Play all audios:
ABSTRACT Fragile X Syndrome results from a loss of Fragile X Mental Retardation Protein (FMRP). We now show that FMRP is a member of a Cav3-Kv4 ion channel complex that is known to regulate
A-type potassium current in cerebellar granule cells to produce mossy fiber LTP. Mossy fiber LTP is absent in _Fmr1_ knockout (KO) mice but is restored by FMRP(1-297)-_tat_ peptide. This
peptide further rapidly permeates the blood-brain barrier to enter cells across the cerebellar-cortical axis that restores the balance of protein translation for at least 24 h and
transiently reduces elevated levels of activity of adult _Fmr1_ KO mice in the Open Field Test. These data reveal that FMRP(1-297)-_tat_ can improve function from the levels of protein
translation to synaptic efficacy and behaviour in a model of Fragile X syndrome, identifying a potential therapeutic strategy for this genetic disorder. SIMILAR CONTENT BEING VIEWED BY
OTHERS MISSENSE MUTATION OF _FMR1_ RESULTS IN IMPAIRED AMPAR-MEDIATED PLASTICITY AND SOCIO-COGNITIVE DEFICITS IN MICE Article Open access 10 March 2021 ADENOSINE A2A RECEPTOR INHIBITION
REDUCES SYNAPTIC AND COGNITIVE HIPPOCAMPAL ALTERATIONS IN FMR1 KO MICE Article Open access 05 February 2021 ASTROGLIAL KIR4.1 POTASSIUM CHANNEL DEFICIT DRIVES NEURONAL HYPEREXCITABILITY AND
BEHAVIORAL DEFECTS IN FRAGILE X SYNDROME MOUSE MODEL Article Open access 27 April 2024 INTRODUCTION The loss of Fragile X mental retardation protein (FMRP) in Fragile X syndrome (FXS) can
interfere with the translation of numerous proteins important for the normal development of synaptic transmission. A growing body of evidence suggests that behavioral disorders in FXS can
involve a disruption in the processing of sensory information in both cortex and cerebellum1,2,3. The mossy fiber projection to cerebellar granule cells represents a major synaptic portal to
the cerebellar cortex where long-term plasticity can shape sensory input4,5,6,7. Any dysfunction at this synapse will thus impair signal processing by the cerebellar cortex at the first
stage of sensory input. Cerebellar granule cells express a Cav3–Kv4 ion channel complex that contributes to long-term potentiation (LTP) of mossy fiber input by increasing the intrinsic
excitability of granule cells in the vermal region of lobule 98,9. FMRP is widely expressed in cerebellum and can modulate the activity of ion channels in the membrane, including Kv4
potassium channels10,11,12,13,14. Given the central role of Kv4 channels in mossy fiber LTP we investigated the potential for FMRP to shape long-term changes in granule cell excitability.
The present study shows that FMRP is a constituent member of the Cav3–Kv4 complex that modulates both Cav3 and Kv4 channels to reduce Kv4 current amplitude. LTP at the mossy fiber-granule
cell input is absent in _Fmr1_ knockout (KO) mice but rescued by infusing an N-terminal fragment of FMRP (FMRP(1–297)) into granule cells. Moreover, a FMRP(1–297)_-tat_ peptide introduced to
_Fmr1_ KO mice by tail vein injection restores Cav3–Kv4 complex function and mossy fiber LTP, reduces the level of activity in adult animals within 1 h, and rescues disrupted translation of
select proteins associated with FXS for at least 24 h, supporting the potential for a _tat_-FMRP conjugate approach to be developed as a therapeutic agent for FXS. RESULTS FMRP RESTORES
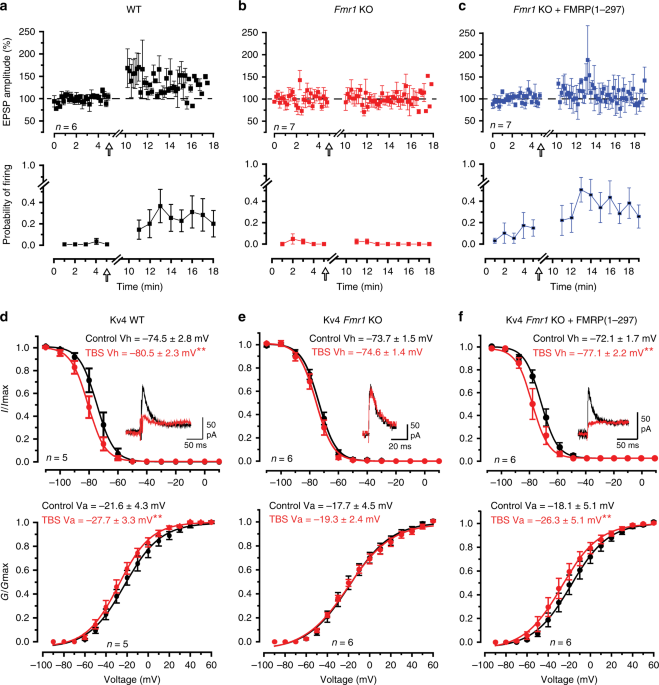
MOSSY FIBER LTP IN _FMR1_ KO MICE The reduction in A-type current in granule cells following a theta burst stimulus (TBS) to mossy fibers was traced to a hyperpolarizing shift in the half
voltage for Kv4 channel inactivation (Vh) (referred to here as a left-shift in Kv4 Vh)8. To determine the potential role for FMRP in regulating Kv4 channels and LTP in granule cells,
whole-cell recordings were obtained in the vermis region of lobule 9 from male P16–P22 wild-type (WT) mice or _Fmr1_ KO mice and mossy fibers were stimulated to evoke a just threshold
excitatory postsynaptic potential (EPSP) (Fig. 1a). In 6/6 cells of WT mice a TBS was followed by an initial peak increase in EPSP amplitude that then decreased to an elevated level of 138.8
± 11.0% (_n_ = 6; _p_ = 0.012) of control 5–13 min post TBS (Fig. 1a). In WT cells just subthreshold synaptic stimulation was associated with little firing probability, but TBS invoked an
increase in firing probability to single pulse synaptic stimuli that persisted for at least 10 min post stimulation (Fig. 1a)8,15. Recordings from granule cells in _Fmr1_ KO mice revealed
similar resting membrane potentials, input resistance and firing threshold as WT mice (Supplementary Table 1). Thus, the loss of FMRP in _Fmr1_ KO animals did not noticeably influence the
basic properties of membrane excitability in granule cells. Yet, in contrast to WT animals, delivering a TBS stimulus to mossy fibers in _Fmr1_ KO mice failed to evoke LTP of either EPSP
amplitude or spike firing probability (Fig. 1b). Previous work has shown that an N-terminal fragment of FMRP (FMRP(1–297)) can modulate select potassium channels11,16,17,18. To test if
FMRP(1–297) could restore plasticity at the mossy fiber-granule cell synapse we included 3 nM FMRP(1–297) in the recording electrode. After 10 min equilibration of FMRP(1–297) EPSP amplitude
exhibited no significant difference from control 10–15 min post TBS (103.6 ± 10.3%, _n_ = 7, _p_ = 0.74) (Fig. 1c). However, TBS now evoked a substantial increase in the firing probability
that persisted 10–15 min post TBS (Fig. 1c). We then tested for any effects of FMRP(1–297) on isolated Kv4 current (see “Methods”)8. In these recordings postsynaptic calcium currents were
not blocked, allowing calcium entering via Cav3 channels to interact with the Kv4–KChIP3 complex8,9,19. In WT mice mossy fiber TBS evoked a significant left-shift in Kv4 Vh or the half
voltage for Kv4 activation (Va) (Fig. 1d). The net effect of these changes on Vh and Va was to produce a 44.2 ± 11.1% (_n_ = 7, _p_ = 0.011) reduction in Kv4 current post TBS, indicating a
more prominent role of the left-shift in Kv4 Vh on current amplitude (Fig. 1d). Repeating these tests in _Fmr1_ KO mice revealed no significant difference in the resting values for Vh or Va
compared to WT mice (Fig. 1e). However, TBS failed to evoke a left-shift in either Kv4 Vh or Va, and no change in Kv4 current amplitude in _Fmr1_ KO mice (107 ± 7% of control, _p_ = 0.40)
(Fig. 1e). We then tested if FMRP(1–297) could restore the TBS-induced left-shift in Kv4 properties associated with LTP. Including 3 nM FMRP(1–297) in the electrode (Fig. 1f) did not affect
the initial control Vh or Va compared to either WT mice (cf Fig. 1d) (Vh, _p_ = 0.51; Va, _p_ = 0.77) or _Fmr1_ KO mice recorded with normal electrolyte (Vh, _p_ = 0.57; Va, _p_ = 0.95)
(Fig. 1e). However, TBS in _Fmr1_ KO cells pre-infused with FMRP(1–297) induced a significant left-shift in Kv4 Vh and Va to reduce Kv4 current by 34 ± 12% (_n_ = 6, _p_ = 0.044) (Fig. 1f).
We found that in tissue slices preincubated for 2 h and recorded in the presence of 20 µM anisomycin to block protein synthesis mossy fiber TBS still evoked a leftward shift in Kv4 Vh and Va
in granule cells of either WT mice or _Fmr1_ KO mice recorded with 3 nM FMRP(1–297) in the electrode, indicating no requirement for protein translation for these effects (Supplementary Fig.
1). These results indicate that reintroducing FMRP(1–297) restores the capacity of mossy fiber TBS to evoke a left-shift in Kv4 Vh and Va, and a long-term increase in the probability of
spike firing that is absent in _Fmr1_ KO animals. FMRP ASSOCIATES WITH CAV3.1 AND KV4.3 CHANNELS To define the association between FMRP and each of Cav3 calcium and Kv4 potassium channels we
conducted protein biochemical and imaging assays. Tests on homogenates of WT whole brain (P30–P40) revealed co-immunoprecipitation (coIP) of both Cav3.1 and Kv4.3 channels with FMRP using
Cav3.1 and Kv4.3 antibodies to immunoprecipitate (IP) and an N-terminus FMRP antibody to immunoblot (Fig. 2a). The reverse coIP could not be completed as the available N-terminus FMRP
antibodies did not function well for IP. We also tested for fluorescence resonance energy transfer (FRET) by preparing constructs with a fluorescent tag on the N-termini of Cav3.1 and Kv4.3,
and on the C-terminus of FMRP or the C-terminal end of FMRP(1–297). Coexpressing a GFP-Cav3.1 construct with FMRP-mKate as donor–acceptor pairs in tsA-201 cells revealed FRET upon
excitation at 457 nm (Fig. 2b, c). Similarly, coexpressing GFP-Kv4.3 and FMRP-mKate evoked FRET with 457 nm activation (Fig. 2d, e). Moreover, companion tests confirmed FRET when either
GFP-Cav3.1 or GFP-Kv4.3 was coexpressed with a FMRP(1–297)-mKate construct (Supplementary Fig. 2). The specificity of FRET was confirmed by a lack of FRET in cells that expressed either
GFP-Cav3.1 or GFP-Kv4.3 together with mKate alone or cells that coexpressed GFP alone with FMRP-mKate or FMRP(1–297)-mKate (Supplementary Fig. 3). These data reveal that FMRP or FMRP(1–297)
can associate with Cav3.1 or Kv4.3 with a proximity of less than 10 nm separation when coexpressed in tsA-201 cells, a result consistent with the coIP tests for native FMRP with these
channels in whole-brain lysates. FMRP MODULATES CAV3.1 AND KV4.3 CHANNELS To test the effects of FMRP(1–297) on channel function we expressed Cav3.1 or Kv4.3 channels in tsA-201 cells and
compared channel properties in normal electrolyte to those when FMRP(1–297) was included or infused into the electrode. We tested Cav3.1 channels expressed in isolation in tsA-201 cells and
found that 30 nM FMRP(1–297) significantly left-shifted Vh and Va (Fig. 3a). While the left shift in Cav3.1 Va should increase channel activation, the amplitude of Cav3.1 current was reduced
by 34.6 ± 8.4% (_n_ = 7, _p_ = 0.0060 with paired-sample _t_ test), indicating a dominant effect on Vh (Fig. 3a). Kv4.3 Vh in tsA-201 cells was also significantly left-shifted in the
presence of FMRP(1–297) but Va was not affected (Fig. 3b). The amplitude of Kv4.3 current in tsA-201 cells was also reduced by 39.1 ± 5.8% (_n_ = 5, _p_ = 0.0026 with two-sample _t_ test)
after FMRP(1–297) infusion (Fig. 3b). These data are important in indicating that FMRP(1–297) can affect the biophysical properties of Cav3.1 and Kv4.3 channels expressed in isolation in
tsA-201 cells. We repeated these tests to determine if FMRP(1–297) can affect the properties of Kv4 current in _Fmr1_ KO granule cells. Including 30 nM FMRP(1–297) in the internal solution
also produced a significant left-shift in Kv4 Vh (_p_ = 0.037, two-sample Student’s _t_ test) and a reduction of Kv4 current amplitude, but no corresponding shift in Va (Control Va, −24.0 ±
3.2 mV, _n_ = 10; Test Va, −22.6 ± 3.6 mV, _n_ = 10; _p_ = 0.78, two-sample Student’s _t_ test) (Fig. 3c). The effects of infusing 30 nM FMRP(1–297) through the electrode (Fig. 3c) did not
involve a change in channel insertion in the membrane given no significant change in current density (pA/pF) when tested from −110 to +60 mV (Supplementary Fig. 4). Previous work established
that Kv4 Vh can be left-shifted by any decrease in Cav3 channel calcium conductance9,20. The ability for FMRP(1–297) to left shift the Vh of Cav3.1 recorded in tsA-201 cells might then
account for the shift in granule cell Kv4 Vh when this protein is infused. We attempted to record Cav3 current in granule cells of WT and _Fmr1_ KO mice but its amplitude in mouse cerebellum
was too small to reliably measure. Instead we compared Kv4 Vh in granule cells of _Fmr1_ KO mice in the presence of TTA-P2 (1 µM) to block Cav3 current to that of a second set of cells
recorded with FMRP(1–297) in the electrode. The cells infused with FMRP(1–297) in the presence of TTA-P2 exhibited a significant left-shift in Kv4 Vh from a mean value of −74 to −78 mV (Fig.
3d). This is important in indicating that FMRP(1–297) can exert at least some of its effects independently of Cav3.1 calcium conductance on one or more subunits of the Cav3–Kv4 complex.
Together these data indicate that FMRP(1–297) can form very close associations with Cav3.1 and Kv4.3 channel subunits both in tsA-201 cells and in situ, with significant biophysical effects
by reducing their availability near resting potential. Despite the rapid effects of FMRP(1–297) infusion on Cav3.1 and Kv4.3 channel Vh (within 10 min) we can not yet attribute these actions
to direct interactions between FMRP(1–297) and the channel subunits, as additional accessory proteins such as KChIP3 and DPP that associate with the Kv4 complex could also be involved19.
FMRP(1–297)_-TAT_ RAPIDLY CROSSES THE BBB The fact that FXS stems from the loss of a single protein raises the possibility that reintroducing FMRP would offset the primary factor underlying
this genetic disorder. One strategy would be to use a cell penetrating peptide to facilitate passage across the blood–brain barrier (BBB) and cell membranes. For this we prepared a _tat_
peptide conjugated to FMRP(1–297) that also included an HA tag for immunocytochemical tests (Fig. 4a). The final FMRP(1–297)-_tat_ protein was ∼35–37 kDa, a size that is within the range for
high efficiency transport across the BBB21. We validated this construct by testing its effects on whole-cell recordings of Kv4 Vh in granule cells of _Fmr1_ KO mice, and found that bath
application of even 10 nM FMRP(1–297)-_tat_ significantly left-shifted Kv4 Vh (_Fmr1_ KO control Kv4 Vh, −71.2 ± 1.9 mV, _n_ = 5; with 10 nM FMRP(1–297)-_tat_, −75.6 ± 2.3 mV, _n_ = 5; _p_ =
0.0072; paired sample Student’s _t_ test). We then examined the pattern of FMRP(1–297)-_tat_ protein distribution after its introduction by tail vein injection. FMRP normally exhibits a
widespread expression across the brain with high levels detected in cell body layers of the cerebellum, cortex, and hippocampus12,13,22. Immunocytochemical tests using an antibody against
the FMRP C-terminus confirmed this expression pattern in WT mice on the FVB.129P2 background. In the cerebellum FMRP immunolabel was apparent across all ten lobules with labeling of the
granule cell layer in WT mice (Fig. 4b)12,13. At higher magnification FMRP immunolabel was detected in virtually all neuronal cell types including granule and Purkinje cells, with label
restricted primarily to the cell body regions (Fig. 4c, d). _Fmr1_ KO mice injected with vehicle and prepared for immunocytochemistry 30 min later produced no labeling when tested with an
antibody against HA (Fig. 4e). We next tail vein injected 1.0 mg/kg HA-FMRP(1–297)_-tat_ in P25–P40 _Fmr1_ KO mice (nominally 500 nM plasma concentration at the time of injection) and 30 min
or 12 h later processed brains for immunocytochemistry. HA immunolabel was apparent in all layers of the cerebellum within 30 min of injecting HA-FMRP(1–297)_-tat_, indicating a rapid
permeation of the BBB and cerebellar cells (Fig. 4f, g). HA immunolabel was detected within granule and Purkinje cell bodies as well as additional labeling in the molecular layer that did
not clearly correspond to Purkinje cell dendrites (Fig. 4f, g). In WT mice an antibody against the FMRP C-terminus detected prominent labeling in the cell body regions of cortical layers,
hippocampus, and dentate gyrus, with no clear dendritic label (Fig. 4h). Tail vein injections of HA-FMRP(1–297)_-tat_ into _Fmr1_ KO mice again labeled primarily cell bodies of pyramidal
cells in the cortex (Fig. 4i–k) and hippocampus (Fig. 4l–n) with exclusion of the nucleus and no apparent labeling of apical or basal dendrites counter labeled with MAP2. As found in
cerebellum additional labeling for HA could be found outside neocortical or hippocampal pyramidal cell somas in regions of synaptic inputs (Fig. 4j, m). In an additional set of tests we tail
vein-injected 1.0 mg/kg HA-FMRP(1–297)_-tat_ and confirmed the presence of detectable HA immunolabel in whole-brain sections reacted after fixation at 2, 12, 24, and 48 h and
processed/imaged under identical conditions to make qualitative comparisons of HA labeling intensities (Fig. 5). HA immunolabel could be detected at 2 h in cerebellum and with even higher
fluorescence intensity in neocortical tissue sections, but not in animals injected with vehicle alone (Fig. 5a, b). By 12 h HA fluorescence was higher in both the cerebellum and neocortex in
being detected as a label in cerebellar Purkinje cells and with additional localization in the neuropil and many cell bodies of neocortical regions. By 24 h HA immunofluorescence
intensities had dropped in both locations and by 48 h immunofluorescence for HA immunolabel was just detectable (Fig. 5a, b). An interaction between HA-FMRP(1–297)-_tat_ and Cav3.1 and Kv4.3
channels in _Fmr1_ KO mice was further verified by obtaining a coIP between HA and these channels 5 h after tail vein injection (_n_ = 3) (Fig. 5c). FMRP(1–297)-_TAT_ IS NONTOXIC TO
CULTURED GRANULE CELLS To test for potential toxicity of the FMRP(1–297)_-tat_ peptide we prepared dissociated cultures of cerebellar granule cells from _Fmr1_ KO mice and at 3 days in vitro
(DIV) applied HA-FMRP(1–297)_-tat_ over a range of 1–500 nM (Fig. 6b, c). Dual labeling for MAP2 and HA confirmed that uptake of FMRP(1–297)_-tat_ by cultured granule cells occurred within
2 h of exposure (Fig. 6a–c). Cells were exposed to treatments for either 24 h or 5 days (culture medium changed every 2 days) before flow cytometric analysis of labeling using a live-dead
cell kit. Exposure to either vehicle alone or up to 500 nM FMRP(1–297)_-tat_ showed no significant increase in the number of compromised cells up to 5 days following the treatment (Fig.
6d–g; Supplementary Fig. 5). These results reveal that FMRP(1–297)-_tat_ peptide can cross the BBB and gain substantial access to cells across the cerebellum and other brain regions within
30 min of injection. Moreover, the FMRP(1–297)-_tat_ peptide did not compromise cell health over time even when applied directly to cultured granule cells at a dose of 500 nM.
FMRP(1–297)-_TAT_ RESTORES MOSSY FIBER-GRANULE CELL LTP To test the effects of FMRP(1–297)-_tat_ on cell activity we tail vein injected FMRP(1–297)-_tat_ peptide into P16–P22 _Fmr1_ KO mice
and 2 h later prepared in vitro slices to test the ability to evoke LTP. Injecting 1.0 mg/kg FMRP(1–297)-_tat_ promoted LTP of the EPSP amplitude 10–15 min post TBS and reliably increased
spike firing probability for at least 15 min following TBS (Fig. 7a, b). Indeed, the degree of EPSP potentiation and increase in firing probability was strikingly similar to that detected in
WT mice (cf. Figs. 1a and 7a), indicating that systemic administration of FMRP(1–297)-_tat_ promoted greater EPSP potentiation than did direct postsynaptic infusion of FMRP(1–297). In
contrast, injecting 0.2 mg/kg FMRP(1–297)-_tat_ did not rescue mossy fiber LTP (Fig. 7c, d), even though FMRP immunolabel can be detected in cerebellum 2 h after tail vein injection
(Supplementary Fig. 6). Similarly, tail vein injection of _tat_ epitope alone (1.0 mg/kg) showed no LTP in response to mossy fiber TBS when slices were prepared 2 h later (Fig. 7e, f). These
data functionally validate the ability for FMRP(1–297)-_tat_ to access cerebellar neurons in vivo and reveal a concentration-dependent effect on its ability to restore synaptic plasticity.
The finding that 1.0 mg/kg FMRP(1–297)-_tat_ injection promoted LTP that was comparable to WT mice is also important in suggesting an additional potential influence of FMRP(1–297)-_tat_ on
presynaptic elements not accessed by postsynaptic infusion of FMRP(1–297). FMRP(1–297)-_TAT_ REDUCES ELEVATED ACTIVITY IN _FMR1_ KO MICE Hyperactivity and anxiety are symptoms exhibited by
FXS patients23,24 that have been assessed in the _Fmr1_ KO mouse model in terms of activity levels in the open field test (OFT)25,26,27. We therefore used OFT to quantify the extent to which
tail vein injection of FMRP(1–297)-_tat_ might rescue elevated levels of activity in _Fmr1_ KO mice. For this we tail vein injected 1.0 mg/kg FMRP(1–297)-_tat_ in male P55–P100 mice. In
agreement with previous work, vehicle-treated _Fmr1_ KO mice traveled a significantly greater distance and with a higher velocity during a 30 min period than vehicle-treated WT mice (Fig.
8). We then injected WT and _Fmr1_ KO animals with different amounts of FMRP(1–297)-_tat_ and conducted OFT 1, 24, or 48 h later. A two-way ANOVA analysis indicated a genotype-dependent
effect of FMRP(1–297)-_tat_ (interaction of genotype and treatment, _p_ = 0.012). One hour after administering 1.0 mg/kg FMRP(1–297)-_tat, Fmr1_ KO mice traveled significantly less than
vehicle injected _Fmr1_ KO animals, while WT mice injected with 1.0 mg/kg FMRP(1–297)-_tat_ showed no significant change (Fig. 8a, left panel). One-way ANOVA revealed a
concentration-dependent effect of FMRP(1–297)-_tat_, with a significant reduction in the distance traveled by _Fmr1_ KO mice for 1.0 mg/kg but not for 0.2 or 2.0 mg/kg FMRP(1–297)-_tat_
(Fig. 8a, right panel). The same pattern was detected at 1 h post injection for measures of velocity (Fig. 8d). Injecting 1.0 mg/kg _tat_ epitope alone to _Fmr1_ KO mice had no effect on
either distance or velocity measurements (Fig. 8a, d, right panels). Twenty-four hours after injecting FMRP(1–297)-_tat_ the distance and velocity were slightly reduced from vehicle-treated
_Fmr1_ KO mice at all doses, but not to significant levels (Fig. 8b, e). Forty-eight hours after FMRP(1–297)-_tat_ injections the levels of activity had returned to levels not significantly
different from vehicle-treated _Fmr1_ KO mice (Fig. 8c, f). FMRP(1–297)-_TAT_ RESTORES PROTEIN LEVELS IN _FMR1_ KO MICE A second established role for FMRP is regulation of protein
translation, where loss of FMRP alters the expression levels of multiple proteins that contribute to circuit dysfunction14,28,29,30,31,32. To determine if FMRP(1–297)-_tat_ could influence
protein translation we used Western blots in lysates of cerebellum or brain to measure the levels of a select set of proteins previously shown to be altered in _Fmr1_ KO mice. These tests
established that _α_CaMKII and the amyloid precursor protein (APP) are significantly elevated in cerebellar and brain lysates of P21–30 _Fmr1_ KO compared to WT mice (Fig. 9a, b). In
contrast, the level of PSD95 protein was lower in _Fmr1_ KO cerebellar (but not brain) lysates compared to WT (Fig. 9c). A single tail vein injection of 1.0 mg/kg FMRP(1–297)-_tat_
significantly reduced these differences in protein levels in both cerebellum and brain samples even 24 h after injection, without affecting the similar baseline values for PSD95 in brain
samples. Interestingly, the levels of all three proteins in WT animals did not significantly change after FMRP(1–297)-_tat_ injections (Fig. 9). DISCUSSION Several studies have attempted to
restore FMRP in _Fmr1_ KO mice through viral injections, _tat_-conjugated peptides, or by targeting the hypermethylation that blocks transcription of the _Fmr1_ gene33,34,35,36,37. Success
was gained by reintroducing FMRP by viral transfection but this was offset by variability in the distribution and expression levels of FMRP, and deleterious effects if overexpressed35,37,38.
Others have used a pharmacological approach to counteract downstream signaling molecules that are disrupted when FMRP is lost39,40. Another strategy is to use a _tat_-conjugate peptide to
increase access across the BBB and cell membranes. The only other study that explored a _tat_-conjugate approach tested a full-length FMRP36 and reported toxicity on cultured fibroblasts by
∼7.5 nM, dampening enthusiasm for a _tat_-conjugate approach to treating FXS. We tested an N-terminal fragment of FMRP that has been shown to affect specific ion channels11,16,17,18. The
results indicate that FMRP(1–297)-_tat_ has the ability to shift the biophysical properties of Cav3.1 and Kv4.3 channels, rescue LTP of mossy fiber input to cerebellum, restore levels of
proteins in _Fmr1_ KO mice for at least 24 h, and reduce elevated levels of activity in _Fmr1_ KO animals at 1 h post-FMRP-_tat_ injection, but not at 24 or 48 h post-injection. No toxicity
was detected up to 5 days after direct exposure of cultured cells to 500 nM FMRP(1-297)-_tat_. Together these data indicate the ability to use a _tat_ peptide-based approach to restore
FMRP-related circuit function in the mouse model of FXS. Our tests using immunocytochemistry indicate that HA-FMRP(1–297)-_tat_ is rapidly taken up following tail vein injection and
distributed widely across the cerebellar-cortical axis. Most of the structures known to label for FMRP in WT mice12,13 were labeled after HA-FMRP(1–297)-_tat_ injection, with a preference
for uptake and/or sequestration at the soma. Additional labeling could be detected outside neuronal somata, such as in layers with a prominent synaptic component (i.e., molecular layer of
cerebellum). The extent to which this labeling reflects uptake by glial or synaptic components is unknown. We also can not ensure that all structures will take up FMRP(1–297)-_tat_ to the
levels required for a desired function, even though mossy fiber LTP was rapidly restored using the concentrations tested here. However, the results are extremely promising in revealing
uptake of FMRP(1–297)-_tat_ across the brain axis in a very short period of time and retention of the HA immunolabel tag or FMRP(1–297) up to 48 h later. Tail vein injection of 1.0 mg/kg
FMRP(1–297)-_tat_ was sufficient to achieve therapeutic actions from the cellular to behavioral level in 1–2 h. We found a progressive increase in HA immunolabel up to 12 h in terms of
fluorescence intensity in tissue sections, with just detectable levels by 48 h. However, we do not know if the molecule remains fully intact or is in the process of degradation/elimination,
as suggested by a lower signal level at 48 h. It is possible that future work will identify suitable shorter length FMRP constructs or modifications to the _tat_ peptide that will further
improve the speed of transport across the BBB or the retention of FMRP(1–297) in situ41,42,43. Fragile X patients exhibit symptoms that have been correlated to the degree of hypoplasia of
the posterior cerebellar vermis3,44,45. The results obtained here on cerebellar synaptic plasticity and cell output are thus relevant to the conditions inherent to patient populations and to
lobule 9 granule cells where the work is centered. The actions of FMRP(1–297)-_tat_ were detected at multiple levels of cell and circuit function. Previous work has shown that intracellular
infusion of the N-terminal fragment FMRP(1–297) can increase activation of Slack, SK2, BK, and Kv1.2 potassium channels11,16,17,18,46. The molecular sites for interaction were identified
between FMRP(1–297) and C-terminal regions of both Kv1.211 and Slack potassium channels16. The ability to detect an interaction with Kv1.2 depends on the state of phosphorylation11,
identifying an additional factor to consider when testing FMRP(1–297) interactions. The current study identifies Cav3.1 calcium and Kv4.3 potassium channels as two previously unrecognized
interactors with the FMRP N-terminus (1–297), raising the number of channel isoforms that can be modified by this FMRP fragment to six. In the case of Kv4.3 the modulation was apparent at
the level of biophysical properties without a change in Kv4.3 channel density. Further work will thus be needed to define how an N-terminal FMRP fragment interacts at the molecular level
with each member of a Cav3–Kv4–KChIP3 complex that together regulate Kv4 current amplitude19,47,48,49. FMRP is known to play a key role in regulating protein translation28,30,31,32,50. Here,
we found that FMRP(1–297)-_tat_ reduced differences in protein levels detected between _Fmr1_ KO and WT mice regardless of whether the levels were initially increased (αCaMKII, APP) or
decreased (PSD95) in _Fmr1_ KO animals. Moreover, the effects of FMRP(1–297)-_tat_ on protein levels were apparent 24 h after a single tail vein injection, indicating actions on cellular
function in both the cerebellum and cortex for an even longer timeframe than the behavioral differences detected in the OFT. These results have broad implications given the number of RNA
binding sites identified for FMRP that might benefit from a _tat_-conjugate approach to reintroducing FMRP(1–297). Our data reveal that FMRP is a constituent member of the Cav3–Kv4 complex
with a role for FMRP in promoting LTP at the mossy fiber synapse, a function it shares with other central synapses by enabling long-term potentiation or depression37,51. Indeed, the short
time required for FMRP(1–297) infusion to restore the capacity to evoke LTP suggests that FMRP is a critical element in the pathways activated by mossy fiber stimulation8. Tail vein
injections of 1.0 mg/kg FMRP(1–297)-_tat_ also had a greater influence on EPSP potentiation and granule cell firing rate than direct infusion of FMRP(1–297) into granule cells. The full
effects of FMRP(1–297)-_tat_ on mossy fiber-evoked LTP must then be exerted on additional elements accessed by the _tat_-conjugate peptide that are not influenced by direct postsynaptic
infusion. It is important to clarify that we can not conclude that the rescue of mossy fiber plasticity by FMRP(1–297)-_tat_ is directly linked to a reduction in elevated levels of activity
in the OFT. Rather, we focused on the mossy fiber-granule cell synapse as a representative synapse to gauge the effectiveness of introducing FMRP(1–297)-_tat_ on ionic and synaptic function.
Activity in the OFT can be interpreted in the context of either hyperactivity or anxiety, both of which are characteristic of Fragile X patients23,24. Further work will be needed to
distinguish between these possibilities. However, measures of activity in the OFT can be assumed to reflect the combined output of multiple brain regions that could be influenced by
HA-FMRP-_tat_ that distributes widely over the cerebellar-cortical axis and restores protein levels in both cerebellar and whole brain lysates. While the full range of sites affected by
HA-FMRP-_tat_ remain to be determined, the results are important in reviving the potential to use a _tat_ peptide-based approach to replace an active fragment of FMRP to rescue circuit
function in FXS. METHODS MOUSE LINES Wild type (Jackson Lab stock #004828, FVB.129P2-_Pde6b__+_ _Tyr__c-ch_/AntJ) and _Fmr1_ knockout (Jackson Lab stock #004624,FVB.129P2-_Pde6b_+
_Tyr__c-ch_ _Fmr1__tm1Cgr_/J) mice on FVB background were purchased from Jackson Lab and maintained in an Animal Resource Center of the University of Calgary in accordance with ethical
guidelines of the Canadian Council of Animal Care reviewed and approved by a Cumming School of Medicine Animal Care Committee. All animals had free access to water and food with a daily
temperature of 20–23 °C and relative humidity of 40–60%. The light cycle was controlled as 12 h light and dark cycle. For electrophysiology and behavioral tests, male mice were used. For
Western blot and coimmunoprecipitation tests samples were harvested from both males and females. BRAIN SLICE PREPARATION Sagittal cerebellar sections for electrophysiological recordings were
prepared from P16 to P22 male mice as previously described19. Briefly, animals were anaesthetized by isoflurane inhalation and the cerebella were dissected out and placed in ice-cold
artificial cerebrospinal fluid (aCSF) composed of (in mM): 125 NaCl, 25 NaHCO3, 25 d-glucose, 3.25 KCl, 1.5 CaCl2, 1.5 MgCl2 bubbled with carbogen (95% O2 and 5% CO2) gas. Tissue slices of
260 µm thickness were cut by vibratome (Leica VT1200 S) and allowed to recover for 20–30 min at 37 °C and then at room temperature (RT) (25 °C) in aCSF bubbled with carbogen gas. For
recordings slices were moved to a recording chamber which temperature was maintained at 31–33 °C on the stage of an Olympus BX51W1 microscope. Unless otherwise indicated all chemicals were
obtained from Sigma-Aldrich. DISSOCIATED GRANULE CELL CULTURE AND FLOW CYTOMETRY Cerebella were dissected out from anesthetized P6 _Fmr1_ KO mice, diced and trypsinized in Hanks'
balanced salt solution/bovine serum albumin (BSA) dissection solutions52. Isolated cells were plated onto 24-well plates coated with poly-d-lysine (1 μg/ml) at a total number of 1 × 106 each
well. Cultured cells were incubated at 37 °C under 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, insulin (5 μg/ml), KCl (25 mM) and 1%
pen–streptomycin. After culture for 24 h, cytosine _β_-d-arabinofuranoside (5 μM) (Sigma-Aldrich) was added to the culture medium for 24 h to inhibit the proliferation of nonneuronal cells
and medium was changed every two days. A single dose of different concentrations of FMRP(1–297)-_tat_ peptide was applied in the granule cell culture medium at 3 DIV or 7 DIV. At 8 DIV cells
were labeled with a live-dead assay kit (ab115347, Abcam) before being analyzed with a flow cytometer BD LSR II (BD Biosciences) at 488 nm excitation wavelength. Emission of labeled dye was
detected at 530 nm for FITC channel and 695 nm for PerCP-CY5-5 channel. Forward scatter, side scatter and doublet discrimination were applied to screen for single neurons and assess the
percentage of live vs dead cells in the population (Supplementary Fig. 5). CEREBELLAR GRANULE CELL ELECTROPHYSIOLOGY Whole-cell patch recordings were obtained from mouse cerebellar granule
cells of lobule 9 near the boundary between mossy fiber input and the granule cell body layer in sagittal tissue sections. Recordings were obtained using a Multiclamp 700B amplifier,
Digidata 1440A and pClamp 10.5 software and digitized at 40 kHz. Glass pipettes of 1.5 mm O.D. (A-M Systems) pulled using a P-95 puller (Sutter Instruments) had 4–8 MΩ resistance. For
whole-cell voltage clamp recordings, series resistance was compensated to at least 70% and leak was subtracted offline in pClamp 10 software. For current clamp recordings cells with lower
than −55 mV resting membrane potential were accepted, and less than 50 pA negative bias current applied to maintain a resting potential at ∼−80 mV. Cells were allowed to equilibrate to the
internal pipette solution for 3–5 min before recordings. Voltage-clamp recordings of Kv4 potassium current used an internal electrolyte of (mM): 140 KCl, 10 HEPES, 2.5 MgCl2, 0.1 EGTA, pH
7.3 via KOH, with 5 di–tris-creatine phosphate, 2 Tris-ATP, and 0.5 Na-GTP added from fresh frozen stock each day. The external medium contained 30 µM CdCl2 to block HVA calcium channels
together with 2 mM CsCl, 5 mM TEA, 1 μM TTX, glutamate receptor blockers DL-AP5 (25 µM) and DNQX (10 µM), and the inhibitory synaptic blockers picrotoxin (50 μM) and CGP55845 (1 μM). In
recordings of Kv4 current before and after mossy fiber TBS the internal solution was composed of (mM) 140 KCl, 10 HEPES, 2.5 MgCl2, 0.15 BAPTA, 0.05 CaCl2, with 200 μM QX-314 internal to
replace external TTX, and 5 di–tris-creatine phosphate, 2 Tris-ATP, and 0.5 Na-GTP added from fresh frozen stock each day, pH 7.3 via KOH. The external medium had no calcium channel
blockers, but contained 2 mM CsCl, 5 mM TEA, and the inhibitory synaptic blockers 50 μM picrotoxin and 1 μM CGP55845. Kv4 or Cav3.1 activation and inactivation plots were calculated from
currents evoked from a holding potential of −110 mV stepped in 10 mV (1000 ms) increments to 60 mV, followed by a return step to −30 mV (500 ms) as the test potential8,9. For tests on the
involvement of protein translation on the shift in Kv4 Vh with LTP, 20 µM anisomycin was pre-applied to tissue slices for 2 h at RT and throughout recordings. Current clamp recordings used
an internal solution modified from Gall et al.53: 126 K-gluconate, 4 NaCl, 15 Glucose, 5 HEPES, 1 MgSO4, 0.15 BAPTA, 0.05 CaCl2, pH 7.3 via KOH, with 5 di–tris-creatine phosphate, 2
Tris-ATP, and 0.5 Na-GTP added from fresh frozen stock each day. The external medium contained 50 μM picrotoxin and 1 μM CGP55845. Mossy fibers were stimulated with a concentric bipolar
electrode (Frederick Haer, CBCMX75 (JL2)) placed near the border of mossy fiber input and the granule cell layer using a Digitimer stimulus isolation unit (0.3 ms pulse). The stimulation
protocol was the same as in Sola et al.54, using a TBS pattern (8 bursts of 10 impulses at 100 Hz, 250 ms interburst interval) at a stimulus intensity that initially evoked a threshold EPSC
or EPSP from a holding potential of −80 mV. For current-clamp recordings, TBS was delivered from a holding potential of ∼−65 mV. For voltage-clamp recordings of Kv4 current the TBS was
paired with a postsynaptic voltage step from −70 to −40 mV for the duration of the TBS synaptic train (3 s total) as in D’Angelo et al.55. While TBS in current-clamp recordings used only
synaptic stimulation. A 5 min period was used to establish baseline synaptic amplitudes before presenting TBS and EPSPs were recorded at 0.1 Hz to monitor LTP. EPSP amplitudes were
calculated only for cases subthreshold to spike discharge and LTP was measured with respect to the mean value of all control records of baseline EPSP amplitude. FMRP AND FUSION OF _TAT_
PEPTIDE TO FMRP Recombinant FMRP(1–297) protein (H000023332-P01, Novus Biologicals, Oakville, ON) was added to the pipette before recordings or infused into the electrode during a recording
(2PK+ Perfusion system; ALA Scientific, Farmingdale, NY). pTrc-HisA-_tat_ vector was a modified pTrc-His A vector (Invitrogen,V360-20, Ottawa, ON) in which the Xpress epitope was replaced by
an 11 aa _tat_ sequence (YGRKKRRQRRR). The FMRP(1–297)-_tat_ construct was made by subcloning FMRP(1–297) cDNA from _Fmr1_ cDNA (Origene, RC222699) into the pTrc-HisA-_tat_ vector with the
following primers: FMR1-1F (5′-CTAGCTAGCATGGAGGAGCTGGTGGTGGAA-3′) and FMR1-297R (5′-CGGGATCCTATTACTTTGCCTACTAAGTT-3′). The construct was transformed and expressed in BL21 _Escherichia coli_
bacteria (Invitrogen, C601003) grown in LB medium supplemented with 1 mg/ml Ampicillin (Sigma-Aldrich) overnight. The bacterial culture was further induced with 500 μM IPTG for 4 h for
protein production. We purified FMRP(1–297)-_tat_ peptide from the bacteria cultures by using the Ni-NTA Fast Start Kit (Qiagen, 30600, Toronto, ON). Aliquots of the peptide were stored at
−80 °C in Elution Buffer of Ni-NTA Fast Start Kit (50 mM Na–phosphate, 300 mM NaCl and 250 mM imidazole at pH 8.0) and a proteinase inhibitor tablet was added to prevent proteolysis (one
tablet in 10 ml solution) (04693124001, Roche). Protein integrity was tested by Coomassie Blue Staining following sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
quantified with the Bradford Protein Assay (Bio-Rad) following purification and before application. A control _tat_ fragment was prepared from the FMRP(1–297)-_tat_ construct by excising the
FMRP(1–297) region and replacing it with the restriction enzyme sites (ELEIC) that existed in the original pTrc-HisA vector. A _tat_-peptide was then purified from BL21 bacteria and stored
in the same way as the FMRP(1–297)-_tat_ peptides for control studies. An HA-FMRP(1–297)-_tat_ construct that included an HA-tag sequence (YPYDVPDYA) on the N-terminus side of
FMRP(1–297)-_tat_ was prepared by using specific primers: HA-FMRP(1-297)-F-NheI, 5′-CTAGCTAGCTACCCATACGATGTTCCAGATTACG CTATGGAGGAGCTGGTGGTGGAA-3′ and HA-FMRP(1-297)-R-BamHI,
5′-CGGGATCCTATTACTT TGCCTACAAGTT-3′. The HA-tagged FMRP(1–297)-_tat_ peptide was purified from BL21 bacteria and stored in the same manner. FMRP(1–297)-_tat_ proteins were delivered once by
tail vein injection to animals under transient isoflurane anesthesia by inhalation (Midmark, Ohio) at 0.2–2.0 mg/kg (approximately 0.1–1 µM in blood upon injection) in 0.9% NaCl to a 200 µl
volume, with an equivalent dilution of Elution Buffer (see above) to prepare vehicle for control groups. Injected animals were provided at least 1 h recovery time before any behavioral tests
or 2 h before in vitro slice experiments were conducted. HETEROLOGOUS EXPRESSION IN TSA-201 CELLS tsA-201 cells were maintained in DMEM supplemented with 10% heat inactivated fetal bovine
serum and 1% pen–streptomycin at 37 °C under 5% CO2. The calcium phosphate based method was used to transiently transfect cDNA20. Cells were washed with fresh medium 16–18 h after
transfection and then transferred to 32 °C under 5% CO2 for 1–2 days. Human Kv4.3 cDNA and human Cav3.1 cDNA was subcloned into the expression vector pCDNA3.1− (Invitrogen). Human FMRP cDNA
in pCMV6-Entry vector was obtained from OriGene and cDNA of both full length and the 1–297 fragment of FMRP were subcloned into mKate-PCDNA3.1−. In electrophysiology studies cells were
transfected as indicated with cDNA of Cav3.1 or Kv4.3, eGFP cDNA was added (1 μg) to identify cells with successful transfection. FRET studies used 1 μg cDNA of GFP-Cav3.1, GFP-Kv4.3, and
FMRP(1–297)-mKate or FMRP-mKate (full length). Transfected cells were incubated at 37 °C (5% CO2) for 24 h and then transferred to a 32 °C incubator (5% CO2) for 48–72 h prior to tests.
TSA-201 CELL ELECTROPHYSIOLOGY Recordings of Kv4 current expressed in tsA-201 cells were carried out at 22 °C with an external solution comprised of (mM): 125 NaCl, 3.25 KCl, 1.5 CaCl2, 1.5
MgCl2, 10 HEPES, 5 TEA, 2 CsCl and 10 d-Glucose (pH adjusted to 7.3 with NaOH). Pipettes were filled with a solution comprised of (mM) 110 potassium gluconate, 30 KCl, 1 EGTA, 5 HEPES, and
0.5 MgCl2, pH 7.3 via KOH, with 5 di–tris-creatine phosphate, 2 Tris-ATP, and 0.5 Na-GTP added from fresh frozen stock each day. Electrode solution could be infused with drugs using the 2PK+
System (ALA Scientific). To record Cav3.1 current pipettes were filled with a solution comprised of (mM) 100 CsCl, 10 EGTA, 10 HEPES, 2.5 MgCl2, pH 7.3 with CsOH, with 5 di–tris-creatine
phosphate, 2 Tris-ATP, and 0.5 Na-GTP added from fresh frozen stock each day. For Cav3.1 recordings the external medium contained (mM) 130 CsCl, 10 HEPES, 1 MgCl2, 2 CaCl2, pH 7.3 with CsOH.
FLUORESCENCE RESONANCE ENERGY TRANSFER (FRET) tsA-201 cells were plated onto poly-d-lysine coated 35 mm glass bottom culture dishes (World Precision Instruments, Sarasota, FL)56. Cells were
transiently transfected with GFP-Cav3.1, GFP-Kv4.3, FMRP(1–297)-mKate or FMRP-mKate constructs to use as donor–acceptor fluorescent pairs56. On the experimental day DMEM was replaced with
imaging medium comprised of (mM): 148 NaCl, 3 KCl, 10 HEPES, 3 CaCl2, 10 d-Glucose, 1 MgCl2 (pH 7.3 with NaOH) at 25 °C. Cells were examined on a Nikon Eclipse C1Si spectral confocal
laser-scanning microscope using a 40×/1.3NA oil immersion objective. Laser lines of 457 nm were used to excite GFP and 561 nm to excite mKate, with emission spectra recorded between 400 and
750 nm. Spectral images were linearly unmixed offline using ImageTrak software56. CO-IMMUNOPRECIPITATION AND WESTERN BLOTTING For protein biochemical tests we define lysates prepared from
whole brain as corresponding to all brain regions including cerebellum and brainstem, Brain to tissue that has cerebellum and hindbrain removed (Fig. 9), and cerebellum to isolated
cerebellum. In no case was olfactory bulb tissue included. All coIP and blotting tests were conducted with the HA-FMRP(1–297)-_tat_ construct for immunodetection. For coIP tests between FMRP
and Cav3.1 and/or Kv4.3 and HA tag experiments, Whole brains (ranging 350–500 mg) were dissected out from P30 to P50 mice after isoflurane anesthesia and homogenized in a lysis buffer
containing (mM): 150 NaCl, 50 Tris, 2.5 EGTA, 1% NP-40, pH 7.5, phosphatase inhibitor (P5726, Sigma-Aldrich) and proteinase inhibitor (04693124001, Roche). For protein level regulation tests
(Fig. 9), brain and cerebellar lysates were prepared separately from WT or KO mice injected with FMRP(1–297)-_tat_ or vehicle and homogenized in the same lysis buffer. The homogenates were
then centrifuged at 13,000_g_ for 10 min at 4 °C. Supernatants were collected and concentrations were measured using the Bradford Protein Assay (Bio-Rad) to provide 500 μg total protein for
immunoprecipitation (IP) with rabbit anti-Cav3.156, rabbit anti-Kv4.3 (ab 65794, Abcam), or rabbit normal IgG (as a control) (ab 172730, Abcam) (antibodies in mixtures 40 µg/ml) and
immunoblotted with a mouse antibody against FMRP N-terminus (1:1000, ab230915, Abcam) (Fig. 2a). Whole brain lysates of _Fmr1_ KO mice injected with HA-FMRP(1–297)-_tat_ underwent IP with
mouse anti-HA antibody (Abcam, ab130275) and mouse normal IgG (as control) (Abcam, ab 37355) (40 μg/ml) and were immunoblotted with rabbit polyclonal anti-Cav3.1 and rabbit polyclonal
anti-Kv4.3 antibodies (1:1000) (Fig. 5c). IP samples were incubated with antibodies for 2 h (4 °C) before the mixtures were incubated with 30 μl Protein G beads (Life Technologies) at 4 °C
overnight. Beads were washed four times with lysis buffer by centrifugation and resuspension. The immuno-complexes were boiled at 95–100 °C for 5 min with 10 μl sample buffer diluted from 4×
sample buffer (mM) 100 Tris, 100 2-mercaptoethanol, 4% SDS, 0.02% bromophenol blue, 20% glycerol, pH 6.8. IP samples in Fig. 2 (30 μl) and Fig. 5c (30 μl) and tissue homogenates (50 μg
total protein) as input were loaded on 6–12% tris-glycine gel and resolved using SDS-PAGE. Samples were transferred to 0.2 µm PVDF membrane (Millipore) and probed with primary antibodies
overnight at 4 °C, followed by goat anti-mouse (1:3000, 62–6520, Invitrogen) or donkey anti-rabbit (1:5000, NA-9340V, GE healthcare) HRP-conjugated secondary antibodies. Blot images were
taken with a ChemiDoc imager and protein densities were analyzed with Image Lab (Bio-Rad). Other primary antibodies used in western blot include: rabbit polyclonal anti-APP (1:1000, A8717,
Sigma-Aldrich), rabbit polyclonal anti-_α_CaMKII (1:3000, ab103840, Abcam), mouse monoclonal anti-PSD95 (1:1000, MABN68, Sigma-Aldrich), rabbit monoclonal anti-GAPDH (1:3000, 2118s, Cell
Signaling Technology), and mouse monoclonal anti-vinculin (1:1000, SAB4200729, Sigma-Aldrich). IMMUNOSTAINING Tissue for immunohistochemistry was obtained from P30 to P60 _Fmr1_ KO or WT
mice8. Animals were anesthetized by isoflurane inhalation until unresponsive to ear pinch and then perfused intracardially with 20 ml 0.1 M phosphate-buffer (PB, pH 7.4) followed by 20 ml 4%
paraformaldehyde (PFA, pH 7.4) at RT. Brains were stored in 4% PFA at RT for 1 h and then overnight at 4 °C. Sagittal sections of 50 μm thickness were cut by vibratome (Leica VT1000 S,
Germany) in PB. Tissue sections were blocked with a solution containing 10% normal goat serum, 0.2% DMSO and 0.1% TWEEN-20 and reacted overnight under 4 °C with the following primary
antibodies diluted in working solution: rabbit monoclonal anti-FMRP C-terminal antibody (1:200, Cell Signaling Technology, 7104S), mouse monoclonal anti-HA (1:300, Abcam, ab130275), and
chicken polyclonal anti-MAP2 (1:500, Abcam, ab92434) in dual labeling experiments. Primary antibodies were omitted in control sections for each experimental series. After washing in PB,
sections were exposed for 1–2 h at RT to AlexaFluor 594-conjugated goat anti-mouse IgG (1:1000, Invitrogen, A11032) or AlexaFluor 488-conjugated goat anti-chicken IgG (1:1000, Invitrogen,
A11039) and AlexaFluor 594-conjugated goat anti-rabbit IgG (1:1000, Invitrogen, A32740). Sections were washed 3 × 10 min in PB, mounted in Molecular Probes gold antifade medium and stored at
−20 °C. Immunocytochemistry in granule cell culture was performed with the same process with minor modifications. Briefly, granule cell cultures were fixed in 4% PFA followed by 3 washes in
PBS. Cells were permeabilized in PBS containing 0.2% Triton X-100 and blocked with 3% BSA in PBST buffer (0.1% Tween-20 in PBS). Imaging was conducted on a Zeiss Axioimager (Zen software)
with Colibri LED illumination. Exposures were constrained to those used for control sections and post processing was restricted to equivalent adjustments of brightness/contrast for
qualitative comparisons (Zen, Photoshop). Tiled montage images of cerebellar tissue sections were compiled from 80 to 90 images at 20× magnification (Zen). OPEN FIELD TEST Different
parameters of animal motion were quantified through an OFT using an overhead acA1300-60 hm camera (Basler, DE) and Noldus Ethovision XT 13 software (Leesburg VA). Male mice of P55-P100 age
were placed in a square plexiglass chamber of 38 cm × 38 cm × 30 cm dimension and recorded during free movement for 30 min. A 4 × 4 grid pattern was applied to quantify different parameters
during the OFT. HA-FMRP(1–297)-_tat_ or vehicle were administered into animals as mentioned above. Analysis of total distance and duration of movement was conducted using Noldus Ethovision
XT 13 and velocity was calculated by dividing total distance with duration for moving. DATA ANALYSIS AND STATISTICAL METHODS Inactivation curves were fitted according to the Boltzmann
equation: _I_/(_I_ − _I_max) = 1/(1 + exp((_Vh_ − _V_)/_k_)), where _Vh_ is the half inactivation potential and _k_ is the slope factor. Activation curves were fit according to the Boltzmann
equation: _G_/(_G_ − _G_max) = 1/(1 + exp((_Va_ − _V_)/_k_)), where _G_ is calculated with equation _G_ = _I_/(_V_ − _V_rev), _Va_ is the half activation potential and _k_ is the slope
factor. Inactivation and activation plots were constructed using Origin 8.0 (OriginLab, Northampton, MA). All figures were prepared and statistical analysis were performed using OriginPro 8
or GraphPad Prism 6, and Adobe Illustrator CC 2018 software. Statistical significance was determined using a two-sample Student’s _t_ test for distinct samples, and a paired-sample Student’s
_t_ tests for the same samples under different conditions. One-way ANOVA followed by post-hoc comparison (Tukey’s multiple comparison) was used to analyze the statistical significance of
more than two groups. In the OFT (Fig. 8) and protein translation test (Fig. 9), two-way ANOVA was applied to analyze the effect of genotype and treatment and their interactions, when
necessary one-way ANOVA followed by post-hoc Tukey’s comparison was applied to test the significance of different treatments in _Fmr1_ KO mice. Normality of data was tested and there was no
exclusion of outlier data points. Two-tailed analysis was chosen for all statistical tests. REPORTING SUMMARY Further information on research design is available in the Nature Research
Reporting Summary linked to this article. DATA AVAILABILITY All relevant data are available without restriction from the authors, with the original values for data underlying Figs. 1–3, 5–9,
Supplementary Table 1, and Supplementary Figs. 1–4 provided as a Source Data file. Figures are also available to the public through a link on Figshare: 10.6084/m9.figshare.12132864. No
accession codes are required. CODE AVAILABILITY ImageTrak software used for FRET imaging measurements is freely available at: http://www.ucalgary.ca/styslab/imagetrak. P.K. Stys, University
of Calgary. REFERENCES * Sinclair, D., Oranje, B., Razak, K. A., Siegel, S. J. & Schmid, S. Sensory processing in autism spectrum disorders and Fragile X syndrome—from the clinic to
animal models. _Neurosci. Biobehav. Rev_. 76, 235–253 (2017). * Fatemi, S. H. et al. Consensus paper: pathological role of the cerebellum in autism. _Cerebellum_ 11, 777–807 (2012). Article
PubMed PubMed Central Google Scholar * Hampson, D. R. & Blatt, G. J. Autism spectrum disorders and neuropathology of the cerebellum. _Front. Neurosci._ 9, 420 (2015). Article
PubMed PubMed Central Google Scholar * Roggeri, L., Rivieccio, B., Rossi, P. & D’Angelo, E. Tactile stimulation evokes long-term synaptic plasticity in the granular layer of
cerebellum. _J. Neurosci._ 28, 6354–6359 (2008). Article CAS PubMed PubMed Central Google Scholar * D’Angelo, E. et al. Long-term potentiation of synaptic transmission at the mossy
fiber-granule cell relay of cerebellum. _Prog. Brain Res._ 148, 69–80 (2005). Article PubMed CAS Google Scholar * Gandolfi, D., Mapelli, J. & D’Angelo, E. Long-term spatiotemporal
reconfiguration of neuronal activity revealed by voltage-sensitive dye imaging in the cerebellar granular layer. _Neural Plast._ 2015, 284986 (2015). Article PubMed PubMed Central CAS
Google Scholar * Garrido, J. A., Ros, E. & D’Angelo, E. Spike timing regulation on the millisecond scale by distributed synaptic plasticity at the cerebellum input stage: a simulation
study. _Front. Comput. Neurosci._ 7, 64 (2013). Article PubMed PubMed Central Google Scholar * Rizwan, A. P., Zhan, X., Zamponi, G. W. & Turner, R. W. Long-term potentiation at the
mossy fiber-granule cell relay invokes postsynaptic second-messenger regulation of Kv4 channels. _J. Neurosci._ 36, 11196–11207 (2016). Article CAS PubMed PubMed Central Google Scholar
* Heath, N. C. et al. The expression pattern of a Cav3-Kv4 complex differentially regulates spike output in cerebellar granule cells. _J. Neurosci._ 34, 8800–8812 (2014). Article PubMed
PubMed Central CAS Google Scholar * Ferron, L. Fragile X mental retardation protein controls ion channel expression and activity. _J. Physiol._ 594, 5861–5867 (2016). Article CAS PubMed
PubMed Central Google Scholar * Yang, Y.-M. et al. Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain. _Mol.
Psychiatry_ https://doi.org/10.1038/s41380-018-0240-0 (2018). * Gholizadeh, S., Halder, S. K. & Hampson, D. R. Expression of fragile X mental retardation protein in neurons and glia of
the developing and adult mouse brain. _Brain Res._ 1596, 22–30 (2015). Article CAS PubMed Google Scholar * Zorio, D. A. R., Jackson, C. M., Liu, Y., Rubel, E. W. & Wang, Y. Cellular
distribution of the fragile X mental retardation protein in the mouse brain. _J. Comp. Neurol._ 525, 818–849 (2017). Article CAS PubMed Google Scholar * Contractor, A., Klyachko, V. A.
& Portera-Cailliau, C. Altered neuronal and circuit excitability in fragile X syndrome. _Neuron_ 87, 699–715 (2015). Article CAS PubMed PubMed Central Google Scholar * Armano, S.,
Rossi, P., Taglietti, V. & D’Angelo, E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. _J. Neurosci._ 20, 5208–5216 (2000).
Article CAS PubMed PubMed Central Google Scholar * Brown, M. R. et al. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. _Nat.
Neurosci._ 13, 819–821 (2010). Article CAS PubMed PubMed Central Google Scholar * Deng, P. Y. et al. FMRP regulates neurotransmitter release and synaptic information transmission by
modulating action potential duration via BK channels. _Neuron_ 77, 696–711 (2013). Article CAS PubMed PubMed Central Google Scholar * Deng, P.-Y. et al. Voltage-independent SK-channel
dysfunction causes neuronal hyperexcitability in the hippocampus of Fmr1 knock-out mice. _J. Neurosci._ 39, 28–43 (2019). Article CAS PubMed PubMed Central Google Scholar * Anderson, D.
et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. _Nat. Neurosci._ 13, 333–337 (2010). Article CAS PubMed Google Scholar * Anderson, D. et al. The Cav3-Kv4
complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations. _J. Neurosci._ 33, 7811–7824 (2013). Article CAS PubMed PubMed Central
Google Scholar * Leibrand, C. R. et al. HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of
transgenic mice. _Neurosci. Lett._ 640, 136–143 (2017). Article CAS PubMed PubMed Central Google Scholar * Bonaccorso, C. M. et al. Fragile X mental retardation protein (FMRP)
interacting proteins exhibit different expression patterns during development. _Int. J. Dev. Neurosci._ 42, 15–23 (2015). Article CAS PubMed Google Scholar * Ciaccio, C. et al. Fragile X
syndrome: a review of clinical and molecular diagnoses. _Ital. J. Pediatr._ 43, 39 (2017). Article PubMed PubMed Central CAS Google Scholar * Wheeler, A. et al. Anxiety, attention
problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. _Am_. _J. Med. Genet. A_ 164A, 141–155 (2014). Article CAS Google Scholar * Spencer, C. M. et al.
Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. _Autism Res._ 4, 40–56 (2011). Article PubMed PubMed Central Google Scholar
* Kazdoba, T. M., Leach, P. T., Silverman, J. L. & Crawley, J. N. Modeling fragile X syndrome in the Fmr1 knockout mouse. _Intractable Rare Dis. Res._ 3, 118–133 (2014). Article
PubMed PubMed Central Google Scholar * Bakker, C. E. et al. Fmr1 knockout mice: a model to study fragile X mental retardation. _Cell_ 78, 23–33 (1994). Google Scholar * Napoli, I. et al.
The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. _Cell_ 134, 1042–1054 (2008). Article CAS PubMed Google Scholar * Schütt, J.,
Falley, K., Richter, D., Kreienkamp, H.-J. & Kindler, S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities.
_J. Biol. Chem._ 284, 25479–25487 (2009). Article PubMed PubMed Central CAS Google Scholar * Sawicka, K. et al. FMRP has a cell-type-specific role in CA1 pyramidal neurons to regulate
autism-related transcripts and circadian memory. _Elife_ 8, e46919 (2019). Article PubMed PubMed Central Google Scholar * Bowling, H. et al. Altered steady state and activity-dependent
de novo protein expression in fragile X syndrome. _Nat. Commun._ 10, 1710 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Muddashetty, R. S., Kelic, S., Gross, C., Xu,
M. & Bassell, G. J. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X
syndrome. _J. Neurosci._ 27, 5338–5348 (2007). Article CAS PubMed PubMed Central Google Scholar * Yrigollen, C. M. & Davidson, B. L. CRISPR to the rescue: advances in gene editing
for the FMR1 gene. _Brain Sci._ 9,17 https://doi.org/10.3390/brainsci9010017 (2019). Article CAS PubMed Central Google Scholar * Lee, B. et al. Nanoparticle delivery of CRISPR into the
brain rescues a mouse model of Fragile X syndrome from exaggerated repetitive behaviours. _Nat. Biomed. Eng._ 2, 497–507 (2018). Article CAS PubMed PubMed Central Google Scholar *
Gholizadeh, S., Arsenault, J., Xuan, I. C., Pacey, L. K. & Hampson, D. R. Reduced phenotypic severity following adeno-associated virus-mediated Fmr1 gene delivery in fragile X mice.
_Neuropsychopharmacology_ 39, 3100–3111 (2014). Article CAS PubMed PubMed Central Google Scholar * Reis, S. A., Willemsen, R., van Unen, L., Hoogeveen, A. T. & Oostra, B. A.
Prospects of TAT-mediated protein therapy for Fragile X syndrome. _J. Mol. Histol._ 35, 389–395 (2004). Article CAS PubMed Google Scholar * Zeier, Z. et al. Fragile X mental retardation
protein replacement restores hippocampal synaptic function in a mouse model of Fragile X syndrome. _Gene Ther._ 16, 1122–1129 (2009). Article CAS PubMed PubMed Central Google Scholar *
Arsenault, J. et al. FMRP expression levels in mouse central nervous system neurons determine behavioral phenotype. _Hum. Gene Ther._ 27, 982–996 (2016). Article CAS PubMed PubMed Central
Google Scholar * Gantois, I. et al. Metformin ameliorates core deficits in a mouse model of Fragile X syndrome. _Nat. Med._ 23, 674–677 (2017). Article CAS PubMed Google Scholar *
Erickson, C. A. et al. Fragile X targeted pharmacotherapy: lessons learned and future directions. _J. Neurodev. Disord._ 9, 7 (2017). Article PubMed PubMed Central Google Scholar *
Youngblood, D. S., Hatlevig, S. A., Hassinger, J. N., Iversen, P. L. & Moulton, H. M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells.
_Bioconjug. Chem._ 18, 50–60 (2007). Article CAS PubMed Google Scholar * Nischan, N. et al. Covalent attachment of cyclic TAT peptides to GFP results in protein delivery into live cells
with immediate bioavailability. _Angew. Chem. Int. Ed. Engl._ 54, 1950–1953 (2015). Article CAS PubMed Google Scholar * Chen, L. L. et al. Increased cellular uptake of the human
immunodeficiency virus-1 Tat protein after modification with biotin. _Anal. Biochem._ 227, 168–175 (1995). Article CAS PubMed Google Scholar * Gothelf, D. et al. Neuroanatomy of Fragile
X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP). _Ann. Neurol._ 63, 40–51 (2008). Article PubMed PubMed Central Google Scholar *
Reiss, A. L., Abrams, M. T., Greenlaw, R., Freund, L. & Denckla, M. B. Neurodevelopmental effects of the FMR-1 full mutation in humans. _Nat. Med._ 1, 159–167 (1995). Article CAS
PubMed Google Scholar * Zhang, Y. et al. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. _J. Neurosci._ 32,
15318–15327 (2012). Article CAS PubMed PubMed Central Google Scholar * Nadal, M. S. et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of
neuronal A-type K+ channels. _Neuron_ 37, 449–461 (2003). Article CAS PubMed Google Scholar * Nadin, B. M. & Pfaffinger, P. J. Dipeptidyl peptidase-like protein 6 is required for
normal electrophysiological properties of cerebellar granule cells. _J. Neurosci._ 30, 8551–8565 (2010). Article CAS PubMed PubMed Central Google Scholar * Carrillo-Reid, L. et al.
Mutant huntingtin enhances activation of dendritic Kv4 K channels in striatal spiny projection neurons. _eLife_ 8, e40818 (2019). * Darnell, J. C. et al. FMRP stalls ribosomal translocation
on mRNAs linked to synaptic function and autism. _Cell_ 146, 247–261 (2011). Article CAS PubMed PubMed Central Google Scholar * Sidorov, M. S., Auerbach, B. D. & Bear, M. F. Fragile
X mental retardation protein and synaptic plasticity. _Mol. Brain_ 6, 15 (2013). Article CAS PubMed PubMed Central Google Scholar * Krämer, D. & Minichiello, L. Cell culture of
primary cerebellar granule cells. _Methods Mol. Biol._ 633, 233–239 (2010). Article PubMed CAS Google Scholar * Gall, D. et al. Altered neuronal excitability in cerebellar granule cells
of mice lacking calretinin. _J. Neurosci._ 23, 9320–9327 (2003). Article CAS PubMed PubMed Central Google Scholar * Sola, E., Prestori, F., Rossi, P., Taglietti, V. & D’Angelo, E.
Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. _J. Physiol._ 557, 843–861 (2004). Article CAS PubMed PubMed
Central Google Scholar * D’Angelo, E., Rossi, P., Armano, S. & Taglietti, V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell
transmission in rat cerebellum. _J. Neurophysiol._ 81, 277–287 (1999). Article PubMed Google Scholar * Asmara, H. et al. A T-type channel-calmodulin complex triggers alphaCaMKII
activation. _Mol. Brain_ 10, 37 (2017). Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge Dr. Q. Long for advice and
discussions on statistics, and J. Forden, M. Kruskic, R. Tobias, and Y.P. Liu for expert technical assistance. This work was supported by grants by a SFARI Explorer grant (R.W.T.), and
grants from the Canadian Institutes for Health Research (R.W.T., J.M. Rho, and G.W.Z.) and Fragile X Canada/FRAXA (R.W.T.). Salary support was provided by the Alberta Children’s Hospital
Foundation (N.C.), and Postdoctoral Fellowships from the Hotchkiss Brain Institute, Cumming School of Medicine and Fragile X Canada/FRAXA (X.Z.), University of Calgary Eyes High (G.S.), and
Alberta Innovates—Health Solutions (AI-HS) (G.S., F.-X.Z.). G.W.Z. holds a Canada Research Chair. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Hotchkiss Brain Institute, University of
Calgary, Calgary, AB, T2N 4N1, Canada Xiaoqin Zhan, Hadhimulya Asmara, Giriraj Sahu, Eduardo Sanchez, Fang-Xiong Zhang, Gerald W. Zamponi, Jong M. Rho & Ray W. Turner * Alberta
Children’s Hospital Research Institute, University of Calgary, Calgary, AB, T2N 4N1, Canada Ning Cheng, Gerald W. Zamponi & Jong M. Rho Authors * Xiaoqin Zhan View author publications
You can also search for this author inPubMed Google Scholar * Hadhimulya Asmara View author publications You can also search for this author inPubMed Google Scholar * Ning Cheng View author
publications You can also search for this author inPubMed Google Scholar * Giriraj Sahu View author publications You can also search for this author inPubMed Google Scholar * Eduardo Sanchez
View author publications You can also search for this author inPubMed Google Scholar * Fang-Xiong Zhang View author publications You can also search for this author inPubMed Google Scholar
* Gerald W. Zamponi View author publications You can also search for this author inPubMed Google Scholar * Jong M. Rho View author publications You can also search for this author inPubMed
Google Scholar * Ray W. Turner View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.Z., R.W.T., H.A., and N.C. designed the experiments, X.Z.
and G.S. conducted electrophysiology experiments, X.Z. and H.A. protein biochemical tests and FRET imaging, N.C., E.S., and X.Z. behavioral tests and analysis, H.A. and F.-X.Z. prepared
fluorescent tagged and _tat_ constructs, R.W.T., X.Z., N.C., and H.A. wrote the paper, R.W.T. and N.C. supervised the study. G.W.Z. and J.R. provided necessary reagents and supplies.
CORRESPONDING AUTHOR Correspondence to Ray W. Turner. ETHICS DECLARATIONS COMPETING INTERESTS The following patent application exists: Applicant: UTI Limited Partnership. Inventors: Turner,
Raymond W Application Number: PCT/CA2019/000001 Status: Application Specific aspect of the paper covered by the patent application: Use of tat-FMRP peptides to treat Fragile X Syndrome.
ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are
available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhan, X., Asmara, H., Cheng, N. _et al._ FMRP(1–297)-tat restores ion channel and
synaptic function in a model of Fragile X syndrome. _Nat Commun_ 11, 2755 (2020). https://doi.org/10.1038/s41467-020-16250-4 Download citation * Received: 25 June 2019 * Accepted: 22 April
2020 * Published: 02 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16250-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative