Play all audios:
ABSTRACT Over 50% of patients with diabetes mellitus, either type 1 or 2, ultimately develop hypertension as a complication. In diabetics, this further increases the incidence of
cardiovascular disease (CVD) by 2- to 3-fold and accelerates the progression of diabetic nephropathy. Arteriosclerosis, a clinical feature of hypertension in diabetics, develops and advances
from a young age. Therefore, in providing treatment, it is necessary to evaluate the degree of arteriosclerosis. Diabetic patients are encouraged to strictly control their blood glucose
levels. Recently developed drugs, such as GLP-1 receptor agonists, DPP-4 inhibitors and SGLT2 inhibitors, also have hypotensive actions, making them ideal for use in diabetics with
hypertension. SGLT2 inhibitors and GLP-1 receptor agonists reportedly suppress the onset and progression of CVD, as well as diabetic nephropathy. The possibility of hypoglycemia triggering
blood pressure elevation and arrhythmia has been noted, so a key point here is not to cause hypoglycemia. In selecting hypotensive agents, we must choose types that do not aggravate insulin
resistance and engage in hypotensive treatment that also considers both nocturnal and morning hypertension. In addition, facing the onset of an aging society, there is a growing need for
treatments that do not cause excessive blood pressure reduction or hypoglycemia. Favorable lifelong blood pressure and glucose control are increasingly important for the treatment of
diabetes accompanied by hypertension. SIMILAR CONTENT BEING VIEWED BY OTHERS RECENT UNDERSTANDINGS ABOUT HYPERTENSION MANAGEMENT IN TYPE 2 DIABETES: WHAT ARE THE ROLES OF SGLT2 INHIBITOR,
GLP-1 RECEPTOR AGONIST, AND FINERENONE? Article 31 May 2023 HYPERTENSION IN DIABETES CARE: EMERGING ROLES OF RECENT HYPOGLYCEMIC AGENTS Article 14 May 2021 DIABETIC VASCULAR DISEASES:
MOLECULAR MECHANISMS AND THERAPEUTIC STRATEGIES Article Open access 10 April 2023 INTRODUCTION It is well known that over 50% of patients with diabetes, either type 1 or type 2, ultimately
develop hypertension in combination. Hypertension as a complication in diabetics further increases the incidence of cardiovascular disease (CVD) by 2- to 3-fold, which is elegantly
summarized in another article in this Review Series [1]. In addition, hypertension as a complication increases the progression of diabetic nephropathy. This article will focus on the
clinical features of hypertension in diabetic patients and compile and summarize past findings while citing Japanese clinical results as much as possible. CAUSE OF DEATH IN DIABETICS In
diabetics, not only are characteristic microvascular disorders triggered, but the advancement of arteriosclerosis also increases the frequency of CVD, including macrovascular disorders, such
as cerebrovascular disorders and heart disease. The Japan Diabetes Society has been investigating the cause and mean age of death of diabetic patients every decade. The latest
investigation, which studied ~45,000 subjects from 2001 to 2010, [2] showed that malignant neoplasms accounted for 38.3% of all deaths, followed by infection at 17.0% and vascular disease at
14.9% (chronic renal failure: 3.5%; ischemic heart disease: 4.8%; and cerebrovascular disorders: 6.6%). Deaths due to CVD have been decreasing in a time-line manner, from 41.5% between 1971
and 1980, to 39.3% between 1981 and 1990, and 26.9% between 1991 and 2000. Moreover, the average age of death in diabetics between 2001 and 2010 was 71.4 years for men and 75.1 years for
women, showing that, similar to the general population, lifespan is showing a tendency to increase every year. However, compared to the average lifespan of the general healthy population of
79.6 years for men and 86.3 years for women, there is a difference of 8.2 years for men and 11.2 years for women. Despite advancements in medicine over the past several decades, these
differences have not narrowed much. Since 1998, diabetic nephropathy has consistently been ranked the leading cause requiring the initiation of hemodialysis. Approximately 44% of the 34,000
patients who newly began dialysis treatment in 2015 suffer this disease. Although the pace of increase has slowed, the number is still growing [3]. In the US, similar to other countries, a
report has been published on the changes over time in the frequency of complications in diabetics from 1990 to 2010 [4]. According to this report, the incidence of acute myocardial
infarction has decreased by 67.8%, from 141.1 per 10,000 individuals in 1990 to 45.5 per 10,000 individuals in 2010, and that of stroke by 52.7%, from 111.8 to 52.9 per 10,000 individuals.
The number of deaths from hyperglycemic emergencies decreased by 64.4%, from 4.2 to 1.5 per 10,000 individuals. By contrast, those dying from end-stage renal failure decreased by only 28.3%,
from 27.9 to 20.0 per 10,000 individuals. As observed, the onset of CVD and deaths observed in diabetics are dramatically decreasing in the US. One reason for this may be the progress in
the treatment of diabetes and hypertension during this period [5]. Compared to 1999, the rate of meeting the blood glucose control goal of “HbA1c < 7.0%” in 2010 increased by 7.9%, from
44.3 to 52.3%; the rate of meeting the blood pressure control goal of “blood pressure <130/80 mmHg” increased by 11.7% from 39.6% to 51.3%; and the rate of meeting the lipid control goal
of “LDL-cholesterol < 100 mg/dL” increased by 20.8%, from 36.0 to 56.8%. However, the share of smokers has dropped only by 1.7%, from 24.0 to 22.3%, and only 14.3% of the subjects met all
the control goals (blood glucose, blood pressure, and cholesterol) and also quit smoking. These goals remain a challenge. THE IMPACT OF HYPERTENSION ON MICROVASCULAR DISORDERS HYPERTENSION
ACCELERATES THE PROGRESSION OF DIABETIC NEPHROPATHY It is well known that patients with diabetes, particularly type 1 diabetes, see their blood pressure elevate, along with the progression
of diabetic nephropathy, and develop hypertension. In type 2 diabetes patients, meanwhile, hypertension is often present as an existing condition. Even if blood pressure was initially
normal, it often rises with the advancement of diabetic nephropathy, resulting in hypertension. In any event, if a diabetic patient develops hypertension as a complication, his or her
progression of diabetic nephropathy is accelerated. In this sense, hypertension can be said to form a vicious cycle with the progression of diabetic nephropathy and declining renal function.
The Multiple Risk Factor Intervention Trial investigated the relationship between blood pressure and end-stage renal failure, although the ratio of diabetics it studied was only 1.5%. The
trial found that the risk of developing end-stage renal failure, if an optimal blood pressure of <120/80 mmHg was used as the reference, increased by 1.9-fold if blood pressure was
high-normal (130–139 mmHg/85–89 mmHg), by 3.1-fold in the case of mild hypertension (140–159 mmHg/90–99 mmHg), and by 11.2-fold in the case of severe hypertension [6]. Generally speaking,
diabetic nephropathy is divided into the following five stages: normoalbuminuria, microalbuminuria, overt albuminuria, end-stage renal failure, and dialysis. The United Kingdom Prospective
Diabetes Study (UKPDS) showed that, first, after a course of 10 years, ~25% of patients developed microalbuminuria [7]. The study also described the annual rate of progression of various
stages of diabetic nephropathy and showed that the rate was 2% from normoalbuminuria to microalbuminuria; 2.8% from microalbuminuria to overt albuminuria; and 2.3% from overt albuminuria to
end-stage renal failure and renal replacement therapy, including dialysis. Thus, albuminuria is a marker for the progression of diabetic nephropathy. Many studies have also revealed
albuminuria to be a predictor of cardiovascular disease [8]. The UKPDS similarly noted that, as albuminuria increased with each stage of diabetic nephropathy, the mortality rate from CVD
markedly increased. In brief, the rate of all-cause deaths and cardiovascular death was 1.4% and 0.7%, respectively, during the normoalbuminuria stage; 3.0 and 2.0%, respectively, during the
microalbuminuria stage; 4.6% and 3.5%, respectively, during the overt albuminuria stage; and 19.2% and 12.1%, respectively, during the end-stage renal failure and renal replacement therapy
stage including dialysis. Although only 2.3% of patients advance from the overt albuminuria stage to the end-stage renal failure and renal replacement therapy stage, including dialysis,
19.2%, or ~8.3 times greater than 2.3%, die mainly of CVD. Therefore, the phenomenon in which CVD increases markedly along with a decline in renal function is called the cardio-renal axis,
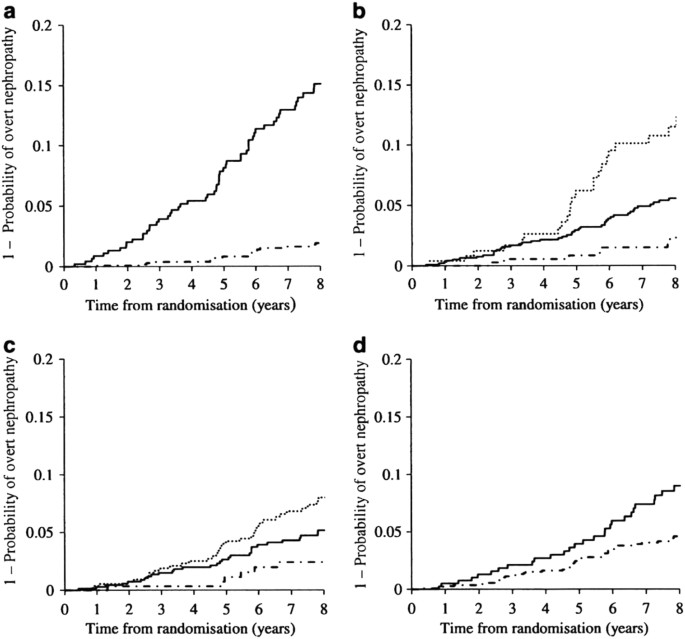
and it is a growing focus of research attention. In a study conducted in Japan on the prevention of the onset of vascular complications in diabetes and suppression of its progression (Japan
Diabetes Complications Study: JDCS), we investigated the onset and progression of diabetic nephropathy (urinary albumin (Alb)/creatinine (Cr) exceeding 300 mg/g·Cr on two consecutive
occasions), targeting 1,558 type 2 diabetics whose urinary Alb/Cr was either normal (< 30 mg/g·Cr) or low-microalbuminuria (30 to < 150 mg/g·Cr) [9]. The rate of onset of nephropathy
up to 8 years later showed a low value of 6.67/1000 person years. Figure 1 reveals that the hazard ratio (HR) of developing nephropathy increases if the initial albuminuria was more than 30
mg/g Cr, if HbAlc was >7%, if systolic blood pressure (SBP) was higher than 120 mmHg, or if smoking could not be discontinued. Conversely, 30.3% of the 452 subjects in the
low–microalbuminuria group at the time of registration saw their Alb/Cr ratio return to normal (remission) of <30 mg/g·Cr. These findings show that, even in Japanese people, who are a
high-risk group for developing nephropathy, early diagnosis during the normoalbuminuria stage or the low-microalbuminuria stage and undergoing treatment using currently available methods in
Japan can minimize the onset and progression of nephropathy. HYPERTENSION PROMOTES THE PROGRESSION OF DIABETIC RETINOPATHY AND NEUROPATHY Many researchers have studied the mechanism by which
hyperglycemia promotes the progression of other diabetic microvascular disorders such as retinopathy and neuropathy. However, few have studied the mechanism by which hypertension aggravates
these microvascular disorders. The JDCS conducted in Japan mentioned earlier showed that the rate of new onset of retinopathy and its rate of worsening in Japan were 38.3 and 21.1 per 1000
person×years, respectively [10]. The risk factors for developing retinopathy are duration of diabetes (HR of 1.26 with a 5-year morbidity period), BMI (HR of 1.05 for every additional 1
kg/m2), SBP (HR of 1.09 for every additional 10 mm Hg), and HbA1c (HR of 1.36 for every additional 1%). Hypertension is clearly a risk factor for developing retinopathy. The UKPDS has
reported that hypotensive treatment suppresses the onset and progression of retinopathy. The DIRECT Study that used candesartan, an ARB, is also well known. It examined candesartan’s effects
of reducing the rate of onset of retinopathy, and of suppressing its progression, in type 1 diabetic patients [11]. Candesartan suppressed the onset of retinopathy (HR 0.82, _P_ = 0.0508)
but did not slow its progression. Conversely, candesartan did not suppress the onset of retinopathy in type 2 diabetic patients, but it significantly improved retinopathy (HR 1.34, _P_ =
0.0091) [12]. The fact that hypertension is a risk factor for neuropathy was reported in the Epidemiology of Diabetes Complications (EDC) Study that observed, over a period of 6 years, 453
type 1 diabetic patients without neuropathy [13]. Hypertension was the greatest risk factor for the onset of neuropathy, followed by the duration of diabetes, hyperglycemia, height, and
smoking. Although this was a small-scale trial, using 41 type 1 or type 2 diabetics, it reported that a 12-month administration of trandolapril, an ACE inhibitor, significantly improved
electrophysiological data such as a reduction in F-wave latency [14]. INFLUENCE OF HYPERTENSION ON MACROVASCULAR DISORDERS Diabetic patients develop CVD at 2- to 3-fold the rate observed in
non-diabetics. Moreover, hypertension is one of the most significant risk factors for arteriosclerosis, and hypertension as a complication in diabetic patients further increases the
frequency of developing CVD by 2- to 3-fold. Ultimately, compared to normotensive non-diabetics, the rate of onset of CVD is 5–7 times higher in diabetic patients with hypertension.
Therefore, early discovery of arteriosclerosis in diabetic patients and the prevention of its progression are extremely important to extend the healthy lifespan of these patients. This
appears to be because a reduction in the production of nitrogen oxide (NO) in the vascular endothelial cells and increased growth of vascular smooth muscle cells are triggered due to
oxidative stress and inflammation based on hyperglycemia and hypertension, generation of advanced glycation end products, and increases in shear stress, etc. For details, please refer to
another article in this Review Series. It should be noted, however, that there are not necessarily many findings of actual investigations of arteriosclerotic lesions in diabetic patients. A
study by McGill et al. [15] showed that HbA1c and obesity are related to arteriosclerotic lesions. Furthermore, the number of individuals with a history of smoking or exposure to four risk
factors (BMI, SBP, hypertriglyceridemia and hyper-LDL-cholesterolemia) is correlated with the degree of either fatty streaks in the aorta or coronary artery, or fibrous plaques [16]. Rizzoni
et al. [17] studied tissue images of arterioles with a diameter of 100–280 µm by dividing the subjects into four small groups of 10–15 subjects each (normotensive individuals, essential
hypertensive patients, normotensive type 2 diabetic patients, and hypertensive type 2 diabetic patients) and collecting subcutaneous fat from their hips. Compared to normotensive
individuals, all other groups had a higher media-to-lumen ratio, and patients with essential hypertension showed no changes in the vascular wall thickness. Although they showed remodeling
that was accompanied by changes in the sequence of vascular smooth muscle cells (eutrophic remodeling), normotensive and hypertensive diabetic patients presented hypertrophic remodeling that
was accompanied by an expansion of vascular wall thickness, along with a 40% and 46% increase in the number of vascular smooth muscle cells, respectively. Compared to the group of
normotensive subjects, the collagen-to-elastin ratio had increased in the essential hypertension group and the diabetic hypertension group. Systolic overload may be said to have a greater
impact on the increase in extracellular matrices. Hypertension is therefore the most important risk factor for arteriosclerosis in diabetics. METHODS OF EVALUATING ARTERIOSCLEROSIS As stated
above, diabetic patients with hypertension often see their arteriosclerosis accelerated, and not only suffer microvascular disorders but also have arteriosclerotic lesions in major blood
vessels such as the coronary artery and cerebral blood vessels. In initiating treatment, it is necessary to take the usual chest X-rays and ECG images, and, in addition, evaluate the degree
of arteriosclerosis in greater detail. PULSE WAVE VELOCITY (PWV) Aortic PWV and forearm vascular resistance calculated by forearm blood flow determined using plethysmography are used as
clinical indicators of arteriosclerosis. Although PWV was used to measure the velocity at which a pulse wave propagates from the carotid artery to the femoral artery in the groin, a device
has recently become widespread that measures, simply and quickly, the PWV of the brachial artery and the tibial artery of the foot (brachial-ankle pulse wave velocity, or baPWV), making it
possible to measure the Ankle Brachial Index (ABI: the ratio between the blood pressure in the lower limb and the upper arm) simultaneously [18]. The results of a systemic review [19] showed
that age and hypertension contributed the most significantly to PWV, at 91% and 90%, respectively. Conversely, diabetes contributed 52%. A systemic review and meta-analysis [20] that
observed 15,877 people for an average of 7.7 years showed that compared to the low-PWV group, the high-PWV group had a 2.26-, 2.02- and 1.90-fold greater relative risk, respectively, of
experiencing CVD events, CVD deaths, and all-cause deaths, and indicated that they were factors for predicting life prognosis. VASCULAR ENDOTHELIAL FUNCTION TESTS: BLOOD FLOW-DEPENDENT
VASODILATION (FLOW MEDIATED DILATION, OR FMD) Methods regarded as the “gold standard” for evaluating the function of vascular endothelial cells include measuring (1) the vasodilation caused
by NO that is generated from the vascular endothelium by invasively injecting acetylcholine inside the blood vessels, and (2) the vasodilation of the non-NO-dependent forearm resistance
vessel when sodium nitroprusside, an NO donor, is injected. Clinically, researchers measured the forearm blood flow and flow-mediated vasodilation (FMD) via plethysmography. A test method
has recently become widespread in which FMD is used to measure the changes in the forearm arterial diameter between before and after reactive hyperemia (produced by forearm ischemia usually
lasting 5 min), employing either the A- or B-mode ultrasonography [21]. As a method to test the vascular endothelial cell function even more simply and conveniently, EndoPAT is increasingly
being used. Compared to healthy controls and essential hypertensives, diabetics show lower forearm blood flow, i.e., a higher vascular resistance, and lower FMD, i.e., a deteriorated
endothelial function. The vascular endothelial functions that have been measured using these techniques reportedly improve when hypotensive drugs such as ACE inhibitors and ARBs; oral
hypoglycemics such as metformin, acarbose, miglitol and pioglitazone; insulin and glucagon-like peptide (GLP)-1 receptor agonists; or statins, are used. Some may be attributable to
hypotensive and hypoglycemic actions. However, the direct mechanism of action is being investigated in some of these agents. OTHER METHODS SUCH AS CAROTID ULTRASONOGRAPHY AND HEAD AND
CORONARY IMAGING It has become an increasingly common practice to check the size, properties and quantities of plaque lesions in the carotid artery via carotid ultrasonography, and/or
measure the carotid artery’s intimal and medial complex thickness (IMT) for use as quantitative indicators of arteriosclerosis. According to a series of studies by Kawamori et al. [22],
diabetic patients have significantly greater IMT than healthy subjects of the same age group. Moreover, a multiple regression analysis revealed that blood glucose control status, smoking,
dyslipidemia and hypertension were the determinant factors of IMT. It has also been shown that IMT correlates with the degree and progression of coronary arterial diseases and can become a
predictive factor for these conditions [23]; thus, in this sense, IMT is an indicator of arteriosclerosis not only in the carotid arteries, but of the entire body. Regardless, head CT and
MRI are useful. In particular, rendering of the intracranial vessels with MRA makes it possible to observe the degree of intracerebral vascular stenosis. Coronary angiography is the “gold
standard” for the diagnosis of coronary arterial disease. The safety and usefulness of coronary arterial CT are gradually being acknowledged as well. THE RELATIONSHIP BETWEEN HYPERGLYCEMIA
AND HYPERTENSION IN DIABETIC PATIENTS HYPERGLYCEMIA ELEVATES BLOOD PRESSURE The leading cause of hypertension in diabetic patients is the greater volume of exchangeable sodium in vivo due to
hyperglycemia than that in non-diabetics. A study of type 1 diabetic patients found their volume of exchangeable sodium to be ~2800–3000 mEq per 1.73 m2, which is approximately 10% higher
than in non-diabetics, and that volume was significantly higher in patients presenting overt albuminuria than in patients presenting normoalbuminuria or microalbuminuria [24]. The study,
however, reportedly found no differences between diabetics and non-diabetics in the volume of circulating plasma. Moreover, the volume of exchangeable sodium in diabetic patients was
positively correlated with mean blood pressure. This is mainly believed to be due to boosted levels of sodium glucose co-transporter (SGLT) 2, increasing both the reabsorption of glucose and
sodium (Na). The glomerular filtration rate (GFR) may increase, or, in other words, hyperfiltration may be manifested [25]. The renin-angiotensin (RA) system is not involved in the excess
of body fluid volume such as this. In other words, most diabetics often show normal or slightly low values of plasma renin activity and aldosterone concentration [24]. Furthermore, their
inactive renin in the blood shows a high value, and the conversion to the active type has declined [26]. Progress in molecular biology, however, is gradually accumulating new knowledge and
findings, such as local intrarenal acceleration of tissue RA systems, the pathophysiological significance of prorenin and (pro)renin receptors, and the mechanism by which mineralocorticoid
receptors are activated inside the kidneys due to high salt intake. For details, please refer to another article in this Review Series. DIABETES IS A SALT-SENSITIVE HYPERTENSION Diabetic
patients are believed to have salt-sensitive hypertension, with high glomerular blood pressure and a flatter pressure-diuresis curve. Hyperinsulinemia caused by insulin resistance is also
involved in accelerating the reabsorption of sodium from the renal tubules [27]. Excessive salt intake inhibits nocturnal blood pressure reduction, or, in other words, nocturnal blood
pressure is set higher than usual to excrete excess sodium, making the patient a non-dipper [28]. Many clinical results show the efficacy of salt reduction, even in patients with diabetes
and those with reduced glucose tolerance, and reducing salt intake is reported to improve vascular endothelial cell functions and insulin resistance. BLOOD PRESSURE DROPS WITH IMPROVEMENT OF
HYPERGLYCEMIA It is well known that blood pressure drops with the improvement of hyperglycemia accompanying educational hospitalization and initiation of hypoglycemic drugs. Metformin and
pioglitazone, which improve insulin resistance, are reported to have slight hypotensive actions. However, some recent hypoglycemic drugs possess hypotensive actions that surpass these drugs.
With GLP-1 receptor agonists, daily administrations of 1.2–1.8 mg of liraglutide, for example, are reported to produce hypotensive actions of between 2.7 and 6.6 mmHg in SBP [29]. One
report claims that DPP-4 inhibitors, which inhibit dipeptidyl peptidase (DPP)-4, show a degree of hypotensive action, although they are weaker than those of GLP-1 receptor agonists. The
mechanism by which GLP-1 and GLP-1 receptor agonists lower blood pressure has not been completely elucidated, leaving room for discussion. However, it appears that in addition to the
aforementioned vasodilating actions, these drugs directly inhibit natrium-hydrogen exchanger 3 (NHE3) in the proximal tubules, resulting in blocking the reabsorption not only of glucose but
also of sodium, and work to promote natriuretic activity [30]. Since NHE3 is created by binding with DPP-4, if DPP-4 inhibitors work from the luminal side, NHE3 activity is inhibited,
triggering natriuresis as a result [30]. Conversely, SGLT2 inhibitors inhibit the reabsorption of glucose in the proximal tubules and inhibit the reabsorption of sodium, triggering
natriuresis as a result. Because of this, body weight decreases, and blood pressure drops. In a phase II or III clinical study conducted in Japan, a 16- to 24-week administration of these
drugs reduced SBP by 2.6–5.6 mmHg and diastolic blood pressure (DBP) by 1.1–2.7 mmHg, as shown in Table 1 [31]. A meta-analysis performed in Europe and the US found that in 21
placebo-controlled studies, SBP had dropped by 3.77 mmHg (95% CI: –4.65 to –2.90), and in 16 studies, DBP had dropped by 1.75 mmHg (95% CI: –2.27 to –1.23) [32]. The results of the EMPA-REG
BP study were recently published, in which empagliflozin had been administered for 12 weeks to 825 type 2 diabetic patients with hypertension [33]. The results showed that in the group given
empagliflozin, daytime SBP fell to approximately 10 mmHg below that of the placebo group. Compared to the placebo group, the empagliflozin group showed a 3.44 mmHg lower 24-hour mean SBP
after the administration of 10 mg/day, and a drop of 4.16 mmHg after the administration of 25 mg/day. The 24-hour mean DBP reduction level at the time was 1.36 and 1.72 mmHg, respectively.
We still recall the surprising results of the EMPA-REG OUTCOME study that used empagliflozin [34]. This was a secondary prevention study in which 7028 type 2 diabetic patients with a history
of CVD were given either a placebo or 10 mg/day of 25 mg/day of empagliflozin. They were then followed up for a period of 3.1 years (mean value). As shown in Table 2, compared to the
placebo group, the groups given empagliflozin saw their HR of primary endpoints (CVD-related deaths, non-fatal myocardial infarction, and non-fatal stroke) drop to 0.86 (_P_ < 0.001 for
noninferiority and _P = _0.04 for superiority). Their HR of CVD-caused deaths, all-cause deaths and hospitalization due to heart failure also decreased to 0.62, 0.68, and 0.65, respectively.
The frequency of myocardial infarction and cerebrovascular disorders in both groups did not show any significant differences, with mainly CVD-related deaths having decreased. The
empagliflozin group began showing a clear reduction in CVD-related deaths approximately three months later; this was most likely not attributable to the improvement in conventional CVD
risks. Although the exact mechanism is unknown, it was probable that empagliflozin had caused improvements in hemodynamics, such as a reduction in circulating plasma volume and a reduction
in blood pressure. The other possibility of SGLT2 inhibitors might be the improvement in a disrupted circadian rhythm of blood pressure, which has been very recently reviewed by Rahman et
al. [35]. An analysis on diabetic nephropathy in the EMPA-REG OUTCOME study [36] also found that, as shown in Table 2, the empagliflozin group’s HR in terms of new onset or worsening of
nephropathy was 0.61. Moreover, eGFR, which had declined during empagliflozin administration, improved after the end of administration, indicating that even after long-term treatment,
glomerular hemodynamics does indeed return to the initial level. In this study, ~80% of the patients were using RA inhibitors; thus, empagliflozin clearly possesses renal protective actions,
even when RA inhibitors are being administered. SGLT2 inhibitors increase the volume of sodium that reaches the distal renal tubules, and with the tubuloglomerular feedback mechanism
mediated by the macula densa, it appears to constrict the afferent glomerular arterioles, reduce intraglomerular pressure, and normalize the glomerular filtration rate [37]. SGLT2 inhibitors
have the potential to be a new strategy to treat nephropathy, particularly in diabetic patients with hyperfiltration. GLP-1 receptor agonists are drawing increasing research attention since
reports suggest that they suppress CVD and curb the advancement of diabetic nephropathy [38, 39]. HYPOGLYCEMIA AND HYPERTENSION/ARRHYTHMIA The Action to Control Cardiovascular Risk in
Diabetes (ACCORD) Study compared strict blood glucose control and standard blood glucose control but was compelled to discontinue the study mid-way since the strict blood glucose control
group showed high mortality rates. The reason for this may have been the large number of serious hypoglycemia cases in the strict blood glucose control group. With this result as a turning
point, a patient-centered approach came to be emphasized, with elderly diabetics with a long disease history and CVD not necessarily being called on to undergo the same type of blood glucose
control as their younger counterparts. Because hypoglycemia activates the sympathetic nervous system, it has a variety of influences on the cardiovascular system. Of the 59,602 patients who
had visited the emergency medicine department, Tsujimoto et al. [40] analyzed 414 individuals who had presented with severe hypoglycemia. He found that type 1 diabetics (_n_ = 88) and type
2 diabetics (_n_ = 326) had blood glucose levels of 32 and 31 mg/dL, respectively, showing no difference between the two groups. The ratio of severe hypertension (≥180/120 mmHg), hypokalemia
(<3.5 mEq/L) and QT prolongation in terms of ECG was 19.8% vs. 38.8% (_P = _0.001); 42.4% vs. 36.3%; and 50.0% vs. 59.9%, respectively. Incidentally, the blood pressure of type 1 and
type 2 diabetics during their hospital visits was 140/76 and 168/80 mmHg, respectively. The level dropped 2 h after the start of hypoglycemic treatment to 134/70 and 140/69 mmHg,
respectively. Furthermore, a detailed analysis was reported of 25 type 2 diabetics on insulin treatment who simultaneously underwent continuous glucose monitoring (CGM) and Holter ECG
measurements [41]. The incidence of bradycardia and atrial/ventricular extrasystole was significantly higher in the nighttime hypoglycemic time zone of <35 mg/dL compared to normoglycemic
time zones. QT prolongation and abnormal T-waves were also observed. Bradycardia observed during hypoglycemic time periods may be due to compensatory tension being applied to the
parasympathetic nervous system, following activation of the sympathetic nervous system. It is clear, however, that arrhythmia also frequently occurs during asymptomatic hypoglycemic periods.
Hypoglycemia’s mortality rate is reported to be 10% and is also called the “dead-in-bed syndrome.” Arrhythmia such as this that occurs during hypoglycemia is likely to be one reason for the
increase in CVD deaths observed during strict blood glucose control. According to a recent systemic review and meta-analysis, the relative risk of CVD occurring during serious hypoglycemia
is reported to be 2.05-fold [42]. Moreover, if hypoglycemic patients are subjected to hyperglycemia, it appears to damage the vascular endothelial function and aggravate the oxidative stress
and inflammation markers [43]. Hypertension and hypoglycemia are also known to reduce cognitive functions. In this sense, it is no exaggeration to say that “high-quality” diabetic treatment
that does not induce hypoglycemia is being sought in diabetic patients, particularly in the growing population of elderly diabetic patients. INFLUENCE OF HYPERTENSION AND HYPOTENSIVE DRUGS
ON GLUCOSE METABOLISM AND INSULIN RESISTANCE Diabetic patients are liable to develop hypertension as a complication because essential hypertension has 42 million patients, and diabetes has
9.5 million patients, showing that they are common diseases that occur at an extremely high frequency and are liable to develop in combination. It is also true, however, that both diseases
have common causes and genetic predispositions and that diabetics are liable to suffer from hypertension. I will leave the details to other articles in this Review Series, and discuss
insulin resistance only, which is commonly observed in obese individuals and in patients with type 2 diabetes and hypertension. ESSENTIAL HYPERTENSION PATIENTS ALSO HAVE INSULIN RESISTANCE
Many patients who have diabetes and hypertension in combination often see hypertension develop prior to diabetes. Hypertensive patients develop diabetes approximately three times more
frequently than normotensive individuals. From ~1985, it became clear that many essential hypertensive patients have glucose metabolism abnormalities and insulin resistance, which is a
disease condition common to both diabetes and obesity. Ferrannini et al. [44] used the hyperinsulinemic-euglycemic clamp technique and revealed that the insulin sensitivity index of young
hypertensive patients had declined compared to controls. Conversely, hypertension develops at a high ratio in patients who suffer impaired glucose tolerance and who show insulin resistance.
In a study in Finland, the authors found that if the blood glucose value one hour after a glucose tolerance test (GTT; loading 1 g of glucose per kilogram of body weight) was divided into
tertiles, the odds ratio of the rate of onset of hypertension 18 years later for the second and third tertiles of blood glucose concentration was shown to be 1.71-fold [45]. In our study, we
observed normotensive subjects who showed borderline types at 75 g GTT over several years and found that the higher the following values at the time of initial GTT, the higher the incidence
of hypertension: fasting insulin (immunoreactive insulin, or IRI) level, total insulin values (∑ IRI) during GTT, and HOMA-R, which is an indicator of insulin resistance. Moreover, the HR
between the uppermost part and the lowermost part, when the glucose level was divided into tertiles, came to be 5- to 10-fold in size [46]. It is clear that insulin resistance is also
involved in the onset of hypertension in the Japanese population. THE IMPACT OF HYPERTENSION AND HYPOTENSIVE DRUGS ON INSULIN RESISTANCE Diabetes develops at higher ratios in hypertensive
patients than in normotensive subjects, particularly in hypertensive patients who are undergoing treatment with diuretics and β-blockers. Conversely, when captopril, an ACE inhibitor, was
administered to type 2 diabetic patients who were taking oral hypotensive drugs, they developed hypoglycemia. This finding, reported in the New England Journal of Medicine in the form of a
Letter [47], prompted a series of investigations into the influence of hypotensive drugs on insulin resistance. First, diuretics aggravate insulin resistance [48]. Researchers note that
thiazide diuretics administered in high doses cause metabolic adverse reactions such as hypokalemia and hyperuricemia and also increase the risk of sudden death. In diabetic patients,
therefore, it is important to use small doses of diuretics to minimize metabolic adverse reactions. Conversely, captopril, an ACE inhibitor, was verified to improve insulin resistance [48].
As one of the mechanisms by which captopril improves insulin resistance, we demonstrated that the drug increases blood flow in the forearm, which decreases postprandially [49]. Later, it was
revealed that ARBs, long-acting calcium antagonists, and α1-blockers also improve insulin resistance and lower blood glucose levels. β-blockers sometimes aggravate insulin resistance and
elevate blood glucose levels and blood levels of LDL cholesterol and triglycerides. However, vasodilative β-blockers improve insulin resistance. When using β-blockers, it is important to
make it less liable for sympathetic nervous stimulation symptoms to appear in hypoglycemia (masking of hypoglycemia). Moreover, since the drugs suppress gluconeogenesis in the liver
(prolongation of hypoglycemia), close attention must be paid to patients taking sulfonylurea and insulin. Recently, it has become possible to investigate, in a large-scale intervention study
of hypertension, the rate of new onset of diabetes when hypotensive drugs have been administered for several years to hypertensive patients who initially did not have diabetes. In a
meta-analysis targeting over 140,000 subjects who were enrolled in 22 studies [50], as shown in Fig. 2, if diuretics were made the criterion, the rate of reduction in the new onset of
diabetes was 43% with ARBs, 33% with ACE inhibitors, and 25% with calcium antagonists. This suggests that the performance of hypotensive drugs, which was examined for their short-term
influence on insulin resistance, has been verified for long-term outcomes in the form of new onset of diabetes. Individuals who had newly developed diabetes after initiating hypotensive
treatment are at a similar degree of risk of developing CVD as are individuals who already have diabetes [51]. Therefore, when providing hypotensive treatment to hypertensive patients,
particularly those who are suffering from metabolic syndrome, it is extremely important to select hypotensive drugs that improve insulin resistance and suppress the new onset of diabetes.
ABNORMAL CIRCADIAN BLOOD PRESSURE VARIATIONS IN DIABETIC PATIENTS OFFICE BLOOD PRESSURE AND HOME BP When measuring blood pressure inside an examination room (“office BP”), we ask the
subjects to take sufficient rest; then, while they are in a sitting position, we measure their blood pressure multiple times at 1–2 min intervals. We take an average of the two measurements
that show the most stable values (measurement values showing a difference of <5 mmHg) and designate this as the blood pressure value. A diagnosis of hypertension, based on office BP, will
be made, using blood pressure values taken at a minimum of two or more different occasions [52]. Home BP (“home BP”) is measured twice on each occasion, in principle. In the morning, blood
pressure is measured within one hour of getting up, after urinating, before taking medication, and before breakfast, in a sitting position after resting for 1–2 min. At night, it is measured
before going to bed, in a sitting position, after resting for 1–2 min. Home BP tends to be lower than office BP, so it should be used as a reference in examination and treatment. The
benchmark value for diagnosing hypertension is 135/85 mmHg or greater as indicated by home BP. It has been reported that blood pressure that has been measured at home, on getting up in the
morning, is more useful for the estimation of the onset of CVD and diabetic microangiopathy than blood pressure measured at the clinic [53, 54]. It is worth special note that with the
accumulation of these results as the basis, the Japanese Society of Hypertension released new guidelines for diagnosing hypertension (JSH2014), which embody the major change that, if office
BP differs from home BP, priority should be given to diagnoses based on home BP [52]. MORNING HYPERTENSION AND CARDIOVASCULAR DISEASE/DIABETIC NEPHROPATHY Kamoi et al. [54] conducted a study
with 170 type 2 diabetes patients and investigated the frequency of CVD and diabetic nephropathy (microalbuminuria or overt nephropathy) and retinopathy, based on the presence or absence of
hypertension at the office (SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg), and the presence or absence of hypertension as indicated by home BP (SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg). Of the patients
who showed hypertension at the office, 56.5% had morning hypertension as indicated by home BP, whereas 59.0% of the patients who had normal blood pressure at the office had morning
hypertension as indicated by home BP. As shown in Fig. 3, regardless of whether the office BP was hypertensive or normotensive, the group of subjects who showed morning hypertension in their
home BP had a significantly higher frequency of developing microvascular complications such as nephropathy and retinopathy. The frequency of the development of cerebrovascular disorders was
also significantly higher. As for heart disease, of the group of subjects who showed hypertension in their office BP, no significant differences were observed in the frequency of developing
heart disease in relation to the presence or absence of morning hypertension (8% vs. 11%, respectively). However, of the group of subjects with normotensive office BP, those with morning
hypertension had a significantly higher frequency of developing heart disease (35% vs. 0%, respectively). The Ibaraki Hypertension Assessment Trial (I-HAT) examined the home BP of 2,554
hypertensive patients including type 2 diabetes patients (20%) who were undergoing hypotensive therapy and found that as glucose tolerance gradually worsened, from normal, abnormal, and
ultimately to diabetes, the subjects’ early morning home BP became significantly higher, from 134.1 and 135.4 mmHg to 137.5 mmHg. Their frequency of morning hypertension ( > 135/85 mmHg)
also rose significantly, from 53.4 and 55.6 to 66.4% [55]. Furthermore, despite undergoing treatment for hypertension, ~60% of diabetic patients still manifested morning hypertension and
eventually developed microangiopathy and cerebrovascular disorders. Incidentally, Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP) is
a large-scale intervention study conducted in Japan that attempted, practically for the first time in the world, to perform hypotensive treatment by using morning home BP as an index. Of the
3518 subjects who participated in this study, 15% were diabetic patients. The study’s findings were recently published as part of a sub-analysis of an Impaired Glucose Metabolism (IGM)
group that targeted glucose tolerance abnormalities and diabetes with fasting blood glucose of over 110 mg/dL or HbA1c of over 5.8% [56]. The cumulative rate of occurrence per 1000
person×years of composite cardiovascular endpoints was 4.88 in Normal Glucose Metabolism (NGM) patients versus 9.95 in IGM patients, or ~2-fold (_P_ = 0.0002). As shown in past reports, on
comparing the predictive capabilities of endpoints by baseline and by blood pressure during hypotensive treatment, home BP was a better predictive factor than office BP. The risk of CVD
showed an increasing tendency with an SBP of >125 mmHg; it was shown to increase significantly with a DBP of >75 mmHg. Even in an analysis using diabetics only, the risk of CVD was
also shown to elevate significantly with an SBP of over 125 mmHg and a DBP of over 75 mmHg. Therefore, a blood pressure level of <125/75 mmHg is believed to be an appropriate hypotensive
goal for home BP in diabetic patients. The fact that a home BP of 125/75 mmHg corresponds to an office BP of 130/80 mmHg is an outcome that supports the target office BP of less than 130/80
mmHg in diabetes patients. Recently, the results were released of a sub-analysis of the “Home BP measurement with Olmesartan-Naive patients to Establish Standard Target blood pressure”
(HONEST) Study that performed hypotensive treatment using olmesartan, an ARB, in diabetic patients (_n_ = 4426) [57]. The incidence of cardiovascular events per 1000 person×years was 11.34
in the diabetic group, which was significantly higher than 5.20 in the non-diabetic group (_n_ = 17,165). If the group of non-diabetics with a morning home BP of less than 125 mmHg was used
as the criterion, the HR of cardiovascular events among the diabetic group tended to show a high value of 1.52-fold in a group of subjects whose morning home SBP was 125–135 mmHg. The HRs
showed significantly higher values of 2.08- and 4.80-fold, respectively, in a group of subjects with SBP of over 135 and <145 mmHg, and in a group with SBP over 155 mmHg. In addition, if
a group of non-diabetics with favorably controlled office and home BP levels was used as the criterion, the HR of CVD in the groups of diabetics with favorably controlled office BP and home
BP (_n_ = 1567 and _n_ = 36.6%) was 1.42-fold, showing no significant differences. By contrast, the HRs of CVD in groups of patients with white coat hypertension (_n_ = 411; 9.6%), masked
hypertension (_n_ = 1164; 27.2%), and poorly-controlled office and home BP levels (_n_ = 1135; 26.5%) were significantly higher at 2.73-, 2.77- and 2.81-fold, respectively. Although this
study is an observational investigation, similar to the HOMED-BP Study, it shows that a slight elevation in home BP can represent a risk for CVD in diabetic patients and may be an outcome
that supports establishing the home SBP goal as below 135 mmHg, or, if possible, below 125 mmHg. Because the HR of cardiovascular events was also high in white coat hypertension and masked
hypertension groups, the study can be said to once again indicate the importance of controlling not only office but also home BP levels. ABNORMALITIES IN CIRCADIAN BLOOD PRESSURE VARIATIONS
IN 24-H AMBULATORY BLOOD PRESSURE MONITORING (ABPM) NOCTURNAL HYPERTENSION, NON-DIPPERS/CARDIOVASCULAR DISEASES, AND ALBUMINURIA Ambulatory Blood Pressure Monitoring (ABPM), which measures
blood pressure under conditions of 24-h free, unrestricted movement, has made it possible to readily obtain a blood pressure profile covering a 24-h period outside the examination room, or,
in other words, blood pressure information in selected time zones such as 24 h, daytime, nighttime, and early morning. The blood pressure of healthy individuals shows circadian variations,
i.e., rising during the day because of physical and mental activities, and decreasing during the night while sleeping. It has become clear that diabetic patients show abnormalities in their
blood pressure circadian variations, such as office hypertension (“white coat hypertension”), morning hypertension (“morning surge”), poor nighttime hypotension (“non-dippers”), blood
pressure elevation (“risers”), and masked hypertension. Initial normotensive type 1 diabetic patients with microalbuminuria had higher 24-h and nocturnal SBP and DBP than healthy subjects
[58], and even normoalbuminuria patients had a high ratio of non-dippers in whom nocturnal blood pressure did not drop sufficiently [59]. It also became clear that, along with the
progression from normoalbuminuria to microalbuminuria, a rise was observed in 24-h SBP and DBP and in nocturnal SBP and that a night-day ratio of higher than 0.9 was a factor for predicting
the progression to microalbuminuria [60]. A study using type 2 diabetics also showed that these subjects had a higher nocturnal BP than healthy individuals, and regardless of their blood
pressure being normal or high, they had a high ratio of non-dippers. Only recently has the definition of a non-dipper of “showing a nocturnal decrease in BP of <10% of daytime BP” become
widespread. Fogari et al. [61] define it as a loss of nocturnal hypotensive action or a rise in nocturnal blood pressure and report that the frequency of non-dippers in type 2 diabetic
patients is ~30%, regardless of whether their blood pressure is normal or high, and that it occurs ~5 times more frequently than in normal subjects. A recent Korean study named The
Assessment of Blood Pressure Control and Target Organ Damage in Patients with Chronic Kidney Disease and Hypertension (APrODiTe) reported that the rate of non-dippers defined as the
night-day ratio of higher than 0.9 was 48.9% in diabetics with CKD stage 2 and 59.3% in diabetics with CKD stage 3–4 [62]. The rate of morning hypertension was also reported to be 22.7 and
23.4% in diabetics with CKD stage 2 and stage 3–4, respectively, which were higher than the rate in non-diabetics (9.5% and 16.1%, respectively). A rise in 24-h blood pressure and nocturnal
blood pressure may be considered to be triggered, either as a cause of diabetic nephropathy, which is a microvascular disorder, and/or as a result of decreased renal function. If neuropathy,
in particular the impairment of the autonomic nervous systems, occurs as a microvascular disorder in diabetes, normal circadian variation disappears. In other words, the difference between
daytime and nighttime blood pressure narrows, and the nighttime excretion volume of albuminuria also increases [63]. These results appear to show that in diabetic patients, the balance
between the sympathetic and parasympathetic systems is lost, and the tension of the sympathetic nervous system becomes relatively stronger during the night. Table 3 summarizes some of the
major results of ABPM that were investigated in diabetic patients in relation to CVD. Nakano et al. [64] performed ABPM in type 2 diabetic patients and observed, over an average period of
four years, a group possessing normal circadian blood pressure variations (_n_ = 201) and a group in which the circadian variations had been reversed (individuals whose blood pressure was
elevated at night: _n_ = 87). The results showed 20 and 56 fatal and non-fatal CVD events, respectively, showing that CVD had occurred more frequently in a group whose circadian variations
have reversed. This group, moreover, performed ABPM in 237 type 2 diabetic patients under the age of 60. The group with a 24-hour pulse pressure of ≥ 53.3 mmHg, rather than nocturnal blood
pressure being high, reportedly had a significantly higher incidence of CVD events compared to the group with a 24-hour pulse pressure below that level (20.7% versus 4.1%) [65]. Eguchi et
al. [66] performed ABPM in 1,268 type 2 diabetic patients, conducted a follow-up observation for an average of 50 months thereafter, and found that the higher the daytime SBP during arousal
and nocturnal SBP during sleep, the higher the incidence of CVD. Regarding the pattern of circadian variations, no differences were observed in the incidence rate of CVD between dippers and
non-dippers. However, the risers, whose blood pressure rose during the night (nighttime SBP > 135 mmHg), reportedly showed a 2.5-fold higher incidence of CVD compared to the group whose
nocturnal SBP was 120–135 mmHg. A study performed involving 1,178 elderly type 2 diabetic patients reported that office pulse rates and albuminuria were the best predictive factors for
all-cause deaths and deaths due to CVD, but 24-hour pulse pressure and nocturnal SBP were also independent predictive factors [67]. MASKED HYPERTENSION The International Database on
Ambulatory blood pressure in relation to the Cardiovascular Outcomes (IDACO) Study, which analyzed the ambulatory blood pressure (ABP) of 9,692 subjects gathered from 11 countries [68],
revealed that diabetics (_n_ = 229) had a higher frequency of masked hypertension than non-diabetics (_n_ = 5,486) (29.3% vs. 18.8%, respectively). The HR of the risk of diabetic patients
with untreated masked hypertension developing composite CVDs was 1.96 times higher than that of normotensive subjects, with the risk being comparable to that of Grade 1 hypertension
(140–159/90–99 mmHg). By way of information, office BP was 129.2 ± 8.0/76.0 ± 7.3 mmHg, and average daytime blood pressure recorded using ABPM was 141.5 ± 9.1/83.7 ± 6.5 mmHg. Conversely,
the risk of composite CVD of diabetic patients with masked hypertension undergoing treatment was equal to that of normotensive subjects. These findings suggest the need to measure home BP
and implement ABPM to detect masked hypertension in diabetic patients, even though their office BP might be normal. If masked hypertension has been diagnosed, a further reduction in office
BP is desired to bring the ABP down to the target blood pressure levels. ADMINISTRATION OF HYPOTENSIVE DRUGS BEFORE GOING TO BED Perhaps in relation to abnormal circadian blood pressure
variations described above, it was reported that if one or more hypotensive drugs were administered to diabetic patients before going to bed, rather than all the hypotensive drugs being
administered in the morning, the subjects’ SBP during sleep dropped from 122.4 to 115.0 mmHg. The ratio of non-dippers also fell from 76.3 to 49.5%, and the hazard ratio of CVD dropped to
0.33 [69]. Since sympathetic nervous systems are blocked, in particular, if doxazosin, which is an α1-blocker, was administered before going to sleep, morning hypertension reportedly
improved and albuminuria dropped [70]. It is therefore necessary to additionally incorporate chronobiological considerations in the treatment of hypertension. Please refer to the recent
review about chronotherapy in patients with resistant hypertension, diabetes mellitus and chronic kidney disease by Hermida et al. [71]. ORTHOSTATIC HYPOTENSION Orthostatic hypotension (OH)
is defined as a reduction in blood pressure of SBP ≥ 20 mmHg and DBP ≥ 10 mmHg that occurs within three minutes of adopting a standing position. In an ACCORD blood pressure study, a post hoc
analysis was made on the frequency of OH and prognosis of CVD [72]. The frequency of OH, examined at the start, one year later and four years later, was 17.8%, 10.4%, and 12.8%,
respectively, and 20% of the patients had manifested OH at least once. Diabetic patients with OH had a significantly higher (1.61-fold) HR of all-cause death and death/hospitalization due to
heart failure (1.85-fold higher). Some diabetics manifest OH along with the progression of diabetic neuropathy, so it is necessary to also measure blood pressure in the recumbent and
standing positions. STRICT BLOOD PRESSURE AND GLUCOSE CONTROL INDUCING MINIMAL FLUCTUATIONS STRICT BLOOD PRESSURE AND GLUCOSE CONTROL Strict blood pressure and glucose control is necessary
in hypertensive diabetic patients. In the ADVANCE Study [73], 11,140 type 2 diabetic patients were randomly allocated, with respect to blood glucose, to a standard treatment group using a
placebo or a strict control group (HbA1c ≤ 6.5%) using a combination of gliclazide MR (30–120 mg/day) and one other drug; with respect to blood pressure, they were allocated to a
conventional control group using a placebo or a routine control group using a fixed-dose combination of perindopril and indapamide (2 mg and 0.625 mg/day). Compared to the standard treatment
group, HbA1c dropped by 0.61% in the strict control group, whereas blood pressure fell 7.1/2.9 mmHg in the group given a fixed-dose combination therapy after an average of 4.3 years.
Combination treatments reduced the risk of new onset or aggravation of nephropathy by 33% and all-cause deaths by 18% compared to no intervention. The efficacy of strict blood glucose
control and routine blood pressure control was mutually independent: in other words, their efficacy was additive in nature. BLOOD PRESSURE AND GLUCOSE CONTROL RESULTING IN SMALL VARIATIONS
The significance of Visit-to-Visit Variability of blood pressure (VVV) still leaves room for discussion, unlike the clinical significance of nocturnal blood pressure and morning
hypertension. It has been revealed, however, that the wider the blood pressure’s VVV, the closer it is related to CVD and microvascular disorders [74]. Conversely, it has also been revealed
that the greater the VVV of blood glucose levels represented in terms of the HbA1c at the time of hospital visit, the higher the incidence of all-cause deaths and CVD [75]. A recent
retrospective survey of 632 type 2 diabetic patients with no past history of CVD [76] showed that groups whose blood pressure VVV and blood glucose VVV were larger than their respective mean
values presented a higher incidence of CVD than the three other groups (HR: 3.08-fold). The survey concluded that the risk of blood pressure VVV and the risk of blood glucose VVV were
additive in nature. BLOOD PRESSURE AND BLOOD GLUCOSE CONTROLS THAT LAST A LIFETIME The UKPDS that used newly diagnosed type 2 diabetic patients found that strict blood pressure control was
as effective as blood glucose control. Strict blood glucose control that was implemented simultaneously did not decrease the incidence of CVD as much as did blood pressure control. However,
if strict blood glucose control is implemented during the study period, its effects are reported to last for an extended period, even if standard treatment is later initiated. This is called
the “legacy effect,” and it is attracting research attention [77]. Unfortunately, the effects of strict blood pressure control to reduce the incidence of microangiopathy and macrovascular
disorders fade with time after changing to a standard treatment [78]. Recently, an observational study followed up all the patients in the ADVANCE Study who had survived for an additional
5.9 years. Its results were published as an ADVANCE-ON Study [79]. The effects of reducing all-cause deaths by hypotensive therapy, which had been noted in the ADVANCE study, had attenuated
in an analysis of the total follow-up period (mean: 9.9 years). However, the HR of all-cause deaths and main major vascular events in the hypotensive treatment group, compared to the placebo
group, had maintained a significant difference of 0.91 and 0.92, respectively: “the carry-forward effect.” The UKPDS reduced the patients’ BP level to 144/82 mmHg as a result of strict
blood pressure control and mainly used ACE inhibitors and β-blockers as hypotensive agents. Conversely, ADVANCE used ACE inhibitors and diuretics, in addition to β-blockers, ARBs and CCBs in
combination, and, as a result, had reduced the BP level to close to 135/75 mmHg. In other words, we can say that “the quality of hypotension” differs between the two. Strict blood pressure
control should be continued throughout the patient’s lifetime. CONCLUSION This paper summarizes the characteristics of hypertension in diabetic patients. Hypertension as a complication
further aggravates microvascular disorders such as diabetic nephropathy and retinopathy, causes arteriosclerosis to advance at an earlier stage, and progressively increases the frequency of
developing CVD. As a treatment for hypertension, physicians must select a hypotensive drug that improves insulin resistance and a type of treatment that also takes morning and nighttime
hypertension into consideration. It has also been revealed that both hypertension and arrhythmia, including QT prolongation, are induced during hypoglycemic periods. With the onset of an
aging society, there is a growing need for treatments that do not cause excessive blood pressure reduction or hypoglycemia. Favorable blood pressure and glucose control throughout a
patient’s lifetime are increasingly understood for the treatment of diabetes accompanied by hypertension. REFERENCES * Tatsumi Y, Ohkubo T. Hypertension with diabetes mellitus: significance
from epidemiological perspectives for Japanese. Hypertens Res. 2017;40:795–806. Article PubMed Google Scholar * Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, Ueki K,
Nakayama T. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: report from the Committee on the cause of death in diabetes
mellitus. J Jpn Diab Soc. 2016;59:667–84. (in Japanese). Google Scholar * Masakane I, Taniguchi M, Nakai S, Tsuchida K, Goto S, Wada A, Ogata S, Hasegawa T, Hamano T, Hanafusa N, Mizuguchi
J, Nakamoto H. An overview of regular dialysis treatment in Japan (as of 31 December 2015). Ther Apher Dial. 2017;50:1–62. (in Japanese). Google Scholar * Gregg EW, Yanfeng L, Wang J,
Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–23. Article CAS PubMed Google
Scholar * Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–24. Article CAS PubMed
Google Scholar * Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–8.
Article CAS PubMed Google Scholar * Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, on behalf of the UKPDS Group. Development and progression of nephropathy in type 2
diabetes: The United Kingdom Prospective. Diabetes Study (UKPDS 64) Kid Intern. 2003;63:225–232. Google Scholar * Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality
in non-insulin dependent diabetes mellitus. A systemic overview of the literature. Arch Intern Med. 1997;157:1413–8. Article CAS PubMed Google Scholar * Katayama S, Moriya T, Tanaka S,
Tanaka S, Yajima Y, Sone H, Iimuro S, Ohashi Y, Akanuma Y, Yamada N, for the Japan Diabetes Complications Study Group. Low transition rate from normo- and low microalbuminuria to proteinuria
in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia. 2011;54:1025–31. Article CAS PubMed PubMed Central Google Scholar * Kawasaki R,
Tanaka S, Tanaka S, Yamamoto T, Sone H, Ohashi Y, Akanuma Y, Yamada N, Yamashita H. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8-year
follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia. 2011;54:2288–94. Article CAS PubMed Google Scholar * Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J,
Parving HH, Bilous R, Sjolie AK, DIRECT Programme Study Group. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes:
randomised, placebo-controlled trials. Lancet. 2008;372:1394–402. Article CAS PubMed Google Scholar * Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi
N, DIRECT Programme Study Group. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet.
2008;372:1385–93. Article PubMed Google Scholar * Forrest KY-Z, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy. Diabetes.
1997;46:665–70. Article CAS PubMed Google Scholar * Malik RA, Williamson S, Abbott C, Carrington AL, Iqbal J, Schacly W. Effect of angiotensin-converting-enzyme (ACE) inhibitor
trandolapril on human diabetic neuropathy: randomized double-blind controlled study. Lancet. 1998;352:1978–81. Article CAS PubMed Google Scholar * McGill C, McMahon CA, Malcom GT,
Oalmann MC, Strong JP. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Arter Thromb Vasc Biol. 1995;15:431–40. Article Google Scholar * Berenson G, Srinivasan SR,
Bao W, Newman WP, Tracy RE, Wattigney WA, for the Bogalusa Heart Study. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J
Med. 1998;338:1650–6. Article CAS PubMed Google Scholar * Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA.
Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–44. Article CAS
PubMed Google Scholar * Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage
and cardiovascular risk. Hypertens Res. 2003;26:615–22. Article PubMed Google Scholar * Cecelja M, Chowienczyk P. Dissociation of aortic pulse velocity with risk factors for
cardiovascular disease other than hypertension. A systemic review. Hypertension. 2009;54:1328–36. Article CAS PubMed Google Scholar * Perticone F, Maio R, Sciacquana A, Andreozzi F,
Ilemma G, Perticone M, Zoccali C, Sesti G. Endothelial dysfunction and C‐reactive protein are risk factors for diabetes in essential hypertension. Diabetes. 2008;57:167–71. Article CAS
PubMed Google Scholar * Tomiyama H, Tsumoto C, Yamada J, Teramoto T, Abe K, Ohta H, Kiso Y, Kawauchi T, Yamashina A. The relationship of cardiovascular disease risk factors to
flow-mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens Res. 2008;31:2019–25. Article PubMed Google Scholar * Kawamori R, Yamasaki Y, Matsushima H,
Nishizawa H, Nao K, Hougaku H, Maeda H, Handa N, Matsumoto M, Kamada T. Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high-resolution B-mode imaging on carotid
arteries. Diabetes Care. 1992;15:1290–4. Article CAS PubMed Google Scholar * Mitsuhashi N, Onuma T, Kubo S, Takayanagi N, Honda M, Kawamori R, Yamasaki Y, Matsushima H, Nishizawa H, Nao
K, Hougaku H, Maeda H, Handa N. Coronary artery disease and carotid artery intima-media thickness in Japanese type 2 diabetic patients. Diabetes Care. 2002;25:1308–12. Article PubMed
Google Scholar * Feldt-Rasmussen B, Mathiesen ER, Deckert T, Giese J, Christensen NJ, Bent-Hansen L, Neilsen MD. Central role for sodium in the pathogenesis of blood pressure changes
independent of angiotensin, aldosterone and catecholamines in type 1 (insulin-dependent) diabetes mellitus. Diabetology. 1987;30:610–17. CAS Google Scholar * Ditzel J, Brochner-Mortensen
J. Tubular reabsorption rates as related to elevated glomerular filtration in diabetic children. Diabetes. 1983;32:28–33. Article PubMed Google Scholar * Luetscher JA, Kraemer FB, Wilson
DM, Schwartz HC, Bryer-Ash M. increased plasma inactive renin in diabetes mellitus: a marker of microvascular complications. N Engl J Med. 1985;312:1412–7. Article CAS PubMed Google
Scholar * Uzu T, Skaguchi M, Yokomaku Y, Kume S, Kanasaki M, Isshiki K, Araki S, Sugimoto T, Koya D, Haneda M, Kashiwagi A. Effects of high sodium intake and diuretics on circadian rhythm
of blood pressure in type 2 diabetic patients treated with an angiotensin II receptor blocker. Clin Exp Nephrol. 2009;13:300–6. Article CAS PubMed Google Scholar * Gans ROB, Bilo HJG,
Nauta JJP, Heine RJ, Donker AJM. Acute hyperinsulinemia induces sodium retention and a blood pressure decline in diabetes mellitus. Hypertension. 1992;20:199–209. Article CAS PubMed
Google Scholar * Mundil D, Cameron-Vendrig A, Husain M. GLP-1 receptor agonist: a clinical perspective on cardiovascular effects. Diab Vasc Dis Res. 2012;9:95–108. Article PubMed Google
Scholar * Tanaka T, Nangaku M, Nishiyama A. The role of incretins in salt-sensitive hypertension: the potential use of dipeptidyl peptidase-IV inhibitors. Curr Opin Nephrol Hypertens.
2015;20:476–81. Article Google Scholar * Katayama S. Glycemic control and blood pressure 2 Hypotensive action of SGLT2 inhibitors. J Blood Press. 2016;23:712–6. (in Japanese) Google
Scholar * Vasilakou D, Karaglannis T, Athanasiadou E, Malnou M, Liakos A, Bekiari E, Sarigianni M, Mathews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitor for type 2 diabetes. A
systemic review and meta-analysis. Ann Intern Med. 2013;159:262–74. Article PubMed Google Scholar * Tikkanen I, Narka K, Zeller C, Green A, Salsali A, Broedl UC, Woerle H on behalf of the
EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–8. Article CAS PubMed Google Scholar *
Zinman B, Arnner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Biomath D, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, for the EMPA-REG OUTCOME Investigators.
Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. Article CAS PubMed Google Scholar * Rahman A, Hitomi H, Nishiyama A.
Cardioprotective effects of SGLT2 inhibitors are possibly associated with normalization of the circadian rhythm of blood pressure. Hypertens Res. 2016;40:535–40. Article Google Scholar *
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Biomath D, Johansen OE, Woerie HJ, Broedl UC, Zinman B, for the EMPA-REG OUTCOME Investigators. Empaglifozin and
progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. Article CAS PubMed Google Scholar * Cherney DZI, Perkins A, Soleymanlou N, Maione M, Lai V, Lee A, Fagan
NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation.
2014;129:587–97. Article CAS PubMed Google Scholar * Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM,
Stockner M, Zinman B, Bergenstal RM, Buse JB, for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N
Engl J Med. 2016;375:311–22. Article CAS PubMed PubMed Central Google Scholar * Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J,
Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med.
2016;375:1834–44. Article CAS PubMed Google Scholar * Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, Kimura A, Kakei M, Noda M. Vital signs, QT-prolongation, and
newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care. 2014;37:217–25. Article CAS PubMed Google Scholar * Chow E,
Bernjak A, Williams S, Fawdry RA, Hilbert S, Freeman J, Sheridan P, Heller S. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk.
Diabetes. 2014;63:1738–47. Article CAS PubMed Google Scholar * Goto A, Ara OA, Goto M, Terauchi Y, Noda M. Sever hypoglycemia and cardiovascular disease: systemic review and
meta-analysis with bias analysis. Br Med J. 2013;347:f4533 https://doi.org/10.1136/bmj.f4533. Article Google Scholar * Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R,
Bonfigli AR, Esposito K, Gingliano D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy
control subjects and subjects with type 1 diabetes. Diabetes. 2012;61:2993–97. Article CAS PubMed PubMed Central Google Scholar * Ferranninini E, Buzzigoli G, Bonadonna R, Giorico MA,
Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–7. Article Google Scholar * Salomaa VV, Strandberg
TE, Vanhanen H, Naukkarinen V, Sarna S, Miettinen TA. Glucose tolerance and blood pressure: long-term follow-up in middle aged men. Br Med J. 1991;302:493–6. Article CAS Google Scholar *
Kashiwabara H, Inaba M, Maruno Y, Morita T, Awata T, Negishi K, Iitaka M, Katayama S. Insulin levels during fasting and glucose tolerance test and HOMA’s index predict subsequent development
of hypertension. J Hypertens. 2000;18:83–88. Article CAS PubMed Google Scholar * Rett K, Wicklmayr M, Dietze GJ. Hypoglycemia in hypertensive diabetic patients treated with
sulfonylureas, biguanides, and captopril (Letter). N Engl J Med. 1988;319:1609. CAS PubMed Google Scholar * Pollare T, Lithell H, Berne C. A comparison of the effects of
hydrochlorothiazide and captopril on glucose and lipid metabolism. N Engl J Med. 1989;321:868–73. Article CAS PubMed Google Scholar * Kodama J, Katayama S, Tanaka K, Itabasgi A, Kawazu
S, Ishii J. Effect of captopril on glucose concentration. Possible role of augmented postprandial forearm blood flow. Diabetes Care. 1990;13:1109–11. Article CAS PubMed Google Scholar *
Eliot WJ, Meyer P. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–07. Article Google Scholar * Verdecchia P, Reboldi G,
Angeli F, Borgioni C, Gattobigio R, Filippucci L, Norgiolini S, Bracco C, Porcellati C. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension.
2004;43:963–9. Article CAS PubMed Google Scholar * Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitdu T, Ito M, Ito S, Itoh H, Iwao H, Kai H,
Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Maruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K,
Shmosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, on behalf of The Japanese Society of Hypertension Committee for Guidelines for the Management of
Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension 2014. Hypertens Res. 2014;37:253–390. Article PubMed Google Scholar * Ohkubo T, Imai Y,
Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekine M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality
than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–5. Article CAS PubMed Google Scholar * Kamoi K, Kaneko S,
Miyakoshi, Nakagawa O, Soda S. Usefulness of home blood pressure measurement in the morning in type 2 diabetic patients. Diabetes Care. 2002;25:2218–23. Article PubMed Google Scholar *
Toyama M, Watanabe S, Miyauchi T, Kuroda Y, Ojima E, Sato A, Seo Y, Aonuma K. Diabetes and obesity are significant risk factors for morning hypertension: from Ibaragi Hypertension Assessment
trial (I-HAT). Life Sci. 2014;104:32–7. Article CAS PubMed Google Scholar * Noguchi Y, Asayama K, Staessen JA, Inaba M, Ohkubo T, Hosaka M, Satoh M, Kamide K, Awata T, Katayama S, Imai
Y, the HOMED-BP study group. Predictive power of home blood pressure and clinic blood pressure in hypertensive patients with impaired glucose metabolism and diabetes. J Hypertens.
2013;31:1593–602. Article CAS PubMed Google Scholar * Kushiro T, Kario K, Saito I, Teramukai S, Sato Y, Okuda Y, Shimada K. Increased cardiovascular risk of treated white coat and masked
hypertension in patients with diabetes and chronic kidney disease: the HONEST Study. Hypertens Res. 2017;40:87–95. Article PubMed Google Scholar * Benhamou PY, Halmi S, De Gaudemaris R,
Boizel R, Pitiot M, Siche JP, Bachelot I, Mallion JM. Early disturbances of ambulatory blood pressure load in normotensive type 1 diabetic patients with microalbuminuria. Diabetes Care.
1992;15:1614–9. Article CAS PubMed Google Scholar * Gilbert R, Phillips P, Clarke C, Jerums G. Day-night blood pressure variation in normotensive, normoalbuminuric type 1 diabetic
subjects. Dippers Non-Dippers Diabetes Care. 1994;17:824–7. Article CAS PubMed Google Scholar * Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in
nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. Article CAS PubMed Google Scholar * Fogari R, Zoppi A, Malamani GD,
Lazzari P, Destro M, Corradi L. Ambulatory blood pressure monitoring in normotensive and hypertensive type 2 diabetics. Preval Impair Diurnal Blood Press Patterns Am J Hypertens. 1993;6:1–7.
CAS Google Scholar * Oh SW, Han SY, Han KH, Cha RH, Kim S, Yoon SA, Rhu DR, Oh J, Lee EY, Kim DK, Kim YS, APrODiTe investigators. Morning hypertension and night non-dipping in patients
with diabetes and chronic kidney disease. Hypertens Res. 2015;38:889–94. Article CAS PubMed Google Scholar * Spallone V, Gambarella S, Maiello MR, Barini A, Frontoni S, Menzinger G.
Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578–84. Article CAS PubMed Google Scholar *
Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal
vascular events in NIDDM subjects. Diabetes. 1998;47:1501–6. Article CAS PubMed Google Scholar * Nakano S, Konishi K, Furuya K, Uehara K, Nishizawa M, Nakagawa A, Kigoshi T, Uchida K. A
prognostic role of mean 24-h pulse pressure level for cardiovascular events in type 2 diabetic subjects under 60 years of age. Diabetes Care. 2005;28:102–7. Article Google Scholar * Eguchi
K, Pickering TG, Hoshiide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular
events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–50. Article PubMed Google Scholar * Palmas W, Pickering TG, Teresi J, Schwartz JE, Moran A, Weinstock RS, Shea
S. Ambulatory blood pressure monitoring and all-cause mortality in elderly people with diabetes mellitus. Hypertension. 2009;53:12–127. Article Google Scholar * Franklin SS, Thijs L, Li
Y, Hansen TW, Boggia J, Liu Y, Asayama K, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Torp-Pederse C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin
Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovský J, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, on behalf of the International Database on Ambulatory blood pressure in relation to
Cardiovascular Outcomes (IDACO) Investigators. Masked hypertension in diabetes mellitus. Treatment implications for clinical practice. Hypertension. 2013;61:964–71. Article CAS PubMed
PubMed Central Google Scholar * Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients
with type 2 diabetes. Diabetes Care. 2011;34:1270–6. Article PubMed PubMed Central Google Scholar * Kamoi K, Ikarashi T. The bedtime administration of doxazosin controls morning
hypertension and albuminuria in patients with type-2 diabetes: Evaluation using home-based blood pressure measurements. Clin Exp Hypertens. 2005;4:369–76. Article Google Scholar * Hermida
RC, Ayala DE, Smolensky MH, Fernández JR, Mojón A, Portaluppi F. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and
stroke risks. Hypertens Res. 2016;39:277–92. Article PubMed Google Scholar * Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Culter JA, Grimm R, Pedley C, Peterson K,
Pop-Busui R, Sperl-Hilelen J, Cushman WC. Orthostatic hypotension in ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial. Prevalence, incidence, and prognostic
significance. Hypertension. 2016;68:888–95. Article CAS PubMed PubMed Central Google Scholar * Zoungas S, deGalan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, MacMahon S, Marre M, Neal
B, Patel A, Woodward M, Chalmers J, on behalf of the ADVANCE Collaborative Group. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and
microvascular outcomes in patients with type 2 diabetes. Diabetes Care. 2009;32:2068–74. Article CAS PubMed PubMed Central Google Scholar * Hata J, Arima H, Rothwell PM, Woodward M,
Zoungas S, Anderson C, Patel A, Neal B, Glasziou P, Hamet P, Mancia G, Poulter N, Williams B, Macmahon S, Chalmers J, ADVANCE Collaborative Group. Effects of visit-to-visit variability in
systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128:1325–34. Article PubMed Google
Scholar * Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y. Fluctuations in HbA1c are associated with a higher incidence of
cardiovascular disease in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:148–55. Article CAS PubMed Google Scholar * Takao T, Matsuyama Y, Suka M, Yanagisawa H,
Iwamoto Y. The combined effect of visit-to-visit variability in HbA1c and systolic blood pressure on the incidence of cardiovascular events in patients with type 2 diabetes. BMJ Open
Diabetes Res Care. 2015;3:e000129 https://doi.org/10.1136/bmjdrc-2015-000129. Article PubMed PubMed Central Google Scholar * Holman RP, Paul SK, Bethel MA, Mathews DR, Neil AW.
10-Year-follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. Article CAS PubMed Google Scholar * Holman RP, Paul SK, Bethel MA, Neil AW, Mathews DR.
Long-term follow up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–76. Article CAS PubMed Google Scholar * Zoungas S, Chalmers J, Neal B, Billliot
L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glaziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Mathews R, Mogensen CE, Perkovic
V, Poulter N, Rogers A, Williams B, MacMahon S, Patel A, Woodward M for the ADVANCE Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J
Med. 2014;371:1392–406. Article PubMed Google Scholar * Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients
with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int 2015; 6:8-18. Article Google Scholar * Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, Langkilde AM. Efficacy and
safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16:1102–10. Article CAS PubMed Google
Scholar * Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a
randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–55. Article CAS PubMed Google Scholar * Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi
Y, Tobe K, Tanizawa Y, Araki E, Ueda M, Suganami H, Watanabe D, Tofogliflozin 003 Study Group. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor
tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc
Diabetol. 2014;13:65. Article PubMed PubMed Central Google Scholar * Rodan M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC on behalf of the EMPA-REG MONO trial
investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Diabetes Endocrinol 2013; 1:208–19. Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Kawagoe Clinic, Saitama Medical University, Saitama, Japan
Shigehiro Katayama * Department of Endocrinology and Diabetes, Faculty of Medicine, Saitama Medical University, Saitama, Japan Masako Hatano & Masashi Issiki Authors * Shigehiro Katayama
View author publications You can also search for this author inPubMed Google Scholar * Masako Hatano View author publications You can also search for this author inPubMed Google Scholar *
Masashi Issiki View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Shigehiro Katayama. ETHICS DECLARATIONS CONFLICT OF
INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International
License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons license, and indicate if changes were made. If you remix, transform, or build upon this article or a part thereof, you must distribute
your contributions under the same license as the original. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Katayama, S., Hatano, M. & Issiki, M. Clinical features and therapeutic perspectives on hypertension in diabetics. _Hypertens Res_
41, 213–229 (2018). https://doi.org/10.1038/s41440-017-0001-5 Download citation * Received: 15 June 2017 * Revised: 20 July 2017 * Accepted: 21 July 2017 * Published: 05 February 2018 *
Issue Date: April 2018 * DOI: https://doi.org/10.1038/s41440-017-0001-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative