Play all audios:
ABSTRACT Idiopathic basal ganglia calcification-1 (IBGC1) is an autosomal dominant disorder characterized by calcification in the basal ganglia, which can manifest a range of
neuropsychiatric symptoms, including parkinsonism. We herein describe a 64-year-old Japanese IBGC1 patient with bilateral basal ganglia calcification carrying a novel _SLC20A2_ variant
(p.Val322Glufs*92). The patient also presented with dopa-responsive parkinsonism with decreased dopamine transporter (DAT) density in the bilateral striatum and decreased cardiac
123I-meta-iodobenzylguanidine uptake. SIMILAR CONTENT BEING VIEWED BY OTHERS BIALLELIC _NAA60_ VARIANTS WITH IMPAIRED N-TERMINAL ACETYLATION CAPACITY CAUSE AUTOSOMAL RECESSIVE PRIMARY
FAMILIAL BRAIN CALCIFICATIONS Article Open access 13 March 2024 COMPUTATIONAL MOLECULAR CHARACTERIZATION OF A NOVEL _SLC20A2_ VARIANT ASSOCIATED WITH PRIMARY FAMILIAL BRAIN CALCIFICATION
Article Open access 28 May 2025 LOSS OF FUNCTION OF _CMPK2_ CAUSES MITOCHONDRIA DEFICIENCY AND BRAIN CALCIFICATION Article Open access 29 November 2022 Idiopathic basal ganglia calcification
(IBGC), also known as Fahr disease or primary familial brain calcification (PFBC), is a disorder characterized by bilateral calcifications in the basal ganglia and other brain regions.
Clinical manifestations of IBGC range from asymptomatic to neuropsychiatric symptoms, including dystonia, parkinsonism, ataxia, and cognitive impairment1. Typically, the inheritance mode of
familial IBGC is an autosomal dominant one and to date, four dominant causal genes of familial IBGC have been identified, including _SLC20A2_ (IBGC1, MIM: #213600), _PDGFRB_ (IBGC4, MIM:
#615007), _PDGFB_ (IBGC5, MIM: #615483), and _XPR1_ (IBGC6, MIM: #616413)2,3,4,5. Recently, _MYORG_ was reported as an autosomal recessive causal gene for IBGC (IBGC7, MIM: #618317)6,7.
Variants in _SLC20A2_, encoding the type III sodium-dependent phosphate transporter 2 (PiT-2), are a major cause of IBGC8,9. Herein, we report an IBGC1 patient with a novel variant in
_SLC20A2_ associated with dopa-responsive parkinsonism. The patient was a 63-year-old Japanese woman who presented to our hospital with a one-month history of lumbago and unsteady gait.
Neurological examination revealed gait disturbance with stooped posture and short steps, but rigidity, tremor, weakness, and cerebellar symptoms were not observed. Computed tomography (CT)
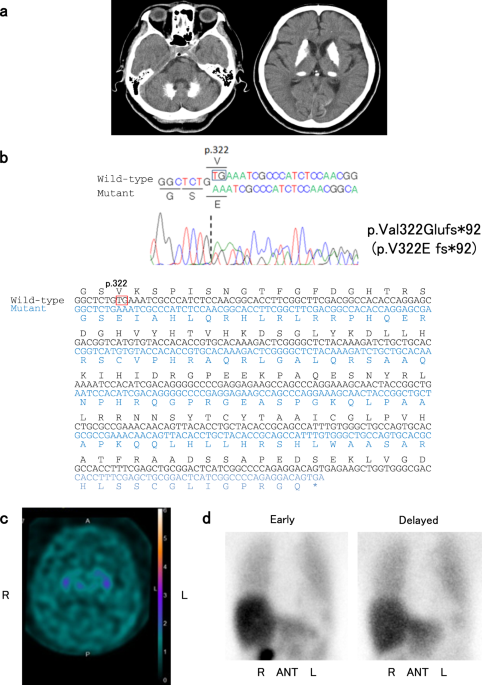
images of her brain revealed marked calcifications in the bilateral basal ganglia, thalami, and dentate nuclei (Fig. 1a). Laboratory tests showed that serum calcium, phosphate, and intact
parathyroid hormone levels were all within the normal ranges. There was no family history of IBGC or parkinsonism. After written informed consent was obtained, we analyzed all the coding
regions of the IBGC causative genes, _SLC20A2_, _PDGFRB_, and _PDGFB_, by Sanger sequencing as previously reported10. We diagnosed her as IBGC1 based on the identification of a novel
heterozygous frameshift variant, p.Val322Glufs*92 (NM_006749.4:c.965_966delTG, exon 8), in _SLC20A2_ (Fig. 1b). The variant was absent in the following genome databases: dbSNP 151
(https://www.ncbi.nlm.nih.gov/projects/SNP/), Integrative Japanese Genome Variation Database (http://ijgvd.megabank.tohoku.ac.jp/), Exome Aggregation Consortium database version 0.3.1
(http://exac.broadinstitute.org/), and Human Gene Mutation Database (HGMD® Professional 2019.1). Ten months after her first visit, she was hospitalized because of difficulties in standing up
without assistance at the age of 64. She showed severe bradykinesia, postural instability, and mild symmetric rigidity without tremor. Her Unified Parkinson Disease Rating Scale part III
(UPDRS-III) score was 43 of 108 on the ninth hospital day. Her Mini-Mental State Examination score was 24 of 30, and her Hasegawa dementia scale revised was 22 of 30. Dopamine transporter
(DAT) single photon emission CT using 123I-ioflupane showed diffusely decreased DAT density in the bilateral striatum (Fig. 1c). The specific binding ratios (SBRs) of both striatum were 0.51
(right) and 0.14 (left). Her 123I-meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy revealed reduced cardiac 123I-MIBG uptake with early and delayed heart to mediastinum (H/M)
rates of 1.995 and 1.585, respectively (Fig. 1d). Levodopa therapy (200 mg/day) was started on the 14th hospital day and was effective against bradykinesia and postural instability. She was
able to walk without assistance in her room. On the 122nd hospital day, she received 600 mg/day of levodopa, and her UPDRS-III score markedly improved from 43 to 11. The variants associated
with IBGC are located widely in _SLC20A2_ among the patients with IBGC, and the correlation of genotype and phenotype remains unclear1,9,11. Parkinsonism is one of the common clinical
symptoms of IBGC. Tadic et al. showed that 13% of patients with _SLC20A2_ or _PDGFRB_ variants presented with parkinsonism1. Another review reported motor improvement with dopatherapy in
five patients with genetically confirmed IBGC12. Genetically confirmed Japanese IBGC1 patients presenting with parkinsonism have also been reported (Table 1)10,13,14. Among the five variants
summarized in Table 1, two variants (c.516+1G>A and c.965_966delTG) are frameshift variants, presumably resulting in loss of function of SLC20A2. In addition, a decreased level of
SLC20A2 protein was described in the case with the missense variant (c.1909A>C, S637R), raising the possibility of unstable mutant protein13. Although the functional investigations were
not reported for the two missense variants (R71H and G90V), loss-of-function variants are considered for the three variants shown in Table 1. Consistent with previous reports, the majority
of variants associated with IBGC are loss-of-function variants8,9, and the present study also suggests that loss-of-function mechanisms are likely involved in at least of the three variants.
The present case demonstrated decreased DAT density in the bilateral striatum and decreased cardiac 123I-MIBG uptake (Fig. 1c, d). The decreased DAT density in the bilateral striatum
suggested presynaptic dopaminergic dysfunction, which was reported in patients with IBGC14,15,16,17. Saito et al. also showed that postsynaptic dopaminergic dysfunction in the bilateral
striatum matched calcified regions16. These findings suggested that basal ganglia calcification might result in dopaminergic dysfunction in IBGC patients. The three cases with reduced DAT
density in the striatum (cases 2, 5, and 7. Table 1) also presented with decreased cardiac 123I-MIBG uptake, which was indistinguishable from that observed in patients with Lewy body
diseases, including idiopathic Parkinson disease (PD)18. Since PD is a relatively common disease in Japan (prevalence of ~150 per 100,000 persons in Japan)19, the coincidental presence of
idiopathic PD and IBGC remains a possibility concerning dopa-responsive parkinsonism of patients with IBGC1. However, it is important to pay attention to patients with IBGC who show
dopa-responsive parkinsonism to provide appropriate treatment. To clarify the etiologies of dopa-responsive parkinsonism occasionally observed in patients with IBGC, further functional
analyses including DAT SPECT and 123I-MIBG myocardial scintigraphy will be required in a larger number of patients with genetically confirmed IBGC. HGV DATABASE The relevant data from this
Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.2603 REFERENCES * Tadic, V. et al. Primary familial brain calcification with known
gene mutations: a systematic review and challenges of phenotypic characterization. _JAMA Neurol._ 72, 460–467 (2015). Article Google Scholar * Wang, C. et al. Mutations in SLC20A2 link
familial idiopathic basal ganglia calcification with phosphate homeostasis. _Nat. Genet_ 44, 254–256 (2012). Article CAS Google Scholar * Keller, A. et al. Mutations in the gene encoding
PDGF-B cause brain calcifications in humans and mice. _Nat. Genet_ 45, 1077–1082 (2013). Article CAS Google Scholar * Nicolas, G. et al. Mutation of the PDGFRB gene as a cause of
idiopathic basal ganglia calcification. _Neurology_ 80, 181–187 (2013). Article CAS Google Scholar * Legati, A. et al. Mutations in XPR1 cause primary familial brain calcification
associated with altered phosphate export. _Nat. Genet_ 47, 579–581 (2015). Article CAS Google Scholar * Yao, X. P. et al. Biallelic mutations in MYORG cause autosomal recessive primary
familial brain calcification. _Neuron_ 98, 1116–1123 (2018). e5. Article CAS Google Scholar * Arkadir, D. et al. MYORG is associated with recessive primary familial brain calcification.
_Ann. Clin. Transl. Neurol._ 6, 106–113 (2019). Article CAS Google Scholar * Hsu, S. C. et al. Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification.
_Neurogenetics_ 14, 11–22 (2013). Article CAS Google Scholar * Lemos, R. R. et al. Update and mutational analysis of SLC20A2: a major cause of primary familial brain calcification. _Hum.
Mutat._ 36, 489–495 (2015). Article CAS Google Scholar * Yamada, M. et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. _Neurology_ 82,
705–712 (2014). Article CAS Google Scholar * Ding, Y. & Dong, H. Q. A novel SLC20A2 mutation associated with familial idiopathic basal ganglia calcification and analysis of the
genotype-phenotype association in Chinese patients. _Chin. Med J. (Engl.)_ 131, 799–803 (2018). Article Google Scholar * Nicolas, G. et al. Phenotypic spectrum of probable and
genetically-confirmed idiopathic basal ganglia calcification. _Brain_ 136, 3395–3407 (2013). Article Google Scholar * Kimura, T. et al. Familial idiopathic basal ganglia calcification:
Histopathologic features of an autopsied patient with an SLC20A2 mutation. _Neuropathology_ 36, 365–371 (2016). Article CAS Google Scholar * Koyama, S. et al. Clinical and radiological
diversity in genetically confirmed primary familial brain calcification. _Sci. Rep._ 7, 12046 (2017). Article Google Scholar * Paschali, A. et al. Dopamine transporter SPECT/CT and
perfusion brain SPECT imaging in idiopathic basal ganglia calcinosis. _Clin. Nucl. Med_ 34, 421–423 (2009). Article Google Scholar * Saito, T. et al. Neuroradiologic evidence of
pre-synaptic and post-synaptic nigrostriatal dopaminergic dysfunction in idiopathic Basal Ganglia calcification: a case report. _J. Neuroimaging_ 20, 189–191 (2010). Article Google Scholar
* Paghera, B., Caobelli, F. & Giubbini, R. 123I-ioflupane SPECT in Fahr disease. _J. Neuroimaging_ 23, 157–158 (2013). Article Google Scholar * Orimo, S., Suzuki, M., Inaba, A. &
Mizusawa, H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. _Park. Relat.
Disord._ 18, 494–500 (2012). Article Google Scholar * Yamawaki, M., Kusumi, M., Kowa, H. & Nakashima, K. Changes in prevalence and incidence of Parkinson’s disease in Japan during a
quarter of a century. _Neuroepidemiology_ 32, 263–269 (2009). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by Grant-in-Aid (No. H26-Jitsuyoka
[Nanbyo]-Ippan-080) from the Ministry of Health, Labour and Welfare, Japan (S.T.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurology, Kyorin University School of
Medicine, Tokyo, Japan Yaeko Ichikawa, Eriko Kurita, Masanori Nakajima, Masaki Tanaka, Chizuko Oishi & Atsuro Chiba * Department of Neurology, The University of Tokyo Hospital, Tokyo,
Japan Masaki Tanaka & Shoji Tsuji * Institute of Medical Genomics, International University of Health and Welfare, Chiba, Japan Masaki Tanaka & Shoji Tsuji * Department of Neurology,
International University of Health and Welfare Mita Hospital, Tokyo, Japan Jun Goto * Department of Molecular Neurology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Shoji Tsuji Authors * Yaeko Ichikawa View author publications You can also search for this author inPubMed Google Scholar * Masaki Tanaka View author publications You can also search for
this author inPubMed Google Scholar * Eriko Kurita View author publications You can also search for this author inPubMed Google Scholar * Masanori Nakajima View author publications You can
also search for this author inPubMed Google Scholar * Masaki Tanaka View author publications You can also search for this author inPubMed Google Scholar * Chizuko Oishi View author
publications You can also search for this author inPubMed Google Scholar * Jun Goto View author publications You can also search for this author inPubMed Google Scholar * Shoji Tsuji View
author publications You can also search for this author inPubMed Google Scholar * Atsuro Chiba View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Yaeko Ichikawa. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ichikawa, Y., Tanaka, M., Kurita, E. _et al._ Novel _SLC20A2_
variant in a Japanese patient with idiopathic basal ganglia calcification-1 (IBGC1) associated with dopa-responsive parkinsonism. _Hum Genome Var_ 6, 44 (2019).
https://doi.org/10.1038/s41439-019-0073-7 Download citation * Received: 01 June 2019 * Revised: 18 July 2019 * Accepted: 25 July 2019 * Published: 04 September 2019 * DOI:
https://doi.org/10.1038/s41439-019-0073-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative