK rolls. Though again in their study a clinical specular microscope was used for quality evaluation after preparation. Whereas our
measurements were not only carried out by an inverse light microscope and automatically analysed by the software for ECD within the square, but also manually screened by a trained technician
to provide the most accurate results. In addition, Rickmann et al. [22] showed that there is a significant difference between inverse light microscopy and specular microscopy in terms of
ECD measurements, concluding that automated light microscopy showed a higher ECD of 31.85% compared to automated specular microscope, and manual light microscopy measurements showed 10.51%
higher ECD compared to specular microscopy. Therefore, both methods cannot be used interchangeably and the early postoperative rapid decrease of ECD might be partly artefactual. To our
understanding regarding the technical basic aspects, there is no fundamental difference between the functioning of inverted light microscopy (ILM) and specular microscopy (SM). Though the
main difference is that inverted light microscopy is suitable for corneas stored in organ culture, while specular microscopy is mostly suitable for corneas stored in hypothermic storage.
Therefore, using ILM it is possible to better differentiate between alive and dead endothelial cells by staining the cells for example with trypan blue. In opposite to SM, ILM does not tend
to overestimation of endothelial cell density by including dead endothelial cells, which cannot be identified as exact (Jirsova et al. 2017) [26], but allows a more precise measurement of
ECD, resulting in values, which reflect the actual number of EC closer to reality. Therefore, we used in our study an inverted light microscope. We are currently performing a study to
compare the accuracy of both types of microscopes and to better investigate their differences, the result of which are still pending. Our ECL values directly after tissue preparation are in
line with the results of Tran et al. [11] who showed an ECL of 9.3%, and the results of Krabcova et al. [27] showing around 5% ECL. More dramatic ECL values after tissue preparation were
also mentioned by Jardine et al. [9], Schallhorn et al. [10], and Downes et al. [28] with respective values of 22.5%, 27% and 29.2%. Despite the rather high level of intraoperative/early
postoperative ECL, all the other clinical outcome measures in the present study were very satisfying. Thus, the sole importance of a high level of ECD postoperatively is questionable. This
hypothesis is supported by the findings of Hammer et al. [29], who came to the conclusion that rather the functionality or quality of all the endothelial cells is crucial for better visual
outcomes than solely the quantity of endothelial cells. An additional source of inaccuracy between different studies regarding the ECL might be the usage of different models of specular
microscopes postoperatively, which are known to vary immensely between manufacturers [30]. Huang et al. [31] discourage to fully trust specular microscopes because of their inaccuracy in
terms of exact ECD counting in the eyes with high polymegethism and/or large cell size, which is unfortunately often the case after DMEK. This indicates that the low postoperative ECD level
might at least in part be artificial, caused by the inaccuracy of specular microscopes themselves. The endothelial cell count was realised during regular clinical follow-ups by well-trained
technicians using the automatic cell count but without manual correction. Clinical specular microscopy in general seems to find a significantly lower ECD (around 32% lower) than transmitted
light microscopy [22], which may lead not only to relatively low postoperative ECD values, but also to the high ECL perioperative. In addition, a standard diameter of our EDML of 7.5 mm as
well as the inclusion of eyes after pars-plana vitrectomy (Aljundi et al. [32]) may contribute to this finding. One issue that might have affected the preoperative accuracy of the ECD
measurement might be the tightness of the EDML roll. This was not tackled by neither Safi et al. [15] nor by our study. However, in our studies we figured that generally ECD measurement of a
loose roll is technically easier than that of a tight roll. And typically, the rolls tend to be loose directly after preparation. In terms of evolution of pachymetry, the literature
described quite different results. Borgardts et al. [2] for instance found an increase in the CT from the pre- to post-operative state, while the majority of the scientific articles stated a
significant decrease in the CT postoperatively [33, 34]. The results of the present study are in line with most of the studies performed for this parameter and showed a significant
reduction of the CT after transplantation, reaching a 10% difference in the early postoperative follow-up and a 14% difference after 1 year postoperatively compared to the preoperative
level. It is worth noting that a preoperative CT > 625 μm was correlated with worse postoperative CT and VA results [35]. Therefore, early treatment of endothelial corneal pathologies
with DMEK is recommended at our Department of Ophthalmology. Our results also showed a significant drop in the CT, between 6 months (539 μm) and 1 year (512 μm) postoperative in contrast to
a publication by Chamberlain et al. [36]. DMEK is known for its good clinical outcomes, especially in terms of fast VA recovery [5]. Starting with an average VA of 0.50 preoperatively, VA
improved to an average of 0,06 at 1 year postoperatively. No events of ocular hypertension were detected postoperatively in the present study, only small variations of the IOP within normal
ranges were observed throughout our study. Indeed, that there are numerous reports of the ECL post DMEK and its impact on intermediate term outcomes. For instance, Hayashi et al. [37] and
Shahnazaryan et al. [38], which as well as our study only treated patients with Fuchs endothelial corneal dystrophy. Not only the indication for DMEK, but also the clinical outcomes after
DMEK – a significant improvement in visual acuity and a significant reduction in terms of pachymetry – unite those mentioned studies with ours. As for Hayashi et al., they even used a quite
similar preparation technique with the very same ‘Moria suction block’. In addition, the postoperative ECD measurements were at the same time 6 months and 1 year after DMEK and performed
with the same clinical specular microscope (‘Tomey specular microscope EM-3000’). However, the essential novelty of our study is, that the non-invasive method of measuring the ECD on the
EDML, introduced in 2020 by Safi et al. [15], was now used in a clinical context. It showed that it can be used directly before DMEK to differentiate the exact ECL caused by the EDML
preparation itself from the intraoperative ECL. This allows us to not only state the ECL from before preparation to after DMEK and its influence on postoperative outcomes, but also to assess
the impact of the preparation of each tissue itself on the postoperative clinical outcomes by noninvasively quantifying the ECL it causes. Neither study Hayashi et al. [37] nor Shahnazaryan
et al. [38] contains this intermediate measurement step, which allows a more accurate understanding of ECL in the whole pre- and postoperative period of DMEK therapy. We noticed a rapid and
significant improvement concerning visual acuity and pachymetry 6 weeks postoperatively, this improvement showed no further statistically significant changes in the following 6 months. Only
after that timepoint and until one year postoperatively did these parameters further significantly improve. The mechanism behind this pattern of improvement remains unexplainable, and
further investigations with confocal microscopy and/or anterior segment OCT might be helpful in the future. In conclusion, we presented in this study a new safe and effective method of
counting the ECD on the rolled, non-manipulated DMEK-grafts preoperatively. Only during preparation, there was an endothelial cell loss of 9%. From 6 weeks to 6 months, the ECD continued to
decrease significantly, only to stabilise after 1 year postoperatively. The higher the early endothelial loss after surgery was, the lower was ECD and the higher was central corneal
thickness at 1 year of follow-up. SUMMARY WHAT WAS KNOWN BEFORE * Descemet-Membrane Endothelial Keratoplasty has become a gold standard therapy for corneal endothelial diseases with good
visual outcomes and low post-operative complication rates. * There is a high variation of reported endothelial Cell Loss after Descemet-Membrane Endothelial Keratoplasty between different
studies. WHAT THIS STUDY ADDS * This study showed a new safe and effective method of counting the endothelial cell density on the rolled, non-manipulated DMEK-grafts preoperatively and was
now used in a clinical context. * It presented only during preparation, there was an endothelial cell loss of 9%. * It showed the higher the early endothelial loss after DMEK-surgery was,
the lower was endothelial cell density and the higher was central corneal thickness at 1 year of follow-up. DATA AVAILABILITY Data is available from the corresponding author on reasonable
request. REFERENCES * Melles GRJ, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25:987–90. PubMed Google Scholar * Borgardts KC,
Spaniol K, Bachmann B, Hellmich M, Geerling G, Maier P, et al. Outcomes after descemet membrane endothelial keratoplasty (DMEK) in a German multicenter study. Invest Ophthalmol Vis Sci.
2019;60:2224. Google Scholar * Flockerzi E, Maier P, Böhringer D, Reinshagen H. Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG-section cornea and its
keratoplasty registry. Am J Ophthalmol. 2018;188:91–8. Article PubMed Google Scholar * Basak SK, Basak S, Pradhan VR. Outcomes of descemet membrane endothelial keratoplasty (DMEK) using
Surgeon’s prepared donor DM-Roll in consecutive 100 Indian eyes. Open Ophthalmol. 2018;12:134–42. Article Google Scholar * Birbal RS, Dhubhghaill SN, Bourgonje VJA, Hanko J, Ham L, Jager
MJ, et al. Five-year graft survival and clinical outcomes of 500 consecutive cases after descemet membrane endothelial keratoplasty. Cornea. 2020;39:290–7. Article PubMed Google Scholar *
Vasiliauskaite I, Oellerich S, Ham L, Dapena I, Baydoun L, van Dijk K, et al. Descemet membrane endothelial keratoplasty: ten-year graft survival and clinical outcomes. Am J Ophthalmol.
2020;217:114–20. Article PubMed Google Scholar * Parekh M, Ruzza A, Romano V, Favaro E, Baruzzo M, Salvalaio G, et al. Descemet membrane endothelial keratoplasty learning curve for graft
preparation in an eye bank using 645 donor corneas. Cornea. 2018;37:767–71. Article PubMed Google Scholar * Altaan SL, Gupta A, Sidney LE, Elalfy MS, Agarwal A, Dua HS. Endothelial cell
loss following tissue harvesting by pneumodissection for endothelial keratoplasty: an ex vivo study. Br J Ophthalmol. 2015;99:710–3. Article PubMed Google Scholar * Jardine GJ, Holiman
JD, Stoeger CG, Chamberlain WD. Imaging and quantification of endothelial cell loss in eye bank prepared DMEK grafts using trainable segmentation software. Curr Eye Res. 2014;39:894–901.
Article CAS PubMed Google Scholar * Schallhorn JM, Holiman JD, Stoeger CG, Chamberlain W. Quantification and patterns of endothelial cell loss due to eye bank preparation and injector
method in descemet membrane endothelial keratoplasty tissues. Cornea. 2016;35:377–82. Article PubMed Google Scholar * Tran KD, Dye PK, Odell K, Galloway J, Stoeger CG, Straiko MD, et al.
Evaluation and quality assessment of prestripped, preloaded descemet membrane endothelial keratoplasty grafts. Cornea. 2017;36:484–90. Article PubMed Google Scholar * Mayko ZM, Benetz BA,
Menegay H, Donovan CP, Stoeger CG, Terry MA, et al. Donor endothelial cell density measurements do not change immediately after DMEK preparation. Cornea. 2016;35:1556–61. Article PubMed
Google Scholar * Menzel-Severing J, Walter P, Plum WJ, Kruse FE, Salla S. Assessment of corneal endothelium during continued organ culture of pre-stripped human donor tissue for DMEK
surgery. Curr Eye Res. 2018;43:1439–44. Article CAS PubMed Google Scholar * Muraine M, Gueudry J, He Z, Piselli S, Lefevre S, Toubeau D. Novel technique for the preparation of corneal
grafts for descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2013;156:851–9. Article CAS PubMed Google Scholar * Safi T, Seitz B, Berg K, Schulz K, Langenbucher A, Daas L.
Reproducibility of non-invasive endothelial cell loss assessment of the pre-stripped DMEK roll after preparation and storage. Am J Ophthalmol. 2020;221:17–26. Article PubMed Google Scholar
* Seitz B, Daas L, Flockerzi E, Suffo S. Descemet membrane endothelial keratoplasty DMEK - Donor and recipient step by step. Ophthalmologe. 2020;117:811–28. Article PubMed Google Scholar
* Seitz B, Daas L, Bischoff-Jung M, Szentmáry N, Suffo S, El-Husseiny M, et al. Anatomy-based DMEK Wetlab in Homburg/Saar: Novel aspects of donor preparation and host maneuvers to teach
descemet membrane endothelial keratoplasty. Clin Anat. 2017;31:16–27. Article PubMed Google Scholar * Bachmann BO, Laaser K, Cursiefen C, Kruse FE. A method to confirm correct orientation
of descemet membrane during descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2010;149:922–925.e2. Article PubMed Google Scholar * Birbal RS, Sikder S, Lie JT, Groeneveld-van
Beek EA, Oellerich S, Melles GRJ. Donor tissue preparation for descemet membrane endothelial keratoplasty: an updated review. Cornea. 2018;37:128–35. Article PubMed Google Scholar * Basak
SK, Basak S, Gajendragadkar N, Ghatak M. Overall clinical outcomes of Descemet membrane endothelial keratoplasty in 600 consecutive eyes: a large retrospective case series. Indian J
Ophthalmol. 2020;68:1044–53. Article PubMed PubMed Central Google Scholar * Pipparelli A, Thuret G, Toubeau D, He Z, Piselli S, Lefèvre S, et al. Pan-corneal endothelial viability
assessment: application to endothelial grafts predissected by eye banks. Invest Ophthalmol Vis Sci. 2011;52:6018–25. Article PubMed Google Scholar * Rickmann A, Boden KE, Wahl S, Jung S,
Boden KT, Szurman P, et al. Significant differences between specular microscopy and corneal bank endothelial cell counts - a pilot study. Acta Ophthalmol. 2019;97:e1077–e1081. Article
PubMed Google Scholar * Inoda S, Hayashi T, Takahashi H, Oyakawa I, Yokogawa H, Kobayashi A, et al. Factors associated with endothelial cell density loss post Descemet membrane endothelial
keratoplasty for bullous keratopathy in Asia. PLoS One. 2020;15:e0234202. Article CAS PubMed PubMed Central Google Scholar * Miron A, Spinozzi D, Bruinsma M, Lie JT, Birbal RS, Baydoun
L, et al. Asymmetrical endothelial cell migration from in vitro Quarter-Descemet membrane endothelial keratoplasty grafts. Acta Ophthalmol. 2018;96:828–33. Article CAS PubMed PubMed
Central Google Scholar * Böhm MS, Wylegala A, Leon P, Tone SO, Ciolino JB, Jurkunas UV. One-year clinical outcomes of preloaded descemet membrane endothelial keratoplasty versus non-
preloaded descemet membrane endothelial keratoplasty. Cornea. 2021;40:311–9. Article PubMed Google Scholar * Jirsová K. Light and specular microscopy of the cornea. Springer.
2017;4:59–74. 5:75-99 Google Scholar * Krabcova I, Studeny P, Jirsova K. Endothelial cell density before and after the preparation of corneal lamellae for Descemet membrane endothelial
keratoplasty with a stromal rim. Cornea. 2011;30:1436–41. Article PubMed Google Scholar * Downes K, Tran KD, Stoeger CG, Chamberlain W. Cumulative endothelial cell loss in descemet
membrane endothelial keratoplasty grafts from preparation through insertion with glass injectors. Cornea. 2018;37:698–704. Article PubMed Google Scholar * Hammer T, Sennewald J, Viestenz
A, editors. DMEK optimiert – aktuelle Aspekte und klinische Resultate. Düsseldorf: German Medical Science GMS Publishing House, 2020. https://doi.org/10.3205/19sag25 * Jbara D, Achiron A,
Antman G, Buhbut O, Hecht I, Tuuminen R, et al. Agreement of corneal endothelial cell analysis between Konan-Noncon Robo SP-6000 and Tomey EM-3000 specular microscopes in healthy subjects.
Eye Contact Lens. 2020;47:191–5. Article Google Scholar * Huang J, Maram J, Tepelus TC, Modak C, Marion K, Sadda SVR, et al. Comparison of manual & automated analysis methods for
corneal endothelial cell density measurements by specular microscopy. J Optom. 2017;11:182–91. Article PubMed PubMed Central Google Scholar * Aljundi W, Abdin A, Suffo S, Seitz B, Daas
L. Descemet membrane endothelial keratoplasty (DMEK) in previously vitrectomized eyes: complications and clinical outcomes. Klin Monbl Augenheilkd. 2021;238:1101–7. Article PubMed Google
Scholar * Kwon RO, Price MO, Price FW, Ambrósio R Jr, Belin MW. Pentacam characterization of corneas with Fuchs dystrophy treated with Descemet membrane endothelial keratoplasty. J Refract
Surg. 2010;26:972–9. Article PubMed Google Scholar * Peraza-Nieves J, Baydoun L, Dapena I, Ilyas A, Frank LE, Luceri S, et al. Two-year clinical outcome of 500 consecutive cases
undergoing descemet membrane endothelial keratoplasty. Cornea. 2017;36:655–60. Article PubMed Google Scholar * Brockmann T, Pilger D, Brockmann C, Maier AKB, Bertelmann E, Torun N.
Predictive factors for clinical outcomes after primary descemet’s membrane endothelial keratoplasty for Fuchs’ endothelial dystrophy. Curr Eye Res. 2019;44:147–53. Article PubMed Google
Scholar * Chamberlain W, Lin C, Austin A, Schubach N, Clover J, McLeod S, Porco T, Lietman T, Rose-Nussbaumer J. Descemet Endothelial Thickness Comparison Trial: A Randomized Trial
Comparing Ultrathin Descemet Stripping Automated Endothelial Keratoplasty with Descemet Membrane Endothelial Keratoplasty. Ophthalmology, 2019;126:19–26. * Hayashi T, Schrittenlocher S,
Siebelmann S, Hung Le VN, Matthaei M, Franklin J, et al. Risk factors for endothelial cell loss after Descemet membrane endothelial keratoplasty (DMEK). Sci Rep. 2020;10:11086. *
Shahnazaryan D, Sese AH, Hollick EJ. Endothelial cell loss after descemet’s membrane endothelial keratoplasty for Fuchs’ endothelial dystrophy: DMEK compared to triple DMEK. Am J Ophthalmol.
2020;218:1–6. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank the Klaus Faber Center for Corneal Diseases, LIONS Eye Bank Saar-Lor-Lux,
Trier/Westpfalz for their help in preserving and measuring the donor corneas. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology, Saarland University Medical Center,
Homburg, Germany Kolja Berg, Tarek Safi, Berthold Seitz & Loay Daas Authors * Kolja Berg View author publications You can also search for this author inPubMed Google Scholar * Tarek Safi
View author publications You can also search for this author inPubMed Google Scholar * Berthold Seitz View author publications You can also search for this author inPubMed Google Scholar *
Loay Daas View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS KB was responsible for writing the manuscript, conducting the search, screening
potentially eligible studies, extracting and analysing data, interpreting results and updating reference lists. TS was responsible for the preclinical trial (published earlier), research and
feedback. BS and LD provided their professional support and feedback to the corresponding author. CORRESPONDING AUTHOR Correspondence to Kolja Berg. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s)
or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Berg, K., Safi, T., Seitz, B. _et al._ Non-invasive endothelial cell density measurement of in toto pre-stripped DMEK-roll – impact of
pre- and intraoperative endothelial cell loss on postoperative midterm clinical outcome. _Eye_ 37, 2956–2962 (2023). https://doi.org/10.1038/s41433-023-02450-x Download citation * Received:
15 May 2022 * Revised: 13 January 2023 * Accepted: 10 February 2023 * Published: 22 February 2023 * Issue Date: October 2023 * DOI: https://doi.org/10.1038/s41433-023-02450-x SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative