Play all audios:
ABSTRACT With time, memories undergo a neural reorganization that is linked to a transformation of detailed, episodic into more semantic, gist-like memory. Traditionally, this reorganization
is thought to involve a redistribution of memory from the hippocampus to neocortical areas. Here we report a time-dependent reorganization within the hippocampus, along its
anterior–posterior axis, that is related to the transformation of detailed memories into gist-like representations. We show that mnemonic representations in the anterior hippocampus are
highly distinct and that anterior hippocampal activity is associated with detailed memory but decreases over time. Posterior hippocampal representations, however, are more gist-like at a
later retention interval, and do not decline over time. These findings indicate that, in addition to the well-known systems consolidation from hippocampus to neocortex, there are changes
within the hippocampus that are crucial for the temporal dynamics of memory. SIMILAR CONTENT BEING VIEWED BY OTHERS TIME-DEPENDENT MEMORY TRANSFORMATION IN HIPPOCAMPUS AND NEOCORTEX IS
SEMANTIC IN NATURE Article Open access 27 September 2023 SCHEMAS PROVIDE A SCAFFOLD FOR NEOCORTICAL INTEGRATION OF NEW MEMORIES OVER TIME Article Open access 02 October 2022 MEMORY ENGRAM
STABILITY AND FLEXIBILITY Article Open access 18 September 2024 INTRODUCTION Memories evolve over time. After initial encoding, new information becomes fixed at a cellular level and
integrated within networks of existing memories1,2. This integration involves a reorganization of memory during which, with time, detailed, episodic memories are transformed into more
semantic, gist-like representations1,3. Although, the neural underpinnings of this time-dependent memory reorganization are at the heart of the neuroscience of memory, the neural evolution
of memories over time remains a topic of much controversy. In particular, whether the hippocampus, a critical hub for initial memory formation4,5,6,7,8, is involved in remote memories or not
has been controversial for decades3,9,10,11,12. The hippocampus can be subdivided into anterior and posterior parts—corresponding to the ventral and dorsal hippocampus, respectively, in
rodents—and these parts differ in function, structure and their connections to cortical and subcortical areas13,14,15. A prominent proposal that was largely based on rodent data linked the
ventral (anterior) hippocampus to emotion, stress, and affect, whereas the dorsal (posterior) hippocampus was implicated in cognitive functions such as learning, memory, and spatial
navigation16. Electrophysiological and lesion studies in rodents, as well as human neuroimaging studies, however, suggest that this view may need to be revised and that both anterior and
posterior hippocampal areas (aHC and pHC, respectively) may contribute to learning and memory processes, although the exact functional specialization is still unclear14,15,17. Further
studies suggest that the aHC and pHC might be differentially involved in recent and remote memories18,19,20, yet whether the transformation of memory over time may be linked to
time-dependent changes in aHC and pHC involvement in memory is completely unknown. Here we determine whether there are time-dependent changes in aHC and pHC contributions to memory and if
so, whether they are associated with the transformation from detailed to gist-like memory. To do so, we combined functional magnetic resonance imaging (fMRI) and multivariate
representational similarity analysis (RSA) with a task probing memory transformation. Participants learned 60 pictures of scenes and objects (30 neutral, 30 negative) and performed a
recognition test for these pictures in the MRI scanner either one day after encoding (1 d group) or four weeks later (28 d group). Critically, the recognition test included, in addition to
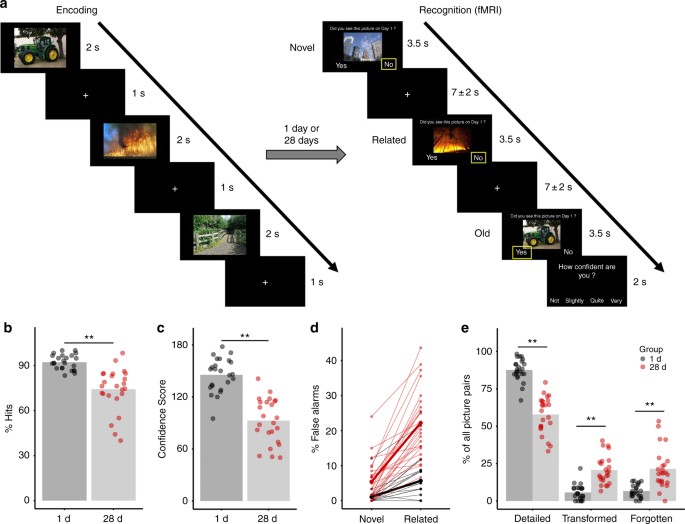
the old pictures learned during encoding and completely novel pictures, related lure pictures that carried the semantic gist of the old pictures but had different details (Fig. 1a). The
endorsement of related pictures as “old” provided a behavioral index of the time-dependent transformation from detailed to more gist-like memory representations. As predicted, we found a
strong increase in the endorsement of related pictures as old in the 28 d group relative to the 1 d group. This finding indicates a time-dependent memory transformation. At the neural level,
this transformation was paralleled by a time-dependent decrease in the aHC, the hippocampal subregion that was directly linked to memory specificity. The pHC, in turn, was not related to
memory specificity and did not decline over time. Further, RSA revealed that activity patterns in the aHC were highly specific and differed between old and new memories in the 1 d group but
not after 28 d. Representations in the pHC became more gist-like after 28 days. Together, these findings show that the aHC that supports memory specificity declines over time, whereas pHC
remains stable over time but carries more gist-like representations. Our data suggest that the time-dependent transformation from detailed to gist-like memory is linked to a reorganization
within the hippocampus. RESULTS TIME-DEPENDENT MEMORY TRANSFORMATION During encoding on experimental day 1, participants of the 1 d- and 28 d groups learned the pictures equally well
(Supplementary Fig. 1). In the recognition test, either 1 day or 28 days after encoding, the hit rate was expectedly lower in the 28 d group than in the 1 d group (main effect Group:
_F_(1,46) = 33.57, _p_ = 5.89e−07, generalized _η_2 = 0.377, _n_ = 48; Fig. 1b), yet memory was still clearly intact after 28 d as reflected by a hit rate of ~75% and a false alarm (FA) rate
for novel lures of only 5%. Most importantly, however, participants in the 28 d group showed a sharp increase in the FA rate specifically for related pictures and to a significantly lesser
extent for novel pictures (Picture Type × Group interaction: _F_(1,46) = 36.31, _p_ = 2.65e−07, generalized _η_2 = 0.155, _n_ = 48; main effect Group: _F_(1,46) = 45.63, _p_ = 2.13e−08,
generalized _η_2 = 0.343, _n_ = 48; main effect Picture Type: _F_(1,46) = 113.18, _p_ = 5.48e−14, generalized _η_2 = 0.363, _n_ = 48; Fig. 1d). For related pictures, the FA rate rose to
almost 25% after 28 days and was thus more than four times higher than the FA rate for novel pictures. This indicates that participants in the 28 d group particularly had difficulties
differentiating between old pictures and related pictures carrying the gist of the old pictures, suggesting a transformation towards more gist-like memory. Additionally, participants were
asked to rate their confidence on a 4-point-scale, whenever they indicated that they had seen the picture before (Fig. 1a), allowing us to calculate a Confidence Score as another measure of
memory specificity. Participants in the 28 d group were, as expected, significantly less confident in their memory than participants in the 1 d group (main effect Group: _F_(1,46) = 63.33,
_p_ = 3.41e−10, generalized _η_2 = 0.550, _n_ = 48; Fig. 1c). We further analyzed the 60 matching picture pairs (i.e., old pictures learned during encoding and their respective related
lures) and categorized memories for them as being either detailed, transformed or forgotten depending on whether participants endorsed solely the old pictures, both the old and related
pictures, or none of them as “old”. Participants in the 28 d group showed, compared to those of the 1 d group, significantly fewer detailed (main effect Group: _F_(1,46) = 102.96, _p_ =
2.55e−13, generalized _η_2 = 0.607, _n_ = 48) and more forgotten memories (main effect Group: _F_(1,46) = 26.88, _p_ = 4.72e−06, generalized _η_2 = 0.335, _n_ = 48), but critically also more
transformed memories (main effect Group: F(1,46) = 45.15, _p_ = 2.41e−08, generalized _η_2 = 0.425, _n_ = 48; Fig. 1e), again in line with the proposed time-dependent memory transformation.
Our behavioral data further suggest that stimulus-related emotional arousal influenced the transformation to gist-like memories: after 28 days significantly fewer negative pictures were
forgotten than neutral ones (paired _t_-test: _t_(23) = −5.00, _p_ = 4.64e−05, Cohen’s _d_ = −1.02, _n_ = 24) and, even more interestingly, negative pictures were significantly more often
transformed than neutral ones (paired _t_-test: _t_(23) = 2.67, _p_ = 0.0138, Cohen’s _d_ = 0.54, _n_ = 24; Supplementary Fig. 2), in line with findings21,22 suggesting that superior memory
for emotional material, indicated here by the slower forgetting rate, comes at the cost of reduced memory for contextual details, reflected here in an increase in transformed memories. AHC
BUT NOT PHC ACTIVITY DECREASES OVER TIME To elucidate the neural underpinnings of the memory dynamics over time, we first analyzed time-dependent changes in the hippocampus as a whole and
other cortical and subcortical areas that have been implicated in episodic memory before. We obtained overall reduced activity in the hippocampus, parahippocampus, and the amygdala in the 28
d group compared to 1 d group (see Supplementary Fig. 3 and Supplementary Tables 1 and 2). In addition, we performed a psychophysiological interaction (PPI) analysis to test whether the
cross-talk of the hippocampus with other areas critical for memory formation changed as a function of time. Our analysis showed specifically reduced functional connectivity between the right
hippocampus and the right amygdala in the 28 d group compared to the 1 d group (Supplementary Fig. 4). This decrease in hippocampal-amygdala connectivity was of particular interest as the
interaction of these areas is commonly linked to vivid memory23. As hippocampal involvement in remote memories has been argued to depend critically on memory vividness3,20, we further
explicitly looked at activity for old items that were recognized with high confidence. Even for those High Confidence Hits, overall hippocampal activity was lower in the 28 d group than in
the 1 d group (Supplementary Fig. 5a). For neocortical areas involved in more semantic or schema-related memory processes, there was, however, no reliable difference in activity in the 28 d
group vs. the 1 d group (when correcting for the number of ROIs; Supplementary Table 2). As we tested memory with a recognition test in which participants directly viewed all pictures, it
may not be surprising that neocortical areas were similarly involved in the 1 d- and 28 d-group, as these areas might just reflect the processing of the currently viewed pictures. The fMRI
findings so far are generally in line with the systems consolidation view, which would predict a decrease of hippocampal involvement in memory, irrespective of the specific picture type,
over time9,12,24. Looking at the hippocampal subregions along the long axis (Fig. 2a), however, revealed that the time-dependent decrease in activity was restricted to the aHC (left aHC main
effect Group: _F_(1,46) = 15.46, _p_ = 0.0003, generalized _η_2 = 0.1546, _n_ = 48) and mid-portion hippocampus (left mHC: _F_(1,46) = 9.24, _p_ = 0.0038, generalized _η_2 = 0.0999, _n_ =
48). Activity in the pHC, however, did not significantly differ between the 1 d- and 28 d-group (left pHC: _F_(1,46) = 1.70, _p_ = 0.1986, generalized _η_2 = 0.0230, _n_ = 48; Fig. 2b).
These differences between the ROIs were underlined by a significant Group × HC Long Axis interaction (_F_(2,92) = 6.07, _p_ = 0.0033, generalized _η_2 = 0.036, _n_ = 48). The decrease in
activity for high-confidence hits was also most pronounced in the aHC (Supplementary Fig. 5b). Moreover, connectivity analysis using the aHC and pHC as seed regions showed that the right
aHC-right amygdala connectivity for related pictures (but not old or novel pictures) was significantly reduced in the 28 d- relative to the 1 d-group (SVC peak level: _x_ = 20, _y_ = −4, _z_
= −14, _t_ = 3.59, _p_(FWE) = 0.0156, _k_ = 15; Fig. 3), while we found no significant differences between the groups in the connectivity to the amygdala when using the pHC as seed region,
suggesting that it might be the connectivity between the aHC and the amygdala that is notably reduced in the 28 d group. In order to further examine whether the decrease in aHC activity
could be directly linked to the change in the nature of remembering, we correlated the activity in the hippocampal subregions with behavioral indices of memory specificity, i.e., the FA rate
for related lures and the Confidence Score. These analyses showed that specifically the aHC was associated with the specificity of memory. In particular, aHC activity for related pictures
was correlated negatively with the FA rate to related pictures (left aHC: _t_(46) = −5.15, _p_ = 5.37e−06, Pearson’s _r_ = −0.60, _n_ = 48; Fig. 2c) and aHC activity for old pictures
correlated positively with the Confidence Score (left aHC: _t_(46) = 3.19, _p_ = 0.0025, Pearson’s _r_ = 0.43, _n_ = 48; Fig. 2d). For the pHC, however, there were no such associations with
memory specificity (left pHC, FA rate to related pictures: _t_(46) = −1.08, _p_ = 0.2879, Pearson’s _r_ = −0.16, _n_ = 48; Confidence Score: _t_(46) = 0.44, _p_ = 0.6645, Pearson’s _r_ =
0.06, _n_ = 48) and the correlations between activity and indicators of memory specificity were significantly distinct in the left aHC and pHC (FA related: Pearson and Filon’s _z_ = −3.46,
_p_ = 0.0005, _n_ = 48; Confidence Score: Pearson and Filon’s _z_ = 2.81, _p_ = 0.0049, _n_ = 48). These correlations across the 1 d- and 28 d-groups indicate that the aHC and pHC are
differentially linked to memory specificity. When we looked at the correlations separately in the 1 d- and 28 d-group, we obtained for the percentage of FA to related items, the key
parameter of memory specificity, a significant correlation with aHC in the 1 d group only (_t_(22) = −2.37, _p_ = 0.027, Pearson’s _r_ = −0.45, _n_ = 24). For the 28 d group, this
correlation did not reach significance (_t_(22) = −1.62, _p_ = 0.121, Pearson’s _r_ = −0.37, _n_ = 24), which might be related to the proposed reduced involvement of the aHC in memory in the
28 d group, although a lack of statistical power might also account for the non-significant correlation in the 28 d group. For the memory Confidence Score, the correlations with aHC
activity did not reach significance in the separate groups (1 d group: _t_(22) = 1.17, _p_ = 0.255, Pearson’s _r_ = 0.24, _n_ = 24; 28 d group: _t_(22) = 1.01, _p_ = 0.326, Pearson’s _r_ =
0.21, _n_ = 24). Although, we found a reduction of activity in the 28 d group in comparison to the 1 d group in the aHC and the mHC, it is important to note that there was no Group × Picture
Type interaction (Supplementary Fig. 6a) in these ROIs. Thus, our univariate results show that the activity in the aHC, but not the pHC, is reduced in the 28 d group compared to the 1 d
group for all picture types, suggesting that the contribution of the aHC in the task in general is reduced. Our brain-behavior correlations further show that the aHC, but not the pHC, is
associated with memory specificity. It is not surprising that aHC activity was reduced irrespective of Picture Type after 28 d because a specific memory representation is required to both
correctly identify an old item as old and to correctly reject novel or related items. SPECIFICITY OF MNEMONIC REPRESENTATIONS IN AHC AND PHC The above univariate analyses showed that it was
specifically the aHC that was associated with memory specificity and that specifically activity in this area decreased at a longer retention interval of 28 days. While univariate analyses
can show a general involvement of an area in a task, multivariate analysis allows the detection of specific patterns of activity across multiple voxels and may be more sensitive to the
changing representations of the different picture types and more informative about the functional organization of memory at different time intervals. Therefore, we ran a RSA (Fig. 4a) to
examine whether the mnemonic representations differed in the aHC, mHC and pHC, whether they changed depending on the retention interval, and to what extent such different representational
patterns can be linked to the proposed memory transformation. We first created average representational dissimilarity matrices (RDMs) in each hippocampal subregion, separately for each group
(Fig. 4b): visualizations of these RDMs suggest the most similar activity patterns for combinations of old pictures in all hippocampal subregions in the 1 d group; whereas, in the 28 d
group the representational pattern was less clear in the aHC and less-specific in the pHC. Note, however, that for the visualizations each RDM was separately rank transformed and scaled into
[0, 1] preventing a direct descriptive comparison across hippocampal subregions. We therefore extracted the mean pattern similarity for each RDM: this showed that the mean similarity was
highest in the pHC and lowest in the aHC (main effect HC Long Axis: _F__(_2,92) = 84.37, _p_ = 1.54e−21, generalized _η_2 = 0.350, _n_ = 48; Fig. 4c), suggesting particularly distinct
activity patterns in the aHC for different pictures which might allow for highly specific memories, whereas in the pHC the higher neural similarity across different pictures may reflect a
larger degree of overlapping representations. We then looked at the similarity of the RDMs across groups and found that the overlap of the representational patterns of the 1 d- and 28
d-groups was significantly higher in the pHC than in the aHC (main effect HC Long Axis: _F_(2,36) = 12.72, _p_ = 6.65e−05, generalized _η_2 = 0.231, _n_ = 19; Fig. 4d). This suggests that
pHC representational patterns changed less over time than aHC representational patterns did. In order to directly test whether the time-dependent changes of the memory representations in
anterior and posterior hippocampal areas were associated with the transformation from detailed to more gist-like memory, we finally compared brain and model-based RDMs. More specifically, we
compared the brain RDMs of the hippocampal subregions with two model RDMs: (1) the model “Old Distinct” expects similar activity patterns for all old pictures that are distinct from
patterns for related or novel pictures, a representation expected in areas that help detecting the old pictures as old and separate them from related pictures; (2) the model “Old and Related
Similar” expects a more similar pattern for the old and related pictures, that is distinct from patterns for the novel pictures, a representation expected in areas that detect the gist as
having been encoded but cannot separate between details (Fig. 4e). Based on the behavioral data, we reasoned that the _“_Old Distinct_”_ model should, in general, fit better in the 1 d group
as these participants still had detailed memories, while the _“_Old and Related Similar_”_ model might fit better in the 28 d group, as for these participants part of the memories had been
transformed to gist-like versions. Our analyses showed that, in the 1 d group, the _“_Old Distinct_”_ model had, compared to the _“_Old and Related Similar_”_ model, indeed a marginally
better fit in the left aHC (one-tailed paired _t_-test: _t_(23) = 1.58, _p_ = 0.0635, _n_ = 24) and a better fit in left mHC (one-tailed paired _t_-test: t(23) = 1.91, _p_ = 0.0345, _n_ =
24), whereas in the left pHC both models were indistinguishable (one-tailed paired _t_-test: _t_(23) = 0.61, _p_ = 0.2737, _n_ = 24). In the 28 d group, on the other hand, the _“_Old and
Related Similar_”_ model had a better fit than the _“_Old Distinct_”_ model in the left mHC (one-tailed paired _t_-test: t(23) = −2.24, _p_ = 0.0174, _n_ = 24) and tended to have a better
fit in the left pHC (one-tailed paired _t_-test: _t_(23) = −1.53, _p_ = 0.0695, _n_ = 24), while in the left aHC both models were indistinguishable (one-tailed paired _t_-test: _t_(23) =
−0.07, _p_ = 0.4740, _n_ = 24) and the respective model fits were generally rather low. The brain data were largely comparable in both hemispheres (see Supplementary Figures 7 and 8 for
results in the right HC). Group differences in the univariate analysis and the RSA were not modulated by stimulus emotionality (see Supplementary Figs. 6b and 9). We did, however, find
time-dependent connectivity changes with hippocampal seed regions that were modulated by stimulus emotionality for some of the ROIs (Supplementary Table 3). DISCUSSION How memories evolve
over time is a fundamental issue of the neuroscience of memory. While previous research focused mainly on time-dependent changes in hippocampal and neocortical contributions to
memory3,9,10,12, here we show a time-dependent reorganization along the hippocampal long axis that is related to the transformation from detailed to gist-like memory. More specifically, our
data indicate that the aHC is involved in memory specificity and represents actually encoded events distinctly from semantically related information at short retention intervals but shows a
marked decrease in activity at longer retention intervals. Activity in the pHC, in turn, was largely unrelated to memory specificity and did not decrease over time, while pHC
representational patterns seemed more gist-like at a longer retention interval. The present data point to a possible involvement of the aHC in the specificity of memory. Previous data in
rodents showed that firing fields of ventral hippocampal (corresponding to aHC in humans) place cells are larger than those in the dorsal hippocampus25, which might translate into more
abstract, large-scale aHC memory representations (26, see also ref. 14). However, the finding that an animals’ exact location can be decoded from ventral hippocampal activity27 is in line
with the role of the aHC in memory specificity that we propose here. In addition, our results fit to a study showing stronger aHC activity for recent memories than for remote memories20, to
a study showing that aHC carries information about memory contexts in immediate and recent (1 day old) memories28 and to studies showing a consistent implication of the aHC in memory of
specific events29. Our results further dovetail with reports showing that the aHC specifically is associated with segregating events30 and with novelty detection17,31, both of which requires
specific memory representations. Whereas the activity of the aHC was reduced after 28 d, no such decline was observed for the pHC, suggesting that not all parts of the hippocampus decrease
in activity over time. The RSA data, however, suggested a time-dependent change of the representational pattern in the pHC. In the 1 d group the pHC representation was already less-specific
than the aHC representation, which corroborates the recent idea that there are complimentary learning systems within the hippocampus with one supporting gist-like representations32,33. In
the 28 d group, the model RSA data even suggested that the representational patterns in the pHC resemble more gist-like patterns. This result suggests a time-dependent decrease in the
specificity of the pHC memory representation. This idea is in line with a recent finding34, showing that neural patterns of overlapping memories were more similar in the pHC after a week of
consolidation. In this study, however, part of the memories were actually overlapping (e.g., same scene with different objects), whereas our study extends this finding by using two different
pictures with only the semantic gist as overlap, thereby pointing to a memory transformation process. Thus, there may be two time-dependent processes that contribute to more gist-like
memory: a decrease in the aHC supporting memory specificity and an increase in the unspecificity of the mnemonic representation in the pHC, whose activity remains rather stable over time.
Whether one process proceeds or follows the other or whether both occur independently remains to be shown. Our findings suggest a functional specialization in which aHC representations
support detailed memories and pHC representations are more gist-like after a longer time delay. Rodent data, however, suggest that the hippocampal long axis is organized along a gradient15.
Most human studies did not address the mHC and rather little is known about the properties of this subregion. Our finding that the mHC was both with respect to its association with memory
specificity and in terms of decreased activity in between the aHC and pHC is in line with the proposed functional gradient along the hippocampal long axis. Yet, how exactly the proposed
different functions of the aHC and pHC are bridged is still unclear and remains a challenge for future research. It is important to note that while we report this time-dependent
reorganization within the hippocampus that was linked to memory transformation, we obtained also evidence for the proposed systems consolidation theory9,12,24. Hippocampal activity during
recognition testing was significantly lower after 28 days than after 1 day, even for items remembered with high confidence. This latter point opposes the transformation hypothesis2, which
would not expect a reduction in hippocampal involvement for high confident, detailed memory. In addition, hippocampus-amygdala connectivity, known to be implicated in vivid memory23,35, was
reduced in the 28 d group compared to the 1 d group. However, this reduction in functional connectivity with the amygdala seemed to be specific to the aHC. This finding is in line with data
suggesting that the aHC is connected to, among other regions, the amygdala, whereas the pHC is connected to areas involved in schematic memory such as the precuneus14,16. Thus, the aHC and
pHC appear to be part of distinct neural networks that are involved in specific vs. gist-like memory and the observed reorganization along the hippocampal long axis is most likely concerted
with the postulated large-scale redistribution (i.e., systems consolidation) of memory. Finally, we would like to point out that the time-dependent changes reported here cannot be
interpreted as a mere indication of a reduction in memory strength. In fact, we have designed this study explicitly to be able to differentiate between a general reduction in memory strength
and memory transformation processes. In particular, we included related pictures that allowed us to probe memory specificity. If only memory strength was reduced after 28 d, this should be
reflected in a comparable increase in the FA rates for related and novel pictures. We observed, however, a much stronger increase of FAs for related pictures than for novel pictures, which
is in sharp contrast to the interpretation of a simple reduction in general memory strength but in line with the proposed transformation from detailed to gist-like memory. In addition, our
model RSA data can also not be explained by a general reduction in memory strength. This view would imply that the memory for specific details and the gist memory decrease to a similar
extent over time so that the relative representation of old and related items remains over time. Our data, however, show that the _“_Old Distinct_”_ model best characterized activity in the
1 d group, whereas after 28 d the two models were indistinguishable in the aHC and the _“_Old and Related Similar_”_ model seemed to fit better in the mHC and pHC. Together, these findings
indicate that, in addition to the well-known decline in memory strength over time, there is also a change in the nature of memory, from detailed to more gist-like. Our findings show that
while the involvement of the hippocampus as a whole in memory decreases over time, this decrease is not present in all parts of the hippocampus. However, although there was a hippocampal
memory representation even long after encoding, the nature (and origin) of the hippocampal contribution to remembering changed significantly with time. To conclude, we suggest here a
time-dependent reorganization within the human hippocampus that is linked to a transformation from detailed to gist-like memory and might operate in tandem with the previously suggested
large-scale reorganization of memory that occurs in the brain over time9,12,24. METHODS PARTICIPANTS We tested 48 healthy, right-handed, young adults (24 men, 24 women; age: mean = 23.85
years, SD = 3.28 years) without a history of any psychiatric or neurological diseases, without medication intake or drug abuse and without circumstances preventing an MRI scan. All
participants gave written informed consent and received monetary compensation for participation. The study protocol was approved by the ethics committee of the German Psychological Society
(DGPs). Participants were pseudo-randomly assigned to the 1 d- or 28 d-group (12 women and 12 men per group). All experiments took place in the afternoon or early evening. The sample size
corresponds to other studies on the neural underpinnings of memory processes and an a-priori power calculation with G* Power (http://www.gpower.hhu.de/; _f_(U) = 0.5, _α_ = 0.05, 1-_β_ =
0.90) for the decisive interaction effect Group × FA Picture Type (see Behavioral data analysis). STUDY DESIGN AND EXPERIMENTAL PARADIGM Testing took place on two experimental days: Day 1,
encoding outside of the scanner and Day 2, recognition memory testing in the MRI scanner. Critically, the time interval between encoding and recognition testing was varied between the two
experimental groups: for participants in the 1 d group recognition testing took place one day after encoding, while for participants in the 28 d group recognition memory was tested 28 days
after encoding. The testing of the two groups was intermixed, so confounds related to changes in, for instance, the technical environment of the scanner over time cannot explain group
differences. STIMULUS MATERIAL We used 180 pictures of natural scenes and objects as stimulus material. About one third of the pictures were taken from the International Affective Picture
System (IAPS36), while the others were taken from open internet platforms. Half of the pictures contained emotionally negative scenes or objects while the other half contained neutral
contents. Participants rated all pictures at the end of the experiment with respect to picture valence (scale from 0 = negative to 100 = positive, with 50 = neutral) and picture arousal
(scale from 0 = not arousing to 100 = very arousing). In retrospect, these data confirmed that neutral pictures (_M_ = 57.38, SEM = 0.79) were perceived as neutral and negative pictures (_M_
= 25.99, SEM = 1.39) as more negative (paired _t_-test: _t_(47) = −18.38, _p_ < 0.0001, Cohen’s _d_ = −2.65, _n_= 48). Furthermore, negative pictures (_M_ = 47.50, SEM = 3.01) had higher
arousal ratings than neutral ones (_M_ = 11.15, SEM = 1.90; paired _t_-test: _t_(47) = 13.42, _p_ < 0.0001, Cohen’s _d_ = 1.94, _n_ = 48). The 180 pictures were divided into three lists
(each 30 negative and 30 neutral pictures): List A and List B contained semantically related pictures, i.e., for each picture in List A there was a matching picture in List B that carried
the same gist (e.g., mowing tractor) but different details (e.g., different brand, color, perspective, and background). List C, on the other hand, contained novel pictures that were not
semantically related to either List A or List B pictures. Half of the participants learned List A during encoding and List B pictures were used as related lures and List C pictures as novel
lures in the recognition test, while the other half of the participants learned List B during encoding and List A pictures were used as related lures and List C pictures as novel lures in
the recognition test. The semantic relatedness of the stimuli was rated by an independent sample (_n_ = 12) on a scale from 1 (“not related”) to 10 (“highly related”). Corresponding pictures
of List A und List B were rated as highly related (_M_ = 8.25, SEM = 0.062), in comparison to List A pictures compared to List C pictures (_M_ = 2.03, SEM = 0.056, paired _t_-test:
_t_(19.84) = 12.86, _p_ < 0.0001, Cohen’s _d_ = 4.18), or List B compared to List C pictures (_M_ = 1.98, SEM = 0.055, paired _t_-test: _t_(11) = 14.85, _p_ < 0.0001, Cohen’s _d_ =
4.49). EXPERIMENTAL DAY 1 (MEMORY ENCODING) On the first experimental day, participants performed three encoding runs. In each run, the 60 pictures from the respective list (either A or B)
were presented to the participant in random order on a computer screen, using MATLAB (www.mathworks.com) with the Psychophysics Toolbox extensions37. Each picture was presented for 2 s
followed by a fixation cross of 1 s. Participants were instructed to memorize the pictures. Immediately after each encoding run a free recall task followed: participants verbally listed all
the pictures they could remember while the investigator checked off the named pictures on a list and prompted the participant to a more detailed description in case the description of a
picture was inconclusive. In total, the encoding session took about 20 min. EXPERIMENTAL DAY 2 (MEMORY TESTING) On the second experimental day, either 1 d or 28 d after encoding,
participants first performed another free recall task outside the scanner and then a recognition task while fMRI measurements were taken. In the recognition task, participants saw the 60 old
pictures, 60 related pictures, i.e., new pictures carrying the gist of the old pictures, and 60 novel pictures in random order. Each picture was shown for 3.5 s and participants were asked
to indicate (“yes” vs. “no”) by button press whether they had seen this picture during the encoding session or not. Critically, participants were informed before the task that some of the
pictures may be similar to the original ones. Participants were further explicitly instructed to answer “Yes” only if they thought the picture was exactly the same as the one learned on
experimental day 1. After participants’ response, their choice was marked by a yellow box around the answer. If they answered “Yes” a confidence rating followed: they were asked to indicate
on a 4-point scale how confident (not at all confident, slightly confident, quite confident, or very confident) they were that they had seen the picture on experimental day 1. This rating
was shown for 2 s, and again their answer was marked by a yellow box. Each trial was followed by a fixation cross with a jittered presentation time of 7 ± 2 s. BEHAVIORAL DATA ANALYSIS To
assess the performance in the recognition task in general we compared the percentages of hits for old pictures in a mixed-design ANOVA with Group (1 d vs 28 d) as between-subject factor and
Emotionality (negative vs neutral) as within-subject factor. In order to assess the specificity of memory, we further analyzed the percentages of FA for related and novel lures in a
mixed-design ANOVA with Group (1 d vs 28 d) as between-subject factor and FA Picture Type (related vs novel) and Emotionality (negative vs neutral) as within-subject factors. To additionally
include information about the confidence of the participants when answering correctly, we calculated a Confidence Score by weighting each hit by the respective confidence (not at all
confident = 0, slightly confident = 1, quite confident = 2 or very confident = 3), resulting in a score of 0–3 for each hit, before summing up overall hits. The maximum Confidence Score is
therefore 180 (60 “very confident” hits). This score was again subjected to a mixed-design ANOVA. We also analyzed the matching picture pairs (old picture and the corresponding related lure
carrying the same gist) by assigning each pair to one of three categories: (1) detailed pairs, for which participants could reliably distinguish between old and related pictures and
therefore correctly identified the old picture as old and correctly rejected the related picture as new. (2) transformed pairs, for which participants could not rely on detailed memories but
still remembered the gist, as reflected by a FA to the related lure (irrespective of the response to the old picture), and (3) forgotten pairs, for which participants may have forgotten the
whole picture (both gist and details), as reflected by a miss for the old picture and a correct rejection for the related picture. Behavioral data analyses were performed with R version
3.3.2 (https://www.r-project.org/). All _p_-values are two-tailed and Welch’s _t_-tests were used as default for between group comparisons38. The Shapiro–Wilk normality test was applied to
the dependent variables: while the Confidence Score (_W_ = 0.96, _p_ = 0.1246) was normally distributed, this was not the case for the Hits (_W_ = 0.87, _p_ = 0.0001) and the FA (_W_ = 0.90,
_p_ = 0.0009). Despite this violation of the normality assumption we applied the above described ANOVAs due to the robustness of these tests against the violation of this assumption39. MRI
ACQUISITION MRI measurements were obtained with a 3T Skyra scanner (Siemens), equipped with a 32-channel head coil. For the functional images, a 3D echoplanar imaging (EPI) sequence (836
volumes) was used with the following parameters: 36 slices, slice thickness = 3 mm, distance factor 20%, repetition time (TR) = 2500 ms, echo time (TE) = 30 ms, voxel size 3.0 mm isotropic.
We additionally acquired a high-resolution T1-weighted anatomical image (TR = 2.5 s, TE = 2.12 ms, 256 slices, voxel size = 0.8 × 0.8 × 0.9 mm) and a magnetic (B0) field map to unwarp the
functional images. DATA PREPROCESSING The fMRI data were preprocessed using MATLAB and SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). The first four functional images (10 s) were discarded from
the rest of the analysis to allow for T1 equilibration. The remaining 832 functional images were first spatially realigned and unwarped using the field maps, then coregistered to the
structural image, followed by a normalization to the MNI space. For the univariate analysis, the images were additionally spatially smoothed using an 8 mm full-width half-maximum Gaussian
kernel. GENERAL LINEAR MODELING AND WHOLE-BRAIN ANALYSIS For the univariate analysis, the data were analyzed using general linear modeling (GLM) as implemented in SPM12. Six separate
regressors for each of the Picture Type × Emotionality combinations were modeled: old negative, old neutral, related negative, related neutral, novel negative, and novel neutral. The onsets
of the confidence ratings were additionally included as a regressor of no interest and all regressors were convolved with the canonical hemodynamic response function. Note that we did not
include movement regressors in the GLM, as we used the SPM unwarp function in the data preprocessing instead. A high-pass filter of 128 s was used to remove low-frequency drifts and serial
correlations in the time series were accounted for using an autoregressive AR(1) model. To look at whole-brain activation differences between the 1 d- and 28 d-groups, we used a two-sample
_t_-test design at second-level modeling. ROI ANALYSIS In addition to the whole-brain analysis, we performed regions of interest analyses that focused on brain areas that have previously
been implicated in detailed and more semantic or schema-related memory processes1,3,9,40. To this end, we used the following anatomical masks from the Harvard-Oxford atlas using a
probability threshold of 50%: hippocampus (left and right), anterior parahippocampal gyrus (left and right), posterior parahippocampal gyrus (left and right), precuneus, angular gyrus (left
and right), anterior cingulate gyrus, inferior frontal gyrus pars opercularis (left and right), inferior frontal gyrus pars triangularis (left and right), temporal pole (left and right), and
the amygdala (left and right). In addition, we used masks created with MARINA (http://www.bion.de/eng/MARINA.php) for the left and right ventromedial prefrontal cortex. The signal within
the ROIs was deconvolved for each of the regressors from the GLM (old negative, old neutral, related negative, related neutral, novel negative, and novel neutral) using a finite impulse
response function (FIR) on the time course averaged across all voxels of the ROI as implemented within MarsBar41 for the first seven repetition times (TRs; 15 s). We choose FIR
deconvolutions here to capture the shape of the HRF and allow for differences in this hemodynamic response across regions and participants. For statistical comparisons in R, we extracted the
peak response from these FIR time courses: as described in refs. 42,43 the peak response was defined as the average signal over time points whose responses (collapsed across all conditions)
did not significantly (_p_ > 0.05) differ from the numerical peak tested across all participants. In most ROIs this procedure resulted in the peak response being the average across the 5
s and the 7.5 s time points (exceptions: left and right posterior parahippocampal gyrus and right angular gyrus = only the 5 s time point; left angular gyrus = 2.5 s and 5 s time points;
precuneous cortex = 7.5 s and 10 s time points; left and right temporal pole = 5 s, 7.5 s and 10 s time points; in the left and right ventromedial prefrontal cortex the numerical peak did
not significantly differ from any of the other time points, as no reliable peak response seems to be present in these two ROIs we did not perform further analysis on this data). Then the
average signal over the respective time points were calculated for each condition separately (old negative, old neutral, related negative, related neutral, novel negative, and novel neutral)
and used in a mixed ANOVA model, with Group (1 d vs 28 d) as between-subject factor and Picture Type (old vs related vs novel) and Emotion (negative vs neutral) as within-subject factors.
In case of violation of the sphericity assumption, Greenhouse-Geisser corrections were applied. The _p_-value threshold was adjusted for multiple comparisons by the numbers of ROIs.
DIFFERENTIATION ALONG THE HIPPOCAMPAL LONG AXIS In order to look at differences across the long (anterior–posterior) axis of the hippocampus, we used the procedure described by ref. 26 to
divide a hippocampal mask into three parts with approximately equal lengths along the long axis, using the WFU pick-atlas44,45: pHC from _Y_ = −40 to −30, mHC from _Y_ = −29 to −19, and aHC
from _Y_ = −18 to −4. For these new hippocampal ROIs, we then deconvolved the signal for each of the regressors using a finite impulse response function (FIR), and extracted the peak
response from these FIR time courses for statistical comparisons in R as described in detail for the other ROIs above. In all of these hippocampal long axis ROIs this procedure resulted in
the peak response being defined as the average across the 5 s and the 7.5 s time points. We then run a mixed ANOVA model on these peak responses, with Group (1 d vs 28 d) as between-subject
factor and Picture Type (old vs related vs novel) and Emotion (negative vs neutral) as within-subject factors in each of the ROIs separately. In addition, we performed another analysis to
see if the delay manipulation (1 d vs 28 d) had a different effect on these three hippocampal ROIs, using an ANOVA with Group as between-subjects factor and HC Long Axis (aHC, mHC, and pHC)
as within-subject factor. COMPARISON OF HIGH- AND LOW-CONFIDENCE HITS In order to assess whether the time-dependent decrease in hippocampal activity was modulated by confidence, we created a
second model that was based on the behavioral responses and confidence ratings of the participants. We modeled five regressors: High-confidence hits (hits with a confidence rating of 3),
low-confidence hits (hits with a confidence rating of 0, 1 or 2), related CR, novel CR, and all incorrect answers (misses + related FA + novel FA + no presses). The onsets of the confidence
ratings were again included as a regressor of no interest and all other procedures were the same as in the first GLM. We then deconvolved the signal for the high-confidence hits and
low-confidence hits in the hippocampal ROIs using a finite impulse response function (FIR) with MarsBar, and extracted the peak response from these FIR time courses for statistical
comparisons in R using the same time points as peak as in the analysis above. We then run a mixed ANOVA model on these peak responses, with Group (1 d vs 28 d) as between-subjects factor and
Hit Type (high vs low) as within-subject factor in each of the ROIs separately. Group effects were followed up by post hoc Welchs _t_-tests comparing the groups (1 d vs 28 d) for each Hit
Type separately. CORRELATION WITH BEHAVIOR We extracted the contrast values from the main effects (condition vs baseline) of the first GLM for each Picture Type (old, related, novel) in each
ROI again using the MarsBar toolbox, and computed correlations between behavioral memory scores (percentage of related FA; Confidence Score) and the contrast values for the respective
picture types (related > baseline, old > baseline) in each of the hippocampal long axis ROIs outside SPM using R for all participants. We then also performed the correlations for each
group (1 d vs 28 d) separately. In a next step, we compared the correlations in the hippocampal long axis ROIs with each other (e.g., correlation in aHC with the correlation in pHC), using
the cocor package from R (http://comparingcorrelations.org/). As the related FA value (or Confidence Score) was used in all three correlations, we report the results of comparisons of two
overlapping correlations based on dependent groups. The reported values are Pearson and Filon’s _z_-scores. FUNCTIONAL CONNECTIVITY ANALYSIS We used a generalized form of context-dependent
psychophysiological interaction (gPPI)46 to measure task-dependent connectivity with either the whole hippocampus (left and right) or the aHC or pHC (left and right) as seed regions. In
contrast to the standard PPI implementation through SPM, the gPPI toolbox allows the inclusion of more than two task conditions in one PPI model and therefore allows a more flexible
analysis: we entered the six task regressors from the first GLM model (old negative, old neutral, related negative, related neutral, novel negative, and novel neutral), plus a PPI
Interaction term for each of these regressors, plus the time course from the respective seed region and the confidence ratings as regressor of no interest into our first-level PPI model. For
second-level modeling, we entered the following contrast files from the first-level PPI analyses (main effects for PPI Old, PPI Related, and PPI Novel, and the differences contrast PPI old
negative > PPI old neutral, PPI related negative > PPI related neutral, PPI novel negative > PPI novel neutral) into two-sample _t_-tests, comparing the 1 d group and 28 d group. We
then applied a small volume correction (SVC) for all our other ROIs (see ROI analysis for a list of the ROIs), to find areas which show a significant difference in their connectivity to the
seed region between the two groups. Voxels were regarded as significant when falling below a corrected voxel threshold of 0.05 (FWE) adjusted for the small volume. All areas with _k_ >
10 significant voxels were reported. REPRESENTATIONAL SIMILARITY ANALYSIS Independent from the univariate analysis, we carried out a RSA47 in the hippocampal long-axis ROIs using the
rsatoolbox48. For each ROI and each subject, brain Representational Dissimilarity Matrices (RDMs) were computed based on a single trial univariate GLM estimated on unsmoothed, normalized
functional images. The response-amplitude beta estimate maps associated with each trial were converted into _t_-maps and used to create vectors of activity patterns for each trial,
separately for each ROI. These activity patterns were used to calculate the dissimilarity between two trials by correlation distances (1−_r_, Pearson linear correlation). Next, the
dissimilarities based on each combination of trials were placed into the respective cells of the 180 × 180 RDMs (Fig. 4a). Due to technical failure, we did not have functional data for all
trials in some of the participants. Thus 8 participants had RDMs of slightly different sizes (in the 1 d group three participants had 179 × 179 RDMs; in the 28 d group two participants had
179 × 179 RDMs, two participants had 178 × 178 RDMs and one participant had a 176 × 176 RDM). For visualization of the RDMS we created average RDMs for each group from the single-subject
RDMs (Fig. 4b). As this required RDMs of the same size, the visualizations only include the data of the 40 participants with 180 × 180 RDMs. COMPARISON OF PATTERN SIMILARITIES ACROSS ROIS We
extracted the mean pattern similarity (_r_) from each single-subject RDM in order to get the overall similarity of activity patterns when performing the recognition task in general,
irrespective of picture types, per ROI. We then compared these mean pattern similarities across the hippocampal long axis by conducting an ANOVA with the factors HC Long Axis (aHC, mHC,
pHC), and Group (1 d vs 28 d), and post hoc Bonferroni corrected paired _t_-tests to compare each region to each of the other regions. Note that for this analysis all 48 participants were
included. COMPARISON OF RDMS BETWEEN GROUPS We next compared the RDMs of the two groups in the respective ROIs: for this we extracted the Spearman correlation for each single-subject RDM of
the 28 d group with the group average RDM of the 1 d group. This similarity of the RDMs between groups was then again compared between the hippocampal long axis ROIs with an ANOVA with the
factor HC Long Axis (aHC, mHC, and pHC), and post hoc Bonferroni corrected paired t-tests to compare each region to each of the other regions. Note that for this analysis only the 40
participants with 180 × 180 RDMs could be included as we aimed to directly compare the RDMs based on single trials, irrespective of picture category. Therefore all RDMs had to be of the same
size for this particular analysis. COMPARISON WITH MODEL RDMS We also compared the brain RDMs to two model RDMs (Fig. 4e) that were based on the expected similarities of the different
picture types: the model “Old Distinct” expects similar activity patterns for all old pictures that are distinct from patterns for related or novel pictures and the model “Old and Related
Similar” expects a similar pattern for the old and related pictures, that is distinct from patterns for the novel pictures. We calculated Spearman’s rank correlation coefficient for each
single-subject brain RDM and these a-priori model RDMs. This rank coefficient is beneficial if it is not possible to assume a direct linear match between the RDMs that are compared47, as is
the case here. We then calculated the mean of these Spearman's _r_’s for each group separately (1 d vs. 28 d) to find the model with the overall best fit in each group and ROI. We then
statistically compared the two models in each ROI per group with one-tailed paired _t_-tests, expecting a better fit for the “Old Distinct” model in the 1 d group and, on the other hand, a
better fit for the “Old and Related Similar” model in the 28 d group. Note that for this analysis all 48 participants could be included by creating model RDMs of the respective size matching
each participants brain RDM. DATA AVAILABILITY All data and codes are available from the corresponding authors upon request. The data are not publicly available yet because they contain
information that could compromise research participant privacy and consent. In the near future, they will be de-identified at the level of contemporary best practices and made publicly
available, together with relevant code, at the corresponding author’s GitHub repository (https://github.com/LarsSchwabeHamburg/transformation). CHANGE HISTORY * _ 24 MAY 2018 In the
originally published version of this Article, the rightmost graph in Fig. 2c was inadvertently replaced with a duplicate of the central panel. This has now been corrected in both the PDF and
HTML versions of the Article. _ REFERENCES * Dudai, Y. The restless engram: consolidations never end. _Annu. Rev. Neurosci._ 35, 227–247 (2012). Article PubMed CAS Google Scholar *
McKenzie, S. & Eichenbaum, H. Consolidation and reconsolidation: two lives of memories? _Neuron_ 71, 224–233 (2011). Article PubMed PubMed Central CAS Google Scholar * Winocur, G.,
Moscovitch, M. & Bontempi, B. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions.
_Neuropsychologia_ 48, 2339–2356 (2010). Article PubMed Google Scholar * Backus, A. R., Bosch, S. E., Ekman, M., Grabovetsky, A. V. & Doeller, C. F. Mnemonic convergence in the human
hippocampus. _Nat. Commun._ 7, 11991 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Chadwick, M. J., Hassabis, D., Weiskopf, N. & Maguire, E. A. Decoding individual
episodic memory traces in the human hippocampus. _Curr. Biol._ 20, 544–547 (2010). Article PubMed PubMed Central CAS Google Scholar * Ekstrom, A. D. & Ranganath, C. Space, time,
and episodic memory: the hippocampus is all over the cognitive map. _Hippocampus_ 00, 1–8 (2017). Google Scholar * Schacter, D. L. & Wagner, A. D. Medial temporal lobe activations in
fMRI and PET studies of episodic encoding and retrieval. _Hippocampus_ 9, 7–24 (1999). Article PubMed CAS Google Scholar * Scoville, W. B. & Milner, B. Loss of recent memory after
bilateral hippocampal lesions. _J. Neurol. Neurosurg. Psychiatry_ 20, 11–21 (1957). Article PubMed PubMed Central CAS Google Scholar * Frankland, P. W. & Bontempi, B. The
organization of recent and remote memories. _Nat. Rev. Neurosci._ 6, 119–130 (2005). Article PubMed CAS Google Scholar * Nadel, L. & Moscovitch, M. Memory consolidation, retrograde
amnesia and the hippocampal complex. _Curr. Opin. Neurobiol._ 7, 217–227 (1997). Article PubMed CAS Google Scholar * Squire, L. R. & Alvarez, P. Retrograde amnesia and memory
consolidation: a neurobiological perspective. _Curr. Opin. Neurobiol._ 5, 169–177 (1995). Article PubMed CAS Google Scholar * Squire, L. R. & Bayley, P. J. The neuroscience of remote
memory. _Curr. Opin. Neurobiol._ 17, 185–196 (2007). Article PubMed PubMed Central CAS Google Scholar * Moser, M. B. & Moser, E. I. Functional differentiation in the hippocampus.
_Hippocampus_ 8, 608–619 (1998). Article PubMed CAS Google Scholar * Poppenk, J., Evensmoen, H. R., Moscovitch, M. & Nadel, L. Long-axis specialization of the human hippocampus.
_Trends Cogn. Sci._ 17, 230–240 (2013). Article PubMed Google Scholar * Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal
longitudinal axis. _Nat. Rev. Neurosci._ 15, 655–669 (2014). Article PubMed CAS Google Scholar * Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally
distinct structures? _Neuron_ 65, 7–19 (2010). Article PubMed PubMed Central CAS Google Scholar * Zeidman, P. & Maguire, E. A. Anterior hippocampus: the anatomy of perception,
imagination and episodic memory. _Nat. Rev. Neurosci._ 17, 173–182 (2016). Article PubMed PubMed Central CAS Google Scholar * Bonnici, H. M. et al. Detecting representations of recent
and remote autobiographical memories in vmPFC and hippocampus. _J. Neurosci._ 32, 16982–16991 (2012). Article PubMed PubMed Central CAS Google Scholar * Bonnici, H. M., Chadwick, M. J.
& Maguire, E. A. Representations of recent and remote autobiographical memories in hippocampal subfields. _Hippocampus_ 23, 849–854 (2013). Article PubMed PubMed Central Google
Scholar * Gilboa, A., Winocur, G., Grady, C. L., Hevenor, S. J. & Moscovitch, M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events.
_Cereb. Cortex._ 14, 1214–1225 (2004). Article PubMed Google Scholar * Kensinger, E. A., Garoff-Eaton, R. J. & Schacter, D. L. Effects of emotion on memory specificity: memory
trade-offs elicited by negative visually arousing stimuli. _J. Mem. Lang._ 56, 575–591 (2007). Article Google Scholar * Waring, J. D. & Kensinger, E. A. How emotion leads to selective
memory: neuroimaging evidence. _Neuropsychologia_ 49, 1831–1842 (2011). Article PubMed PubMed Central Google Scholar * Richardson, M. P., Strange, B. A. & Dolan, R. J. Encoding of
emotional memories depends on amygdala and hippocampus and their interactions. _Nat. Neurosci._ 7, 278–285 (2004). Article PubMed CAS Google Scholar * Squire, L. R., Genzel, L., Wixted,
J. T. & Morris, R. G. Memory consolidation. _Cold Spring Harb. Perspect. Biol._ 7, a021766 (2015). Article PubMed PubMed Central CAS Google Scholar * Kjelstrup, K. B. et al. Finite
scale of spatial representation in the hippocampus. _Science_ 321, 140–143 (2008). Article ADS PubMed CAS Google Scholar * Collin, S. H., Milivojevic, B. & Doeller, C. F. Memory
hierarchies map onto the hippocampal long axis in humans. _Nat. Neurosci._ 18, 1562–1564 (2015). Article PubMed PubMed Central CAS Google Scholar * Keinath, A. T. et al. Precise spatial
coding is preserved along the longitudinal hippocampal axis. _Hippocampus_ 24, 1533–1548 (2014). Article PubMed PubMed Central Google Scholar * Ritchey, M., Montchal, M. E., Yonelinas,
A. P., Ranganath, C. Delay-dependent contributions of medial temporal lobe regions to episodic memor retrieval. _eLife_ 4, e05025 (2015). Article PubMed Central Google Scholar * Svoboda,
E., McKinnon, M. C. & Levine, B. The functional neuroanatomy of autobiographical memory: a meta-analysis. _Neuropsychologia_ 44, 2189–2208 (2006). Article PubMed PubMed Central Google
Scholar * Milivojevic, B., Vicente-Grabovetsky, A. & Doeller, C. F. Insight reconfigures hippocampal-prefrontal memories. _Curr. Biol._ 25, 821–830 (2015). Article PubMed CAS Google
Scholar * Kumaran, D. & Maguire, E. A. Which computational mechanisms operate in the hippocampus during novelty detection? _Hippocampus_ 17, 735–748 (2007). Article PubMed Google
Scholar * Schapiro, A. C., Kustner, L. V. & Turk-Browne, N. B. Shaping of object representations in the human medial temporal lobe based on temporal regularities. _Curr. Biol._ 22,
1622–1627 (2012). Article PubMed PubMed Central CAS Google Scholar * Schapiro, A. C., Turk-Browne, N. B., Botvinick, M. M., Norman, K. A. Complementary learning systems within the
hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. _Philos_. _Trans_. _R_. _Soc_. _Lond_. _B_ _Biol_. _Sci_. 372, 20160049 (2017).
Article PubMed PubMed Central Google Scholar * Tompary, A. & Davachi, L. Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal
cortex. _Neuron_ 96, 228–241 (2017). e225. Article PubMed CAS PubMed Central Google Scholar * Kensinger, E. A., Addis, D. R. & Atapattu, R. K. Amygdala activity at encoding
corresponds with memory vividness and with memory for select episodic details. _Neuropsychologia_ 49, 663–673 (2011). Article PubMed PubMed Central Google Scholar * Lang, P. J., Bradley,
M. M. & Cuthbert, B. N. _International Affective Picture System (IAPS): Technical Manual and Affective Ratings_ (National Institute of Mental Health Center for the Study of Emotion and
Attention, Gainesville, 1997). * Brainard, D. H. The psychophysics toolbox. _Spat. Vis._ 10, 433–436 (1997). Article PubMed CAS Google Scholar * Delacre, M., Lakens, D. & Leys, C.
Why psychologists should by default use Welch’s t-test instead of Student’s t-test. _Int. Rev. Social. Psychol._ 30, 92 (2017). Article Google Scholar * Tabachnick B. G., Fidell L. S.
_Using Multivariate Statistics_ 6th edn (Pearson Education, Boston, 2013). * van Kesteren, M. T., Ruiter, D. J., Fernandez, G. & Henson, R. N. How schema and novelty augment memory
formation. _Trends Neurosci._ 35, 211–219 (2012). Article PubMed CAS Google Scholar * Brett, M., Anton, J. L., Valabregue, R. & Poline, J. B. Region of interest analysis using an SPM
toolbox. In _8th International Conference on Functional Mapping of the Human Brain_. Available in_ NeuroImage_ 16, abstr. 497 (Sendai, Japan, 2002). * Turk-Browne, N. B., Simon, M. G. &
Sederberg, P. B. Scene representations in parahippocampal cortex depend on temporal context. _J. Neurosci._ 32, 7202–7207 (2012). Article PubMed PubMed Central CAS Google Scholar *
Turk-Browne, N. B., Yi, D. J. & Chun, M. M. Linking implicit and explicit memory: common encoding factors and shared representations. _Neuron_ 49, 917–927 (2006). Article PubMed CAS
Google Scholar * Lancaster, J. L. et al. Automated Talairach atlas labels for functional brain mapping. _Hum. Brain Mapp._ 10, 120–131 (2000). Article PubMed CAS Google Scholar *
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. _Neuroimage_
19, 1233–1239 (2003). Article PubMed Google Scholar * McLaren, D. G., Ries, M. L., Xu, G. & Johnson, S. C. A generalized form of context-dependent psychophysiological interactions
(gPPI): a comparison to standard approaches. _Neuroimage_ 61, 1277–1286 (2012). Article PubMed PubMed Central Google Scholar * Kriegeskorte, N., Mur, M. & Bandettini, P.
Representational similarity analysis - connecting the branches of systems neuroscience. _Front. Syst. Neurosci._ 2, 4 (2008). Article PubMed PubMed Central Google Scholar * Nili, H. et
al. A toolbox for representational similarity analysis. _PLoS Comput. Biol._ 10, e1003553 (2014). Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS
The authors thank Daniel Kutzner, Wiebke Schmidt, Sandra Erbach, Kristin Medel, and Amina Shah for assistance during data collection and Dr. Lynn Nadel as well as Dr. Tobias Sommer for
helpful comments on a previous version of this manuscript. This work was supported by the German Research Foundation (DFG; SCHW1357/12-1). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Cognitive Psychology, University of Hamburg, 20146, Hamburg, Germany Lisa C. Dandolo & Lars Schwabe Authors * Lisa C. Dandolo View author publications You can also search
for this author inPubMed Google Scholar * Lars Schwabe View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.C.D. collected the data, analyzed
the data, and wrote the manuscript. L.S. conceived and designed the experiment, supervised the project, and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Lars Schwabe. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION (PDF 1823 KB) PEER REVIEW FILE(PDF 647 KB) RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dandolo, L.C., Schwabe, L.
Time-dependent memory transformation along the hippocampal anterior–posterior axis. _Nat Commun_ 9, 1205 (2018). https://doi.org/10.1038/s41467-018-03661-7 Download citation * Received: 22
September 2017 * Accepted: 02 March 2018 * Published: 23 March 2018 * DOI: https://doi.org/10.1038/s41467-018-03661-7 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative