Play all audios:
ABSTRACT The intense arms race between bacteria and phages has led to the development of diverse antiphage defense systems in bacteria. Unlike well-known restriction-modification and
CRISPR-Cas systems, recently discovered systems are poorly characterized. One such system is the Thoeris defense system, which consists of two genes, _thsA_ and _thsB_. Here, we report
structural and functional analyses of ThsA and ThsB. ThsA exhibits robust NAD+ cleavage activity and a two-domain architecture containing sirtuin-like and SLOG-like domains. Mutation
analysis suggests that NAD+ cleavage is linked to the antiphage function of Thoeris. ThsB exhibits a structural resemblance to TIR domain proteins such as nucleotide hydrolases and Toll-like
receptors, but no enzymatic activity is detected in our in vitro assays. These results further our understanding of the molecular mechanism underlying the Thoeris defense system,
highlighting a unique strategy for bacterial antiphage resistance via NAD+ degradation. SIMILAR CONTENT BEING VIEWED BY OTHERS PHAGES CARRY ORPHAN ANTITOXIN-LIKE ENZYMES TO NEUTRALIZE THE
DARTG1 TOXIN-ANTITOXIN DEFENSE SYSTEM Article Open access 13 February 2025 ARCHITECTURE AND ACTIVATION MECHANISM OF THE BACTERIAL PARIS DEFENCE SYSTEM Article 07 August 2024 THE DARTG
TOXIN-ANTITOXIN SYSTEM PROVIDES PHAGE DEFENCE BY ADP-RIBOSYLATING VIRAL DNA Article 20 June 2022 INTRODUCTION Bacteriophages (phages) are viruses that infect bacteria1. They are the most
abundant biological entities in the biosphere and coexist with their host2. The virus-rich environment and the constant exposure to viral infection led to the intense arms race between
bacteria and phages3,4, resulting in the development of diverse and sophisticated antiphage defense systems in bacteria5. Bacterial antiviral systems use various phage resistance mechanisms
and function during distinct stages of the phage infection cycle6,7. Some of them have been thoroughly characterized for many years, including the restriction–modification and CRISPR-Cas
systems8,9. Deciphering their detailed molecular mechanisms not only has contributed to our fundamental understanding of host–parasite interactions in microbiology but also has provided
useful biotechnology tools such as restriction enzymes and Cas9 nuclease10,11. Systematic pangenomic analyses and subsequent experimental verification recently allowed the discovery of
previously unknown bacterial defense systems12,13,14,15. Because antiphage defense genes tend to be clustered within specific loci termed “defense islands” in microbial genomes, candidate
defense systems located near previously known defense genes were identified and functionally validated for their antiviral activities12,13,14,15. Among the newly discovered systems, the
Thoeris defense system, named after a deity in Egyptian mythology, has been identified in more than 2000 microbial genomes with broad phylogenetic distribution14. It was detected in nine
different taxonomic phyla, including a wide variety of bacteria and archaea14. The Thoeris system is composed of two genes, _thsA_ and _thsB_. The first gene in the system, _thsA_, contains
a domain (often annotated as sirtuin-like domain or macro domain) that binds to nicotinamide adenine dinucleotide (NAD) or its metabolites16,17. Sirtuins are a family of protein deacetylases
whose activity is dependent on NAD hydrolysis16. They are widely distributed from bacteria to higher eukaryotes18. Its founding member, yeast Silent information regulator 2 (Sir2) is a
histone deacetylase involved in a variety of cellular regulation16. CobB is a well-characterized bacterial sirtuin that regulates the function of acyl-CoA synthetase by lysine
deacetlyation19. The macro domain is a widespread and conserved module of ~190 residues that can bind NAD metabolites or related molecules17. It is named after the C-terminal domain of
macroH2A, a variant of histone H2A containing an extensive C-terminal nonhistone tail20. In several pathogenic bacteria, the connections between macro domains and pathogenesis have been
suggested21. In some instances, _thsA_ has an N-terminal transmembrane domain14. The second gene, _thsB_, contains a Toll/interleukin-1 receptor (TIR) motif and, in more than 50% of cases,
is present in multiple, diverse copies around the single _thsA_ gene14. Bacterial TIR-containing proteins have initially been hypothesized to function in pathogenesis but have also been
implicated in other diverse mechanisms such as nucleic acid metabolism22. In several recent studies, the TIR domains of bacterial and eukaryotic proteins exhibited NAD+ (the oxidized form of
NAD) cleavage activities23,24, suggesting that the antiphage function of the Thoeris system is related to NAD+ binding and/or processing14. Two instances of the Thoeris system have been
experimentally validated for their antiphage function14. One is from _Bacillus cereus_ MSX-D12. The _thsA_ gene and its downstream _thsB_ gene conferred resistance against myophage infection
when engineered into the model bacterium _Bacillus subtilis_ BEST7003, which is devoid of an intrinsic Thoeris system14. _B_. _cereus_ ThsA contains the sirtuin-like domain and lacks the
N-terminal transmembrane domain and thus is predicted to be cytoplasmic14. Deletion experiment revealed that both ThsA and ThsB are required for the antiphage activity of the _B_. _cereus_
Thoeris system14. The other validated Thoeris system is from _Bacillus amyloliquefaciens_ Y214. In this system, ThsA includes the macro domain with the additional N-terminal transmembrane
domain and a shorter C-terminal region compared with the _B. cereus_ homologue14. In the present study, we report structural and functional analyses of the ThsA and ThsB proteins from the
_B_. _cereus_ Thoeris defense system. Crystal structures of ThsA and ThsB were determined to resolutions of 2.5 and 1.8 Å, respectively. The enzymatic activities of the Thoeris proteins were
also tested in the presence of NAD+ and other nucleotides. We found that ThsA exhibited robust NAD+ cleavage activity. Combined with previous findings, these results advance our
understanding of the molecular mechanism underlying the Thoeris defense system and suggest that NAD+ degradation is a previously unknown strategy for bacterial antiphage resistance. RESULTS
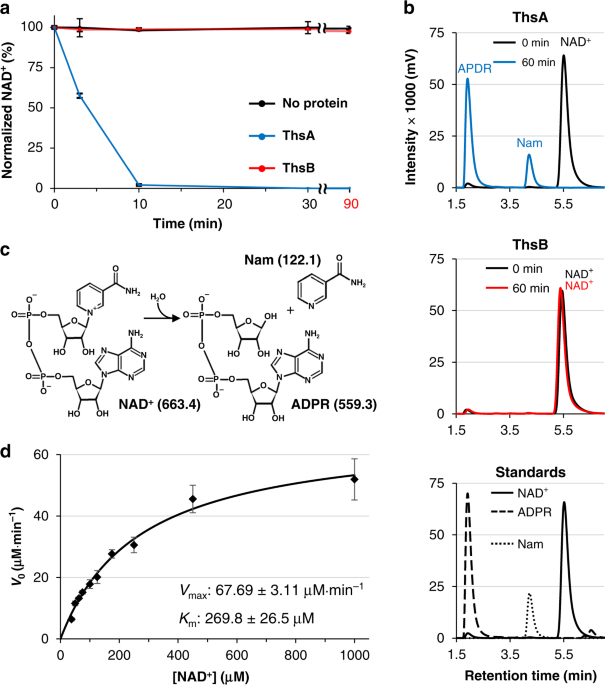
THSA IS AN NAD+-CLEAVING ENZYME Since the Thoeris system was suggested to function via NAD+ binding and hydrolysis14, we performed analytical size-exclusion chromatography (SEC) to test the
interaction between NAD+ and purified recombinant _B_. _cereus_ Thoeris proteins. We observed that the chromatographic peak of NAD+ was divided into two parts in the presence of ThsA
(Supplementary Fig. 1a), suggesting that ThsA processes NAD+. We further assessed whether the Thoeris proteins can cleave NAD+. NAD+ was incubated with the _B_. _cereus_ ThsA or ThsB, and
the amount of remaining NAD+ was measured at different time points using high-performance liquid chromatography (HPLC). In this NAD+ cleavage assay, NAD+ was rapidly degraded by ThsA, but
not by ThsB (Fig. 1a), indicating that ThsA is an NAD+-consuming enzyme. Using liquid chromatography–mass spectrometry (LC-MS), we identified nicotinamide (Nam) and
adenosine-5ʹ-diphosphoribose (ADPR) as products of NAD+ degradation by ThsA (Fig. 1b and Supplementary Fig. 2), implying that ThsA cleaves NAD+ into Nam and ADPR by hydrolyzing the
Nam–ribosyl bond of NAD+ (Fig. 1c). Notably, the NAD+ cleavage activity was also observed in sirtuin proteins catalyzing NAD+-dependent protein deacetylation, in which NAD+ is used as a
co-substrate16. The kinetic analysis revealed that the _B_. _cereus_ ThsA exhibited a _K_m of 270 μM and a _k_cat of 33.9 min−1 (Fig. 1d). The specificity constant (_k_cat_/K_m) of ThsA was
calculated to be 2091 M−1 s−1. This falls within the range observed for NAD+ cleavage by eukaryotic sirtuin proteins including the yeast Sir2 (3.5–8205 M−1 s−1)25. It is also noteworthy that
the intracellular NAD+ level of a bacterium _Escherichia coli_ was previously estimated to be ~0.64 mM26, which is within the same order of magnitude as the _K_m value of ThsA. THSA
CONTAINS SIRTUIN-LIKE AND SMF/DPRA-LOG (SLOG)-LIKE DOMAINS To further investigate the role of ThsA in Thoeris-mediated phage defense, we determined the crystal structure of _B_. _cereus_
ThsA (Supplementary Table 1). The asymmetric unit contains four ThsA protomers (chains A–D), the structures of which are essentially identical. The root-mean-square deviation (RMSD) values
of the Cα atomic positions between the protomers range from 0.5 to 0.8 Å. The protomers form two dimeric assemblies (AB and CD), which are very similar to each other (Supplementary Fig. 3a).
The RMSD value of the Cα atoms between the two dimers is only 0.7 Å. The analysis using PISA27 predicted tetrameric or octameric states formed with symmetry molecules (A2B2, C2D2, or
A2B2C2D2) as probable quaternary structures of ThsA (Supplementary Fig. 3b). Consistent with this prediction, SEC with multi-angle light scattering (MALS) analysis confirmed that the
tetrameric state of ThsA is predominant in a solution containing a trace amount of octamer (Supplementary Fig. 3c). The protomer structure of ThsA revealed a two-domain architecture that
consists of an N-terminal sirtuin-like domain (residues 1–283) and a C-terminal SLOG-like domain (residues 284–476) (Fig. 2a)28. The N-terminal domain contains the Rossmann-like fold, in
which an extended seven-stranded parallel β-sheet (β3–β4–β2–β1–β5–β6–β7) is sandwiched between two helical layers (α1, α5, α6, and α11 on one side and α7–α10 on the other), comprising a
three-layered globular structure (Fig. 2b). Three additional α-helices (α2–α4) form a small triangular structural module protruding from the Rossmann-like fold. This domain displays a
structural similarity with sirtuin proteins, especially in the portion corresponding to the Rossmann-like fold (Fig. 2c). When the N-terminal domain of ThsA was structurally aligned with
sirtuin proteins, the RMSD values ranged from 2.7 to 3.6 Å for ~170 Cα atoms (Supplementary Table 2). However, structural differences were also noted in the periphery of the Rossmann-like
fold (Supplementary Fig. 4). The acetyl substrate-binding pocket of sirtuins is not available in the N-terminal domain of ThsA due to the blocking by its α7 helix (Supplementary Fig. 4),
excluding the possibility that ThsA serves as an NAD+-dependent deacetylase like sirtuins. ThsA also lacks the insertion element for zinc coordination present in sirtuins (Fig. 3a and
Supplementary Fig. 4), the disruption of which results in loss of the NAD+ hydrolysis function of sirtuins16. Thus, the N-terminal domain of ThsA does not completely share the structural and
functional characteristics of sirtuins. The C-terminal domain of ThsA also possesses the Rossmann-like fold, which contains a five-stranded parallel β-sheet and three α-helices with a
combination of βαβαβ (β8–α12–β9–α13–β10) and βαβ (β11–α16–β14) motifs (Fig. 2d). The residues connecting β11 and α16 form a β-hairpin structure. Additional α-helices (α14, α15, α17–α19)
surround the edges of the β-sheet. A structural analysis indicated that this domain could be classified into the Sir2/TIR-associating SLOG (STALD) family within the SLOG superfamily28.
Members of this protein family were predicted to function as sensors of nucleotides or related ligands, which are likely processed or modified by associating effectors28. The C-terminal
domain of ThsA shares common features of the STALD family at the putative ligand-binding site, including secondary structural elements and conserved residues28. These residues include Ser288
and Phe357 in the loops following β8 and β10, respectively, and Arg371 and Glu403 of the helices (α15 and α16, respectively) in the ligand-binding pocket (Fig. 2e). NAD+ CLEAVAGE BY THSA IS
LINKED TO ANTIPHAGE ACTIVITY In a previous study, a point mutation (N112A) in ThsA resulted in complete loss of antiphage protection by the Thoeris system14, indicating that Asn112 of ThsA
plays a crucial role in the defense mechanism of Thoeris. In our crystal structure of ThsA, this residue is found within the putative NAD+ binding site in its N-terminal domain (Fig. 3b and
Supplementary Fig. 5). This strongly suggests a direct connection between the NAD+ cleavage by ThsA and the protective function of the Thoeris system. To confirm this linkage, we generated
ThsA mutants and tested their NAD+ cleavage activities. Mutations were introduced at Asn112 and another residue in the putative NAD+ binding pocket, His152 (Fig. 3b and Supplementary Fig.
5). These residues are also conserved in sirtuin proteins (Fig. 3c and Supplementary Fig. 6). In the NAD+-bound sirtuin structure (Fig. 3b), the residues are located adjacent to the
Nam–ribosyl portion of NAD+, with the Asn and His side chains interacting with the Nam and ribose moieties, respectively. In sirtuins, the Asn residue is involved in substrate
recognition29,30, and the His side chain serves as a general base31. Both of the purified ThsA mutants, N112A and H152A, exhibited circular dichroism (CD) spectra similar to that of the wild
type (Supplementary Fig. 7), which is characteristic of folded proteins. In analytical SEC, the chromatogram of the N112A mutant was essentially identical to that of the wild type control,
but the H152A mutant was eluted significantly later (Supplementary Fig. 8a). These results suggest that the N112A mutant retains the overall folding of the wild type as well as its
oligomeric state, but the H152A mutation disrupts the proper oligomerization of ThsA. In subsequent binding assays using analytical SEC, neither of the mutants bound NAD+ (Supplementary Fig.
8b). In our enzyme assays, both N112A and H152A abolished the NAD+ cleavage activity of ThsA (Fig. 3d). Based on these observations, we concluded that the Asn112 residue is critical for
NAD+ hydrolysis of ThsA. For the H152A mutant, it cannot be ruled out that the inactivation is caused by the disruption of the functionally relevant oligomeric state. Thus, the same point
mutation (N112A) that destroyed the NAD+-cleavage activity of ThsA resulted in complete inactivation of the Thoeris system14. Consequently, combined with the result from a previous study14,
our mutation analysis suggests that NAD+ degradation by ThsA is linked to the antiphage function of the Thoeris defense system. THSB EXHIBITS STRUCTURAL SIMILARITY TO TIR DOMAIN PROTEINS The
second gene in the Thoeris defense system, _thsB_, contains a TIR domain, and ThsB was previously implicated in NAD+ hydrolysis14; several TIR domain proteins have been shown to exhibit
NAD+-cleavage activity23,24. However, in our in vitro assays, NAD+ was cleaved by ThsA, but not by ThsB (Fig. 1a). In the TIR NAD+ hydrolases, NAD+-cleavage activity was found to be
dependent on self-association32,33; mutations disrupting functionally relevant oligomeric states abolished the activity32,33 and addition of macromolecular crowding agents simulating the
intracellular environment stimulated NAD+ degradation32. By contrast, our SEC-MALS analysis indicated that ThsB exists as a monomer even at high concentrations (~1 mM; Supplementary Fig. 9).
No stimulating effect by a macromolecular crowding agent (PEG 400) was observed for ThsB (Supplementary Fig. 10). It is also unlikely that ThsB alters the function of ThsA or catalyzes the
potential pathway(s) downstream of NAD+ cleavage because ThsA was still active in the presence of ThsB, which did not further process the cleavage products Nam and ADPR (Supplementary Fig.
11). We also suspected the possibility that ThsB may function as a component of a larger multi-protein complex formed with ThsA. However, in our analytical SEC experiment, ThsB was eluted
separately from ThsA with and without NAD+ (Supplementary Fig. 1b, c). When expressing (His)6-maltose binding protein (MBP)-tagged ThsA together with untagged ThsB in _E. coli_ cells, ThsB
did not co-purify with ThsA in nickel-affinity chromatography (Supplementary Fig. 12). These observations suggest that ThsB does not form a stable complex with ThsA. To gain structural
insight into the role of ThsB in the Thoeris defense system, we determined the crystal structure of _B_. _cereus_ ThsB (Supplementary Table 1). Two ThsB molecules were found in the
asymmetric unit, and the RMSD value of the Cα atoms was only 0.9 Å, indicating high similarity. ThsB exhibited a four-layered structure in which α-helices and β-sheets were alternately
stacked (Fig. 4a). A five-stranded parallel β-sheet (β2–β1–β3–β4–β10) and five α-helices comprise the Rossmann-like fold, with α1, α2, and α5 on the concave side of the β-sheet and α3 and α4
on the convex side (Fig. 4b). An additional antiparallel β-sheet (β5–β9) is located adjacent to the α4 helix in an approximately parallel orientation (Fig. 4c). A search for structural
neighbors using the Dali server34 did not identify a significant match to the entire ThsB structure. However, excluding the additional antiparallel β-sheet, the Rossmann-like fold portion of
ThsB revealed a structural resemblance to TIR domain proteins such as human sterile alpha and TIR motif containing 1 (SARM1), nucleoside monophosphate hydrolases, and toll-like receptors
(Supplementary Table 3 and Fig. 4d). SARM1 is an NAD+-cleaving TIR family protein32. Despite the high structural similarity to SARM1, ThsB did not cleave NAD+ in our in vitro assay (Fig.
1a). If ThsB is a nucleoside monophosphate hydrolase, the conservation of Phe6 in the potential active site strongly suggests that ThsB would prefer substrates containing 2′-ribosyl groups
to those containing 2′-deoxyribosyl moiety (Supplementary Fig. 13a, b)35. On this basis, we tested the hydrolase activity of ThsB in the presence of various ribonucleotides such as AMP, GMP,
UMP, and CMP, but no hydrolysis was detected (Supplementary Fig. 13c). This raises other possibilities; the enzymatic activity of ThsB may be triggered by an unknown in vivo mechanism, or
ThsB may have nonenzymatic function(s) as a toll-like receptor. DISCUSSION In this study, we demonstrated experimentally that the ThsA protein in the Thoeris defense system is an
NAD+-cleaving enzyme. This is consistent with the previous proposition that NAD+ binding and hydrolysis are essential for Thoeris-mediated antiphage resistance14. Our mutation analysis also
revealed a direct connection between NAD+ cleavage by ThsA and the protective activity of the Thoeris system. The N112A point mutation of ThsA, which was shown to neutralize the Thoeris
defense system in a previous plaque assay14, abolished the NAD+ hydrolase activity of ThsA in our experiment (Fig. 3d). These results suggest that the Thoeris system uses NAD+ degradation as
an antiphage strategy, indicating a previously unknown bacterial defense mechanism against phage infection. It cannot be completely ruled out that the binding to NAD+, not its cleavage, is
important for the antiphage function since the N112A mutant did not bind NAD+. To further study structural aspect of NAD+ cleavage by ThsA, we tried to crystallize NAD+-bound complexes of
both wild-type and mutant ThsA proteins, but our attempts were not successful. In addition to the sirtuin-like N-terminal domain, ThsA contains a C-terminal SLOG-like domain, the function of
which is less well understood. SLOG superfamily proteins are involved in various nucleotide-related processes28, including the modification of nucleotides for cytokinin hormone production
in plants36,37, interaction with single-stranded DNA during bacterial transformation38, and binding to molybdenum cofactors, which often share structural similarities to nucleotides39.
_Streptococcus pneumonia_ DprA and its _B. subtilis_ ortholog (Smf) belong to the SLOG superfamily and have been previously shown to bind single-stranded DNA of random sequence38. However,
the DNA binding affinity was not observed for ThsA in our electrophoretic mobility shift assay (EMSA; Supplementary Fig. 14). In a previous bioinformatics study, certain versions of
SLOG-like domains were identified as components of nucleotide-centric systems involved in biological conflicts and were predicted to serve as sensors recognizing nucleotides or the enzymes
that process them28. Recently, _B. subtilis_ YpsA, a bacterial member of the SLOG superfamily, was implicated in the response to oxidative stress and regulation of cell division40. It is
also noteworthy that ~30% of ThsA homologues do not contain the SLOG-like domain but rather an additional N-terminal multi-transmembrane domain14. These findings suggest that the SLOG-like
domain of ThsA is involved in the recognition and/or transmission of nucleotide-related or other molecular signals. Thus, the C-terminal domain of ThsA may play a role in the regulation or
signaling associated with the NAD+ cleavage activity of the N-terminal domain. Nevertheless, the exact function of the SLOG-like domain in the Thoeris defense system awaits further
investigation. In the structural and functional analyses, we could not establish a role for ThsB in the Thoeris defense system. ThsB possesses a TIR domain and was originally predicted to be
an NAD+-cleaving enzyme14, since several TIR domain-containing proteins show NAD+ cleavage activity23,24. However, ThsB did not exhibit NAD+ cleavage activity in our experiment (Fig. 1a and
Supplementary Fig. 10). It is possible that the enzymatic activity of ThsB is regulated by an unidentified in vivo mechanism, or we failed to recreate functionally relevant reaction
condition(s) for ThsB in our in vitro assays. Alternatively, ThsB may have a function different from NAD+ cleavage since ThsA is responsible for cleaving NAD+ in the Thoeris system. Based on
the presence of _thsB_ in multiple, diverse copies in more than 50% of Thoeris systems, it was previously proposed that ThsB participates in recognition of phage infection with various ThsB
proteins sensing different phage components14. In _B. cereus_ MSX-D12, the second _thsB_ gene is found upstream of the pair of _thsA_ and _thsB_ genes characterized in this study. The amino
acid sequence identity is only 18% between the two ThsB homologues, indicating the diversity of multiple ThsB copies in Thoeris defense system. Notably, the single _B. cereus thsA_ gene and
its downstream _thsB_ gene were sufficient to confer antiphage resistance14, suggesting the dispensability of the upstream _thsB_ gene. NAD+ is an important metabolic component in all forms
of life, and its cleavage has previously been implicated in various biological conflicts. _Staphylococcus aureus_ TirS virulence factor induces NAD+ loss in mammalian cells23.
_Mycobacterium tuberculosis_ secretes NAD+-hydrolyzing toxin to trigger macrophage necroptosis41. The plant commensal bacterium _Pseudomonas protegens_ employs NAD+-degrading effectors that
exhibit interbacterial antagonism42. In plants, NAD+ cleavage, triggered by the recognition of pathogen effectors, induces localized cell death through a yet unknown downstream mechanism to
restrict pathogen infection32,33. NAD+ depletion also mediates local axonal degeneration after injury as an intrinsic self-destruction program24,32,43. Based on these observations, we
carefully raise the possibility that the Thoeris system is an “altruistic suicide” defense system that kills phage-infected cells via NAD+ degradation to prevent parasite transmission to
neighboring bacteria. It is unclear whether the NAD+ cleavage by the Thoeris system is sufficient to cause cell death by NAD+ depletion. During the expression of recombinant ThsA and/or ThsB
in _E. coli_, we did not observe growth arrest or toxicity. This suggests that the Thoeris system does not deplete the intracellular NAD+ to drive direct cell death and is a part of a more
elaborate defense mechanism requiring downstream signaling after NAD+ cleavage as in the case of the plant TIR NAD+ hydrolases32,33. The more precise molecular mechanism of bacterial
antiphage protection involving the Thoeris defense system remains to be determined, including the regulation of ThsA and the function of ThsB. METHODS CLONING, EXPRESSION, AND PURIFICATION
Synthetic _thsA_ and _thsB_ genes of _B_. _cereus_ MSX-D12 were cloned into pET28a vectors with an N-terminal (His)6-MBP tag and a tobacco etch virus protease cleavage site. Mutant genes
were generated by polymerase chain reaction using mismatched primers (Supplementary Table 4). _E. coli_ BL21(DE3) cells (Enzynomics) transformed with these constructs were cultured in LB
medium at 37 °C until the optical density at 600 nm reached 0.7. Protein expression was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG), followed by incubation
at 17 °C for 16 h. The cells were harvested by centrifugation and resuspended in lysis buffer (300 mM NaCl, 10% (w/v) glycerol, 5 mM β-mercaptoethanol, 0.3 mM phenylmethylsulfonyl fluoride,
0.3 mM Triton X-100, 20 mM Tris-HCl pH 7.5). After sonication and centrifugation, the supernatant was loaded onto a 5-mL HisTrap HP column (GE Healthcare) pre-equilibrated with purification
buffer (300 mM NaCl, 10% (w/v) glycerol, 5 mM β-mercaptoethanol, 30 mM imidazole, 20 mM Tris-HCl pH 7.5). After washing the column with purification buffer, the bound proteins were eluted by
applying a linear gradient of imidazole (up to 450 mM). The (His)6-MBP tag was cleaved via the tobacco etch virus protease and separated using a 5-mL HisTrap HP column (GE Healthcare).
Proteins were further purified using the HiLoad 16/60 Superdex 200 column (GE Healthcare) equilibrated with buffer (200 mM NaCl, 2 mM dithiothreitol (DTT), 20 mM HEPES pH 7.5). ENZYME
ACTIVITY ASSAY Proteins (2.5 μM) were incubated with 250 μM substrate (NAD+, AMP, GMP, UMP, and CMP) in 100 μL buffer (63 mM NaCl, 14 mM Tris-HCl pH 7.5) at 25 °C. Reactions were stopped by
the addition of 100 μL of ice-chilled 1 M HClO4, followed by neutralization with 33.4 μL of 3 M K2CO3 on ice. After centrifugation of the sample, 99 μL of the supernatant was mixed with 11
μL of 0.5 M potassium phosphate buffer (pH 7.0) for further analysis. HPLC Reaction products were analyzed using the 1100 HPLC system (Agilent) with a diode array detector at 260 nm. Sample
(5 μL) was loaded onto a YMC-Triart C18 column (100 × 2.0 mm, S-3 μm, 12 nm; YMC) equilibrated with mobile phase A (0.1% (v/v) formic acid and 5 mM ammonium formate in water). Separation was
performed at a flow rate of 0.2 mL min−1 using the gradient program for mobile phase B (0.1% (v/v) formic acid and 5 mM ammonium formate in methanol): 0–10 min, 0%; 13 min, 50%; 16–20 min,
95%; 25–40 min, 0%. LC-MS LC-MS was performed using the Nexera X2 UHPLC system coupled to the LCMS-8040 triple quadrupole mass spectrometer (Shimadzu). LC was conducted using the same column
and the conditions described for HPLC with the exception of the gradient program for mobile phase B, which was as follows: 0–3 min, 0%; 10 min, 50%; 13–15 min, 95%; 18–23 min, 0%. In the MS
system, ionization of target analytes was performed in the electrospray ionization positive mode. Mass spectra of the samples were obtained by scanning between _m/z_ 100 and 800. The
desolvation line and heat block temperature were 250 and 400 °C, respectively. The nebulizing (nitrogen) and drying gas (nitrogen) flow were 3 and 15 L min−1, respectively. As LC-MS
software, LabSolutions (ver. 5.60; Shimadzu) was used for data processing. DETERMINATION OF ENZYME KINETIC PARAMETERS Kinetic parameters (_K_m and _V_max) were determined using 2.0 μM ThsA
based on the initial velocity measurement of NAD+ consumption during the first 60 s of the reaction. The remaining NAD+ amount was measured using HPLC as described above. Data were fitted to
the Michaelis–Menten equation using SigmaPlot (Systat Software). CRYSTALLIZATION AND STRUCTURE DETERMINATION The selenomethionyl proteins were expressed in _E_. _coli_ BL21 (DE3) cells
grown in minimal medium supplemented with selenomethionine (SeMet)44. The cells transformed with the pET28a vectors containing the _thsA_ and _thsB_ genes were cultured in M9 medium at 37 °C
until the optical density at 600 nm reached 0.6. To inhibit Met biosynthesis, the culture was supplemented with other amino acids (0.5 mM Lys, 0.8 mM Val, 0.8 mM Thr, 0.6 mM Phe, 0.8 mM
Leu, 0.8 mM Ile). After incubation at 17 °C for 25 min, the cells were further supplemented with 0.25 mM SeMet. Protein expression was induced by the addition of 0.5 mM IPTG, followed by
incubation at 17 °C for 20 h. The selenomethionyl proteins were purified as the native proteins described above. The incorporation of SeMet was monitored by mass spectrometry. Seven and two
Met residues out of eight and two possible sites were substituted with SeMet for ThsA and ThsB, respectively. ThsA crystals were obtained at 20 °C by the sitting-drop vapor diffusion method
from 43 mg mL−1 protein solution in buffer (200 mM NaCl, 7 mM DTT, 20 mM HEPES pH 7.5) mixed with an equal amount of reservoir solution (22% (v/v) polypropylene glycol 400, 0.2 M MgCl2, 0.1
M MES pH 6.5). ThsB crystals were obtained at 20 °C by the sitting-drop method from 23 mg mL−1 protein solution in buffer (200 mM NaCl, 2 mM DTT, 20 mM HEPES pH 7.5) mixed with an equal
amount of reservoir solution (3 M NaCl, 0.1 M Tris pH 8.5). The crystals were flash-frozen in liquid nitrogen without additional cryoprotecting reagents. Diffraction data were collected at
the beamline 7A of the Pohang Accelerator Laboratory at 100 K. Diffraction images were processed using HKL200045. The determinations of selenium positions, density modification, and initial
model building were conducted using PHENIX46. The structures were completed using alternate cycles of manual fitting in COOT47 and refinement in PHENIX and REFMAC548. The stereochemical
quality of the final models was assessed using MolProbity49. ANALYTICAL SEC Analytical SEC was performed using the Superdex 200 10/300 GL column (GE Healthcare). The column was equilibrated
with buffer (150 mM NaCl, 1 mM DTT, 20 mM HEPES pH 7.5). Proteins (20 μM) and NAD+ (40 μM) were incubated at 4 °C for 1 h and loaded onto the column at a flow rate of 0.5 mL min−1. Elution
fractions were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by Coomassie staining. SEC-MALS SEC-MALS analysis was performed on the Superdex 200
Increase 10/300 GL column (GE Healthcare) coupled with the DAWN HELEOS II (18-angle) and Optilab T-rEX instruments (Wyatt Technology). The column was equilibrated with buffer (200 mM NaCl, 2
mM DTT, 20 mM HEPES pH 7.5), after which ThsA (11 mg mL−1) and ThsB (24 mg mL−1) were loaded onto the column at a flow rate of 0.5 mL min−1 at 25 °C. Data were analyzed using ASTRA 6
software (Wyatt Technology). CD SPECTROSCOPY CD spectra were measured with protein samples (0.8 μM) in 500 μL buffer (10 mM potassium phosphate pH 7.5) at 25 °C using a J-815 CD
spectropolarimeter (Jasco). EMSA DNAs (0.5 μM) were incubated with proteins (2.5 μM) and NAD+ (1 mM) in reaction buffer (100 mM NaCl, 20 mM HEPES pH 7.5) at 20 °C for 30 min. The samples
were analyzed on 9% acrylamide gels and visualized by ethidium bromide staining. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting
Summary linked to this article. DATA AVAILABILITY The atomic coordinates and structure factors for ThsA and ThsB were deposited in the Protein Data Bank50 with the accession codes 6LHX
[https://doi.org/10.2210/pdb6LHX/pdb] and 6LHY [https://doi.org/10.2210/pdb6LHY/pdb], respectively. The source data underlying Figs. 1a, b, d and 3d and Supplementary Figs. 1a–c, 3c, 7–11a,
12a, b, 13c and 14a are provided as Source Data file. Other data are available from the corresponding author upon reasonable request. Source Data are provided with this paper. REFERENCES *
Ofir, G. & Sorek, R. Contemporary phage biology: from classic models to new insights. _Cell_ 172, 1260–1270 (2018). Article CAS PubMed Google Scholar * Summers, W. C. In the
beginning. _Bacteriophage_ 1, 50–51 (2011). Article PubMed PubMed Central Google Scholar * Weinbauer, M. G. Ecology of prokaryotic viruses. _FEMS Microbiol. Rev._ 28, 127–181 (2004).
Article CAS PubMed Google Scholar * Fuhrman, J. A. Marine viruses and their biogeochemical and ecological effects. _Nature_ 399, 541–548 (1999). Article ADS CAS PubMed Google Scholar
* Rostol, J. T. & Marraffini, L. (ph)ighting phages: how bacteria resist their parasites. _Cell Host Microbe_ 25, 184–194 (2019). Article PubMed PubMed Central CAS Google Scholar
* Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. _Nat. Rev. Microbiol._ 8, 317–327 (2010). Article CAS PubMed Google Scholar * Dy, R. L., Richter,
C., Salmond, G. P. & Fineran, P. C. Remarkable mechanisms in microbes to resist phage infections. _Annu. Rev. Virol._ 1, 307–331 (2014). Article PubMed CAS Google Scholar * Tock, M.
R. & Dryden, D. T. The biology of restriction and anti-restriction. _Curr. Opin. Microbiol._ 8, 466–472 (2005). Article CAS PubMed Google Scholar * Hille, F. et al. The biology of
CRISPR-Cas: backward and forward. _Cell_ 172, 1239–1259 (2018). Article CAS PubMed Google Scholar * Petty, N. K., Evans, T. J., Fineran, P. C. & Salmond, G. P. Biotechnological
exploitation of bacteriophage research. _Trends Biotechnol._ 25, 7–15 (2007). Article CAS PubMed Google Scholar * Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications
of CRISPR-Cas9 for genome engineering. _Cell_ 157, 1262–1278 (2014). Article CAS PubMed PubMed Central Google Scholar * Goldfarb, T. et al. Brex is a novel phage resistance system
widespread in microbial genomes. _EMBO J._ 34, 169–183 (2015). Article CAS PubMed Google Scholar * Ofir, G. et al. Disarm is a widespread bacterial defence system with broad anti-phage
activities. _Nat. Microbiol_ 3, 90–98 (2018). Article CAS PubMed Google Scholar * Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. _Science_
359 eaar4120 (2018). Article PubMed PubMed Central CAS Google Scholar * Cohen, D. et al. Cyclic gmp-amp signalling protects bacteria against viral infection. _Nature_ 574, 691–695
(2019). Article ADS CAS PubMed Google Scholar * North, B. J. & Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. _Genome Biol._ 5, 224 (2004). Article PubMed
PubMed Central Google Scholar * Karras, G. I. et al. The macro domain is an ADP-ribose binding module. _EMBO J._ 24, 1911–1920 (2005). Article CAS PubMed PubMed Central Google Scholar
* Greiss, S. & Gartner, A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. _Mol. Cells_ 28, 407–415 (2009). Article CAS PubMed Google Scholar *
Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D. & Escalante-Semerena, J. C. Sir2-dependent activation of acetyl-coa synthetase by deacetylation of active lysine. _Science_ 298,
2390–2392 (2002). Article ADS CAS PubMed Google Scholar * Aravind, L. The wwe domain: a common interaction module in protein ubiquitination and ADP ribosylation. _Trends Biochem. Sci._
26, 273–275 (2001). Article CAS PubMed Google Scholar * Rack, J. G., Perina, D. & Ahel, I. Macrodomains: structure, function, evolution, and catalytic activities. _Annu. Rev.
Biochem._ 85, 431–454 (2016). Article CAS PubMed Google Scholar * Spear, A. M., Loman, N. J., Atkins, H. S. & Pallen, M. J. Microbial TIR domains: not necessarily agents of
subversion? _Trends Microbiol._ 17, 393–398 (2009). Article CAS PubMed Google Scholar * Essuman, K. et al. Tir domain proteins are an ancient family of NAD(+)-consuming enzymes. _Curr.
Biol._ 28, 421–430.e4 (2018). Article CAS PubMed PubMed Central Google Scholar * Essuman, K. et al. The sarm1 toll/interleukin-1 receptor domain possesses intrinsic NAD(+) cleavage
activity that promotes pathological axonal degeneration. _Neuron_ 93, 1334–1343.e5 (2017). Article CAS PubMed PubMed Central Google Scholar * Du, J., Jiang, H. & Lin, H.
Investigating the ADP-ribosyltransferase activity of sirtuins with nad analogues and 32p-NAD. _Biochemistry_ 48, 2878–2890 (2009). Article CAS PubMed Google Scholar * Zhou, Y. et al.
Determining the extremes of the cellular NAD(h) level by using an _Escherichia coli_ NAD(+)-auxotrophic mutant. _Appl. Environ. Microbiol._ 77, 6133–6140 (2011). Article CAS PubMed PubMed
Central Google Scholar * Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. _J. Mol. Biol._ 372, 774–797 (2007). Article CAS PubMed Google
Scholar * Burroughs, A. M., Zhang, D., Schaffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological
conflicts, immunity and signaling. _Nucleic Acids Res._ 43, 10633–10654 (2015). Article CAS PubMed PubMed Central Google Scholar * Min, J., Landry, J., Sternglanz, R. & Xu, R. M.
Crystal structure of a Sir2 homolog-NAD complex. _Cell_ 105, 269–279 (2001). Article CAS PubMed Google Scholar * Sanders, B. D., Zhao, K., Slama, J. T. & Marmorstein, R. Structural
basis for nicotinamide inhibition and base exchange in Sir2 enzymes. _Mol. Cell_ 25, 463–472 (2007). Article CAS PubMed PubMed Central Google Scholar * Sauve, A. A., Wolberger, C.,
Schramm, V. L. & Boeke, J. D. The biochemistry of sirtuins. _Annu. Rev. Biochem._ 75, 435–465 (2006). Article CAS PubMed Google Scholar * Horsefield, S. et al. NAD(+) cleavage
activity by animal and plant tir domains in cell death pathways. _Science_ 365, 793–799 (2019). Article ADS CAS PubMed Google Scholar * Wan, L. et al. Tir domains of plant immune
receptors are NAD(+)-cleaving enzymes that promote cell death. _Science_ 365, 799–803 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Holm, L., Kaariainen, S.,
Rosenstrom, P. & Schenkel, A. Searching protein structure databases with dalilite v.3. _Bioinformatics_ 24, 2780–2781 (2008). Article CAS PubMed PubMed Central Google Scholar *
Sikowitz, M. D., Cooper, L. E., Begley, T. P., Kaminski, P. A. & Ealick, S. E. Reversal of the substrate specificity of cmp n-glycosidase to DCMP. _Biochemistry_ 52, 4037–4047 (2013).
Article CAS PubMed Google Scholar * Kurakawa, T. et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. _Nature_ 445, 652–655 (2007). Article CAS PubMed
Google Scholar * Samanovic, M. I. et al. Proteasomal control of cytokinin synthesis protects _Mycobacterium tuberculosis_ against nitric oxide. _Mol. Cell_ 57, 984–994 (2015). Article CAS
PubMed PubMed Central Google Scholar * Mortier-Barriere, I. et al. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA.
_Cell_ 130, 824–836 (2007). Article CAS PubMed Google Scholar * Fischer, K. et al. Function and structure of the molybdenum cofactor carrier protein from chlamydomonas reinhardtii. _J.
Biol. Chem._ 281, 30186–30194 (2006). Article CAS PubMed Google Scholar * Brzozowski, R. S. et al. Deciphering the role of a slog superfamily protein ypsa in gram-positive bacteria.
_Front. Microbiol._ 10, 623 (2019). Article PubMed PubMed Central Google Scholar * Pajuelo, D. et al. NAD(+) depletion triggers macrophage necroptosis, a cell death pathway exploited by
_Mycobacterium tuberculosis_. _Cell Rep._ 24, 429–440 (2018). Article CAS PubMed PubMed Central Google Scholar * Tang, J. Y., Bullen, N. P., Ahmad, S. & Whitney, J. C. Diverse
nadase effector families mediate interbacterial antagonism via the type VI secretion system. _J. Biol. Chem._ 293, 1504–1514 (2018). Article CAS PubMed Google Scholar * Gerdts, J.,
Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. Sarm1 activation triggers axon degeneration locally via NAD(+) destruction. _Science_ 348, 453–457 (2015). Article ADS CAS
PubMed PubMed Central Google Scholar * Mark, B. L. et al. Crystallographic evidence for substrate-assisted catalysis in a bacterial beta-hexosaminidase. _J. Biol. Chem._ 276, 10330–10337
(2001). Article CAS PubMed Google Scholar * Otwinowski, Z. & Minor, W. Processing of x-ray diffraction data collected in oscillation mode. _Method Enzymol._ 276, 307–326 (1997).
Article CAS Google Scholar * Adams, P. D. et al. Phenix: a comprehensive python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol. Crystallogr._ 66, 213–221
(2010). Article CAS PubMed PubMed Central Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D Biol. Crystallogr._ 60,
2126–2132 (2004). Article CAS PubMed Google Scholar * Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. _Acta
Crystallogr. D Biol. Crystallogr._ 53, 240–255 (1997). Article CAS PubMed Google Scholar * Chen, V. B. et al. Molprobity: all-atom structure validation for macromolecular
crystallography. _Acta Crystallogr. D Biol. Crystallogr._ 66, 12–21 (2010). Article CAS PubMed Google Scholar * Berman, H. M. et al. The protein data bank. _Nucleic Acids Res._ 28,
235–242 (2000). Article ADS CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the staff of the beamline 7A of the Pohang Accelerator Laboratory for
their support with the data collection, Gyujin Lee for assistance in activity assay, and Jasung Koo for help in CD spectroscopy. This work was supported by the National Research Foundation
of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C1086298) and the BK21 Plus Program of the Department of Agricultural Biotechnology, Seoul National University. AUTHOR
INFORMATION Author notes * These authors contributed equally: Donghyun Ka, Hyejin Oh. AUTHORS AND AFFILIATIONS * Department of Agricultural Biotechnology, Seoul National University, Seoul,
08826, Korea Donghyun Ka, Hyejin Oh, Eunyoung Park, Jeong-Han Kim & Euiyoung Bae * Department of Applied Biology and Chemistry, Seoul National University, Seoul, 08826, Korea Hyejin Oh *
Research Institute of Agriculture and Life Sciences, Seoul National University, Seoul, 08826, Korea Jeong-Han Kim & Euiyoung Bae Authors * Donghyun Ka View author publications You can
also search for this author inPubMed Google Scholar * Hyejin Oh View author publications You can also search for this author inPubMed Google Scholar * Eunyoung Park View author publications
You can also search for this author inPubMed Google Scholar * Jeong-Han Kim View author publications You can also search for this author inPubMed Google Scholar * Euiyoung Bae View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.K. and E.B. conceived the study. D.K. and H.O. performed the protein purification, crystallization,
enzymatic assays, SEC, and EMSA. D.K. determined the crystal structures. D.K., H.O., E.P., and J.-H.K. conducted the HPLC and LC-MS analyses. H.O. performed CD spectroscopy. D.K., H.O., and
E.B. analyzed the data, and wrote the paper with input from all authors. CORRESPONDING AUTHOR Correspondence to Euiyoung Bae. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Joseph Bondy-Denomy, Ryan Jackson and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ka, D., Oh, H., Park, E. _et al._ Structural and
functional evidence of bacterial antiphage protection by Thoeris defense system via NAD+ degradation. _Nat Commun_ 11, 2816 (2020). https://doi.org/10.1038/s41467-020-16703-w Download
citation * Received: 13 February 2020 * Accepted: 18 May 2020 * Published: 04 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16703-w SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative