Play all audios:
ABSTRACT CD8+ T cells play a critical role in specific immunity. In recent years, cell therapy has been emerging rapidly. The specific cytotoxic capabilities of these cells enable them to
precisely identify and kill cells presenting specific antigens. This has demonstrated promise in the treatment of autoimmune diseases and cancers, with wide-ranging applications and value.
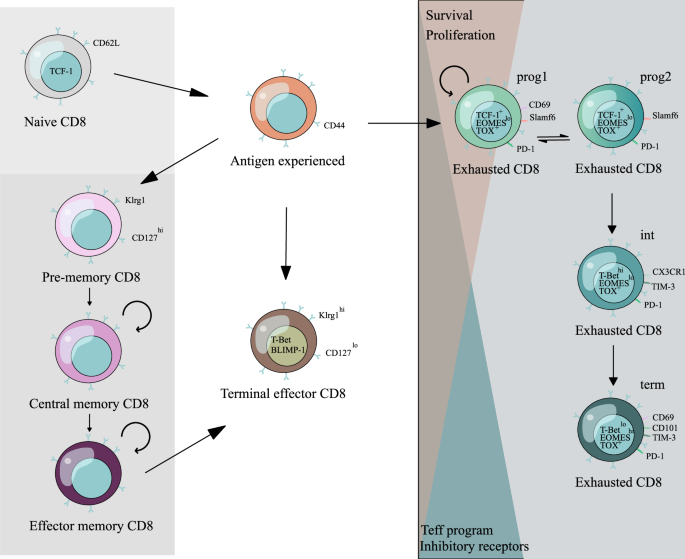
However, in some diseases, such as tumors and chronic infections, T cells may adopt an exhausted phenotype, resulting in a loss of cytotoxicity and limiting their further application.
Epigenetics plays a significant role in the differentiation and regulation of gene expression in cells. There is extensive evidence indicating that epigenetic remodeling plays an important
role in T cell exhaustion. Therefore, further understanding its role in CD8+ T cell function can provide insights into the programmatic regulation of CD8+ T cells from a genetic perspective
and overcome these diseases. We attempted to describe the relationship between the activation, function, and exhaustion mechanisms of CD8+ T cells, as well as epigenetics. This understanding
makes it possible for us to address the aforementioned issues. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Subscribe to this journal Receive 6 digital issues and online access to articles $119.00 per year only $19.83 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE EPIGENETIC LANDSCAPE OF FATE DECISIONS IN T CELLS Article 19 March
2025 EPIGENETIC REGULATION OF T CELL EXHAUSTION Article 27 May 2022 T CELLS IN HEALTH AND DISEASE Article Open access 19 June 2023 REFERENCES * Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al.
CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001. Article CAS PubMed PubMed Central Google
Scholar * Rudloff MW, Zumbo P, Favret NR, Roetman JJ, Detrés Román CR, Erwin MM, et al. Hallmarks of CD8+ T cell dysfunction are established within hours of tumor antigen encounter before
cell division. Nat Immunol. 2023;24:1527–39. Article CAS PubMed PubMed Central Google Scholar * Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. Article CAS PubMed Google
Scholar * Huang Y, Si X, Shao M, Teng X, Xiao G, Huang H. Rewiring mitochondrial metabolism to counteract exhaustion of CAR-T cells. J Hematol Oncol. 2022;15:38. Article CAS PubMed
PubMed Central Google Scholar * Beltra J-C, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals
Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity. 2020;52:825. Article CAS PubMed PubMed Central Google Scholar * Belk JA, Daniel B, Satpathy AT.
Epigenetic regulation of T cell exhaustion. Nat Immunol. 2022;23:848–60. Article CAS PubMed PubMed Central Google Scholar * McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion
During Chronic Viral Infection and Cancer. In: Yokoyama WM, editor. Annu Rev Immunol. 2019;37:457–95. Annual Review of Immunology. 372019 Article CAS PubMed Google Scholar * Wherry EJ,
Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. Article CAS PubMed PubMed Central Google Scholar * Hu Y, Zhou Y, Zhang M, Ge W, Li Y,
Yang L, et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2021;27:2764–72.
Article CAS PubMed Google Scholar * Ledford H. CAR-T Therapy Forces Autoimmune Diseases into Remission. Nature. 2023;624:483–4. Article CAS PubMed Google Scholar * Arnold C.
Autoimmune disease is the next frontier for CAR T cell therapy. Nat Med. 2024;30:6–9. Article CAS PubMed Google Scholar * Horste EL, Fansler MM, Cai T, Chen X, Mitschka S, Zhen G, et al.
Subcytoplasmic location of translation controls protein output. Mol cell. 2023;83:4509–23.e11. Article CAS PubMed PubMed Central Google Scholar * Wang Z, Wu Z, Liu Y, Han W. New
development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53. Article PubMed PubMed Central Google Scholar * Papp F, Hajdu P, Tajti G. et al. Periodic Membrane Potential and Ca2+
Oscillations in T Cells Forming an Immune Synapse. Int J Mol Sci. 2020;21:1568. Article PubMed PubMed Central Google Scholar * Green DR, Droin N, Pinkoski M. Activation-induced cell
death in T cells. Immunological Rev. 2003;193:70–81. Article CAS Google Scholar * Denton AE, Wesselingh R, Gras S, Guillonneau C, Olson MR, Mintern JD, et al. Affinity thresholds for
naive CD8+ CTL activation by peptides and engineered influenza A viruses. J Immunol. 2011;187:5733–44. Article CAS PubMed Google Scholar * Duan H, Jing L, Jiang X, Ma Y, Wang D, Xiang J.
et al. CD146 bound to LCK promotes T cell receptor signaling and antitumor immune responses in mice. J Clin Invest. 2021;131:e148568 Article CAS PubMed PubMed Central Google Scholar *
Kasmani MY, Zander R, Chung HK. et al. Clonal lineage tracing reveals mechanisms skewing CD8+ T cell fate decisions in chronic infection. J Exp Med. 2023;220:e20220679. Article PubMed
Google Scholar * Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying
Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity. 2020;52:825–41.e8. Article CAS PubMed PubMed Central Google Scholar * Blank CU, Haining WN, Held W, Hogan PG,
Kallies A, Lugli E, et al. Defining ‘T cell exhaustion. Nat Rev Immunol. 2019;19:665–74. Article CAS PubMed PubMed Central Google Scholar * Schietinger A, Philip M, Krisnawan VE, Chiu
EY, Delrow JJ, Basom RS, et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45:389–401. Article
CAS PubMed PubMed Central Google Scholar * Siddiqui I, Schaeuble K, Chennupati V, Marraco SAF, Calderon-Copete S, Ferreira DP, et al. Intratumoral Tcf1 + PD-1 + CD8 + T Cells with
Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity. 2019;50:195. Article CAS PubMed Google Scholar * Ozga AJ, Chow MT,
Lopes ME, Servis RL, Di Pilato M, Dehio P, et al. CXCL10 chemokine regulates heterogeneity of the CD8 + T cell response and viral set point during chronic infection. Immunity. 2022;55:82.
Article CAS PubMed Google Scholar * Pritykin Y, van der Veeken J, Pine AR, Zhong Y, Sahin M, Mazutis L, et al. A unified atlas of CD8 T cell dysfunctional states in cancer and infection.
Mol Cell. 2021;81:2477. Article CAS PubMed PubMed Central Google Scholar * McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome
in autoimmunity and infection. Nature. 2015;523:612. Article CAS PubMed PubMed Central Google Scholar * Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, et
al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell. 2019;176:775. Article CAS PubMed Google Scholar * Miller BC, Sen DR, Al
Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8 + T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326. Article
CAS PubMed PubMed Central Google Scholar * Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, et al. The transcription factor Runx3 guards cytotoxic CD8 + effector T cells against
deviation towards follicular helper T cell lineage. Nat Immunol. 2017;18:931. Article CAS PubMed PubMed Central Google Scholar * Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et
al. CXCR5 + follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17:1187. Article CAS PubMed Google Scholar * Weng NP, Akbar AN, Goronzy J. CD28(-)
T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. Article CAS PubMed PubMed Central Google Scholar * Seyda M, Elkhal A, Quante M,
Falk CS, Tullius SG. T Cells Going Innate. Trends Immunol. 2016;37:546–56. Article CAS PubMed PubMed Central Google Scholar * Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only
age-related cells but a subset of regulatory T cells. Cell Mol Immunol. 2018;15:734–6. Article CAS PubMed PubMed Central Google Scholar * Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ,
Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–8. Article CAS PubMed Google Scholar * Huff WX, Kwon JH, Henriquez M,
Fetcko K, Dey M. The Evolving Role of CD8+CD28− Immunosenescent T Cells in Cancer Immunology. Int J Mol Sci. 2019;20:2810. * Dai D, Pei Y, Zhu B, Wang D, Pei S, Huang H, et al.
Chemoradiotherapy-induced ACKR2+ tumor cells drive CD8+ T cell senescence and cervical cancer recurrence. Cell Rep Med. 2024;5:101550. * Guan Y, Zhang C, Lyu G, Huang X, Zhang X, Zhuang T,
et al. Senescence-activated enhancer landscape orchestrates the senescence-associated secretory phenotype in murine fibroblasts. Nucleic Acids Res. 2020;48:10909–23. Article CAS PubMed
PubMed Central Google Scholar * Pomp W, Meeussen JVW, Lenstra TL. Transcription factor exchange enables prolonged transcriptional bursts. Mol Cell. 2024;84:1036–1048.e9. Article CAS
PubMed PubMed Central Google Scholar * Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–402. Article CAS PubMed Google Scholar * Jurkowska RZ, Jurkowski
TP, Jeltsch A. Structure and Function of Mammalian DNA Methyltransferases. Chembiochem. 2011;12:206–22. Article CAS PubMed Google Scholar * Ghanty U, Wang T, Kohli RM. Nucleobase
Modifiers Identify TET Enzymes as Bifunctional DNA Dioxygenases Capable of Direct N-Demethylation. Angew Chem-Int Ed. 2020;59:11312–5. Article CAS Google Scholar * DNMT3A DNA-Binding
Residues Provide Specificity for CpG DNA Methylation. Cancer discovery. 2018;8:OF14-OF. * Zhang Z-M, Lu R, Wang P, Yu Y, Chen D, Gao L, et al. Structural basis for DNMT3A-mediated de novo
DNA methylation. Nature. 2018;554:387. Article CAS PubMed PubMed Central Google Scholar * Nunez JK, Chen J, Pommier GC, Cogan JZ, Replogle JM, Adriaens C, et al. Genome-wide
programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 2021;184:2503. Article CAS PubMed PubMed Central Google Scholar * Greer EL, Shi Y. Histone methylation: a
dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–57. Article CAS PubMed PubMed Central Google Scholar * Kschonsak M, Haering CH. Shaping mitotic chromosomes:
From classical concepts to molecular mechanisms. Bioessays. 2015;37:755–66. Article CAS PubMed PubMed Central Google Scholar * Eustermann S, Patel AB, Hopfner K-P, He Y, Korber P.
Energy-driven genome regulation by ATP-dependent chromatin remodellers. Nat Rev Mol Cell Biol. 2023;25:309–32. * Song J, Gooding AR, Hemphill WO, Kasinath V, Cech TR. Structural basis for
inactivation of PRC2 by G-quadruplex RNA. Science. 2023;381:1331–7. * Skvortsova K, Iovino N, Bogdanovic O. Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Mol Cell
Biol. 2018;19:774–90. Article CAS PubMed Google Scholar * Roundtree IA, Luo G-Z, Zhang Z, Wang X, Zhou T, Cui Y, et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated
mRNAs. eLife. 2017;6:e31311. * Fitz-James MH, Cavalli G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat Rev Genet. 2022;23:325–41. Article CAS PubMed Google Scholar
* Jain N, Zhao Z, Koche RP, Antelope C, Gozlan Y, Montalbano A, et al. Disruption of SUV39H1-Mediated H3K9 Methylation Sustains CAR T-cell Function. Cancer Discov. 2024;14:142–57. Article
CAS PubMed PubMed Central Google Scholar * Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu ZY, et al. Acquisition of full effector function in vitro paradoxically
impairs the in vivo antitumor efficacy of adoptively transferred CD8 + T cells. J Clin Investig. 2005;115:1616–26. Article CAS PubMed PubMed Central Google Scholar * Pantaleo G, Harari
A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–23. Article CAS PubMed Google Scholar * Baessler A, Vignali
DAA. T Cell Exhaustion. Annu Rev Immunol. 2024;42:179–206. Article CAS PubMed Google Scholar * Yu B, Zhang K, Milner JJ, Toma C, Chen R, Scott-Browne JP, et al. Epigenetic landscapes
reveal transcription factors that regulate CD8(+) T cell differentiation. Nat Immunol. 2017;18:573–82. Article CAS PubMed PubMed Central Google Scholar * Yang R, Mele F, Worley L,
Langlais D, Rosain J, Benhsaien I, et al. Human T-bet Governs Innate and Innate-like Adaptive IFN-gamma Immunity against Mycobacteria. Cell. 2020;183:1826–47 e31. Article CAS PubMed
PubMed Central Google Scholar * Zhao X, Shan Q, Xue H-H. TCF1 in T cell immunity: a broadened frontier. Nat Rev Immunol. 2021;22:147–57. Article PubMed Google Scholar * Gattinoni L,
Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8 + memory stem cells. Nat Med. 2009;15:808–U129. Article CAS
PubMed PubMed Central Google Scholar * Sun Q, Cai D, Liu D, Zhao X, Li R, Xu W, et al. BCL6 promotes a stem-like CD8(+) T cell program in cancer via antagonizing BLIMP1. Sci Immunol.
2023;8:eadh1306. Article CAS PubMed Google Scholar * Jung IY, Narayan V, McDonald S, Rech AJ, Bartoszek R, Hong G, et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate
antitumor CAR T cell stemness and exhaustion. Sci Transl Med. 2022;14:eabn7336. Article CAS PubMed PubMed Central Google Scholar * Li Q, Zhang L, You W, Xu J, Dai J, Hua D, et al.
PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells. Nat Commun. 2022;13:7677. Article CAS PubMed PubMed Central Google
Scholar * Stairiker CJ, Thomas GD, Salek-Ardakani S. EZH2 as a Regulator of CD8+ T Cell Fate and Function. Front Immunol. 2020;11:593203. Article CAS PubMed PubMed Central Google
Scholar * Abadie K, Clark EC, Valanparambil RM, Ukogu O, Yang W, Daza RM, et al. Reversible, tunable epigenetic silencing of TCF1 generates flexibility in the T cell memory decision.
Immunity. 2024;57:271–86.e13. Article CAS PubMed Google Scholar * Wang Y, Qiu F, Xu Y, Hou X, Zhang Z, Huang L, et al. Stem cell-like memory T cells: The generation and application. J
Leukoc Biol. 2021;110:1209–23. Article CAS PubMed Google Scholar * Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, et al. TOX and TOX2 transcription factors
cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci USA. 2019;116:12410–5. Article CAS PubMed PubMed Central Google Scholar * Ferreira DP,
Silva JG, Wyss T, Marraco SAF, Scarpellino L, Charmoy M, et al. Central memory CD8 + T cells derive from stem-like Tcf7 hi effector cells in the absence of cytotoxic differentiation.
Immunity. 2020;53:985. Article Google Scholar * Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–9. Article CAS
PubMed Google Scholar * Doan AE, Mueller KP, Chen AY, Rouin GT, Chen Y, Daniel B, et al. FOXO1 is a master regulator of memory programming in CAR T cells. Nature. 2024;629:211–8. Article
CAS PubMed PubMed Central Google Scholar * McCutcheon SR, Swartz AM, Brown MC, Barrera A, McRoberts Amador C, Siklenka K, et al. Transcriptional and epigenetic regulators of human CD8(+)
T cell function identified through orthogonal CRISPR screens. Nat Genet. 2023;55:2211–23. Article CAS PubMed PubMed Central Google Scholar * Yang R, Mele F, Worley L, Langlais D,
Rosain J, Benhsaien I, et al. Human T-bet Governs Innate and Innate-like Adaptive IFN-γ Immunity against Mycobacteria. Cell. 2020;183:1826–47.e31. Article CAS PubMed PubMed Central
Google Scholar * Hertweck A, Evans CM, Eskandarpour M, Lau JC, Oleinika K, Jackson I, et al. T-bet Activates Th1 Genes through Mediator and the Super Elongation Complex. Cell Rep.
2016;15:2756–70. Article CAS PubMed PubMed Central Google Scholar * Li Y, Han M, Wei H, Huang W, Chen Z, Zhang T, et al. Id2 epigenetically controls CD8(+) T-cell exhaustion by
disrupting the assembly of the Tcf3-LSD1 complex. Cell Mol Immunol. 2024;21:292–308. Article CAS PubMed PubMed Central Google Scholar * Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin
CY, Kagey MH, et al. Master Transcription Factors and Mediator Establish Super-Enhancers at Key Cell Identity Genes. Cell. 2013;153:307–19. Article CAS PubMed PubMed Central Google
Scholar * Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, et al. Super-Enhancers in the Control of Cell Identity and Disease. Cell. 2013;155:934–47. Article CAS PubMed
Google Scholar * Danilo M, Chennupati V, Silva JG, Siegert S, Held W. Suppression of Tcf1 by Inflammatory Cytokines Facilitates Effector CD8 T Cell Differentiation. Cell Rep.
2018;22:2107–17. Article CAS PubMed Google Scholar * Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H. Differentiation and Persistence of Memory CD8 + T Cells Depend on T Cell
Factor 1. Immunity. 2010;33:229–40. Article CAS PubMed PubMed Central Google Scholar * Miller CH, Klawon DEJ, Zeng S, Lee V, Socci ND, Savage PA. Eomes identifies thymic precursors of
self-specific memory-phenotype CD8 + T cells. Nat Immunol. 2020;21:567. Article CAS PubMed PubMed Central Google Scholar * Llao-Cid L, Roessner PM, Chapaprieta V, Oeztuerk S, Roider T,
Bordas M, et al. EOMES is essential for antitumor activity of CD8+T cells in chronic lymphocytic leukemia. Leukemia. 2021;35:3152–62. Article CAS PubMed PubMed Central Google Scholar *
Beltra J-C, Abdel-Hakeem MS, Manne S, Zhang Z, Huang H, Kurachi M, et al. Stat5 opposes the transcription factor Tox and rewires exhausted CD8+ Tcells toward durable effector-like states
during chronic antigen exposure. Immunity. 2023;56:2699–718.e11. Article CAS PubMed PubMed Central Google Scholar * Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, et al.
Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552:404–9. Article CAS PubMed PubMed Central Google Scholar * Herek TA, Bouska A, Lone W, Sharma S,
Amador C, Heavican TB, et al. DNMT3A mutations define a unique biological and prognostic subgroup associated with cytotoxic T cells in PTCL-NOS. Blood. 2022;140:1278–90. Article CAS PubMed
PubMed Central Google Scholar * Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci
Transl Med. 2021;13:eabh0272. * Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL
Partner TET1. Science. 2009;324:930–5. Article CAS PubMed PubMed Central Google Scholar * Dixon G, Pan H, Yang D, Rosen BP, Jashari T, Verma N, et al. QSER1 protects DNA methylation
valleys from de novo methylation. Science. 2021;372:eabd0875. * Bamezai S, Demir D, Pulikkottil AJ, Ciccarone F, Fischbein E, Sinha A, et al. TET1 promotes growth of T-cell acute
lymphoblastic leukemia and can be antagonized via PARP inhibition. Leukemia. 2021;35:389–403. Article CAS PubMed Google Scholar * Whiteside SK, Grant FM, Alvisi G, Clarke J, Tang L,
Imianowski CJ, et al. Acquisition of suppressive function by conventional T cells limits antitumor immunity upon Treg depletion. Sci Immunol. 2023;8:eabo5558.– eabo Article CAS PubMed
PubMed Central Google Scholar * Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An Interleukin-21-Interleukin-10-STAT3 Pathway Is Critical for Functional Maturation of Memory CD8 + T Cells.
Immunity. 2011;35:792–805. Article CAS PubMed PubMed Central Google Scholar * Sun Q, Zhao X, Li R, Liu D, Pan B, Xie B, et al. STAT3 regulates CD8 + T cell differentiation and
functions in cancer and acute infection. J Exp Med. 2023;220:e20220686. * Klysz DD, Fowler C, Malipatlolla M, Stuani L, Freitas KA, Chen Y, et al. Inosine induces stemness features in CAR-T
cells and enhances potency. Cancer Cell. 2024;42:266–82 e8. Article CAS PubMed Google Scholar * Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase
I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. Article CAS PubMed PubMed Central Google
Scholar * Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599–611. Article CAS PubMed Google Scholar * Chang CH, Qiu J,
O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. Article CAS PubMed
PubMed Central Google Scholar * Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52.
Article CAS PubMed Google Scholar * Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. Article PubMed PubMed
Central Google Scholar * Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Sig Transduct Targeted Ther. 2021;6:128. * Tsvetkov P, Coy S, Petrova B,
Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254. Article CAS PubMed PubMed Central Google Scholar
* Liu X, Nie L, Zhang Y, Yan Y, Wang C, Colic M, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol. 2023;25:404. Article CAS PubMed
PubMed Central Google Scholar * Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–61. Article CAS PubMed
PubMed Central Google Scholar * Xiao L, Ma X, Ye L, Su P, Xiong W, Bi E, et al. IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced
antitumor activity. Journal of Clinical Investigation. 2022;132:e153247. * Vignali PDA, DePeaux K, Watson MJJ, Ye C, Ford BR, Lontos K, et al. Hypoxia drives CD39-dependent suppressor
function in exhausted T cells to limit antitumor immunity. Nat Immunol. 2023;24:267. Article CAS PubMed Google Scholar * Alizadeh D, Wong RA, Yang X, Wang D, Pecoraro JR, Kuo CF, et al.
IL15 Enhances CAR-T Cell Antitumor Activity by Reducing mTORC1 Activity and Preserving Their Stem Cell Memory Phenotype. Cancer Immunol Res. 2019;7:759–72. Article CAS PubMed PubMed
Central Google Scholar * MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. Article CAS PubMed PubMed Central Google Scholar
* Wang F, Cheng F, Zheng F. Stem cell like memory T cells: A new paradigm in cancer immunotherapy. Clin Immunol. 2022;241:109078. Article CAS PubMed Google Scholar * Chang CH, Curtis
JD, Maggi LB Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. Article CAS PubMed
PubMed Central Google Scholar * Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2020;20:55–70. Article CAS PubMed Google
Scholar * Franco F, Jaccard A, Romero P, Yu Y-R, Ho P-C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2:1001–12. Article CAS PubMed Google Scholar * Pearce
EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. Article PubMed PubMed Central Google Scholar *
Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–61. Article CAS PubMed PubMed Central Google Scholar * Ma X, Bi
E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143–56.e5. Article CAS PubMed PubMed Central Google
Scholar * Cao Y, Trillo-Tinoco J, Sierra RA, Anadon C, Dai W, Mohamed E, et al. ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet
repression. Nature Communications. 2019;10:1280. * Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations
Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45:358–73. Article CAS PubMed PubMed Central Google Scholar * Ogando J, Sáez
ME, Santos J, Nuevo-Tapioles C, Gut M, Esteve-Codina A, et al. PD-1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8(+) T lymphocytes. J Immunother
Cancer. 2019;7:151. Article PubMed PubMed Central Google Scholar * Chen SW, Zhu SQ, Pei X, Qiu BQ, Xiong D, Long X, et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell
dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20:144. Article PubMed PubMed Central Google Scholar * Yang C, Wu S, Mou Z, Zhou Q, Dai
X, Ou Y, et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol Ther. 2022;30:1054–70. Article CAS PubMed
PubMed Central Google Scholar * Mahat DB, Tippens ND, Martin-Rufino JD, Waterton SK, Fu J, Blatt SE, et al. Single-cell nascent RNA sequencing unveils coordinated global transcription.
Nature. 2024;631:216–23. * Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583. Article
CAS PubMed PubMed Central Google Scholar * Goverde CA, Wolf B, Khakzad H, Rosset S, Correia BE. De novo protein design by inversion of the AlphaFold structure prediction network.
Protein Sci. 2023;32:e4653. * Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature.
2024;630:493–500. * Wang J, Lisanza S, Juergens D, Tischer D, Watson JL, Castro KM, et al. Scaffolding protein functional sites using deep learning. Science. 2022;377:387–94. Article CAS
PubMed PubMed Central Google Scholar * Fudge JB. Diffusion model expands RoseTTAFold’s power. Nat Biotechnol. 2023;41:1072. PubMed Google Scholar * Roth SY, Denu JM, Allis CD. Histone
acetyltransferases. Annu Rev Biochem. 2001;70:81–120. Article CAS PubMed Google Scholar * Gao S, Liang X, Wang H, Bao B, Zhang K, Zhu Y, et al. Stem cell-like memory T cells: A
perspective from the dark side. Cell Immunol. 2021;361:104273. Article CAS PubMed Google Scholar * Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: a key player in cancer development. J
Hematol Oncol. 2020;13:151. Article PubMed PubMed Central Google Scholar * Han P, Chang CP. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015;12:1094–8. Article PubMed
PubMed Central Google Scholar * Chen D, Lei EP. Function and regulation of chromatin insulators in dynamic genome organization. Curr Opin Cell Biol. 2019;58:61–8. Article CAS PubMed
PubMed Central Google Scholar * Wei GH, Liu DP, Liang CC. Chromatin domain boundaries: insulators and beyond. Cell Res. 2005;15:292–300. Article CAS PubMed Google Scholar *
Phillips-Cremins Jennifer E, Corces Victor G. Chromatin Insulators: Linking Genome Organization to Cellular Function. Mol Cell. 2013;50:461–74. Article CAS PubMed PubMed Central Google
Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Yanjing Medical College, Capital Medical University, 101300, Beijing, China Hao Zu & Xiaoqin Chen Authors * Hao
Zu View author publications You can also search for this author inPubMed Google Scholar * Xiaoqin Chen View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Hao Zu, as the first author, proposed the research topic and conducted the literature search, analysis, illustration creation, and writing of the manuscript. Xiaoqin Chen, as
the corresponding author, provided guidance throughout the writing process, integrated feedback, and reviewed and revised the final manuscript, as well as offering support with formatting
revisions. CORRESPONDING AUTHOR Correspondence to Xiaoqin Chen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society
or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of
this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zu, H., Chen, X. Epigenetics
behind CD8+ T cell activation and exhaustion. _Genes Immun_ 25, 525–540 (2024). https://doi.org/10.1038/s41435-024-00307-1 Download citation * Received: 06 March 2024 * Revised: 29 October
2024 * Accepted: 31 October 2024 * Published: 14 November 2024 * Issue Date: December 2024 * DOI: https://doi.org/10.1038/s41435-024-00307-1 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative