Play all audios:
ABSTRACT Imbuing bio-inspired sensory devices with intelligent functions of human sensory organs has been limited by challenges in emulating the preprocessing abilities of sensory organs
such as reception, filtering, adaptation, and sensory memory at the device level itself. Merkel cells, which is a part of tactile sensory organs, form synapse-like connections with afferent
neuron terminals referred to as Merkel cell-neurite complexes. Here, inspired by structure and intelligent functions of Merkel cell-neurite complexes, we report a flexible, artificial,
intrinsic-synaptic tactile sensory organ that mimics synapse-like connections using an organic synaptic transistor with ferroelectric nanocomposite gate dielectric of barium titanate
nanoparticles and poly(vinylidene fluoride-trifluoroethylene). Modulation of the post-synaptic current of the device induced by ferroelectric dipole switching due to triboelectric-capacitive
coupling under finger touch allowed reception and slow adaptation. Modulation of synaptic weight by varying the nanocomposite composition of gate dielectric layer enabled tuning of
filtering and sensory memory functions. SIMILAR CONTENT BEING VIEWED BY OTHERS AN ARTIFICIAL NEUROMORPHIC SOMATOSENSORY SYSTEM WITH SPATIO-TEMPORAL TACTILE PERCEPTION AND FEEDBACK FUNCTIONS
Article Open access 13 August 2022 A TACTILE SENSOR SYSTEM WITH SENSORY NEURONS AND A PERCEPTUAL SYNAPTIC NETWORK BASED ON SEMIVOLATILE CARBON NANOTUBE TRANSISTORS Article Open access 11
December 2020 BIO-INSPIRED ARTIFICIAL MECHANORECEPTORS WITH BUILT-IN SYNAPTIC FUNCTIONS FOR INTELLIGENT TACTILE SKIN Article 28 April 2025 INTRODUCTION Sensory organs enable animals to
gather information for perception and to have ability for lives, such as conducting skilled movements and seeking protection from hazardous situations. Sensory organs consist of
stimuli-sensitive cells such as photoreceptors for vision1,2, chemoreceptors for olfaction and gustation3, and mechanoreceptors for audition4, all of which have synapse-like connections to
afferent neurons. Synapses in the brain allow information processing in parallel with high energy efficiency, and there is some evidence that sensory organs with synapse-like connections
with neurons also preprocess information via intelligent functions including adaptation, filtering, amplification, and memory before transmitting the information to the brain5,6,7,8,9,10.
Merkel cells are cutaneous mechanosensitive cells that form synapses with afferent neurons, and these complexes are referred to as Merkel cell-neurite complexes (MCNCs)11. There have been
some reports on artificial tactile sensors mimicking mechanoreceptors with slowly adapting (SA) firing analysis12,13. Even though much effort has been made to mimic mechanoreceptors in the
human body14,15,16,17,18, emulation of their intelligent functions to extend sensory reception has not been widely successful due to difficulty in adding synaptic connection between
receptors and neuron terminal at the device level. Several bio-inspired tactile reception systems have recently been developed. For example, an artificial sensory nerve was developed by
connecting a piezoresistive pressure sensor, a ring oscillator generating action potential, and an ion-gel-based synaptic transistor19. A tactile perceptual learning system was realized by
integration of a piezoresistive touch sensor, an ionic cable, and an ion-gel-based synaptic transistor20. These bio-inspired tactile reception systems were developed by integrating discrete
synapse devices and sensors mimicking the reception function of mechanoreceptors such as Meissner’s or Pacinian corpuscles. However, the sensors themselves are not intelligent, in contrast
to mechanoreceptors in the human body, and complicated fabrication processes are required to generate the discrete components of the systems. Herein, we demonstrate a flexible, artificial,
intrinsic-synaptic tactile sensory organ (AiS-TSO) that mimics the synapse-like connections of MCNCs, conferring intrinsic-synaptic properties to the unit sensor for conducting further
intelligent work. This AiS-TSO is based on a flexible ferroelectric organic field-effect transistor (Fe-OFET) gated by a triboelectric-capacitive coupling effect. Touch stimulation induces
alignment of dipoles in the ferroelectric gate dielectric by triboelectric-capacitive coupling effect which causes the post-synaptic current signal to be modulated, thereby allowing tactile
information to be imparted to the signal in a self-energy transducing manner. The synaptic function of the device enables the output signal to be pre-processed though the multiple functions
of SA, filtering and memory in a self-energy transducer manner. Tunability of SW in the AiS-TSO was achieved by varying the composition of the nanocomposite ferroelectric layer of barium
titanate nanoparticles (BT NPs) and poly(vinyledenedifluoride–trifluoroethylene) (P(VDF-TrFE)). We also emulated sensory memory in a 2 × 2 sensor array by recognizing the number and order of
touch without additional signal processing after all stimuli ceased. We believe that the concept of AiS-TSO represents a new paradigm in sensor technology for artificial intelligence (AI)
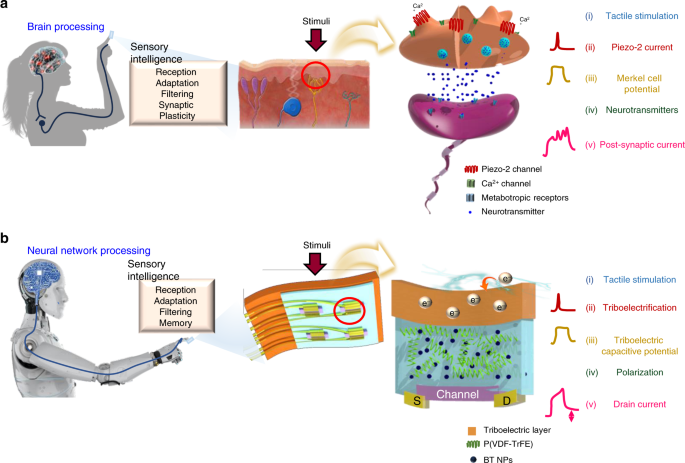
and autonomous systems requiring real-time decisions and highly energy-efficient sensing. RESULTS MIMICKING HUMAN TACTILE SENSORY ORGAN Even though a well-integrated theory of the reception
mechanism of MCNCs is lacking21,22,23, influx of Ca2+ ions through mechanoresponsive Ca2+ gating ion channels (Piezo-2) in Merkel cells increases membrane potential and release of
neurotransmitters, resulting in SA sensations with modulation of SW (Fig. 1a)23,24. By emulating the synapse-like connections of a sensory organ, biomimetic tactile sensors with information
preprocessing ability at the device level could be used as intrinsically intelligent devices in future intelligent systems. For an explanation of the analogy between MCNCs and AiS-TSO, see
Supplementary Table 1 and Supplementary Note 1. A schematic illustration of an AiS-TSO mimicking the structure and functions of an MCNC in the human body is provided in Fig. 1. To mimic the
stimuli reception of Piezo-2 channels in Merkel cells that convert mechanical energy to potential, we exploited a triboelectric-capacitive coupling effect on the receptive part (substrate)
of the device. Touch cause triboelectric charge pumping to occur from the finger to the receptive part, similar to the inflow of Ca2+ ions through the Piezo-2 channel. The tribo-capacitance
of the receptive part, which increases in response to pumped electrons, induces a polarization change of the ferroelectric layer, which is analogous to neurotransmitter release from the
Merkel cell. The change in orientation of permanent dipoles in the ferroelectric gate dielectric modulates the channel conductivity of an Fe-OFET, acting as SW control. The drain current of
the device is equivalent to the post-synaptic current (PSC, _I_PSC), which is controlled by the generated tribo-capacitive potential (receptor potential, _V_rec). SYNAPTIC PROPERTIES OF
FE-OFET We first fabricated and characterized an Fe-OFET device with BT NP(20 wt%)/P(VDF-TrFE) nanocomposite gate dielectric (thickness of 0.6 μm) and Ni gate electrode on polyimide (PI)
substrate using pentacene as organic semiconductor channel to investigate the synaptic properties of the AiS-TSO (Fig. 2a). Notably, the tribo-capacitive potential induced by touch in the
tactile reception experiment was replaced with biasing of the gate electrode (also represented as _V_rec) for full characterization of the synaptic characteristics. Details of the
fabrication process of the Fe-OFET are provided in the Methods section. Fundamental output characteristics, transfer characteristics and gate leakage current of the Fe-OFET are shown in
Supplementary Fig. 1. The schematic in Fig. 2b illustrates the principles of synaptic functions. When we applied a negative _V_rec to the gate electrode, dipoles in the ferroelectric layer
aligned in a downward direction, resulting in increased accumulation of hole carriers in the p-type channel and, in turn, an increase in drain current, i.e., PSC (excitatory PSC). In
contrast, when we applied positive _V_rec to the gate electrode, dipoles in the ferroelectric layer changed their orientation to the opposite direction, resulting in a decrease in PSC
(inhibitory PSC). When Vrec was removed, polarization in the same direction as with _V_rec was generated or not depending on the nature of _V_rec. The state change of polarization can be
considered analogous to the memory process while restoration of channel conductance can be considered analogous to the forgetting process. To characterize the synaptic properties of the
Fe-OFET, we defined the synaptic weight (SW) as the current change ratio of initial PSC (_I_PSC,i) to the relaxed current after 15 s (_I_sw), which we expressed as (_I_sw –
_I_PSC,i)/_I_PSC,i (Fig. 2c). Depending on the resulting varied retention time of polarization, we classified SW as short-term or long-term plasticity (STP/LTP). To obtain STP synaptic
properties, we applied a _V_rec of −10 V with a frequency of 0.1 Hz and pulse width of 0.5 s to the device; the peak PSC (IPSC) value was approximately −6 nA, and it decayed stably (∼−2 nA)
within ∼5 s (Fig. 2d). In contrast, LTP was observed at a higher frequency of 1.42 Hz with the same pulse width of 0.5 s (Fig. 2e). Synaptic functions of STP and LTP were effectively
realized in low (0.1–1.42 Hz, pulse width of 0.5 s) and high (1–8 Hz, pulse width of 0.1 s) frequency ranges at amplitude of −10 V for potentiation (Supplementary Fig. 2 and Supplementary
Note 2). We fabricated an OFET using polyvinylpyrrolidone (PVP) to demonstrate that the SW generation mechanism of Fe-FET is due to partial polarization. SW in the OFET with PVP was
negligible compared to that in the Fe-OFET with BT NP(20 wt%)/P(VDF-TrFE). Other mechanism such as charge trapping in organic semiconductor25,26 may not be not be enough to induce SW in the
OFET with PVP (Supplementary Fig. 3 and Supplementary Note 3). Therefore, generation of SW in the Fe-OFET is mainly attributed to ferroelectric partial polarization switching. Another
parameter of SW is the paired pulse ratio (PPR), which is defined as the ratio of two peak currents (A2/A1), as shown in Fig. 2c. PPR is representative of STP, which is the subsequent output
enhanced by previous stimuli27,28,29. To emulate the PPR of a biological synapse, we applied two successive _V_rec values with different pulse widths (Supplementary Fig. 4). The PPR value
decreased as the time interval between two successive pulses (Δ_t_rec) at both pulse widths increased. Release of neurotransmitters varies depending on membrane potential, which is related
to changes in SW. We demonstrated spike amplitude-dependent plasticity (SADP) by increasing the amplitude of _V_rec (−1, −3, −5, −7, −10, −15, and −20 V) with the same pulse width of 0.5 s
(Fig. 2f). The SW value of 9.95 at a _V_rec of −20 V was larger by a factor of 17 than that at a _V_rec of −7 V (SW = 0.56), while the SW at a _V_rec of −1 V of was negative (SW = −0.30).
This means that there was no change in polarization for _V_rec = −1 and −3 V (SW = −0.01) or −5 V (SW = −0.05), indicating STP properties. Furthermore, characteristics of spike
number-dependent plasticity (SNDP) were obtained by increasing the number of pulses while keeping the amplitude and pulse width of _V_rec constant at −10 V and 0.5 s, respectively. The value
of _I_PSC gradually increased as the spiking number increased from 1 to 100 (Fig. 2g). Further investigation of SW indicated that SW values increased as the frequency (spike rate-dependent
plasticity, SRDP), duration time (spike duration time-dependent plasticity, SDDP), and number of pulses increased (Supplementary Fig. 5). In addition, we demonstrated depression of SW under
the same conditions except positive _V_rec amplitude (Supplementary Fig. 5). Also, we measured the _I_PSC of Fe-OFET by applying _V_rec pulses with their amplitude and number consecutively
increased or decreased after full recovery to confirm repeatability in the synaptic characteristics of Fe-OFET. The _I_PSC values measured were almost similar when measured with increasing
or decreasing amplitude or number of _V_rec pulses (Supplementary Fig. 6 and Supplementary Note 4). Summing up the phenomena presented in Fig. 2, the state change of polarization controlling
the synaptic properties was dependent on the nature of the applied _V_rec. The larger, longer, and more repetitive was the applied _V_rec, the larger was the polarization generated.
Generation of SW in the AiS-TSO with ferroelectric nanocomposite can be explained by partial polarization switching at low electric field, similarly to previous investigations on
polarization switching dynamics of minor loops in ferroelectric materials30,31,32,33,34,35. We investigated the partial polarization in minor loops of BT NP(20 wt%)/P(VDF-TrFE) thin film
with measurement of P–E curves (Supplementary Fig. 7 and Supplementary Note 5). We usually use the pulse amplitude of ±10 V using minor loops of ferroelectric layer rather than the
saturation loop since we could get synaptic properties which can be used for sensory memory8,23,24 by controlling the partial polarization switching in ferroelectric gate dielectric layer
and so control the STP and LTP properties by varying the duration, number and frequency of pulses (Fig. 2). Of course, when we compare the memory retention, the retention time was much
smaller (∼68 min) with −10 V of _V_rec pulses applied than that with the pulse amplitude of −30 V was applied (∼1814 min) (Supplementary Fig. 8 and Supplementary Note 6). The results are
consistent with the concerns about using minor-loop of ferroelectric materials addressed in the previous reports33,36,37,38,39. However, high linearity of PSC was advantageous when we use
the −10 V than −30 V as _V_rec pulse amplitude as shown in Supplementary Fig. 8. Since we used partial polarization of ferroelectric gate insulating layer, we did not conduct poling process.
In case of generating SW by using the partial polarization, poling process on the device which makes the saturated polarization switching resulting in formation the internal field in gate
insulating layer was disadvantageous for generation of SW with both potentiation and depression. (Supplementary Fig. 9 and Supplementary Note 7). After _V_rec was turned off, the increased
polarization slowed the recovery of the PSC and generated SW. The stronger was the polarization, the slower was the decay of the PSC due to a larger SW. The decay (retention) process was
analyzed quantitatively using the exponential decay function shown in Eq. (1): $$I_{{\mathrm{PSC}}}\left( t \right) = I_{\mathrm{PSCi}} + \left( {I_{{\mathrm{PSC}}}\left( 0 \right) -
I_{{\mathrm{PSC,i}}}} \right){\mathrm{exp}}\left( { - t/\tau } \right)$$ (1) Here, _t_ is the time after applying _V_rec, _I_PSC(_t_) is IPSC at time _t_, and _I_PSC (0) the drain current at
_t_ = 0. _τ_ refers to the decay time constant that depends on the frequency, duration, and number of _V_rec pulses. As the frequency, number of pulses, and duration increased, _τ_ values
increased and were larger for the device with BT NP(20 wt%)/P(VDF-TrFE) than for that with P(VDF-TrFE) only (Supplementary Fig. 10). These results indicate that repetitive, frequent, and
longer stimuli can be memorized with longer decay time constants, similar to biological consolidation processes in which sensory memory transitions to long-term memory (LTM) due to SW40.
Mechanical flexibility is another required characteristic for soft and intelligent bio-mimic electronics. Therefore, evaluation of synaptic functions and endurance was carried out under
static and cyclic bending tests. Comparison of _I_PSC values after 100,000 bending cycles at 1.25% tensile strain showed that variation in the PPR value of the device after cyclic bending
was <0.1 (Fig. 2h). Relative variations in PPR, SW, ΔPPR/PPR0, and ΔSW/SW0, where PPR0 and SW0 indicate PPR and SW under no mechanical strain, respectively, were also negligibly small
under static tensile strain up to 1.88% (Fig. 2i). Additional results from flexibility tests indicated that variation in synaptic characteristics was also negligible under compressive strain
(Supplementary Fig. 11). In addition, we were able to tune the SW of the device by controlling the fraction of BT NPs in the nanocomposite gate dielectric. We found that the BT NP(20
wt%)/P(VDF-TrFE) nanocomposite has a smaller coercive field (48.83 MV/m) than that of pure P(VDF-TrFE) (88.2 MV/m), which implies easier polarization switching in the nanocomposite than in
P(VDF-TrFE). Nevertheless, the coercive field of our P(VDF-TrFE) layer is significantly higher than reported elsewhere41,42. Therefore, the Fe-OFET device with BT NP(20 wt%)/P(VDF-TrFE)
nanocomposite had a higher hysteresis than the device with no BT NP (Supplementary Fig. 1). Because the dielectric constant of BT NPs/P(VDF-TrFE) nanocomposites is higher than that of
P(VDF-TrFE), the PSC of the Fe-OFET with the ferroelectric nanocomposite was greatly enhanced due to the increased dielectric constant (Fig. 2j). In addition, the device with 20 wt% BT NP
showed a larger rise in PSC (∼3.7 nA) than that without BT NPs (∼0.8 nA), indicating an increase in polarization (_V_rec amplitude of −10 V and pulse width of 1 s). The SW data in Fig. 2k
show that the SW value at different frequencies depends on the fraction of BT NPs. When _V_rec at a frequency of 1 Hz (amplitude of −10 V and pulse width of 0.1 s) was applied, the SWs of
the devices with nanocomposite (20 wt% BT NP) and only P(VDF-TrFE) were 4.67 and −0.52, respectively. The SW of the nanocomposite device at a _V_rec of 8 Hz was 35.50, which was seven times
higher compared with that of the P(VDF-TrFE) device. Investigation of synaptic characteristics by comparing the devices with nanocomposite (20 wt% BT NP) or P(VDF-TrFE) gate dielectrics
showed that increasing duration, frequency, and number of stimuli increased the SW value (Supplementary Fig. 5). Furthermore, the degree of change in SW could be tuned by varying the
fraction of BT NPs in the nanocomposite ferroelectric layer. Comparison of τ for the devices with nanocomposite (20 wt% BT NP) or P(VDF-TrFE) gate dielectrics revealed that the nanocomposite
device had a longer polarization retention time (Supplementary Fig. 10). These results indicate that tunability of the synaptic properties and SW of the Fe-OFET used in our AiS-TSO confer
it with intelligent properties as well as controllability of its sensory functions. One of factors affecting SW is the device scaling which includes changes in the thickness of ferroelectric
layer and channel length or width43,44,45,46,47,48 in Fe-FET. Decrease in the channel length of our Fe-OFET enhanced IPSC (Supplementary Fig. 12). However, increase in the channel length
enhanced SW due to larger retention time which might be related to slower polarization switching44,46,49. SYNAPTIC FUNCTIONS OF AIS-TSO There is still active research about structural and
phenomenological observations on MCNCs11,23,24,50 including the relationship between the number and spatial density of MCNCs and SA perception51. Although the exact mechanism of SA firing in
MCNCs has not been discovered, it is obvious that there are complex interactions between mechanosensitive Piezo-2 channels, cell membrane potentials, and synergistic synapses of MCNCs that
allow Merkel cells to initiate Aβ afferent pulses to encode tactile information9,22,52. This suggests that the functions of reception and information preprocessing in our body are not
independent of each other. Therefore, we developed an AiS-TSO by imitating an MCNC’s united synaptic functions of reception and preprocessing of tactile information. As shown in Fig. 3a, the
AiS-TSO mimics both the structure and functionality of MCNCs. The photograph of AiS-TSO and TEM image of cross-sectional view of Fe-OFET are shown in Supplementary Fig. 13, and image of
experimental setting for touch measurement is shown in Supplementary Fig. 14. The gate electrode of the AiS-TSO is the receptive part to which touch were applied (Methods section). The IPSC
response of AiS-TSO to prolonged tactile stimulation was induced by tribo-capacitive potential by finger touch (Fig. 3b); the corresponding working principle of AiS-TSO is shown
schematically in Fig. 3c. In step I (I in Fig. 3b, c), mechanical contact of skin with the receptive part induces movement of negative charges from the skin to the receptive part because of
the difference in electron affinity between them. Indeed, the current increases or decreases depending on the triboelectric properties of the material in contact with the receptive part due
to different electron affinities (Supplementary Fig. 15 and Supplementary Note 8). Receptor potential of a Merkel cell accepting Ca2+ ions forms continuously from the beginning to the end of
the stimulation due to the high resistance of the membrane and is the key factor responsible for SA sensation23. Similar to this, in step II (II in Fig. 3b, c), the receptive part receives
the pumped triboelectric charge until equilibrium is reached, resulting in tribo-capacitive potential; thus, the _I_PSC increases with a decreasing slope throughout stimulation, akin to SA
sensation. When the tactile stimulus is removed in step III (III in Fig. 3b, c), the equilibrium is disrupted, and the negative charges on the triboelectric layer induce greater alignment of
dipoles in the same direction to compensate for the removed positive charge on the skin, causing the IPSC to increase for a moment. The IPSC then decreases slowly, exhausting the
tribo-capacitance stored in the receptive part. Even though the stimulus is removed, there is polarization because the ferroelectricity of the nanocomposite modulates the channel conductance
and, in turn, generation of SW (IV in Fig. 3b, c). The SA properties of the Fe-OFET were confirmed by applying a sustained Vrec with varying amplitude for ∼10 s (Supplementary Fig. 16).
Here, the SW of the AiS-TSO, which we ascribe to polarization switching, is similar to neurotransmitter release at synapse-like connections in MCNCs. The SA characteristics of the AiS-TSO
endow it with synapse-like connections, expanding the basic reception functions of the sensor. Also, to confirm that the main mechanism of AiS-TSO is triboelectric-capacitive coupling
effect, we investigated the response of AiS-TSO to varying temperature during touch and mechanical bending strain. The results indicated that the pyroelectric or piezoelectric effects were
negligible compared to triboelectric-capacitive coupling effect (Supplementary Fig. 17 and Supplementary Note 9). In addition, functions of AiS-TSO are clearly different from those of
conventional piezoelectrically-coupled tactile sensor such as piezoelectric oxide semiconductor FET (POSFET)53,54. More detailed explanation is given in supplementary information
(Supplementary Fig. 18 and Supplementary Note 10). To demonstrate the synapse-like characteristics of AiS-TSO, we touched with different forces (as measured by a hand force gauge),
durations, and frequencies to the device with a nanocomposite (20 wt% BT NP) layer (Fig. 3d–f, respectively). Both _I_PSC and SW during stimulation (different forces but same duration of 1
s) increased as tactile force increased, indicating a transition from STP (SW at ≈0.3 kPa force = 0.15) to LTP (SW at ≈3 kPa force = 1.86) (Fig. 3d). A larger force resulted in a larger
contact area between the triboelectric layer and skin so that tribo-capacitive potential increased (Supplementary Fig. 19)15,16,18,55,56,57,58. These results are consistent with the
electrical analysis results shown in Fig. 2. Furthermore, the effects of duration of stimulation were detected by monitoring both IPSC and SW. When touch with the same force (≈1 kPa) but
different durations (1 s/5 s/ 10 s) were applied, the SW for a stimulus duration of 10 s (SW = 2.4) was five times larger than that for a duration of 1 s (SW = 0.42) (Fig. 3e). The longer
the stimulus was applied, the greater was the triboelectric charge pumped from the skin to the receptive part, which resulted in greater alignment of ferroelectric dipoles and hence a larger
SW. In biological synapses, SRDP is related to the boosted signal from consecutive pulses with a narrow interval by residual neurotransmitters released as a result of the previous pulse59.
We expect that MCNCs have a similar characteristic as this would help distinguish significant and repetitive stimuli from noise. Therefore, we created an AiS-TSO filtering function by
setting a SW threshold and performing measurements (Fig. 3f). When we touched the device at a frequency of 0.5, 1, 3, or 5 Hz for a duration of about 5 s under manual forcing at ≈1 kPa, SW
increased. If the threshold filtering frequency is set using SW value, the AiS-TSO can be utilized as an intelligent sensor at the unit device level. We further investigated the
characteristics of AiS-TSO such as PSC change ratio (Δ_I_PSC/_I_PSC,i) by repetitive touch with different retention time and PPR with different time interval of touch (Supplementary Fig.
20). We observed that the shorter retention time made the Δ_I_PSC/_I_PSC,i larger. Also, PPR was increased when the time interval of two consecutive touches was decreased. By the way, since
the triboelectric effect is highly influenced by humidity60,61, we investigated the PSC response of AiS-Tso by finger touch in environments with different humidity levels. The results
indicated that the _I_PSC value was decreased with the humidity level increased (Supplementary Fig. 21). In addition, we were able to tune the filtering functions of AiS-TSO by varying the
fraction of BT NPs in the nanocomposite. BT NPs in the various fraction ferroelectric nanocomposites were well dispersed (Supplementary Fig. 22). When we applied 0.3 kPa (duration of 1 s),
the SW of the device with P(VDF-TrFE) only was −0.32. However, the SW of the nanocomposite (40 wt% BT NP) device was 2.22 (Fig. 3g). Therefore, if the threshold of noise filtering is set to
be a positive SW value, the signal at the applied force of 0.3 kPa is filtered out as noise for the device with P(VDF-TrFE) only. However, in the nanocomposite (40 wt% BT NP) device, both
signals from the device at forces of ≈0.3 and ≈1 kPa (SW = 3.19) were treated as meaningful. To investigate the dependency of the filtering function on fraction of BT NPs, we touched the
device with a pressure around 1 kPa for 1, 5, or 10 s. As shown in Fig. 3h, SW increased as the weight fraction of BT NPs increased from 0 to 20 to 40 wt% (SW values of −0.14, 0.42, and
0.55, respectively) for a stimulus duration of 1 s. When we set the criterion of STP to SW = 0, the device with P(VDF-TrFE) only showed STP for a duration of stimulation of 1 s. For all
three cases, SW values with 10 s stimulation were 1.6, 2.4, and 3.8 for 0 wt%, 20 wt%, and 40 wt% BT NPs, respectively, which were 5 times higher than those obtained for a 1-s stimulation.
When we set the criterion for LTP to SW = 3, only the AiS-TSO with BT NPs (40 wt%)/P(VDF-TrFE) showed LTP characteristics. Dependence of filtering function on the frequency of touches for
devices with different fractions of BT NPs was observed (Fig. 3i). When we set the criteria of noise filtering to a positive SW, no signals of the device with a BT NP(40 wt%)/P(VDF-TrFE)
layer were filtered (SW = 1.8, 1.9, and 3.3 for 1, 2, and 5 Hz, respectively). For the device with P(VDF-TrFE) only, however, signals from 1 Hz (SW = −0.34) were treated as noise. These data
indicate that a wide range of filtering parameters can be developed based on the tunability of the SW of AiS-TSO. Using different SW change depending on the concentration of BT NPs in the
nanocomposites, therefore, we could set the different criteria to be used for noise filtering or getting specific range of information similarly to biological mechanoreceptors which transfer
signals to brain depending on characteristics of the receptor cells or number and distribution of connections between afferent neurons12. In human sensory perception systems, synapses play
an important role in memory functions. As seen from the schematic in Fig. 4a, the learning and memory model proposed by Atkinson and Shiffrin classifies memory into three steps: sensory
memory (SM), short-term memory (STM), and LTM62. SM is a memory temporarily stored in the human brain about incoming sensory information that is initially processed and then transferred to
short-term storage. STM is related to the STP of the SW and is responsible for moment-to-moment awareness of environment changes. If the stimulation is repeated, then it becomes an LTM in
the cerebral cortex, a process referred to as consolidation62. Many researchers have investigated the functions of STM or LTM using synaptic devices based on brain synapses32,63,64,65,66,67,
but there is a lack of research into sensory perception based on sensory neural synapses. Here, we mimicked this memory process using synapse-like connections in our sensor, which is a very
promising first-step to realizing memory functions in bio-inspired artificial sensory systems, which would mitigate the requirement for further information processing. Based on the
structure of the sensory organs, we implemented a memory function in an AiS-TSO without any other storage device based on synaptic plasticity. Even without knowledge of the real-time signal
of IPSC during touching, the number of touches of similar force can be inferred by analyzing signals after touching (Fig. 4b). _I_PSC after a touch increased as the number of touches
increased from 1 to 30 (≈1 kPa and frequency of 1 Hz) (left, Fig. 4c). Here, SW values ranged from 0.6 to 4.2 when the number of touches increased from 1 to 30 (right, Fig. 4c). Therefore,
SNDP in AiS-TSO observed with finger touch (Fig. 4c and Supplementary Fig. 23) shows potential to be used as a sensing device embedded with memory function at unit device level without the
need for an additional memory device. To emulate the memory process of a sensory organ, tracking the order of touch is also important. We constructed a 2 × 2 array of AiS-TSO pixels (Fig.
4d) and touched three pixels in sequence with the same force for 5 s with an interval of 15 s between the different pixels. We then analyzed the degree of memory extracted from the IPSC
before (IPSC,0) and after touch (_I_PSC,mem), described as (_I_PSC,0 – _I_PSC,mem)/_I_PSC,0. Because the pixels were touched sequentially, not simultaneously, there were differences in the
time interval between _I_PSC,0 and _I_PSC,mem depending on the order of touch (∼77, ∼67, and ∼57 s for the 1st, 2nd, and 3rd pixels touched). The memory strength for the non-touched pixel
was smallest, and that of the last touched pixel was the largest because the decrease in IPSC was shortest for the last pixel. Based on an analysis of the strength of memory, we derived the
order of touch from the stored tactile information in SW. As shown in Fig. 4e, the strength of memory of each pixel from 1 to 4 was 4.7, 1.6, 1.3, and 11, respectively. Therefore, we
postulated that pixels were touched in the order of pixel 2 → 1 → 4, and that pixel 3 was not touched. The results shown in Fig. 4f indicate that the touch order of the pixels was in fact 4
→ 3 → 2, and that pixel 1 was not touched. This result corresponds to the strength of memory of pixels 1, 2, 3, and 4, which was 1, 5.3, 4.2, and 3.1, respectively. Also, we measured the
memory strength three times with the touch order of pixel 2 → 1 → 4, without touching of pixel 3, showing the same tendency for all three measurements (Supplementary Fig. 24). DISCUSSIONS
Our AiS-TSO successfully imitates the intelligent functions of a human touch sensory organ, namely the MCNC, due to a simple synapse-like connection at the unit sensor level. We utilized a
tribo-capacitive coupling effect in a ferroelectric transistor to endow the sensor with synaptic functions. We were able to control the synaptic characteristics of the AiS-TSO by tuning SW
through variation of the BT NP fraction in the ferroelectric nanocomposite gate dielectric layer. This study shows a simple demonstration of filtering function by fabricating Fe-OFETs with
the ferroelectric nanocomposite with the same concentration of BT NPs on a single substrate. AiS-TSO would be more practical if it is possible to fabricate an array of devices patterned with
nanocomposites of different of BT NP concentrations by using an additive printing process of improved nanocomposite solutions. Since AiS-TSO is flexible, it can be applied to soft
biomimetic devices in applications such as intelligent soft electronic skins. We demonstrated that our AiS-TSO device has adaptation, filtering, and memory functions and shows parallel
spatiotemporal reception and preprocessing of tactile information by SW control using the partial polarization of minor loops in ferroelectric layer. We can also consider saturation loop of
ferroelectric layer for future work to get synaptic properties with longer retention time. We successfully mimicked the synapse-like connection of receptor cells and afferent neuron
terminals in a sensory organ, thereby endowing the sensor itself with intrinsic intelligence without linking it to a neuronal processor. We believe that this flexible AiS-TSO inspired by the
MCNC represents a new paradigm in sensor technology for neuro-robotics, autonomous systems, intelligent electronic skins and better AI technologies that decreases the requirement for
information processing and facilitates greater device integration and energy efficiency. METHODS PREPARATION OF MATERIALS AND DEVICE FABRICATION Materials used in the ferroelectric
nanocomposites were P(VDF-TrFE) (65 mol% VDF) purchased from Piezotech S.A. and BT NPs purchased from Sigma Aldrich. The BT NPs were ball-milled for 30 min to enhance their dispersion in
solution. Then, the BT NPs were dissolved in a 3-aminopropyltriethoxy silane (APTES)/ethanol solution. The solution was adjusted to a pH of 4–5 using acetic acid and stirred for 12 h. The
solution was then centrifuged, and the BT NPs were washed with ethanol to remove residual APTES. The filtered BT NPs were cured at 110 °C for 10 min in a small amount of ethanol and then
mixed with _N_,_N_-dimethylformamide (DMF, purchased from Sigma Aldrich). After mixing, the solution was centrifuged again to obtain a solvent-particle mixture with BT NPs. BT NPs at a
specified fraction (wt%) with respect to P(VDF-TrFE) were dispersed in DMF with P(VDF-TrFE). To fabricate Fe-OFET, a 100-nm-thick Ni gate electrode was deposited on a clean PI substrate (75
μm-thick) by electron beam evaporation. The prepared ferroelectric nanocomposite solution was spin-coated as the gate dielectric layer onto the Ni gate electrode, followed by drying at 110
°C to remove the DMF solvent. Next, the gate dielectric layer was annealed at 200 °C to melt it completely. Annealing was continued in nitrogen ambient at 140 °C for β-phase crystallization
of P(VDF-TrFE), which contributes to polarization. We did not conduct poling process for gate insulator. Pentacene as a channel layer (70 nm) and Au (70 nm) as the S/D of the Fe-OFET were
deposited by thermal evaporation. The process of AiS-TSO fabrication was the same except that the Fe-OFET and the gate electrode were not formed and a thinner PI substrate (50 μm-thick) was
used to enhance the triboelectric effect. MEASUREMENTS Electrical characteristics and the PSC in response to applied electrical pulses and finger touch stimuli were measured using a
semiconductor parameter analyzer (Keysight, B1500). Touch stimuli force was measured using a hand force gauge (Algol instrument, HF-10), and the output voltage of triboelectrification with
finger and PI was measured with an oscilloscope (Tektronix, TDS 3014B). Dynamic and static flexibility tests of devices were carried out using a custom-built bending system. Dispersion of
loaded BT NPs in the ferroelectric nanocomposite and the thickness of nanocomposite were examined by field-emission scanning electron microscopy (FE-SEM, JEO JSM-6500F). Capacitance
measurement with MIM device was conducted using a semiconductor parameter analyzer (Agilent B1500). DATA AVAILABILITY The data that support the findings of this study are available from the
corresponding author upon request. REFERENCES * Cao, Y. et al. Mechanism for selective synaptic wiring of rod photoreceptors into the retinal circuitry and its role in vision. _Neuron_ 87,
1248–1260 (2015). Article CAS PubMed PubMed Central Google Scholar * Katz, L. C. & Shatz, C. J. Synaptic activity and the construction of cortical circuits. _Science_ 274, 1133–1138
(1995). Article ADS Google Scholar * Wicher, D. & Marion-poll, F. Editorial: function and regulation of chemoreceptors. _Fron. Cell_ 12, 10–12 (2018). Google Scholar * Water Van De,
T. R. Determinants of neuron-sensory receptor cell interaction during development of the inner ear. _Hear. Res._ 22, 265–277 (1986). Article Google Scholar * Terre, V., Ashmore, J. F.,
Lamb, T. D. & Menini, A. Transduction and adaptation in sensory receptor cells CT. _J. Neurosci._ 75, 7757–7768 (1995). Article Google Scholar * Wark, B., Lundstrom, B. N. &
Fairhall, A. Sensory adaptation. _Curr. Opin. Neurobiol._ 17, 423–429 (2007). Article CAS PubMed PubMed Central Google Scholar * Ricci, A. J., Kennedy, H. J., Crawford, A. C. &
Fettiplace, R. The transduction channel filter in auditory hair cells. _J. Neurosci._ 25, 7831–7839 (2005). Article CAS PubMed PubMed Central Google Scholar * Hao, J., Bonnet, C.,
Amsalem, M., Ruel, J. & Delmas, P. Transduction and encoding sensory information by skin mechanoreceptors. _Pflug. Arch._ 467, 109–119 (2015). Article CAS Google Scholar * Wu, J.,
Lewis, A. H. & Grandl, J. Touch, tension, and transduction—the function and regulation of piezo ion channels. _Trends Biochem. Sci._ 42, 57–71 (2017). Article PubMed CAS Google
Scholar * Adrian, E. D., F. R. S. & O. M. Sensory discrimination: with some recent evidence from the olfactory organ. _Br. Med. Bull._ 6, 330–332 (1950). Article CAS PubMed Google
Scholar * Council, R. et al. Merkel cell receptors: structure and transducer function. _Science_ 214, 183–186 (1981). Article Google Scholar * Hu, J., Zhao, Q., Jiang, R., Wang, R. &
Ding, X. Responses of cutaneous mechanoreceptors within fingerpad to stimulus information for tactile softness sensation of materials. _Cogn. Neurodyn_ 7, 441–447 (2013). Article PubMed
PubMed Central Google Scholar * Hu, J. et al. Analysis of fingertip / fabric friction-induced vibration signals toward vibrotactile rendering. _J. Text. Inst._ 10, 7967–7975 (2016). Google
Scholar * Chun, K. Y., Son, Y. J., Jeon, E. S., Lee, S. & Han, C. S. A Self-powered sensor mimicking slow- and fast-adapting cutaneous mechanoreceptors. _Adv. Mater._ 30, e1706299
(2018). Article PubMed CAS Google Scholar * Ha, M. J. et al. Skin-inspired hierarchical polymer architectures with gradient stiffness for spacer-free, ultrathin, and highly-sensitive
triboelectric sensors. _ACS Nano_ 12, 3964–3974 (2018). Article CAS PubMed Google Scholar * Lin, L. et al. Triboelectric active sensor array for self-powered static and dynamic pressure
detection and tactile imaging. _ACS Nano_ 7, 8266–8274 (2013). Article CAS PubMed Google Scholar * Wang, N. et al. Tactile sensor from self-chargeable piezoelectric supercapacitor. _Nano
Energy_ 56, 868–876 (2018). Article CAS Google Scholar * Meng, Y. et al. Mechanosensation-active matrix based on direct-contact tribotronic planar graphene transistor array. _ACS Nano_
12, 9381–9389 (2018). Article CAS PubMed Google Scholar * Kim, Y. et al. A bioinspired flexible organic artificial afferent nerve. _Science_ 360, 998–1003 (2018). Article ADS CAS
PubMed Google Scholar * Wan, C. et al. An artificial sensory neuron with tactile perceptual learning. _Adv. Mater._ 30, e180129 (2018). Google Scholar * Abraira, V. E. & Ginty, D. D.
The sensory neurons of touch. _Neuron_ 79, 618–639 (2013). Article CAS PubMed Google Scholar * Ikeda, R. et al. Merkel cells transduce and encode tactile stimuli to drive Ab-afferent
impulses. _Cell_ 157, 664–675 (2014). Article CAS PubMed PubMed Central Google Scholar * Woo, S., Lumpkin, E. A. & Patapoutian, A. Merkel cells and neurons keep in touch. _Trends
Cell Biol._ 25, 74–81 (2015). Article PubMed Google Scholar * Maksimovic, S., Baba, Y. & Lumpkin, E. A. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a
gentle-touch receptor. _Ann. N. Y. Acad. Sci._ 1279, 13–21 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Tello, B. M., Chiesa, M., Duffy, C. M. & Sirringhaus, H.
Charge trapping in intergrain regions of pentacene thin film transistors. _Adv. Funt. Mater._ 18, 3907–3913 (2008). Article CAS Google Scholar * Ha, R. & Batlogg, B. Gate bias stress
in pentacene field-effect-transistors: Charge trapping in the dielectric or semiconductor. _Appl. Phys. Lett._ 99, 083303 (2011). Article ADS CAS Google Scholar * Zucker, R. S. &
Regehr, W. G. Short-term synaptic plasticity. _Annu. Rev. Physiol._ 64, 355–405 (2002). Article CAS PubMed Google Scholar * Schall, J. D. Neural basis of deciding, choosing and acting.
_Nat. Rev. Neurosci._ 2, 33–42 (2001). Article CAS PubMed Google Scholar * Bardoni, R. et al. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the
spinal cord dorsal horn. _Neuron_ 81, 1312–1327 (2011). Article CAS Google Scholar * Tomas, J., Bellaiche, L. & Bibes, M. Learning through ferroelectric domain dynamics in
solid-state synapses. _Nat. Commun._ 8, 14736 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Hu, L., Dalgleish, S., Matsushita, M. M., Yoshikawa, H. & Awaga, K.
Storage of an electric field for photocurrent generation in ferroelectric-functionalized organic devices. _Nat. Commun._ 5, 3279 (2014). Article ADS PubMed CAS Google Scholar * Duiker,
H. M. et al. Fatigue and switching in ferroelectric memories: Theory and experiment fatigue and switching in ferroelectric memories. _J. Appl. Phys._ 68, 5783 (1990). Article ADS CAS
Google Scholar * Jerry, M., Dutta, S., Kazemi, A., Ni, K. & Zhang, J. A ferroelectric field effect transistor based synaptic weight cell. _J. Phys. D. Appl. Phys._ 51, 433001 (2018).
Article CAS Google Scholar * Kim, M. & Lee, J. Ferroelectric analog synaptic transistors. _Nanolett_ 19, 2044–2050 (2019). Article ADS CAS Google Scholar * Kyunys, B., Lurchuk,
V., Meny, C., Majjad, H. & Doudin, B. Sub-coercive and multi-level ferroelastic remnant states with resistive readout. _Appl. Phys. Lett._ 104, 232905 (2014). Article ADS Google
Scholar * Kim, E. J., Kim, K. A. & Yoon, S. M. Investigation of the ferroelectric switching behavior of P(VDF-TrFE)-PMMA blended films for synaptic device applications. _J. Phys. D.
Appl. Phys._ 49, 075105 (2016). Article ADS CAS Google Scholar * Zhao, D. et al. Retention of intermediate polarization states in ferroelectric materials enabling memories for multi-bit
data storage. _Appl. Phys. Lett._ 108, 232907 (2016). Article ADS CAS Google Scholar * Oh, S., Kim, T., Kwak, M., Song, J. & Woo, J. HfZrOx -Based ferroelectric synapse device with
32 levels of conductance states for neuromorphic applications. _IEEE Electron Device Lett._ 38, 732–735 (2017). Article ADS CAS Google Scholar * Yoon, S., Kim, E., Kim, Y. &
Ishiwara, H. Adaptive-learning functions of ferroelectric field-effect transistors for synaptic device applications. In _Proc. 2017 International Symposium on Nonlinear Theory and Its
Applications_ 314–317 (The NOLTA Society, 2017). * Atkinson, R. C. & Shiffrin, R. M. Storage and retrieval processes in long-term memory. _Psychol. Rev._ 76, 179–193 (1969). Article
Google Scholar * Furukawa, T. Structure and functional properties of ferroelectric polymers. _Adv. Colloid Interface Sci._ 71-72, 183–208 (1997). Article CAS Google Scholar * Stadlober,
B., Zirkl, M. & Irimia-Vladu, M. Route towards sustainable smart sensors: ferroelectric polyvinylidene fluoride-based materials and their integration in flexible electronics. _Chem. Soc.
Rev._ 48, 1787–1825 (2019). Article CAS PubMed Google Scholar * Yurchuk, E. et al. HfO2 based ferroelectric field-effect transistors with 260 nm channel length and long data retention.
In _Proc. 4th IEEE International Memory Workshop_ (IEEE, 2012). * Park, B. E. et al. Practical characteristics of organic ferroelectric-gate FETs: Ferroelectric-gate field effect transistors
with flexible substrates. In _Ferroelectric- Gate Field Effect Transistor Memories_ 227–262 (Springer, 2016). * Yurchuk, E. et al. Impact of scaling on the performance of HfO2 based
ferroelectric field effect transistors. _IEEE Trans. Electron Devices_ 61, 3699–3706 (2014). Article ADS CAS Google Scholar * Muller, J. et al. Nanosecond polarization switching and long
retention in a novel MFIS-FET based on ferroelectric HfO2. _IEEE Electron Device Lett._ 33, 185–187 (2012). Article ADS CAS Google Scholar * Xiao-Wen, J. et al. MoS2 field effect
transistors with Lead Zirconate Titanate ferroelectric gating. _IEEE Electron Device Lett._ 36, 784–786 (2015). Article ADS CAS Google Scholar * Unni, K. N. N., Bettignies, R., Seignon,
S. D. & Nunzi, J. M. A nonvolatile memory element based on an organic field-effect transistor. _Appl. Phys. Lett._ 85, 1823–1825 (2004). Article ADS CAS Google Scholar * Sugano, R.
et al. Switching time in ferroelectric organic field-effect transistors. _Adv. Sci._ 215, 1701059 (2018). Google Scholar * Maksimovic, S. et al. Epidermal Merkel cells are mechanosensory
cells that tune mammalian touch receptors. _Nature_ 509, 617–621 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Yao, M. & Wang, R. Neurodynamic analysis of Merkel
cell–neurite complex transduction mechanism during tactile sensing. _Cogn. Neurodyn_ 13, 293–302 (2019). Article PubMed Google Scholar * Zimmerman, A., Bai, L. & Ginty, D. D. The
gentle touch receptors of mammalian skin. _Science_ 346, 950–954 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Dahiya, R. S., Adami, A., Collini, C. & Lorenzelli,
L. POSFET tactile sensing arrays using CMOS technology. _Sens. Actuators A. Phys._ 202, 226–232 (2013). Article CAS Google Scholar * Adami, A., Dahiya, R. S., Collini, C., Cattin, D.
& Lorenzelli, L. POSFET touch sensor with CMOS integrated signal conditioning electronics. _Sens. Actuators A. Phys._ 188, 75–81 (2012). Article CAS Google Scholar * Zou, H. et al.
Quantifying the triboelectric series. _Nat. Commun._ 10, 1427 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Yang, Y. et al. Human skin based triboelectric
nanogenerators for harvesting biomechanical energy and as self-powered active tactile sensor system. _ACS Nano_ 7, 9213–9222 (2013). Article CAS PubMed Google Scholar * Yu, A., Zhu, Y.,
Wang, W. & Zhai, J. Progress in triboelectric materials: toward high performance and widespread applications. _Adv. Funct. Mater._ 29, 1900098 (2019). Article CAS Google Scholar * Hu,
W., Zhang, C. & Wang, Z. L. Recent progress in piezotronics and tribotronics. _Nanotechnology_ 30, 042001 (2019). Article ADS CAS PubMed Google Scholar * Dittman, J. S., Kreitzer,
A. C. & Regehr, W. G. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. _J. Neurosci._ 20, 1374–1385 (2000). Article CAS PubMed PubMed
Central Google Scholar * Nguyen, V., Zhu, R. & Yang, R. Environmental effects on nanogenerators. _Nano Energy_ 14, 49–61 (2014). Article CAS Google Scholar * Nguyen, V. & Yang,
R. Effect of humidity and pressure on the triboelectric nanogenerator. _Nano Energy_ 2, 604–608 (2013). Article CAS Google Scholar * Mcgaugh, J. L. Memory—a century of consolidation.
_Science_ 287, 248–251 (2000). Article ADS CAS PubMed Google Scholar * Wang, H. et al. A ferroelectric/electrochemical modulated organic synapse for ultraflexible, artificial
visual-perception system. _Adv. Mater._ 30, e1803961 (2018). Article PubMed CAS Google Scholar * Van De Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage
artificial synapse for neuromorphic computing. _Nat. Mater._ 16, 414–418 (2017). Article ADS PubMed CAS Google Scholar * Lai, Q. et al. Ionic/electronic hybrid materials integrated in a
synaptic transistor with signal processing and learning functions. _Adv. Mater._ 22, 2448–2453 (2010). Article CAS PubMed Google Scholar * Xu, W., Min, S.-Y., Hwang, H. & Lee, T.-W.
Organic core-sheath nanowire artificial synapses with femtojoule energy consumption. _Sci. Adv._ 2, e1501326 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Yang, K. et
al. Tunable flexible artificial synapses: a new path toward a wearable electronic system. _npj Flex. Electron_ 2, 20 (2018). Article Google Scholar Download references ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program (2020R1A2C3013480 & 2019R1A6A1A03033215) through the National Research Foundation (NRF). The work was also partially
supported by Samsung Electronics. We would like to thank J.-Y. Kim, Y.-I. Choi, and M.-J. Choi at SKKU and Dr. S. Siddiqui at BUITEMS for their help with material preparation and
characterization. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Advanced Materials Science & Engineering, Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon-si,
Gyunggi-do, 16419, Korea Yu Rim Lee, Tran Quang Trung, Byeong-Ung Hwang & Nae-Eung Lee * SKKU Advanced Institute of Nano Technology (SAINT), Sungkyunkwan University, 2066 Seobu-ro,
Jangan-gu, Suwon-si, Gyunggi-do, 16419, Korea Nae-Eung Lee * Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu,
Suwon-si, Gyunggi-do, 16419, Korea Nae-Eung Lee * Institute of Quantum Biophysics (IQB), Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon-si, Gyunggi-do, 16419, Korea Nae-Eung Lee *
Biomedical Institute for Convergence at SKKU (BICS), Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon-si, Gyunggi-do, 16419, Korea Nae-Eung Lee Authors * Yu Rim Lee View author
publications You can also search for this author inPubMed Google Scholar * Tran Quang Trung View author publications You can also search for this author inPubMed Google Scholar * Byeong-Ung
Hwang View author publications You can also search for this author inPubMed Google Scholar * Nae-Eung Lee View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Y.R.L. and N.-E.L. designed the experiments. Y.R.L. conducted experiments, fabricated devices, and analyzed data. Y.R.L. and T.Q.T. measured the electrical properties of
devices. Y.R.L and B.-U.H. conducted flexibility tests of the devices. All authors contributed to the manuscript by discussion of the results. Y.R.L. and N.-E.L. wrote this article.
CORRESPONDING AUTHOR Correspondence to Nae-Eung Lee. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION
_Nature Communications_ thanks Rubin Wang, and the other, anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Lee, Y.R., Trung, T.Q., Hwang, BU. _et al._ A flexible artificial intrinsic-synaptic tactile sensory organ. _Nat Commun_ 11, 2753 (2020). https://doi.org/10.1038/s41467-020-16606-w
Download citation * Received: 30 May 2019 * Accepted: 11 May 2020 * Published: 02 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16606-w SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative