Play all audios:
ABSTRACT Establishing multi-colour patterning technology for colloidal quantum dots is critical for realising high-resolution displays based on the material. Here, we report a solution-based
processing method to form patterns of quantum dots using a light-driven ligand crosslinker, ethane-1,2-diyl bis(4-azido-2,3,5,6-tetrafluorobenzoate). The crosslinker with two azide end
groups can interlock the ligands of neighbouring quantum dots upon exposure to UV, yielding chemically robust quantum dot films. Exploiting the light-driven crosslinking process, different
colour CdSe-based core-shell quantum dots can be photo-patterned; quantum dot patterns of red, green and blue primary colours with a sub-pixel size of 4 μm × 16 μm, corresponding to a
resolution of >1400 pixels per inch, are demonstrated. The process is non-destructive, such that photoluminescence and electroluminescence characteristics of quantum dot films are
preserved after crosslinking. We demonstrate that red crosslinked quantum dot light-emitting diodes exhibiting an external quantum efficiency as high as 14.6% can be obtained. SIMILAR
CONTENT BEING VIEWED BY OTHERS DIRECT PATTERNING OF COLLOIDAL QUANTUM DOTS WITH ADAPTABLE DUAL-LIGAND SURFACE Article 11 August 2022 LARGE-AREA PATTERNING OF FULL-COLOR QUANTUM DOT ARRAYS
BEYOND 1000 PIXELS PER INCH BY SELECTIVE ELECTROPHORETIC DEPOSITION Article Open access 29 July 2021 HIGHLY EFFICIENT PRINTED QUANTUM DOT LIGHT-EMITTING DIODES THROUGH ULTRAHIGH-DEFINITION
DOUBLE-LAYER TRANSFER PRINTING Article 02 August 2024 INTRODUCTION Colloidal quantum dots (QDs), nanocrystalline semiconductors with dimensions residing in the quantum confinement
regime1,2,3,4, exhibit ultrahigh colour purity and near-unity luminescence quantum yield5,6,7. These excellent characteristics of QDs have led the materials to be successfully incorporated
into commercial display devices, positioned to challenge organic light-emitting diode (OLED) technology. These commercial devices, as of now, harness the photoluminescence (PL)
characteristics of QDs; PL from QDs in these devices contributes to forming a high-quality white light source for the display, whereby the white light is split into primary colours through
liquid crystal layers and colour filters before being conceived by the human eye8,9,10. The technology is now driven to launch displays based on the electroluminescence (EL) of
QDs11,12,13,14,15,16,17,18,19,20,21,22, akin to what has been achieved from OLEDs. Among the issues to be resolved, the development of processing methods to precisely locate red (_R_), green
(_G_) and blue (_B_) QDs at a given position in the pixel over a large area is one of the critical challenges in realising EL displays based on QDs. The challenge arises from the fact that
QDs are processed from solutions, unlike organic luminophores used for OLEDs that are typically processed by thermal evaporation23,24,25. While the solution processability of QDs allows
low-cost production of films over a large area, it prevents conducting a secondary solution process on top of the underlying QD film. This indicates that conventional photoresist-based
patterning methods are hardly applicable, unless the surface of QDs is delicately managed26; the QD films would be soluble to the solvent used to apply the photoresist. Furthermore, forming
patterns of different colour QDs (e.g., _R_, _G_, _B_ patterns of QDs) side-by-side by conducting consecutive cycles of solution processing is challenging, because processing one of the QD
layers is likely to damage the underlying QD patterns. Han and colleagues27 employed an additional layer of positively charged polyelectrolyte underneath the film of QDs modified with
negatively charged ligands. High-resolution patterns of QDs could be successfully prepared, but the luminescence characteristics of QDs could not be preserved completely. Alternative
patterning methods for QD films have been developed extensively, including ink-jet printing28,29,30 and micro-contact printing31,32,33. These methods, however still require further
development for industrial-scale usage in terms of the achievable uniformity, resolution and throughput rate. The utilisation of light-driven chemical/physical transformation of QD films for
patterning is a promising strategy that can meet these practical requirements. Manna and colleagues34 demonstrated that aliphatic ligands of QDs can be activated under X-ray exposure to
form chemically crosslinked QD films. A similar approach was done by Liao and colleagues35 using an Ar plasma as the irradiation source. Despite the success of patterning, the use of a
high-energy X-ray or plasma source is likely to cause loss of PL, which prevents the use of this process for luminescent applications. Talapin and colleagues36,37 designed inorganic ligand
molecules anchored on the surface of QDs, which can be transformed upon exposure to various wavelengths of ultraviolet (UV)–Visible (Vis) light (254–450 nm) and even to _e_-beam. As the
solubility of the QD film alters as the surface properties of ligand molecules change under irradiation, QD film could be patterned by selectively removing either the irradiated region or
the un-exposed region with an appropriate developer solvent. Consequently, they demonstrated micrometre-sized QD patterns and multi-layered patterns of _RGB_ QDs by repeating the patterning
process. However, the luminescence properties of the resulting QD patterns have not been investigated comprehensively, which are critical to their optical or optoelectronic applications.
Here, we report a simple yet effective method to form high-resolution patterns of QDs that preserves the inherent luminescent properties of the material using a light-driven ligand
crosslinker (LiXer). UV exposure on a blended film prepared from QD-LiXer mixed solutions galvanises the chemical reaction between azides and the alkyl chain of QD surface ligands to
construct a chemically robust QD network. Because of the excellent crosslinking efficiency of fluorinated phenyl azides we used38,39,40, QD patterns are readily achieved with a small amount
of LiXer (less than 5 wt%) using a handheld UV-lamp (254 nm, 0.4 mW cm−2) over a short period of time (5 s). As the resulting crosslinked QD films are structurally robust against subsequent
solution processes, multiple patterns of QDs can be formed through consecutive cycles of solution-based film deposition and photo-patterning processes. Based on this strategy, we
successfully fabricate QD line patterns with a minimum feature size of 3 μm and _RGB_ QD patterns with a sub-pixel size of 4 μm × 16 μm that corresponds to a resolution of >1400 pixels
per inch (p.p.i.). Owing to the little contents of LiXer and benign processing conditions, degradation in the PL characteristics of QDs during the patterning process and the associated EL
characteristics of the QD-LEDs could be avoided. Consequently, QD-LEDs yielding an external quantum efficiency (E.Q.E.) of 14.6% could be obtained from the crosslinked QD layer, which is a
comparable value achievable from pristine QD layer. The simple strategy presented here will make a significant impact on the production of high-resolution, large area, full-colour QD-LEDs,
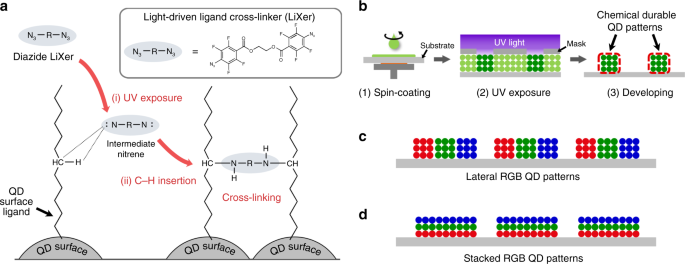
which are intensively explored across the scientific community to industry. RESULTS DESCRIPTION OF THE PHOTO-PATTERNING METHOD BASED ON LIXER Figure 1 describes the core of the
high-resolution photo-patterning method for QDs. The method utilises ethane-1,2-diyl bis(4-azido-2,3,5,6-tetrafluorobenzoate) as the LiXer that contains two fluorinated perfluorophenyl azide
groups at both ends of the molecule41,42,43,44. The chemical structure of ethane-1,2-diyl bis(4-azido-2,3,5,6-tetrafluorobenzoate) is shown in Fig. 1a. Fluorinated aryl azide is a
well-known photo-active moiety forming reactive nitrene intermediate upon exposure to UV (254 nm), which can easily undergo C–H insertion reaction in the presence of alkyl chains
nearby45,46,47. In our scheme, the crosslinker with two fluorinated phenyl azide terminals is intended to undergo C–H insertion reaction into the long aliphatic chains of the ligands (i.e.,
oleic acids or alkyl thiols) that passivate the surface of QDs. Therefore, it allows crosslinking the ligands of neighbouring QDs under exposure to UV. Unlike previous methods36,37, the new
method can directly utilise high-quality QDs typically terminated with long alkyl chains without undergoing additional ligand modification, which often degrades the luminescence quantum
yield of the materials. The patterns of QDs can be formed by (i) simple spin-coating of a solution mixture of QDs and LiXer onto a substrate, (ii) irradiation of UV and (iii) simple
developing step using conventional organic solvents (Fig. 1b), all of which are well-established procedures used for photolithography in the semiconductor industry. Moreover, owing to the
crosslinked nature of the resulting QD films, they should be chemically robust against subsequent solution processing, even using the very same solvent that was used to cast the given QD
films. This allows for a large degree of freedom in realising QD patterns of multiple colours. Since the processing of different coloured QDs can be conducted repeatedly as many times as
needed, lateral patterns of _RGB_ QDs (Fig. 1c) as well as the vertical or tandem stacking of different QD layers (Fig. 1d) should also be possible by repeating the procedures. Figure 2a
shows a series of Fourier-transform infrared spectroscopy (FT-IR) spectra for QD films containing the LiXer that was prepared by spin-casting a mixture of 13-nm sized CdSe/CdZnS QDs and
LiXer (5 wt%) in toluene. Transmission electron microscope (TEM) images, X-ray diffractograms and absorbance/PL spectra of the QDs used in this work are shown in Supplementary Figs. 1, 2 and
3, respectively. The black spectrum is from the film obtained before it was exposed to UV. The peaks at 2130 cm−1 and 1250 cm−1 reflect the presence of the N3-moieties in the mixture film.
The red spectrum is obtained from the same film after UV exposure to a handheld UV source (254 nm, 0.4 mW cm−2). Clearly, the peaks associated with the N3-moieties were reduced after the
exposure to UV, suggesting that chemical reaction described in Fig. 1a has proceeded. Although the formation of the reactive nitrene intermediate and the final secondary amine moiety
resulting from the C–H insertion reaction could not be confirmed directly from the FT-IR spectrum43, we conjecture that the N3-moieties of LiXer underwent the C–H insertion reaction with the
long alkyl ligands of neighbouring QDs, which formed the chemically crosslinked QD assembly. Quantitative description on the photon-to-crosslink conversion process of LiXer is provided in
Supplementary Information (Supplementary Fig. 4 and Supplementary Discussion). The crosslinking did not reduce the interparticle distance between the QDs. As shown in Fig. 2b, the GI-SAXS
pattern of a 13-nm sized CdSe/CdZnS QD added with LiXer (5 wt%) but not exposed to a UV source showed a peak at _q_xy = 0.04386 Å−1, corresponding to the mean centre-to-centre distance (_d_)
for neighbouring QDs of 14.3 nm. The same film that underwent the crosslinking reaction (red) also showed a peak with a similar _q_xy value (0.04394 Å−1), indicating that no discernible
change in _d_ has occurred upon the photo-crosslinking process. Crosslinking the ligands awards the ability to withstand a structural failure against consecutive solution processing to QD
films. Figure 2c shows the photographs of the crosslinked versus pristine QD films when dipped into the toluene. Note that toluene was the mother solvent that was used to cast the QD film.
The submerged part of the pristine QD film dissolved immediately upon dipping to toluene. By contrast, noticeable change in the crosslinked film was not found even after dipping for a long
period (Fig. 2d, e). This supports the idea to devise multi-coloured QD patterns either placed side-by-side or stacked vertically simply by repeating the photo-crosslinking reaction of QD
films with LiXer and rinsing steps. In fact, only 2 wt% of LiXer in 13-nm sized CdSe/CdZnS QD solution was sufficient to ensure the structural robustness of the QD film. LATERAL AND TANDEM
PATTERING OF QDS By exploiting the structural robustness of the crosslinked QD films, we could fabricate QD patterns by selective UV exposure through a patterned photomask. Figure 3a, b show
sets of optical and fluorescence (inset) images of dot and line patterns based on red QDs, respectively. The patterns were achieved by (i) spin-coating the mixture solution of QD and LiXer
in toluene onto a Si/SiO2 wafer, (ii) exposing the film to UV irradiation (254 nm, 0.4 mW cm−2) through a photomask and (iii) removing the uncrosslinked regime of the film by rinsing with
toluene. Dot patterns with a diameter of 2 μm and a spacing of 3 μm as well as line patterns with a width of 3 μm and a spacing of 4 μm could be successfully attained (atomic force
microscopy (AFM) image and height profile in Fig. 3a, b). The line edge roughness (LER) of these line patterns was 0.14 μm (Supplementary Fig. 5a). Dot and line patterns could also be formed
based on green-emitting CdSe/CdZnSeS QDs (Supplementary Fig. 6). LER of these green-emitting QD line patterns was 0.15 μm (Supplementary Fig. 5b). Repeated cycles of QD film deposition,
photo-patterning and rinsing processes allowed to achieve well-defined high-resolution patterns of different colour QDs. In fact, one should remember that the development of scalable
processing methods to precisely locate _RGB_ QDs in designated positions within a given pixel is one of the critical challenges in realising EL displays based on QDs. Figure 3c, d displays
fluorescence images of the resulting _RGB_ QD patterns after repeating photo-patterning steps. The size of a single pattern in the image is 4 µm by 16 µm. The three _RGB_ patterns with a
spacing of 2 µm constitute a single 18 µm by 18 µm _RGB_ square pixel, which corresponds to >1400 p.p.i. in terms of the display pixel resolution. This value is higher than the value that
is currently used in commercial OLED displays. In addition to the patterns placed side-by-side, exploiting the structural robustness of the crosslinked QD film allowed the vertical stacking
of different colour patterns of QDs. Figure 3e shows a fluorescence image of “the three primary colours of light” expressed through _RGB_ QD patterns, formed by repeating the
photo-crosslinking process three times. The resulting pattern nicely demonstrates that the photo-crosslinked QD films can be stacked vertically, as well as horizontally. Furthermore,
μm-thick layers of QDs can be produced by repeating multiple rounds of film-casting and photo-crosslinking steps, which can be exploited for colour conversion layer applications of QDs
(Supplementary Fig. 7). PHOTOPHYSICAL PROPERTIES OF CROSSLINKED QDS WITH LIXER The adoption of reactive radicals in patterning QD films is often regarded as a double-edged sword. The low
activation energy to generate radicals with high reactivity enables effective chemical crosslinking of QD solids at a mild reaction condition, but simultaneously the reactive radicals could
attack the QD surface and create surface trap states, leading to the reduction of PL QY of the crosslinked films48. Given that the reaction of azide radicals with the surface of QDs is
primarily responsible for the drawback, optimising the content of LiXer in QD films is acute in achieving well-defined QD patterns with high luminance efficiency. Figure 4a shows PL QYs of
13-nm sized red-emitting CdSe/CdZnS QD films as a function of added LiXer contents. QD films prepared with LiXer contents below 2 wt% are readily devastated during the rinsing processes
(Fig. 4b). LiXer contents >2 wt% are sufficient to form a robust network of QDs, but high LiXer contents accompany PL QY loss of QD films during the photo-crosslinking (Fig. 4a). To avoid
unwanted outcome by the presence of excess azide radicals, we cut the content of LiXer in QD solutions down to the limit that ensures the structural robustness of QD films against rinsing
steps. The optimum LiXer content varies depending on the QD dimension; greater LiXer contents for smaller QDs and smaller LiXer contents for larger QDs (Fig. 4c and corresponding PL decay
curves in Supplementary Fig. 8). QD films with the optimum content of LiXer retain their PL spectra (peak emission wavelength and the spectral linewidth) and PL QY throughout the
photo-crosslinking and the rinsing steps (Fig. 4d, e), representing that the present approach is indeed non-destructive; the photophysical properties of the QD materials could be
well-preserved. Resulting QD films emit colour-saturated primary colours solely originating from the band-edge transition in QDs. We note that the colour space achieved with the resulting
_RGB_ spectra of crosslinked QD films far surpasses the standards of the up-to-date commercial displays (sRGB or DCI-P3) (Fig. 4f). ELECTROLUMINESCENCE CHARACTERISTICS OF CROSSLINKED QDS
WITH LIXER As an ultimate achievement, we exemplify QD-LEDs with the photo-crosslinked QD films. The devices were constructed in an inverted structure employing hybrid charge transport
layers (Fig. 5a)16. For effective electron injection from the indium tin oxide (ITO) cathode to the QD layer, a transparent zinc oxide nanoparticle (ZnO NP) layer was adopted as an electron
transport layer (ETL). The 4,4-bis(N-carbazolyl)-1,1-biphenyl (CBP) layer was used as the hole transport layer (HTL) for holes injected from the MoO3/Al anode. A 30 nm-thick crosslinked QD
emissive layer was prepared from red-emitting CdSe/CdZnSe/ZnSeS QD (diameter = 20 nm) dispersion containing 1 wt% of LiXer. Figure 5b shows a cross-sectional TEM image of the QD-LED and Fig.
5c depicts the energy diagram of the device at a static condition (_V_applied = 0 V). We note that the deposition of the QD emission layer, UV crosslinking and rinsing, were all carried out
in an inert atmosphere. Figure 5d–f compare the performances of QD-LEDs incorporating a pristine QD layer (black) versus a crosslinked QD layer (red). Apparently, no significant difference
was observed in the electrical and optoelectronic characteristics of devices. Specifically, the turn-on voltage (_V_T, defined as the voltage yielding at 1 nit) and the peak E.Q.E. of both
red-emitting devices were identical as 2.4 V and 14.6%, respectively. (Fig. 5d, e). The peak position of EL spectra (_λ_EL, max) and full-width-at-half-maximum (FWHM) of the EL spectra for
both the pristine and crosslinked QD-LEDs also matched well. The performance of green- and blue-emitting QD-LEDs, as well as their EL spectra could also be preserved upon exploiting the
crosslinked emissive layer (Supplementary Fig. 9). In addition, both red-emitting devices displayed similar device operation stability under the same applied current density (30 mA cm−2)
(Fig. 5f). These results coherently attest that the emission and electrical characteristics of the QD films are well-preserved even after they are photo-crosslinked, and hence imply that the
present approach is indeed applicable to nearly all optical and optoelectronic applications requiring patterned QD arrays. Finally, _RG_ pixelated QD-LED were successfully fabricated
through consecutive the photo-crosslinking process. (inset in Fig. 5f) The dimension of a single rectangular pattern in the image is 10 μm × 38 μm. This result demonstrates the possibility
of realising QD EL displays using LiXer. DISCUSSION A straightforward and effective photo-patterning method of colloidal QDs is reported. QD films with LiXer, which is designed to crosslink
the alkyl ligands of neighbouring QDs in films upon exposure to UV, are awarded to retain their structure against exposure to subsequent solution processes. Well-defined QD patterns with
feature sizes of a few micrometres are, thus, easily attained by conducting the process cycle of QD/LiXer film deposition, UV exposure and rinsing. Ultimately, tandem processing of the
present method permits us to achieve laterally resolved _RGB_ QD patterns (>1400 p.p.i.), as well as vertically stacked _RGB_ layers at a high resolution. Unlike previous photo-patterning
methods, suppression in the PL characteristics of QDs during the patterning process and the associated EL characteristics of the QD-LEDs can be avoided. As only mixing between QDs and LiXer
is required, the method should be valid to nearly all QDs that entail alkyl ligands without any delicate secondary steps to prepare a precursor solution for patterning. Thus, the highest
quality QDs, holding high luminescence efficiency prepared from state-of-the-art synthetic methods, should be easily applicable to this method. Overall, the approach here is expected to
catalyse the practicable use of QDs in production of high-resolution, large-area down-conversion or EL displays. METHODS MATERIALS FOR SYNTHESIS Zinc acetate (Zn(ac)2, 99.99%), sulphur (S,
99.99%), selenium (Se, 99.99%), oleic acid (OA, 99%) and 1-octadecene (ODE, 99%) were purchased from Uniam. _n_-Trioctylphosphine (TOP, technical grade, 90%), 1-dodecanethiol (DDT, 98%),
ethylene glycol and dichloromethane (anhydrous, >99.8%) were purchased from Sigma Aldrich. Myristic acid (MA, 90%), cadmium oxide (CdO, 99.9%) were purchased from Alfa Aesar.
4-Azido-2,3,5,6-tetrafluorobenzoic acid (>98%) and triethyl amine (TEA, 99%) were purchased from TCI. All chemicals are used without further purification. Thionyl chloride and the rest of
other solvents were purchased from Daejung and used as received. MATERIALS FOR DEVICE FABRICATION 4,4-Bis(N-carbazolyl)-1,1-biphenyl (CBP, 99.9%) were purchased from OSM. Molybdenum oxide
(MoO3, 99.95%) and aluminium (Al, 99.999%) metal source were purchased from Taewon Scientific Co. (TASCO). SYNTHESIS OF LIXER The synthesis of LiXer (ethane-1,2-diyl
bis(4-azido-2,3,5,6-tetrafluorobenzoate)) was done by modifying the process described previously by Keana and colleagues41. 4-Azido-2,3,5,6-tetrafluorobenzoic acid (931.6 mg, 3.9626 mmol)
and thionyl chloride (1 M in dichloromethane) (23.8 mL, 23.77 mmol) were dissolved in anhydrous dichloromethane (30.6 mL), and this solution was heated for 19 h at 70 °C. The reaction
mixture was cooled down to room temperature, and then the organic solvents were removed by distillation at a reduced pressure. The resulting acyl chloride compound was dissolved in anhydrous
dichloromethane (12 mL) and transferred dropwise to a mixture of ethylene glycol (102.48 mg, 1.6511 mmol) and TEA (400.975 mg, 0.8507 mmol) in anhydrous dichloromethane (18 mL). This
reaction mixture was stirred for 12 h at room temperature and quenched by the addition of 1-M HCl(aq) (25 mL). The resulting solution was extracted with dichloromethane (16 mL × 3). The
dichloromethane phases were washed with brine (60 mL) and dried over anhydrous MgSO4. After filtration, the organic solvent was removed from the filtrate using a rotary evaporator at a
reduced pressure. The obtained crude product was purified by silica gel column chromatography, using an ethyl acetate/_n_-hexane (1/5 to 1/3) eluent. Pure white solid of LiXer was obtained
(439 mg, 54%). 1H-NMR (400 MHz, CDCl3): _δ_ = 4.68 (s, 4H); 19F-NMR (376 MHz, CDCl3): _δ_ = −138.17 to −138.26 (m), −150.71 to −150.81 (m); 13C-NMR (100 MHz, CDCl3): _δ_ = 159.11‒159.01,
146.90‒146.67, 144.33‒144.10, 141.82‒141.58, 139.32‒139.09, 123.96‒123-72, 107.09‒106.79, 63.39; gas chromatography (GC)/mass spectroscopy (MS) calculated for C16H4F8N6O4 M+: 496.0166,
found: _m_/_z_ 496.1. Nuclear magnetic resonance (NMR) and GC/MS spectra of LiXer are given in Supplementary Information. (Supplementary Figs. 10–14) SYNTHESIS OF RED-EMITTING QDS Stock
solutions of 0.5-M cadmium oleate (Cd(OA)2) diluted in ODE, 0.5-M zinc oleate (Zn(OA)2) in ODE, 2-M TOPSe and 2-M TOPS were prepared for use in the synthesis of QDs. CdSe/CdZnS QDs were
synthesised referring to the method used by Lim et al_._49 with minor modification. For a typical synthesis of CdSe/CdZnS QDs, a mixture of 1 mmol of CdO, 3 mmol of MA and 15 mL of ODE were
degassed at 110 °C for 2 h, followed by heating to 270 °C to form a clear solution. Meanwhile, 0.25 mL of 2-M TOPSe solution was rapidly injected into the reaction flask and reacted for 3
min at 300 °C to the formation of CdSe cores. To grow the CdZnS shell, 4 mL of 0.5-M Zn(OA)2 solution was added to the flask before 1.5 mmol of DDT was added dropwise. After 30 min of
reaction at the elevated temperature, Cd(OA)2, Zn(OA)2 and TOPS (total 8 mmol, 17 mmol and 23 mmol, respectively) were injected repeatedly to grow CdZnS shell in a desired thickness.
Synthesised QDs was purified repeatedly via typical precipitation/redispersion method over ten times with various anti-solvents (e.g., ethanol and acetone) before use. Weakly bound TOP on
the surface QDs is likely to be removed during this step. 20-nm sized CdSe/CdZnSe/ZnSeS QDs with composition gradient in the two shell layers were synthesised referring to the method used by
Lim et al.18 CdZnSe shell was grown on CdSe cores by continuous injection of Cd(OA)2, Zn(OA)2 and TOPSe. CdSe cores were prepared via the method analogous to the recipe for the core of
CdSe/CdZnS QDs. For growth of CdZnSe shell, 56 mL of 0.5-M Zn(OA)2 solution was added to the flask followed by the continuous injection of a mixture of 12 mL of 0.5-M Cd(OA)2 solution, 12 mL
of 2-M TOPSe solution and 24 mL of ODE with injection rate of 24 mL h−1. Additional ZnSeS shell was grown on the exterior of CdSe/CdZnSe QDs by continuous injection of 80 mmol of Zn(OA)2,
32 mmol of TOPSe and 34 mmol of TOPS. Synthesised CdSe/CdZnSe/ZnSeS QDs were purified repeatedly via typical precipitation/redispersion method. Synthetic schemes for green and blue coloured
QDs are detailed in Supplementary Methods. DEVICE FABRICATION Photo-patterned QD-LEDs were fabricated in an inverted structure. Typical processes are described as follows. Glass substrates
pre-patterned with indium tin oxide (ITO) electrodes were first cleaned with isopropyl alcohol, acetone and distilled water in an ultrasonic bath for 15 min each. Then, 20 mg mL−1 of ZnO NPs
dispersed in 1-butanol was spin-coated onto the substrate at 2000 rpm for 40 sec, and the films were annealed at 100 °C for 30 min under a nitrogen atmosphere. Dispersions of QDs in toluene
added with different contents of LiXer were spin-coated at 4000 rpm for 30 sec. The resulting films were irradiated with a handheld UV-light source (254 nm, 0.4 mW cm−2) for 5 sec to derive
the crosslinking reaction between QDs and LiXer. After the irradiation process, the films were developed by rinsing the films with toluene. This process effectively removed the QDs in the
areas not exposed to UV (Supplementary Fig. 15). CBP (60 nm), MoO3 (10 nm) and Al (130 nm) were consecutively deposited using a thermal evaporator. CHARACTERISATION 1H-NMR, 13C-NMR and
19F-NMR were measured by a Bruker Avance III HD at 400, 100 and 376 MHz, respectively, with deuterated chloroform (CDCl3) as solvent, which was purchased from Cambridge Isotope Laboratories.
GC/MS was measured by a Bruker 450-GC and 320-MS at the UNIST Central Research Facilities Centre (UCRF), Ulsan, Korea. UV–Vis absorption and fluorescence spectra of QD films were measured
using a V-770 UV–Vis–NIR spectrophotometer (Jasco) and FS-2 fluorescence spectrometer (Scinco), respectively. The measurements of time-resolved photoluminescence (TRPL) were conducted with
time-correlated single photon counting (TCSPC) system from Horiba-Jovin Yvon with resolution of about 100 ps. The samples were excited at 3.06 eV at a repetition rate of 500 kHz. FT-IR
spectra were collected in a reflectance mode under ambient condition using an iS10 FT-IR spectrometer (Thermo Fisher Scientific). Samples for FT-IR analysis were prepared on bare Si wafer
substrates. AFM images were taken with a Park NX10 AFM system (Park Systems) under ambient conditions through a non-contact mode. The thickness of the crosslinked QD films were measured
using a Dektak XT-E stylus profiler. X-ray diffraction (XRD) measurements were performed using an Ultima IV X-ray diffractometer (Rigaku) (_λ_CuK_α_1 = 1.5406 Å, 40 kV, 30 mA). Transmission
electron microscopy (TEM) images were collected using a JEM-3010 (JEOL) equipped with a Gatan digital camera (MSC-794) (point resolution of 0.17 nm) at the National Centre for
Inter-University Research Facilities (NCIRF), Seoul National University. Scanning electron microscopy (SEM) images were obtained using a GeminiSEM 300 (Zeiss). Electrical characteristics of
the photo-crosslinked QD films were analysed using a Keithley 236 source measure unit and a Keithley 2000 multimeter connected to calibrated Si photodiodes. Electroluminescence (EL) spectra
were measured with a Konica-Minolta CS-2000 spectroradiometer. High-resolution single and multi-colour patterns of QDs were fabricated by using a Karl Süss MA-6 Mask Aligner. Grazing
incidence small-angle X-ray scattering (GI-SAXS) measurements were performed at the 9 A U-SAXS beamline of Pohang Light Source-II (PLS-II) in Republic of Korea. The X-ray coming from the
in-vacuum undulator (IVU) are monochromated using Si (111) double crystals and focused at the detector position using K-B type mirror. Two-dimensional (2D) scattering patterns were recorded
with a 2D CCD (Rayonix MX170-HS). The wavelength of X-ray and sample-to-detector distance were set to be 1.12 Å and 2.5 m, respectively. DATA AVAILABILITY All data generated or analysed
during this study are included in this article (and its Supplementary Information files). REFERENCES * Ekimov, A. I., Efros, A. L. & Onushchenko, A. A. Quantum size effect in
semiconductor microcrystals. _Solid State Commun._ 56, 921–924 (1985). Article ADS CAS Google Scholar * Brus, L. Electronic wave functions in semiconductor clusters: experiment and
theory. _J. Phys. Chem._ 90, 2555–2560 (1986). Article CAS Google Scholar * Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. _Science_ 271, 933–937 (1996).
Article ADS CAS Google Scholar * Norris, D. J. & Bawendi, M. G. Measurement and assignment of the size-dependent optical spectrum in CdSe quantum dots. _Phys. Rev. B_ 53, 16338–16346
(1996). Article ADS CAS Google Scholar * Hines, M. A. & Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. _J. Phys. Chem._ 100,
468–471 (1996). Article CAS Google Scholar * Jeong, B. G. et al. Colloidal spherical quantum wells with near-unity photoluminescence quantum yield and suppressed blinking. _ACS Nano_ 10,
9297–9305 (2016). Article CAS PubMed Google Scholar * Zhang, F. et al. Super color purity green quantum dot light-emitting diodes fabricated by using CdSe/CdS nanoplatelets. _Nanoscale_
8, 12182–12188 (2016). Article ADS CAS PubMed Google Scholar * Jang, E. et al. White-light-emitting diodes with quantum dot color converters for display backlights. _Adv. Mater._ 22,
3076–3080 (2010). Article CAS PubMed Google Scholar * Sun, J.-Y. et al. Facile two-step synthesis of all-inorganic perovskite CsPbX3 (X = Cl, Br, and I) zeolite-Y composite phosphors for
potential backlight display application. _Adv. Funct. Mater._ 27, 1704371 (2017). Article CAS Google Scholar * Lai, C.-F., Tien, Y.-C., Tong, H.-C., Zhong, C.-Z. & Lee, Y.-C.
High-performance quantum dot light-emitting diodes using chip-scale package structures with high reliability and wide color gamut for backlight displays. _RSC Adv._ 8, 35966–35972 (2018).
Article CAS Google Scholar * Colvin, V. L., Schlamp, M. C. & Alivisatos, A. P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. _Nature_
370, 354–357 (1994). Article ADS CAS Google Scholar * Coe, S., Woo, W.-K., Bawendi, M. G. & Bulović, V. Electroluminescence from single monolayers of nanocrystals in molecular
organic devices. _Nature_ 420, 800–803 (2002). Article ADS CAS PubMed Google Scholar * Mueller, A. H. et al. Multicolor light-emitting diodes based on semiconductor nanocrystals
encapsulated in GaN charge injection layers. _Nano Lett._ 5, 1039–1044 (2005). Article ADS CAS PubMed Google Scholar * Sun, Q. et al. Bright, multicoloured light-emitting diodes based
on quantum dots. _Nat. Photonics_ 1, 717–722 (2007). Article ADS CAS Google Scholar * Anikeeva, P. O., Halpert, J. E., Bawendi, M. G. & Bulović, V. Quantum dot light-emitting devices
with electroluminescence tunable over the entire visible spectrum. _Nano Lett._ 9, 2532–2536 (2009). Article ADS CAS PubMed Google Scholar * Kwak, J. et al. Bright and efficient
full-color colloidal quantum dot light-emitting diodes using an inverted device structure. _Nano Lett._ 12, 2362–2366 (2012). Article ADS CAS PubMed Google Scholar * Dai, X. et al.
Solution-processed, high-performance light-emitting diodes based on quantum dots. _Nature_ 515, 96–99 (2014). Article ADS CAS PubMed Google Scholar * Lim, J., Park, Y.-S., Wu, K., Yun,
H. J. & Klimov, V. I. Droop-free colloidal quantum dot light-emitting diodes. _Nano Lett._ 18, 6645–6653 (2018). Article ADS CAS PubMed Google Scholar * Chang, J. H. et al.
Unraveling the origin of operational instability of quantum dot based light-emitting diodes. _ACS Nano_ 12, 10231–10239 (2018). Article CAS PubMed Google Scholar * Won, Y.-H. et al.
Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. _Nature_ 575, 634–638 (2019). Article ADS CAS PubMed Google Scholar * Khan, Q. et al. Structure optimization
of perovskite quantum dot light-emitting diodes. _Nanoscale_ 11, 5021–5029 (2019). Article CAS PubMed Google Scholar * Rhee, S. et al. “Positive incentive” approach to enhance the
operational stability of quantum dot-based light-emitting diodes. _ACS Appl. Mater. Interfaces_ 11, 40252–40259 (2019). Article CAS PubMed Google Scholar * Tian, P. F. et al. Precise,
scalable shadow mask patterning of vacuum-deposited organic light emitting devices. _J. Vac. Sci. Technol._ 17, 2975–2981 (1999). Article ADS CAS Google Scholar * Lim, J. et al.
Perspective on synthesis, device structures, and printing processes for quantum dot displays. _Opt. Mater. Express_ 2, 594–628 (2012). Article ADS Google Scholar * Kwon, J. H., Yoo, S.,
Lampande, R. & Kim, S. Vacuum Deposition. in _Handbook of Organic Light-Emitting Diodes_ (eds Adachi, C., Hattori, R., Kaji, H. & Tsujimura, T.) (Springer Japan, 2019). * Shulga, A.
G. et al. Patterned quantum dot photosensitive FETs for medium frequency optoelectronics. _Adv. Mater. Technol._ 4, 1900054 (2019). Article CAS Google Scholar * Park, J.-S. et al.
Alternative patterning process for realization of large-area, full-color, active quantum dot display. _Nano Lett._ 16, 6946–6953 (2016). Article ADS CAS PubMed Google Scholar * Wood, V.
et al. Inkjet-printed quantum dot–polymer composites for full-color AC-driven displays. _Adv. Mater._ 21, 2151–2155 (2009). Article CAS Google Scholar * Kim, B. H. et al. High-resolution
patterns of quantum dots formed by electrohydrodynamic jet printing for light-emitting diodes. _Nano Lett._ 15, 969–973 (2015). Article ADS CAS PubMed Google Scholar * Yang, P., Zhang,
L., Kang, D. J., Strahl, R. & Kraus, T. High-resolution inkjet printing of quantum dot light-emitting microdiode arrays. _Adv. Opt. Mater._ 8, 1901429 (2019). Article CAS Google
Scholar * Kim, T.-H. et al. Full-colour quantum dot displays fabricated by transfer printing. _Nat. Photonics_ 5, 176 (2011). Article ADS CAS Google Scholar * Choi, M. K. et al.
Wearable red–green–blue quantum dot light-emitting diode array using high-resolution intaglio transfer printing. _Nat. Commun._ 6, 7149 (2015). Article ADS CAS PubMed PubMed Central
Google Scholar * Keum, H. et al. Photoresist contact patterning of quantum dot films. _ACS Nano_ 12, 10024–10031 (2018). Article CAS PubMed Google Scholar * Palazon, F., Akkerman, Q.
A., Prato, M. & Manna, L. X-ray lithography on perovskite nanocrystals films: from patterning with anion-exchange reactions to enhanced stability in air and water. _ACS Nano_ 10,
1224–1230 (2016). Article CAS PubMed Google Scholar * Wang, L. et al. Giant stability enhancement of CsPbX3 nanocrystal films by plasma-induced ligand polymerization. _ACS Appl. Mater.
Interfaces_ 11, 35270–35276 (2019). Article CAS PubMed Google Scholar * Wang, Y., Fedin, I., Zhang, H. & Talapin, D. V. Direct optical lithography of functional inorganic
nanomaterials. _Science_ 357, 385–388 (2017). Article ADS CAS PubMed Google Scholar * Wang, Y., Pan, J.-A., Wu, H. & Talapin, D. V. Direct wavelength-selective optical and
electron-beam lithography of functional inorganic nanomaterials. _ACS Nano_ 13, 13917–13931 (2019). Article CAS PubMed Google Scholar * Reiser, A. & Leyshon, L. J. Spin state of
photogenerated phenylnitrene. _J. Am. Chem. Soc._ 93, 4051–4052 (1971). Article CAS Google Scholar * Leyva, E., Platz, M. S., Persy, G. & Wirz, J. Photochemistry of phenyl azide: the
role of singlet and triplet phenylnitrene as transient intermediates. _J. Am. Chem. Soc._ 108, 3783–3790 (1986). Article CAS Google Scholar * Keana, J. F. W. & Cai, S. X. New reagents
for photoaffinity labeling: synthesis and photolysis of functionalized perfluorophenyl azides. _J. Org. Chem._ 55, 3640–3647 (1990). Article CAS Google Scholar * Cai, S. X., Glenn, D.
J., Kanskar, M., Wybourne, M. N. & Keana, J. F. W. Development of highly efficient deep-UV and electron beam mediated cross-linkers: synthesis and photolysis of bis(perfluorophenyl)
azides. _Chem. Mater._ 6, 1822–1829 (1994). Article CAS Google Scholar * Khong, S.-H. et al. General photo-patterning of polyelectrolyte thin films via efficient ionic bis(fluorinated
phenyl azide) photo-crosslinkers and their post-deposition modification. _Adv. Funct. Mater._ 17, 2490–2499 (2007). Article CAS Google Scholar * Png, R.-Q. et al. High-performance polymer
semiconducting heterostructure devices by nitrene-mediated photocrosslinking of alkyl side chains. _Nat. Mater._ 9, 152 (2009). Article ADS PubMed CAS Google Scholar * Wang, G.-J. N.
et al. Tuning the cross-linker crystallinity of a stretchable polymer semiconductor. _Chem. Mater._ 31, 6465–6475 (2019). Article CAS Google Scholar * Fleet, G. W. J., Porter, R. R. &
Knowles, J. R. Affinity labelling of antibodies with aryl nitrene as reactive group. _Nature_ 224, 511–512 (1969). Article ADS CAS Google Scholar * Poe, R., Schnapp, K., Young, M. J.
T., Grayzar, J. & Platz, M. S. Chemistry and kinetics of singlet pentafluorophenylnitrene. _J. Am. Chem. Soc._ 114, 5054–5067 (1992). Article CAS Google Scholar * Liu, L.-H. &
Yan, M. Perfluorophenyl azides: new applications in surface functionalization and nanomaterial synthesis. _Acc. Chem. Res._ 43, 1434–1443 (2010). Article CAS PubMed PubMed Central Google
Scholar * Giansante, C. & Infante, I. Surface traps in colloidal quantum dots: a combined experimental and theoretical perspective. _J. Phys. Chem. Lett._ 8, 5209–5215 (2017). Article
CAS PubMed PubMed Central Google Scholar * Lim, J. et al. Influence of shell thickness on the performance of light-emitting devices based on CdSe/Zn1−xCdxS core/shell heterostructured
quantum dots. _Adv. Mater._ 26, 8034–8040 (2014). Article ADS CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Basic Science Programme
(NRF-2017R1C1B2006789 and 2020R1A2C2011478), the Creative Materials Discovery Programme (NRF-2019M3D1A1078299), and the Engineering Research Centre Programme (NRF-2018R1A5A1025594) through
the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Korea. AUTHOR INFORMATION Author notes * These authors contributed equally: Jeehye Yang, Donghyo
Hahm, Kyunghwan Kim. AUTHORS AND AFFILIATIONS * Department of Chemical and Biomolecular Engineering, Sogang University, Seoul, 04107, Republic of Korea Jeehye Yang, Seunghan Kim, Hye Won
Park, Minkyoung Lee, Hyeokjun Kim & Moon Sung Kang * SKKU Advanced Institute of Nanotechnology (SAINT), School of Nano Science & Technology, Sungkyunkwan University (SKKU), Suwon,
16419, Republic of Korea Donghyo Hahm, Jun Hyuk Chang & Wan Ki Bae * Department of Electrical and Computer Engineering, Inter-University Semiconductor Research Center, Seoul National
University, Seoul, 08826, Republic of Korea Kyunghwan Kim, Seunghyun Rhee, Jeonghun Kwak & Changhee Lee * Department of Chemistry, Korea University, Seoul, 02841, Republic of Korea
Myeongjae Lee * Department of Energy Science, Center for Artificial Atoms, Sungkyunkwan University (SKKU), Suwon, 16419, Republic of Korea Jaehoon Lim * Pohang Accelerator Laboratory,
POSTECH, Pohang, 37673, Republic of Korea Joohee Bang & Hyungju Ahn * Department of Chemical and Biomolecular Engineering, Yonsei University, Seoul, 03722, Republic of Korea Jeong Ho Cho
* Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919, Republic of Korea BongSoo Kim Authors * Jeehye Yang View author publications You can
also search for this author inPubMed Google Scholar * Donghyo Hahm View author publications You can also search for this author inPubMed Google Scholar * Kyunghwan Kim View author
publications You can also search for this author inPubMed Google Scholar * Seunghyun Rhee View author publications You can also search for this author inPubMed Google Scholar * Myeongjae Lee
View author publications You can also search for this author inPubMed Google Scholar * Seunghan Kim View author publications You can also search for this author inPubMed Google Scholar *
Jun Hyuk Chang View author publications You can also search for this author inPubMed Google Scholar * Hye Won Park View author publications You can also search for this author inPubMed
Google Scholar * Jaehoon Lim View author publications You can also search for this author inPubMed Google Scholar * Minkyoung Lee View author publications You can also search for this author
inPubMed Google Scholar * Hyeokjun Kim View author publications You can also search for this author inPubMed Google Scholar * Joohee Bang View author publications You can also search for
this author inPubMed Google Scholar * Hyungju Ahn View author publications You can also search for this author inPubMed Google Scholar * Jeong Ho Cho View author publications You can also
search for this author inPubMed Google Scholar * Jeonghun Kwak View author publications You can also search for this author inPubMed Google Scholar * BongSoo Kim View author publications You
can also search for this author inPubMed Google Scholar * Changhee Lee View author publications You can also search for this author inPubMed Google Scholar * Wan Ki Bae View author
publications You can also search for this author inPubMed Google Scholar * Moon Sung Kang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
M.S.K., W.K.B. and J.H.C. (Jeong Ho Cho) conceived the core strategy of the patterning method and designed the experiment. J.Y., K.K., S.K., H.W.P., M.L. (Minkyoung Lee) and H.K. carried out
the patterning experiment and thin film characterisation. J.Y., K.K., S.R., J.K. and C.L. fabricated the QD-LEDs and analysed their performance. D.H., J.H.C. (Jun Hyuk Chang), and J.L.
synthesised the QDs. D.H., J.H.C. (Jun Hyuk Chang) and W.K.B. analysed the luminescent characteristics of the patterned QDs. J.B. and H.A. analysed the structural characterisation of QD
films. M.L. (Myeongjae Lee) and B.K. synthesised LiXer. J.Y., D.H., W.K.B. and M.S.K. mainly prepared the manuscript. CORRESPONDING AUTHORS Correspondence to Wan Ki Bae or Moon Sung Kang.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Hunter McDaniel and the
other, anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, J., Hahm, D., Kim, K. _et al._ High-resolution patterning of colloidal
quantum dots via non-destructive, light-driven ligand crosslinking. _Nat Commun_ 11, 2874 (2020). https://doi.org/10.1038/s41467-020-16652-4 Download citation * Received: 15 December 2019 *
Accepted: 12 May 2020 * Published: 08 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16652-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative