Play all audios:
ABSTRACT Most organisms experience variable and sometimes suboptimal environments in their lifetime. While stressful environmental conditions are normally viewed as a strong selective force,
they can also impact directly on the genetic basis of traits such as through environment-dependent gene action. Here, we used the _Drosophila melanogaster_ Genetic Reference Panel to
investigate the impact of developmental temperature on variance components and evolutionary potential of cold tolerance. We reared 166 lines at five temperatures and assessed cold tolerance
of adult male flies from each line and environment. We show (1) that the expression of genetic variation for cold tolerance is highly dependent on developmental temperature, (2) that the
genetic correlation of cold tolerance between environments decreases as developmental temperatures become more distinct, (3) that the correlation between cold tolerance at individual
developmental temperatures and plasticity for cold tolerance differs across developmental temperatures, and even switches sign across the thermal developmental gradient, and (4) that
evolvability decrease with increasing developmental temperatures. Our results show that the quantitative genetic basis of low temperature tolerance is environment specific. This conclusion
is important for the understanding of evolution in variable thermal environments and for designing experiments aimed at pinpointing candidate genes and performing functional analyses of
thermal resistance. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS CHILL COMA ONSET AND RECOVERY FAIL TO REVEAL TRUE VARIATION
IN THERMAL PERFORMANCE AMONG POPULATIONS OF _DROSOPHILA MELANOGASTER_ Article Open access 25 May 2021 EVOLUTION OF CROSS-TOLERANCE IN _DROSOPHILA MELANOGASTER_ AS A RESULT OF INCREASED
RESISTANCE TO COLD STRESS Article Open access 14 November 2022 SPECIES-SPECIFIC EFFECTS OF THERMAL STRESS ON THE EXPRESSION OF GENETIC VARIATION ACROSS A DIVERSE GROUP OF PLANT AND ANIMAL
TAXA UNDER EXPERIMENTAL CONDITIONS Article 06 July 2020 INTRODUCTION Stressful environmental conditions not only act as a strong selective force, but also directly affect the genetic
architecture of traits in several ways. Firstly, stressful conditions can influence the nature of environmental variation, _V_E (Hoffmann and Merilä 1999; Ørsted et al. 2018a). Secondly,
these conditions can directly affect expression of additive genetic variation, _V_A (e.g., Czesak et al. 2006; Wilson et al. 2006). Both changes in _V_E and _V_A will influence heritability
estimates (Gebhardt-Henrich and van Noordwijk 1991; Charmantier and Garant 2005; Wilson et al. 2006; Visscher et al. 2008; Dingemanse et al. 2009; Kristensen et al. 2015). Thirdly,
environment-dependent gene action may exist, where the function of genes or gene networks, rather than overall genetic variation, change under stressful conditions (Telonis-Scott et al.
2009). This can lead to a situation where genes influencing a trait in one environment may not have much impact on that trait in a different environment (Sgrò and Hoffmann 2004), which in
turn will alter genetic correlations between environments (de Jong 1990; Stearns et al. 1991; Vieira et al. 2000; Stinchcombe et al. 2010). The impact of these environmental effects on
genetic variation can be important for evolutionary trajectories (Hoffmann and Merilä 1999; Hercus and Hoffmann 2000), but these mechanisms are rarely studied, as they are typically regarded
as a nuisance in quantitative genetic models (but see Wilson et al. 2006; Husby et al. 2011; Wood and Brodie 2016). Environmental conditions are likely to have particularly large effects on
the genetic architecture of ecological traits critical for responding to variable environmental conditions. For this class of traits, the environment can influence _V_A, _V_E and gene
action (Sgrò and Hoffmann 2004; Kristensen et al. 2015), affecting the extent to which organisms respond to selection and hence persist in the long run. Tolerance to stressful temperatures
in ectotherms, especially in _Drosophila_, provides examples of traits whose genetic architecture is strongly affected by environmental variation, such as the impact of developmental
temperature on the resistance to cold extremes (Schou et al. 2017; Ørsted et al. 2017). The ways in which the environment influences trait expression across genotypes is often characterised
through genotype-by-environment (GxE) interactions, where the direction and magnitude of GxE can depend on genotype (Rohde et al. 2017; Ørsted et al. 2018a) and/or as genetic correlations of
a trait measured in several environments (Vieira et al. 2000; Sgrò and Hoffmann 2004; Agrawal and Stinchcombe 2009). Genotypic responses to environmental variation are usually expressed as
differences in phenotypic plasticity, which is commonly quantified with linear reaction norms, i.e., the slope of trait values under continuous variation in an environmental variable
(Valladares et al. 2006). However, while environmental effects on gene action have typically been investigated by comparing two environments (Telonis-Scott et al. 2009; Ellers and Driessen
2011; Levine et al. 2011; Ketola et al. 2012; Gerken et al. 2015), patterns across environmental gradients are not well established. If the slopes of different genotypes along a gradient
vary, there will be GxE interactions and genetic correlations will be less than one. Under a continuous environmental gradient, a polygenic trait might be expected to show declining genetic
correlations (increasing GxE interactions) as environments become more dissimilar, assuming that there is an increase in variance due to environment-specific gene expression (Via and Lande
1985; Sgrò and Blows 2004; Spichtig and Kawecki 2004). This could lead to a linear or non-linear decrease in genetic correlations with the environmental variable. Surprisingly, this rather
simple prediction has rarely been studied experimentally. Knowledge of the effects of environmental similarity on genetic correlations of stress resistance traits can prove valuable when
trying to predict adaptive responses in heterogeneous environments (Sheldon et al. 2003; Sgrò and Blows 2004), such as those arising from anthropogenic climate change (Etterson and Shaw
2001; Agrawal and Stinchcombe 2009). Environment-dependent gene action also raises the question of how plasticity is linked to stress tolerance in a particular environment. Phenotypic
plasticity can be costly, because it requires energy and flexibility of the organism on a number of biological levels (Auld et al. 2010; Tonsor et al. 2013; Murren et al. 2015), and might
become maladaptive if environments change rapidly and in unpredictable ways (Kristensen et al. 2008). Several studies have found an inverse association between stress resistance in one
environment (here defined as “basal” tolerance) and the capacity for plasticity (Stillman 2003; Nyamukondiwa et al. 2011; Gerken et al. 2015; Comte and Olden 2017; Noh et al. 2017),
suggesting a trade-off between the ability to cope with current conditions and performance in an altered environment in the future (Murren et al. 2015). However, other studies have not found
evidence for such trade-offs (Kellett et al. 2005; Calosi et al. 2008; Franke et al. 2012; Gunderson et al. 2015; Ørsted et al. 2018a). Thus, it remains unclear if plasticity constrains
basal thermal tolerance and _vice versa_. Adding to the ambiguity, some studies have found that trade-offs are species-specific even across related taxa (Nyamukondiwa et al. 2011; Strachan
et al. 2011; Comte and Olden 2017), while others have found seasonal variation in trade-offs (Noh et al. 2017), highlighting the influence of environmental conditions on these patterns.
Here, we investigate the effect of developmental temperature on the genetic architecture of cold tolerance in _Drosophila melanogaster_. The aims were to explore the environmental dependency
of quantitative genetic parameters of cold tolerance, and potential trade-offs between cold tolerance in each environment and plasticity in this trait. We used the _Drosophila melanogaster_
Genetic Reference Panel (DGRP; Mackay et al. 2012; Huang et al. 2014), which was established from a natural population of _D. melanogaster_ from Raleigh, USA. The DGRP consists of a
collection of lines that has been inbred to an expected inbreeding coefficient of F ~1, resulting in extremely high homozygosity throughout the genome within each line. Thus, by assessing
cold tolerance of the same DGRP genotype reared in different thermal environments, knowledge about the genetic aspects of cold tolerance, and the association between cold tolerance at
individual temperatures and plasticity, can be obtained. Low temperatures limit the geographical distribution of many species more than warm temperatures (Sunday et al. 2011; Williams et al.
2014), and cold tolerance is therefore a good predictor of present and future distributions of species (Kimura 2004; Overgaard et al. 2011; Araújo et al. 2013). The temperature regimes
employed in this study were well within the range of what the cosmopolitan _D. melanogaster_ will experience in its natural habitats (Hoffmann et al. 2003), thus providing ecological
relevance to our experimental setup. However, it can be difficult to ascertain which of the thermal environments are optimal and which imposes a level of stress, as this is highly dependent
on the biological context and traits investigated (David et al. 1997; Hoffmann 2010). We reared 166 DGRP lines at five different developmental temperatures and quantified cold tolerance in
adults from each developmental temperature. We performed quantitative genetic analyses to estimate genetic and environmental variance components, heritabilities, evolvabilities and
phenotypic/genetic correlations of cold tolerance in each developmental environment and for (slope-based) plasticity. This allowed three main questions to be answered. (1) To what extent is
cold tolerance phenotypically and genetically correlated between developmental environments, and are these correlations dependent on environmental similarity? (2) Do the developmental
temperatures impact on variance components, heritability and evolvability of cold tolerance within developmental temperatures, and on plastic cold tolerance? (3) Is there a trade-off between
cold tolerance at individual developmental temperatures and the plasticity of cold tolerance, and is this trade-off dependent on the environment? MATERIALS AND METHODS EXPERIMENTAL SETUP
This study included 166 lines from the _D. melanogaster_ Genetic Reference Panel (DGRP) obtained from Bloomington _Drosophila_ Stock Centre (NIH P40OD018537), maintained at 23 °C, 50%
relative humidity and a 12:12 h photoperiod on a standard oatmeal–sugar–agar–yeast medium for two generations before starting the experiment. The experimental procedure follows the setup
previously described in Ørsted et al. (2018a). In summary, we reared each DGRP line at five different temperatures: 17, 20, 23, 26 and 29 °C, from eggs to adults in vials containing 7 mL
standard medium (for details on medium composition see Ørsted et al. 2018b). Eggs were laid by approximately 20 adult flies in three 12 h periods. Vials were checked daily and eclosed flies
were sexed under CO2 anaesthesia. Males were transferred to vials containing fresh medium, and kept at the respective developmental temperature for 48 h. At age 60 ± 12 h, the cold tolerance
of around 10 males (for exact numbers see Table S1) per DGRP line per temperature were assessed using the dynamic measure Critical Thermal Minimum (CTmin; Overgaard et al. 2012). In this
standardised procedure, individual flies were placed in glass vials in a water bath pre-set to 23 °C. Water in the bath was gradually cooled at a rate of 0.1 °C/min. The temperature at which
a fly enters chill coma, i.e., when all movement ceases, was recorded and defines CTmin. In total, 7690 flies were scored for CTmin by the same person to reduce potential scoring bias. We
chose males because virginity is not likely to impact on males to the same extent as it does to females (Goenaga et al. 2012), and we were not able to assure that all flies were virgins when
assessed. Phenotypic plasticity of cold tolerance was estimated as the regression coefficient of a linear regression, i.e., slope of CTmin on rearing temperature. Linear regression analysis
provided the best fit compared to polynomial regression, as assessed by the coefficient of determination (results not shown). In addition, linear norms-of-reaction are the most commonly
used in studies of phenotypic plasticity (Valladares et al. 2006), and especially for the relationship between CTmin and developmental temperature, which is typically linear in _Drosophila_
(Schou et al. 2017; Ørsted et al. 2018a). QUANTITATIVE GENETIC ANALYSES Genetic analyses was performed with the average information restricted maximum-likelihood (AI-REML) procedure (Madsen
et al. 1994) utilising the R package QGG (http://psoerensen.github.io/qgg/) that links to DMU (http://dmu.agrsci.dk/DMU/). We estimated variance components, genetic correlations and broad
and narrow sense heritabilities (_H_2 and _h_2, respectively) based on multi-trait linear mixed models. For CTmin we fitted a five-trait multi-trait model including all developmental
temperatures, and to estimate genetic correlations between CTmin at individual temperatures and plasticity we fitted a bivariate model. The multi-trait model including _i_-traits was
generalised as: $$\left[ {\begin{array}{*{20}{c}} {{\mathbf{y}}_1} \cr \vdots \cr {{\mathbf{y}}_i} \end{array}} \right] = \left[ {\begin{array}{*{20}{c}} {{\mathbf{X}}_1} \cr \vdots \cr
{{\mathbf{X}}_i} \end{array}\begin{array}{*{20}{c}} {{\mathbf{b}}_1} \cr \vdots \cr {{\mathbf{b}}_i} \end{array}} \right] + \left[ {\begin{array}{*{20}{c}} {{\mathbf{Z}}_1} \cr \vdots \cr
{{\mathbf{Z}}_i} \end{array}\begin{array}{*{20}{c}} {{\mathbf{g}}_1} \cr \vdots \cr {{\mathbf{g}}_i} \end{array}} \right] + \left[ {\begin{array}{*{20}{c}} {{\mathbf{e}}_1} \cr \vdots \cr
{{\mathbf{e}}_i} \end{array}} \right]$$ where, Y_i_ was a vector of phenotypic observations (i.e., either temperature specific CTmin or plasticity), X_i_ and Z_i_ are design matrices linking
fixed and random genetic effects to the phenotypes. The B_i_ is a vector of fixed effects (experimental day, water bath, time on day (for CTmin), _Wolbachia_ infection status, and five
major chromosomal inversions (only when estimating the additive genetic variance), and _E__i_ is a vector of random residuals (\(\boldsymbol {e}_i\sim N\left( {0, \boldsymbol {I}\sigma
_{e_i}^2} \right)\)). Estimates of the genetic variance components (and therefore _H_2) were obtained by assuming the DGRP lines to be independent, which we modelled by an identity block
matrix (_I__L_) as (co)variance structure of the genetic values, \(g{\mathrm{\sim }}N\left( {0,{{\boldsymbol {I}}}_L\sigma _g^2} \right)\). To estimate the additive genetic variance
components (including _h_2) we estimated the realised relationship among DGRP lines from publicly available single nucleotide polymorphisms (SNPs). Thus _h_2 was the proportion of phenotypic
variance captured by common segregating SNPs within the DGRP. We used standard filtering for the DGRP, i.e., only considering biallelic SNPs with minor allele frequencies ≥0.05, and for
which the Phred scaled variant quality was greater than 500, and the genotype call ≥0.8 (Mackay et al. 2012; Huang et al. 2014). This yielded 1,725,755 genetic markers distributed on the six
chromosome arms (2L, 2R, 3L, 3R, 4 and X). The notation is similar as above, however, here we included five major polymorphic inversions present in the DGRP system; _I2Lt_, _I2Rns_, _I3Rp_,
_I3Rk_ and _I3RMo_), _G__i_ was a vector of random genetic effects, \({\boldsymbol{g}}_i{\mathrm{\sim }}N\left( {0,{\boldsymbol{G}}\sigma _{g_i}^2} \right)\), where _G_ was the additive
genomic relationship matrix, and _E__i_ was a random residual, \({\boldsymbol{e}}_i{\mathrm{\sim }}N\left( {0,{\boldsymbol{I}}\sigma _{e_i}^2} \right)\). The _G_ matrix was computed as _G_ =
_WW_′/_m_ (VanRaden 2008), where _m_ was the number of genetic markers, and _W_ was a centred and scaled genotype matrix. Each column vector of _W_, \({\boldsymbol{w}}_i =
\frac{{{\boldsymbol{a}}_i - 2p_i}}{{\sqrt {2p_i\left( {1 - p_i} \right)} }}\), _pi_ was the frequency of the minor allele at locus _i_, and _A__i_ was the _i_th column vector of the allele
count matrix, _A_, with the genotypes encoded as 0, 2, referring to the number of the minor allele. The SNP data, including karyotype inversions and _Wolbachia_ infection status, can be
obtained from the DGRP2 website (http://dgrp2.gnets.ncsu.edu). To achieve a replicated structure of the plasticity measures, which was needed to obtain accurate estimates of the genetic
parameters, we grouped the CTmin data within each DGRP line into three groups of individuals according to day of assay, time of day, and water bath. Based on these replicate groups, we
obtained three replicates measures of slope per DGRP line. The slope estimates were robust against re-sampling of random individuals into groups of the same size (for details see Ørsted et
al. 2018a). Phenotypic correlations were computed as Pearson’s correlation using within DGRP line means, and standard errors were estimated as: \({\mathrm{SE}}_{r} = \sqrt {\frac{{1 -
{r}^2}}{{n - 2}}}\), where _n_ was the sample size. We also computed correlations between the rank order of the DGRP lines (_r__r_), in which lines are ranked within rearing regimes from 1
to 166. All variance components were tested under the null hypothesis of being zero using Wald’s test. In addition, we tested if correlations differed from zero, i.e., the estimate deviated
more than 1.645 × SE from 0 (_p_ < 0.05). We performed pairwise comparisons of genetic correlations, _r__g_ among CTmin in the five rearing temperatures (e.g., _r__g_ (17, 20 °C) vs.
_r__g_ (17, 23 °C)), as well as between correlations between CTmin in each environment and plasticity across environments. These comparisons were based on Fisher’s _z_ transformation.
Evolvability (_I_A) was computed at each developmental temperature as: \({I}_{\mathrm{A}} = \widehat {V}_{\mathrm{A}}/\left( {\overline {{\mathrm{CT}}} _{{\mathrm{min}}}} \right)^2 \times
100\), where \(\overline {{\mathrm{CT}}} _{{\mathrm{min}}}\) is the mean CTmin across all DGRP lines in a given environment (Houle 1992; Hoffmann et al. 2016). All analyses were done within
the R programming environment (R Core Team 2017). RESULTS HERITABLE VARIATION FOR COLD TOLERANCE AT THE INDIVIDUAL REARING TEMPERATURES AND PLASTICITY We observed a significant increase in
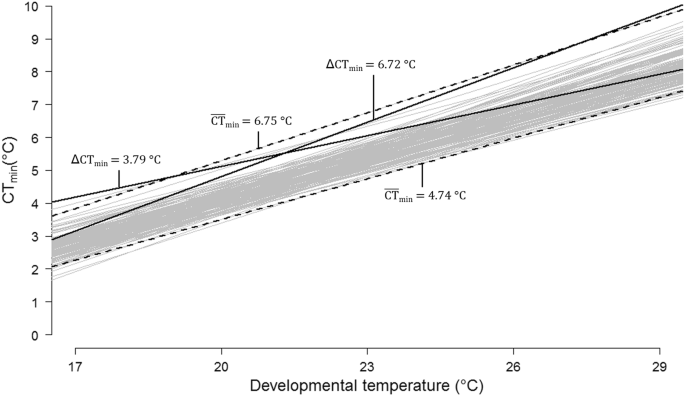
line mean CTmin, i.e., a decrease in cold tolerance, with increasing rearing temperature (Fig. 1, Table 1, _F_4,804 = 3956; _p_ < 0.001). Average CTmin (±SE) across DGRP lines spanned
from 2.81 ± 0.03 °C for flies reared at 17 to 7.80 ± 0.04 °C for flies reared at 29 °C, with considerable variation among DGRP lines within rearing environment (Fig. 2, Table 1 and Table
S1). The estimates of _H_2 and _h_2 for CTmin were consistent across the developmental temperatures, with estimates in the range of 0.41–0.48 and 0.27–0.34 for _H_2 and _h_2, respectively
(Table 2). Plasticity of cold tolerance was estimated as the slope of a linear regression of CTmin as a function of developmental temperature. The slope was significantly different from zero
in all DGRP lines (_t_(3–21) > 2.13; _p_ < 0.001). A significant interaction between line and developmental temperature (two-way ANOVA; _F_(165,477) = 2.22, _p_ < 0.001) indicated
a GxE interaction, reflecting differences among DGRP lines in slope ranging from 0.32 to 0.57 °C in CTmin per 1 °C increase in developmental temperature. The heritability estimates for
plasticity of CTmin were 0.65 and 0.51 for _H_2 and _h_2, respectively. We observed a higher total genetic variance \(\left( {\widehat {V}_{\mathrm{G}}} \right)\), additive genetic variance
\(\left( {\widehat {V}_{\mathrm{A}}} \right)\) and environmental variance \(\left( {\widehat {V}_{\mathrm{E}}} \right)\) of CTmin at 29 °C than at 26 °C (Table 2). At 17, 20 and 23 °C, we
observed a decrease in all variance components as compared to 29 °C (Table 2). In contrast, for evolvability (_I_A; mean corrected \(\widehat {V}_{\mathrm{A}}\)) of CTmin, we observed higher
values at lower temperatures, i.e., from 0.67 at 17 to 0.18 at 29 °C (Table 2). CORRELATIONS BETWEEN CTMIN FROM DIFFERENT DEVELOPMENTAL TEMPERATURES INCREASED WITH ENVIRONMENTAL SIMILARITY
We found strong positive genetic correlations (_r__g_) between CTmin for DGRP lines at the different rearing temperatures (Fig. 2). The _r__g_ values were inversely proportional to the
difference between developmental temperatures, i.e., correlations between similar developmental temperatures were larger than between dissimilar developmental temperatures. This pattern was
confirmed by statistical pairwise comparisons of _r__g_. The phenotypic correlations (_r__p_) followed the same pattern, decreasing with increasing environmental dissimilarity (Fig. 2).
Similarly, the rank order of the DGRP lines was more positively correlated across thermal environments that were more similar to each other (Fig. 2). TRADE-OFFS BETWEEN CTMIN AT INDIVIDUAL
DEVELOPMENTAL TEMPERATURES AND PLASTIC COLD TOLERANCE DEPENDED ON THE ENVIRONMENT The phenotypic (_r__p_) correlations between CTmin at the individual temperatures and slope of CTmin across
temperatures depended on the environment. At 17 °C, the correlation was significantly negative (Fig. 3), i.e., in this environment genotypes with a high cold tolerance (low CTmin) tended to
have a high plasticity of cold tolerance. However, we did not find this association at 20 °C. At higher temperatures, correlations were significantly positive (Fig. 3), thus genotypes with a
high cold tolerance (low CTmin) tended to have low plasticity of cold tolerance, suggesting a trade-off between inherent cold tolerance at these developmental temperatures and plasticity.
The genetic correlations (_r__g_) followed the same pattern, particularly when involving CTmin scores following development at 17, 26 and 29 °C (Fig. 3). DISCUSSION In the present study, we
investigated the effects of developmental temperature on quantitative genetic parameters of cold tolerance, which is a key stress tolerance trait. We showed that environmental conditions can
directly influence the quantitative genetic basis of cold tolerance by affecting variance components, especially \(\widehat {V}_{\mathrm{A}}\). It is expected that if standing genetic
variation is environmentally dependent, selection responses will also be affected (Roff 2002; Bijlsma and Loeschcke 2005; Wilson et al. 2006; Husby et al. 2011; Wood and Brodie 2016). This
has been demonstrated in recent studies showing that genetic variation and evolutionary potential of stress resistance, morphological traits and life-history traits depend on the
environmental conditions (van Heerwaarden and Sgrò 2011; Bubliy et al. 2012; Kristensen et al. 2015; Bastide et al. 2016; Zhao et al. 2016; Feldman et al. 2017). Such findings can be
explained by environment-specific gene expression, i.e., the impact of genes on a trait can depend on prior environmental exposures. This highlights the need to perform experiments and field
studies under environmental conditions that are relevant to organisms at the time traits are under selection (Hoffmann and Parsons 1991; Hoffmann and Merilä 1999). This is important not
only from an ecological and evolutionary perspective (Bay et al. 2017) but also for animal and plant breeding, in which effects of the environment on the genetic basis of production traits
pose a challenge, such as for genomic selection in heterogeneous environments (Kadarmideen et al. 2006; Zhao et al. 2016). It has long been debated whether some types of environmental
conditions, and particularly stressful or benign conditions, affect heritable variation in distinct ways (Hoffmann and Parsons 1991; Møller and Swaddle 1998). Here we showed an increase in
\(\widehat {V}_{\mathrm{A}}\) of cold resistance with increasing developmental temperature, and heritability estimates being similar across developmental temperatures (Table 2). However, for
mean corrected \(\widehat {V}_{\mathrm{A}}\), (i.e., evolvability (_I_A)), we found higher values at 17 and 20 °C than in the range 23–29 °C, suggesting that the evolutionary capacity for
increasing cold tolerance might be more restricted in warm than in cold environments. This is supported by recent _Drosophila_ studies showing that the potential for evolution is lower in
relatively warmer conditions (Schou et al. 2014; Kristensen et al. 2015). In nature, individuals reared under cold conditions are more likely to encounter subsequent extreme cold conditions,
therefore stronger selection for cold tolerance might be expected in natural populations experiencing low developmental temperatures compared to in hotter environments (Ayrinhac et al.
2004). Thus, in the case of cold tolerance, developmental environments with low temperatures might be considered more optimal than those with high temperatures. However, because we did not
have a direct measure of stress in each thermal environment, we cannot confidently determine whether the patterns in \(\widehat {V}_{\mathrm{A}}\) and _I_A are associated with unfavourable
environmental conditions (Hoffmann and Merilä 1999; Swindell and Bouzat 2006; Talloen et al. 2009). The environment can also affect genetic variation via environment-dependent gene action,
where genes influencing a certain trait in one environment may not be important for that trait in a different environment. In such cases selection responses can be slowed or even become
maladaptive (Steinger et al. 2003). This will often be expressed as altered genetic correlations either between different traits in one environment or between the same trait in a range of
environments (Vieira et al. 2000; Sgrò and Hoffmann 2004; Agrawal and Stinchcombe 2009). Here we found positive genetic and phenotypic correlations between cold tolerance across
environments, i.e., genotypes with high cold tolerance when developed at one temperature also tended to have high cold tolerance when developed at other temperatures, implying an overlap in
the sets of alleles influencing cold tolerance across developmental thermal environments (Via and Lande 1985; Falconer and Mackay 1996; Agrawal and Stinchcombe 2009). However, we also showed
that the magnitude of correlations between thermal environments decreased as developmental temperatures diverged. This suggests a shared genetic basis for cold tolerance between rearing
environments, which contributes less to genetic variation as environments become more dissimilar. Although evidence for such patterns has rarely been investigated, a few studies have found
similar results; Sgrò and Blows (2004) found declining genetic correlations with environmental dissimilarity in three life-history traits in _Drosophila serrata_, while Stinchcombe et al.
(2010) found declining genetic correlations with environmental distance for growth rate in jewelweed. Our findings are in line with other studies suggesting that the genetic architecture of
fitness components is specific to an environment (Bourret and Garant 2015; Rellstab et al. 2017). This represents a challenge when testing for adaptation at specific loci which often seem to
be environment and population specific (Zhao et al. 2011; Manenti et al. 2016). Knowledge about the genetic architecture of many complex traits has been rapidly accumulating recently, aided
by developments in genomics and quantitative genetics. Typically, such studies identify genes, variance components and heritabilities for a given trait in one environment to predict
evolutionary trajectories. In animal and plant breeding, such knowledge may be used to predict selection responses and devise genomic selection procedures. However, our results show that the
architecture of an important stress resistance trait is highly dependent on environmental conditions, challenging the notion that there are candidate genes and a particular architecture
applicable across a gradient of environmental conditions. An important issue for the evolution of thermal resistance is whether an organism’s thermotolerance is constrained by plasticity,
particularly in response to anthropogenic climate change (Stillman 2003; Chown et al. 2010; Levine et al. 2011; Gunderson et al. 2015; Comte and Olden 2017). Some studies point to a negative
association between an organism’s thermal tolerance and plasticity in tolerance (Hoffmann et al. 2003; Kellett et al. 2005; Nyamukondiwa et al. 2011; Gerken et al. 2015; Noh et al. 2017).
Here we showed that any association between cold tolerance and plasticity depend on the environment; both the phenotypic and genetic correlations shifted in sign as developmental
temperatures shifted from 17 to 29 °C (Fig. 3). Given that the lowest CTmin occurred at the lowest developmental temperature, genotypes with a flat slope would likely have a fitness
advantage when exposed to low temperatures, as they would remain the most cold tolerant (i.e., maintain relatively lower CTmin at higher developmental temperatures). However, as indicated by
the negative correlation between slope and CTmin at 17 °C, DGRP lines with low CTmin reared at this temperature, typically had a high CTmin (low cold tolerance) when they developed at
higher temperatures, indicative of a trade-off (Fig. 3a). At low developmental temperatures, lines with low CTmin and a steep slope could be considered “specialists”; i.e., they performed
relatively better in one environment, but relatively poorly across environments. At higher developmental temperatures (23, 26 and 29 °C), we found a positive correlation between CTmin and
the plastic response, suggesting that high cold tolerance (low CTmin) at high developmental temperatures was associated with low plasticity (Fig. 3c–e). Thus, genotypes with low CTmin at
higher developmental temperatures could also be considered as the “specialists” because they performed relatively better in one environment. This shift in sign of the correlations reflects a
reversal in the ordering of genotypes for cold resistance across the thermal gradient, with a tipping point at 20 °C, when there was no correlation between cold tolerance and plasticity.
These genotypic differences in constraints may reflect differential environmental sensitivity and suggests that the fitness effect of plasticity depends heavily on the environment where
genotypes are reared. Thus, the fitness landscape of cold tolerance needs to be considered in the context of environmental frequencies, i.e., how likely are individuals reared under one set
of thermal conditions to encounter subsequent extreme cold conditions (Ayrinhac et al. 2004)? Our findings support those of Levine et al. (2011) who suggested a “directionality” of
plasticity in gene expression depending on the thermal environment commonly encountered in the native range of populations of _D. melanogaster_. This has implications for understanding
developmental processes in variable environments (Stearns et al. 1991), and also for understanding local adaptation in thermal tolerances in populations with a broad geographic distribution
along an environmental cline (Ayrinhac et al. 2004; Levine et al. 2011; Cooper et al. 2012; Kellermann et al. 2012; Kristensen et al. 2015). Our results indicate that the quantitative
genetic basis of basal cold tolerance and plastic cold tolerance is highly environment specific, which is important for predicting selection responses in natural and variable environments
(Sgrò and Hoffmann 2004; Agrawal and Stinchcombe 2009). Most studies investigating phenotypic and genetic correlations and/or candidate genes between thermal environments focus on only two
temperatures (Guerra et al. 1997; Ellers and Driessen 2011; Ketola et al. 2012) or investigate two settings such as field _vs_. lab conditions (Kristensen et al. 2008; Overgaard et al.
2010), and thus do not provide a comprehensive picture of changing genetic correlations and architectures across environments. A meta-analysis by Charmantier and Garant (2005) specifically
called for approaches that consider genetic variability under multiple conditions, and ideally along a continuous environmental gradient such as in the present study. Because correlations
within and between traits can change between environments or even shift in sign (Sgrò and Hoffmann 2004; Agrawal and Stinchcombe 2009) as evident in this study, we reiterate this call and
caution about genetic constraints and trade-offs deduced from studies that consider only one or two environments. In conclusion, we showed that the expression of additive genetic variation
for cold tolerance was environment dependent, and that evolvability of cold resistance decreased with increasing developmental temperatures. We also showed that the relationship between
basal and plastic cold tolerance was affected by the environment, and even switched sign across a gradient of developmental temperatures. We further provided evidence for an evolutionary
trade-off between cold tolerance in one environment and cold tolerance across environment. It will be interesting to test for these patterns in relevant ecological settings, such as in
populations exposed to strong diurnal and seasonal temperature fluctuations. REFERENCES * Agrawal AF, Stinchcombe JR (2009) How much do genetic covariances alter the rate of adaptation? Proc
R Soc B Biol Sci 276:1183–1191 Article Google Scholar * Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL (2013) Heat freezes niche evolution. Ecol Lett
16:1206–1219 Article PubMed Google Scholar * Auld JR, Agrawal AA, Relyea RA (2010) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc B Biol Sci 277:503–511
Article Google Scholar * Ayrinhac A, Debat V, Gibert P, Kister A-G, Legout H, Moreteau B et al. (2004) Cold adaptation in geographical populations of _Drosophila melanogaster_: phenotypic
plasticity is more important than genetic variability. Funct Ecol 18:700–706 Article Google Scholar * Bastide H, Lange JD, Lack JB, Yassin A, Pool JE (2016) A variable genetic architecture
of melanic evolution in _Drosophila melanogaster_. Genetics 204:1307–1319 Article CAS PubMed PubMed Central Google Scholar * Bay RA, Rose N, Barrett R, Bernatchez L, Ghalambor CK,
Lasky JR et al. (2017) Predicting responses to contemporary environmental change using evolutionary response architectures. Am Nat 189:463–473 Article PubMed Google Scholar * Bijlsma R,
Loeschcke V (2005) Environmental stress, adaptation and evolution: an overview. J Evol Biol 18:744–9 Article CAS PubMed Google Scholar * Bourret A, Garant D (2015) candidate
gene-environment interactions and their relationships with timing of breeding in a wild bird population Ecol Evol 5:3628–3641 Article PubMed PubMed Central Google Scholar * Bubliy OA,
Kristensen TN, Kellermann V, Loeschcke V (2012) Humidity affects genetic architecture of heat resistance in _Drosophila melanogaster_. J Evol Biol 25:1180–1188 Article CAS PubMed Google
Scholar * Calosi P, Bilton DT, Spicer JI (2008) Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol Lett 4:99–102 Article PubMed Google Scholar *
Charmantier A, Garant D (2005) Environmental quality and evolutionary potential: lessons from wild populations. Proc R Soc B Biol Sci 272:1415–1425 Article Google Scholar * Chown SL,
Hoffmann AA, Kristensen TN, Angilletta MJ, Stenseth N, Pertoldi C (2010) Adapting to climate change: a perspective from evolutionary physiology. Clim Res 43:3–15 Article Google Scholar *
Comte L, Olden JD (2017) Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob Change Biol 23:728–736 Article Google Scholar * Cooper BS,
Tharp JM, Jernberg II, Angilletta MJ (2012) Developmental plasticity of thermal tolerances in temperate and subtropical populations of _Drosophila melanogaster_. J Therm Biol 37:211–216
Article Google Scholar * Czesak ME, Fox CW, Wolf JB (2006) Experimental evolution of phenotypic plasticity: how predictive are cross-environment genetic correlations? Am Nat 168:323–35
PubMed Google Scholar * David JR, Gibert P, Gravot E, Petavy G, Morin JP, Karan D et al. (1997) Phenotypic plasticity and developmental temperature in drosophila: analysis and significance
of reaction norms of morphometrical traits. J Therm Biol 22:441–451 Article Google Scholar * Dingemanse NJ, Van der Plas F, Wright J, Reale D, Schrama M, Roff DA et al. (2009) Individual
experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc R Soc B Biol Sci 276:1285–1293 Article Google Scholar *
Ellers J, Driessen G (2011) Genetic correlation between temperature-induced plasticity of life-history traits in a soil arthropod. Evol Ecol 25:473–484 Article Google Scholar * Etterson
JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 294:151–154 Article CAS PubMed Google Scholar * Falconer DS, Mackay TFC (1996) Introduction to
quantitative genetics, 4th edn. Longman Publishing Group, Harlow, UK Google Scholar * Feldman MJ, Paul RE, Banan D, Barrett JF, Sebastian J, Yee MC et al. (2017) Time dependent genetic
analysis links field and controlled environment phenotypes in the model C4 grass Setaria. PLOS Genet 13:e1006841 * Franke K, Dierks A, Fischer K (2012) Directional selection on cold
tolerance does not constrain plastic capacity in a butterfly. BMC Evol Biol 12:235 Article PubMed PubMed Central Google Scholar * Gebhardt-Henrich SG, van Noordwijk AJ (1991) Nestling
growth in the Great Tit I. Heritability estimates under different environmental conditions. J Evol Biol 4:341–362 Article Google Scholar * Gerken AR, Eller OC, Hahn DA, Morgan TJ (2015)
Constraints, independence, and evolution of thermal plasticity: probing genetic architecture of long- and short-term thermal acclimation. Proc Natl Acad Sci USA 112:4399–4404 Article CAS
PubMed PubMed Central Google Scholar * Goenaga J, Mensch J, Fanara JJ, Hasson E (2012) The effect of mating on starvation resistance in natural populations of _Drosophila melanogaster_.
Evol Ecol 26:813–823 Article Google Scholar * Guerra D, Cavicchi S, Krebs R, Loeschcke V (1997) Resistance to heat and cold stress in _Drosophila melanogaster_: intra and inter population
variation in relation to climate. Genet Sel Evol 29:497–510 Article PubMed Central Google Scholar * Gunderson AR, Stillman JH (2015) Plasticity in thermal tolerance has limited potential
to buffer ectotherms from global warming. Proc R Soc B Biol Sci 282:1–8 * van Heerwaarden B, Sgrò CM (2011) The effect of developmental temperature on the genetic architecture underlying
size and thermal clines in _Drosophila melanogaster_ and _D. simulans_ from the east coast of Australia. Evolution 65:1048–1067 Article PubMed Google Scholar * Hercus MJ, Hoffmann AA
(2000) Maternaland grandmaternal age influence offspring fitness in Drosophila. Proc R Soc B Biol Sci 267:2105–2110 Article CAS Google Scholar * Hoffmann AA (2010) Physiological climatic
limits in _Drosophila_: patterns and implications. J Exp Biol 213:870–880 Article CAS PubMed Google Scholar * Hoffmann AA, Merilä J (1999) Heritable variation and evolution under
favourable and unfavourable conditions. Trends Ecol Evol 14:96–101 Article CAS PubMed Google Scholar * Hoffmann AA, Merilä J, Kristensen TN (2016) Heritability and evolvability of
fitness and nonfitness traits: lessons from livestock. Evolution 70:1770–1779 Article PubMed Google Scholar * Hoffmann AA, Parsons PA (1991) Evolutionary genetics and environmental
stress. Oxford University Press, Oxford, UK * Hoffmann AA, Sørensen JG, Loeschcke V (2003) Adaptation of _Drosophila_ to temperature extremes: bringing together quantitative and molecular
approaches. J Therm Biol 28:175–216 Article Google Scholar * Houle D (1992) Comparing evolvability and variability of quantitative traits. Genetics 130:195–204 CAS PubMed PubMed Central
Google Scholar * Huang W, Massouras A, Inoue Y, Peiffer J, Ràmia M, Tarone AM et al. (2014) Natural variation in genome architecture among 205 _Drosophila melanogaster_ Genetic Reference
Panel lines. Genome Res 24:1193–1208 Article CAS PubMed PubMed Central Google Scholar * Husby A, Visser ME, Kruuk LEB (2011) Speeding up microevolution: the effects of increasing
temperature on selection and genetic variance in a wild bird population. PLOS Biol 9:e1000585 * de Jong G (1990) Quantitative genetics of reaction norms. J Evol Biol 3:447–468 Article
Google Scholar * Kadarmideen HN, Von Rohr P, Janss LLG (2006) From genetical genomics to systems genetics: potential applications in quantitative genomics and animal breeding. Mamm Genome
17:548–564 Article CAS PubMed PubMed Central Google Scholar * Kellermann V, Overgaard J, Hoffmann AA, Fløjgaard C, Svenning J-C, Loeschcke V (2012) Upper thermal limits of _Drosophila_
are linked to species distributions and strongly constrained phylogenetically. Proc Natl Acad Sci USA 109:16228–33 Article CAS PubMed PubMed Central Google Scholar * Kellett M, Hoffmann
AA, Mckechnie SW (2005) Hardening capacity in the _Drosophila melanogaster_ species group is constrained by basal thermotolerance. Funct Ecol 19:853–858 Article Google Scholar * Ketola T,
Kellermann V, Kristensen TN, Loeschcke V (2012) Constant, cycling, hot and cold thermal environments: strong effects on mean viability but not on genetic estimates. J Evol Biol 25:1209–1215
Article CAS PubMed Google Scholar * Kimura MT (2004) Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 140:442–449 Article
PubMed Google Scholar * Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V (2008) Costs and benefits of cold acclimation in field-released _Drosophila_. Proc Natl
Acad Sci USA 105:216–221 Article CAS PubMed Google Scholar * Kristensen TN, Overgaard J, Lassen J, Hoffmann AA, Sgrò C (2015) Low evolutionary potential for egg-to-adult viability in
_Drosophila melanogaster_ at high temperatures. Evolution 69:803–814 Article PubMed Google Scholar * Levine MT, Eckert ML, Begun DJ (2011) Whole-genome expression plasticity across
tropical and temperate _Drosophila melanogaster_ populations from eastern Australia. Mol Biol Evol 28:249–256 Article CAS PubMed Google Scholar * Mackay TFC, Richards S, Stone EA,
Barbadilla A, Ayroles JF, Zhu D et al. (2012) The _Drosophila melanogaster_ Genetic Reference Panel. Nature 482:173–178 Article CAS PubMed PubMed Central Google Scholar * Madsen P,
Jensen J, Thompson R (1994). Estimation of (co)-variance components by REML in multivariate mixed linear models using average of observed and expected information. In: FifthWorld Congress of
Genetics Applied to Livestock Production, Guelph, Ontario, Canada, pp 455–462. * Manenti T, Sørensen JG, Moghadam NN, Loeschcke V (2016) Few genetic and environmental correlations between
life-history and stress resistance traits affect adaptation to fluctuating thermal regimes. Heredity 117:149–154 Article CAS PubMed PubMed Central Google Scholar * Murren CJ, Auld JR,
Callahan H, Ghalambor CK, Handelsman CA, Heskel MA et al. (2015) Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115:293–301
Article CAS PubMed PubMed Central Google Scholar * Møller A, Swaddle K (1998) Asymmetry, developmental stability and evolution. Oxford University Press, Oxford, UK Google Scholar * Noh
S, Everman ER, Berger CM, Morgan TJ (2017) Seasonal variation in basal and plastic cold tolerance: adaptation is influenced by both long- and short-term phenotypic plasticity. Ecol Evol
7:5248–5257 Article PubMed PubMed Central Google Scholar * Nyamukondiwa C, Terblanche JS, Marshall KE, Sinclair BJ (2011) Basal cold but not heat tolerance constrains plasticity among
_Drosophila_ species (Diptera: Drosophilidae). J Evol Biol 24:1927–1938 Article CAS PubMed Google Scholar * Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA (2011) Thermal tolerance
in widespread and tropical _Drosophila_ species: does phenotypic plasticity increase with latitude? Am Nat 178:80–96 Article Google Scholar * Overgaard J, Kristensen TN, Sørensen JG (2012)
Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLOS ONE 7:1–7 Article CAS Google Scholar * Overgaard J, Sørensen JG, Jensen LT, Loeschcke V,
Kristensen TN (2010) Field tests reveal genetic variation for performance at low temperatures in _Drosophila melanogaster_. Funct Ecol 24:186–195 Article Google Scholar * R Core Team
(2017) R: a language and environment for statistical computing. Version 3.4.0. R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org/ Google Scholar * Rellstab
C, Fischer MC, Zoller S, Graf R, Tedder A, Shimizu KK et al. (2017) Local adaptation (mostly) remains local: reassessing environmental associations of climate-related candidate SNPs in
_Arabidopsis halleri_. Heredity 118:193–201 Article CAS PubMed Google Scholar * Roff DA (2002) Life history evolution. Sinauer Associates, Sunderland, UK Google Scholar * Rohde PD,
Gaertner B, Wards K, Sørensen P, Mackay TFC (2017) Genomic analysis of genotype-by-social environment interaction for _Drosophila melanogaster_ aggressive behavior. Genetics 206:1969–1984
Article CAS PubMed PubMed Central Google Scholar * Schou MF, Kristensen TN, Kellermann V, Schlötterer C, Loeschcke V (2014) A _Drosophila_ laboratory evolution experiment points to low
evolutionary potential under increased temperatures likely to be experienced in the future. J Evol Biol 27:1859–1868 Article CAS PubMed Google Scholar * Schou MF, Mouridsen MB, Sørensen
JG, Loeschcke V (2017) Linear reaction norms of thermal limits in _Drosophila_: predictable plasticity in cold but not in heat tolerance. Funct Ecol 31:934–945 Article Google Scholar *
Sgrò CM, Blows MW (2004) The genetic covariance among clinal environments after adaptation to an environmental gradient in _Drosophila serrata_. Genetics 167:1281–1291 Article PubMed
PubMed Central Google Scholar * Sgrò CM, Hoffmann AA (2004) Genetic correlations, trade-offs and environmental variation. Heredity 93:241–248 Article PubMed Google Scholar * Sheldon BC,
Kruuk LEB, Merilä J (2003) Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution 57:406–420 Article CAS PubMed Google Scholar *
Spichtig M, Kawecki TJ (2004) The maintenance (or not) of polygenic variation by soft selection in heterogeneous environments. Am Nat 164:70–84 Article PubMed Google Scholar * Stearns S,
de Jong G, Newman B (1991) The effects of phenotypic plasticity on genetic correlations. Trends Ecol Evol 6:122–126 Article CAS PubMed Google Scholar * Steinger T, Roy BA, Stanton ML
(2003) Evolution in stressful environments II: adaptive value and costs of plasticity in response to low light in _Sinapis arvensis_. J Evol Biol 16:313–323 Article CAS PubMed Google
Scholar * Stillman JH (2003) Acclimation capacity underlies susceptibility to climate change. Science 301:65 Article CAS PubMed Google Scholar * Stinchcombe JR, Izem R, Heschel MS,
McGoey BV, Schmitt J (2010) Across environment genetic correlations and the frequency of selective environments shape the evolutionary dynamics of growth rate in impatiens capensis.
Evolution 64:2887–2903 PubMed Google Scholar * Strachan LA, Tarnowski-Garner HE, Marshall KE, Sinclair BJ (2011) The evolution of cold tolerance in _Drosophila_ larvae. Physiol Biochem
Zool 84:43–53 Article PubMed Google Scholar * Sunday JM, Bates AE, Dulvy NK (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc R Soc B Biol Sci 278:1823–1830
Article Google Scholar * Swindell WR, Bouzat JL (2006) Associations between environmental stress, selection history, and quantitative genetic variation in _Drosophila melanogaster_.
Genetica 127:311–20 Article PubMed Google Scholar * Talloen W, Van Dongen S, Van Dyck H, Lens L (2009) Environmental stress and quantitative genetic variation in butterfly wing
characteristics. Evol Ecol 23:473–485 Article Google Scholar * Telonis-Scott M, Hallas R, McKechnie SW, Wee CW, Hoffmann AA (2009) Selection for cold resistance alters gene transcript
levels in _Drosophila melanogaster_. J Insect Physiol 55:549–555 Article CAS PubMed Google Scholar * Tonsor SJ, Elnaccash TW, Scheiner SM (2013) Developmental instability is genetically
correlated with phenotypi plasticity, constraining heritability, and fitness. Evolution 67:2923–2935 PubMed Google Scholar * Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative
estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116 Article Google Scholar * VanRaden PM (2008)
Efficient methods to compute genomic predictions. J Dairy Sci 91:4414–4423 Article CAS PubMed Google Scholar * Via S, Lande R (1985) Genotype-environment interaction and the evolution of
phenotypic plasticity. Evolution 39:505–522 Article PubMed Google Scholar * Vieira C, Pasyukova EG, Zeng ZB, Hackett JB, Lyman RF, Mackay TF (2000) Genotype-environment interaction for
quantitative trait loci affecting life span in _Drosophila melanogaster_. Genetics 154:213–227 CAS PubMed PubMed Central Google Scholar * Visscher PM, Hill WG, Wray NR (2008)
Heritability in the genomics era—concepts and misconceptions. Nat Rev Genet 9:255–266 Article CAS PubMed Google Scholar * Williams CM, Henry HAL, Sinclair BJ (2014) Cold truths: how
winter drives responses of terrestrial organisms to climate change. Biol Rev 90:214–235 Article PubMed Google Scholar * Wilson AJ, Pemberton JM, Pilkington JG, Coltman DW, Mifsud DV,
Clutton-Brock TH et al. (2006) Environmental coupling of selection and heritability limits evolution. PLoS Biol 4:1270–1275 Article CAS Google Scholar * Wood CW, Brodie ED (2016)
Evolutionary response when selection and genetic variation covary across environments. Ecol Lett 19:1189–1200 Article PubMed Google Scholar * Zhao K, Tung C-W, Eizenga GC, Wright MH, Ali
ML, Price AH et al. (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in _Oryza sativa_. Nat Commun 2:467 Article PubMed CAS Google Scholar *
Zhao W, Wang X, Wang H, Tian J, Li B, Chen L et al. (2016) Genome-wide identification of QTL for seed yield and yield-related traits and construction of a high-density consensus map for QTL
comparison in _Brassica napus_. Front Plant Sci 7:17 PubMed PubMed Central Google Scholar * Ørsted M, Malmendal A, Muñoz J, Kristensen TN (2018a) Metabolic and functional phenotypic
profiling of _Drosophila melanogaster_ reveals reduced sex differentiation under stressful environmental conditions. Biol J Linn Soc 123:155–162 Article Google Scholar * Ørsted M, Rohde
PD, Hoffmann AA, Sørensen P, Kristensen TN (2018b) Environmental variation partitioned into separate heritable components. Evolution 72:136–152 Article PubMed Google Scholar * Ørsted M,
Schou MF, Kristensen TN (2017) Biotic and abiotic factors investigated in two _Drosophila_ species—evidence of both negative and positive effects of interactions on performance. Sci Rep
7:40132 Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS DGRP stocks were obtained from the Bloomington _Drosophila_ Stock Centre (NIH P40OD018537).
We thank Iben R. Jensen, Helle Blendstrup, Susan M. Hansen, and Iben V. Nielsen for laboratory assistance. This research was financed by the Danish Natural Science Research Council through
a Sapere aude stipend to TNK (DFF—4002-00036), the Science Industry Endowment Fund and a grant from the Velux Visiting Professor programme to AAH, and by the Danish Strategic Research
Council (GenSAP: Centre for Genomic Selection in Animals and Plants, contract 12-132452) to PS. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry and Bioscience, Section
of Biology and Environmental Science, Aalborg University, Aalborg E, 9220, Denmark Michael Ørsted, Ary Anthony Hoffmann & Torsten Nygaard Kristensen * Department of Bioscience, Section
of Genetics, Ecology and Evolution, Aarhus University, Aarhus C, 8000, Denmark Michael Ørsted & Torsten Nygaard Kristensen * School of Biosciences, Bio21 Molecular Science and
Biotechnology Institute, The University of Melbourne, Parkville, Victoria, 3010, Australia Ary Anthony Hoffmann * Department of Molecular Biology and Genetics, Center for Quantitative
Genetics and Genomics, Aarhus University, Tjele, 8830, Denmark Palle Duun Rohde & Peter Sørensen Authors * Michael Ørsted View author publications You can also search for this author
inPubMed Google Scholar * Ary Anthony Hoffmann View author publications You can also search for this author inPubMed Google Scholar * Palle Duun Rohde View author publications You can also
search for this author inPubMed Google Scholar * Peter Sørensen View author publications You can also search for this author inPubMed Google Scholar * Torsten Nygaard Kristensen View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Michael Ørsted. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare
that they have no conflict of interest. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY TABLE OF CT<SUB>MIN</SUB> RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Ørsted, M., Hoffmann, A.A., Rohde, P.D. _et al._ Strong impact of thermal environment on the quantitative genetic basis of a key stress tolerance trait. _Heredity_
122, 315–325 (2019). https://doi.org/10.1038/s41437-018-0117-7 Download citation * Received: 08 March 2018 * Revised: 20 June 2018 * Accepted: 21 June 2018 * Published: 26 July 2018 * Issue
Date: March 2019 * DOI: https://doi.org/10.1038/s41437-018-0117-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative