Play all audios:
ABSTRACT Uranus and Neptune are generally assumed to have helium only in their gaseous atmospheres. Here, we report the possibility of helium being fixed in the upper mantles of these
planets in the form of NH3–He compounds. Structure predictions reveal two energetically stable NH3–He compounds with stoichiometries (NH3)2He and NH3He at high pressures. At low
temperatures, (NH3)2He is ionic with NH3 molecules partially dissociating into (NH2)− and (NH4)+ ions. Simulations show that (NH3)2He transforms into intermediate phase at 100 GPa and 1000 K
with H atoms slightly vibrate around N atoms, and then to a superionic phase at ~2000 K with H and He exhibiting liquid behavior within the fixed N sublattice. Finally, (NH3)2He becomes a
fluid phase at temperatures of 3000 K. The stability of (NH3)2He at high pressure and temperature could contribute to update models of the interiors of Uranus and Neptune. SIMILAR CONTENT
BEING VIEWED BY OTHERS MELTING CURVE OF SUPERIONIC AMMONIA AT PLANETARY INTERIOR CONDITIONS Article 29 May 2023 EVIDENCE OF HYDROGEN−HELIUM IMMISCIBILITY AT JUPITER-INTERIOR CONDITIONS
Article 26 May 2021 DOUBLE SUPERIONICITY IN ICY COMPOUNDS AT PLANETARY INTERIOR CONDITIONS Article Open access 21 November 2023 INTRODUCTION Knowledge of the interior compositions of planets
is crucial to understanding the processes of their formation and evolution. Various methods have been used to investigate the Earth’s interior, while studies of the composition and
structure of the solar system’s ice giants, Uranus and Neptune, are limited by using only global observable properties such as gravitational and magnetic moments1. Uranus and Neptune are
generally assumed to have a three-layer structure: a rocky core, an ice mantle (contains an upper mantle and a lower mantle), and a gas atmosphere1,2,3,4,5,6. Although many studies have
focused on the interiors of Uranus and Neptune, their internal compositions remain to be fully understood7,8,9,10,11,12. A widely accepted model for each of these planets is that the upper
mantle comprises a mixture of ionized H2O, NH3, and CH45,8,11, whereas the lower mantle consists of metallic H2O, NH35,6 Much effort has been devoted to determine the ratio of the components
in the interior of the ice planets8,10,11, however, no consensus were reached. To understand realistic compositions of Uranus and Neptune, researchers have focused on high-pressure and
high-temperature phases of NH3 and H2O, and mixtures of the two13,14,15. Cavazzoni et al16. performed molecular dynamic simulations to estimate the phase diagram of water and ammonia at
pressures and temperatures in the range of 30– 300 GPa and 300–7000 K. They found that water and ammonia exhibited a superionic phase (at about 100 GPa, 1500 K) between the ionic solid phase
and ionic fluid phase. However, for the component CH4, the results of equation of state have shown that it would dissociate into hydrocarbons at the extreme conditions17. A computational
search undertaken by Pickard and Needs in 2008 found that ammonia transformed into an ionic phase consisting of (NH2)− and (NH4)+ ions at pressures above 90 GPa13; the transformation was
subsequently confirmed by experiment14,15. By combining empirical and theoretical results, Ninet et al18. found that a novel superionic conductive phase of ammonia becomes stable at about 70
GPa and 8500 K. Using Raman spectroscopy and synchrotron X-ray diffraction, Laniel et al19. found two unusual ionic N–H stoichiometries, (N2)6(H2)7 and N2(H2)2, which are stable at about
50–GPa. While for water, it has a rich phase diagram, with at least 17 solid phases identified experimentally20,21,22, and seven other high-pressure phases predicted by theoretical
studies23,24,25,26. Ninet et al18. and Millot et al27. proposed that superionic water ice can exist in the mantles of the ice giants as a result of shock compression. Recently, Huang et
al28. found that H2O can react with H2 and form a novel superionic compound of H3O under high pressure and high temperature. For a 2:1 mixture of NH3 and H2O, Robinson et al29. predicted a
novel ionic compound, O2−(NH4+)2, to form at pressures above 65 GPa. Recent theoretical and experimental studies have shown that NH3H2O decomposes into ammonia and water at 120 GPa30,31.
Bethkenhagen et al32. used an evolutionary random structure search code to propose a superionic phase of NH3H2O at 800 GPa and high temperature (1000–6000 K). An unusual layered ionic phase
of NH3(H2O)2 was predicted for a 1:2 mixture of NH3 and H2O; it was then modeled to transform into a superionic phase at high pressure and high temperature (41 GPa and 600 K)33. These
findings contribute to our understanding of the interiors of the giant ice planets. The above results have led to the assumption that the elements (i.e., C, H, and N) in the ice giants’
gaseous atmospheres except He appear in their solid mantles. Helium is generally considered likely to remain only in the atmosphere and not form solid compounds in the mantle, because it is
the most chemically inert element due to its stable closed-shell electronic configuration. In fact, Nettelmann et al11. have proposed a three-layer structure model, in which the considering
of small amount of He/H in the outer core of the planets reproducing well the gravitational moments of the ice giants. Recent studies have indicated that high pressure can induce He to form
compounds such as HeN434,Na2He35, FeHe36, MgOHe37, H2OHe38,39, and FeO2He40. Specially, the compound of H2OHe2 exhibited a superionic property under high pressure and high temperature and
then transformed into fluid39. These results inspired us to explore whether some of the abundant elemental He from the planets’ atmospheres could be trapped inside the mantles of Uranus and
Neptune. Therefore, we carried out calculations to search for stable compounds in NH3–He systems at high pressure and high temperature. Our results show that He can react with NH3 to form
(NH3)2He under extreme conditions, to a certain extent corresponding to the upper mantles of Uranus and Neptune, thereby providing information essential to the understanding of the interior
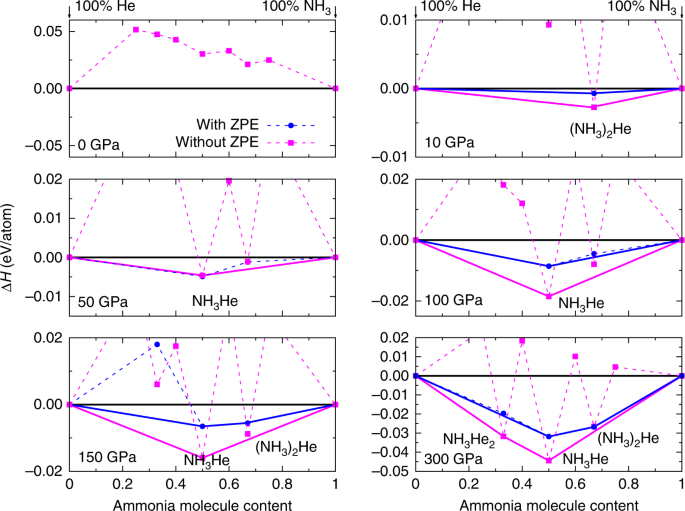
models of these planets. RESULTS STABLE NH3–HE COMPOUNDS AT HIGH PRESSURE The formation enthalpies of the energetically most-stable structures of (NH3)_x_He_y_ (_x_ = 1 ~ 3, _y_ = 1 ~ 3) as
compared to mixtures of NH3 and He at selected pressures are summarized in Fig. 1. The phases lying on the convex hull are thermodynamically stable against decomposition into other
compositions. The figure also shows the effects of zero-point vibrational energy (ZPE). The positive formation enthalpies show that, as expected, no thermodynamically stable compositions
were found at ambient pressure. However, static-lattice enthalpy calculations revealed three stable compositions at high pressures: (NH3)2He at 10 and 300 GPa, NH3He at 50, 100, and 150 GPa,
and NH3He2 at 300 GPa (Supplementary Fig. 1). The inclusion of ZPE alters significantly the stability of (NH3)2He and NH3He2, i.e., (NH3)2He becomes energetically stable also at 150 and 300
GPa, while NH3He2 turns to be unstable at all pressures. Figs. 2 and 3, respectively present detailed stable pressure ranges for the three obtained compositions and their corresponding
crystal structures. Optimized lattice parameters for all the structures at selected pressures are listed in Supplementary Table 1. The results indicate that (NH3)2He with space group _I_4,
labeled here as _I_4–(NH3)2He, becomes energetically stable with respect to NH3 and He at pressures as low as 9 GPa (Fig. 2). Tetragonal _I_4–(NH3)2He consists of isolated NH3 molecules and
He atoms. Figure 3a depicts the NH3 layers of this structure in the _a_–_b_ planes, with He atoms located in the pockets formed by neighboring NH3 molecules. Interestingly, the _I_4
structure displays unique channels formed by NH3 molecules that are arranged parallel to the _c_-axis, and linear He chains localize within the interstices formed by four neighboring
channels (Fig. 3b). To our knowledge, this is the first report of such a channel-bearing NH3 structure. (NH3)2He remains energetically stable up to 40 GPa, above which it decomposes into a
mixture of NH3 and He. However, (NH3)2He re-emerges as energetically stable phase at 110 GPa with the formation of an orthorhombic _F__m__m_2 structure. A similar
combination-decomposition-recombination pattern has previously been reported for CaLi244. Partial dissociation of NH3 molecules into (NH2)− and (NH4)+ ions is found to accompany the
formation of _F__m__m_2–(NH3)2He. Bader analysis demonstrates the strongly ionic nature of the species, with Bader charges of −0.57 _e_− and 0.53 _e_− for (NH2)− and (NH4)+, respectively,
similar to those observed in the ionic phase of pure NH313. The _F__m__m_2 phase is also layered, consisting of layers formed by NH3, (NH2)−, and (NH4)+ units in the _a_–_c_ planes. The
spacing between neighboring layers is 2.07 Å. Viewing the structure along the _a_-axis reveals unique channels formed by NH3, (NH2)−, and (NH4)+ units, with He atoms located in the
interstices. NH3He composition becomes energetically stable at 35 GPa, as shown in Fig. 2, adopting an orthorhombic _P__m__m__a_ structure. The _P__m__m__a_ phase is the most stable
configuration over a large pressure range up to 180 GPa for NH3He, above which it transforms into the _P_212121 structure. The _P_212121–NH3He phase will remain stable up to 300 GPa, which
is the maximum pressure considered in this study. Both the _P__m__m__a_ and the _P_212121 structures of NH3He are composed of isolated NH3 molecules and He atoms, and there is no evidence of
dissociation of NH3 in the whole pressure range studied here. The calculated phonon dispersions confirm the dynamical stability of all these structures in their energetically stable
pressure ranges (Supplementary Fig. 3). ELECTRONIC PROPERTIES To examine the interactions among N, H, and He atoms in the two compounds, we calculate electronic properties including the
electronic localization function (ELF) and Bader charges. The ELF is a quantum chemistry tool to visualize covalent bonds; values close to 1 corresponding to strong covalent bonding. The ELF
results rule out covalent bonds between N–H units (NH3, (NH2)−, (NH4)+) and He atoms, given the absence of any ELF local maxima between them (Supplementary Fig. 5). Interestingly, Bader
analysis indicates a slight charge transfer from N–H units to He atoms. Table 1 lists the Bader charge of one He atom in _I_4–(NH3)2He as ~ -0.02 _e_− at 10 GPa, which increases to -0.03
_e_− when the _F__m__m_2 structure is adopted at 120 GPa. Similar to that in (NH3)2He, each He atom in NH3He and NH3He2 gains nearly 0.03 _e_− from the NH3 molecules. The Bader charge of a
He atom in the three NH3–He compounds is similar to the charges predicted for H2O–He, MgF2He, MgOHe, and FeO2He (between −0.02 _e_− and -0.07 _e_−)36,37,38,40. The current results indicate
the three compounds have an ionic nature and that He atoms could serve as a Coulomb shield in stabilizing them at high pressure. Electronic band structures show that all three compounds are
insulators (Supplementary Fig. 4). At the PBE-GGA level, the band gap of (NH3)2He is calculated to be 6.0 eV at 10 GPa, which increases to 7.5 eV at 180 GPa. For NH3He, the band gaps is
calculated to be 7.2 eV at 35 GPa. SUPERIONIC PHASES OF (NH3)2HE The stable pressure and temperature regions of _F__m__m_2–(NH3)2He cover the geotherms in the upper mantle of Neptune and
Uranus. We, therefore, performed ab initio molecular dynamics simulations at the pressure of 100 GPa, 200 GPa, and 300 GPa, respectively, to examine the formation of _F__m__m_2–NH3)2He
inside Neptune and Uranus. The calculated mean squared displacement (MSD) of the atomic positions and the behaviors of three different atoms of _F__m__m_2–(NH3)2He are shown in the Fig. 4.
At _P_ = 100 GPa and _T_ = 200 K, _F__m__m_2–(NH3)2He is a solid phase with all atoms vibrating around their lattice positions and with diffusion coefficients (_D_H = _D_He = _D_N = 0). When
the temperature increasing to 1000 K, the H atoms seems diffusive with _D_H = 1.4 × 10−6 cm2 s−1. However, from the atomic trajectories shown in Fig. 4b, one can find that H atoms in NH3
become diffuse while H atoms in (NH2)− and (NH4)+ keep vibrating around their lattice positions. This means that the H atoms in NH3 units become considerable vibrate with a fixed N position
at this condition. With the temperature further increased to 2000 K, _F__m__m_2–(NH3)2He transforms into a real superionic phase with fully diffusive H atoms (_D_H = 2.0 × 10−4 cm2 s−1)
within the fixed N and He framework. With the temperature increased to 3000 K, all atoms including N, He, and H are diffusive with high diffusion coefficients (_D_N = 4.4 × 10−5 cm2 s−1,
_D_He = 2.1 × 10−5 cm2 s−1 and _D_H = 4.5 × 10−4 cm2 s−1). This result reveals that at this conditions the superionic _F__m__m_2–(NH3)2He phase transformed into a fluid phase. Here, we found
the diffusion of H atoms occurs prior to that of He atom, which is opposite to that found for He2(H2O)39, where He atoms diffuse firstly. Generally, lighter atoms are easier to diffuse. The
abnormal diffusive behavior in He2(H2O) was explained by that the H atoms has higher diffusion barrier than He atoms because of the strong covalent H-O bonds39. In fact, He atoms in
He2(H2O) share large space that allows the free diffusion, as shown in Supplementary Fig. 6. As compared to He2(H2O), although form weak interaction with N–H units, He atoms are trapped in
cages formed by NH3, (NH2)− and (NH4)+ units, this makes helium atoms are more difficult to diffuse. While for _P_ = 200 GPa and _T_ = 300 K, _F__m__m_2–(NH3)2He keeps its solid property.
With the temperature increasing to 1000 K and up to 4000 K, _F__m__m_2–(NH3)2He becomes to a superionic phase and then turns in to a fluid when the temperature is above 4200 K, as shown in
Supplementary Fig. 7. Under pressure of 300 GPa, the trend is similar to that under 200 GPa, but the critical point of the superionic phase to fluid is at the temperature of 4600 K, as shown
in Supplementary Fig. 8. Figure 5 presents the pressure–temperature (P–T) phase diagram for the mixture of NH3 and He, showing the (NH3)2He and NH3He phases. Temperature has a significant
effect on the system: _I_4–(NH3)2He and NH3He decompose at high temperature (Fig. 5 and Supplementary Fig. 2). Their maximum temperatures of stability vary, being >700 K for
_I_4–(NH3)2He (which decomposes fully to NH3 and He), >1000 K for NH3He (for full decomposition to NH3 and He at _P_ < 100 GPa and decomposition into _F__m__m_2–(NH3)2He and He at _P_
> 100 GPa). In contrast, _F__m__m_2–(NH3)2He has a large stability field and thermodynamically stable in pressure range of 80–300 GPa and at any temperature in the tested range (0–5000
K). Figure 5 also presents estimated geotherms for the interiors of Uranus and Neptune. We also pointed the phase states of _F__m__m_2–(NH3)2He in the Fig. 5. Our calculation show that the
_F__m__m_2–(NH3)2He phase presents superionic and fluid properties at the condition which is close to the geotherms in the upper mantle of Neptune and Uranus. This suggests that He could be
trapped as superionic (NH3)2He inside the upper mantles of these planets with the mixture of superionic and fluid forms during their formation. Previous studies have assumed the presence of
NH3, CH4, H2O, and H2 inside the giant ice planets. Our predicted stability of superionic (NH3)2He as well as the recent reported superionic H2OHe239 under the P–T conditions corresponding
to the ice giants’ upper mantles indicate that helium could be remained inside the planets during their formation. Coincidently, the stability of NH3–He and H2O–He compounds provide an
evidence to support the new three-layer model suggested by Nettelmann11, in which helium was considered as a small component in outer core of the planets. Therefore, the current results are
essential to the understanding of the interior models of these planets. Moreover, CH4 and H2 are another two main components in upper mantle of these planets, therefore, there is a high
possibility that helium could react with CH4 or H2 at high pressures to form new compounds, which deserves further investigation. In our submission process, we were aware of the work by Liu
et al.46 predicting plastic and superionic helium-ammonia compounds at extreme condition. They predicted three stable stoichiometries and eight new stable phases of He–NH3 compounds under
pressures up to 500 GPa and found that the predicted He–NH3 compounds exhibit superionic behavior at high pressure and high temperature. These similar results further provide knowledge for
our understanding of the composition of the planet’s interior. In summary, a combination of first-principles calculations and crystal structure predictions was carried out to search for
stable compounds in the NH3–He systems under high-_P_–_T_ conditions. Calculations at 0 K revealed two compounds ((NH3)2He and NH3He) that are energetically stable relative to the equivalent
mixture of solid NH3 and He at high pressures. Specially, (NH3)2He remains energetically stable under the extreme conditions corresponding to the upper mantles of Uranus and Neptune. The
current results provide evidence that He could be trapped inside these planets as NH3–He compounds with the mixture of superionic and fluid properties, in contrast to the current view that
He occurs only in their atmospheres. Molecule dynamic simulations results show that the _F__m__m_2-(NH3)2He phase will transform into a superionic solid and then to a fluid with the
increasing temperature. METHODS STRUCTURAL PREDICTIONS Structure predictions for NH3–He compounds were performed using a particle-swarm optimization algorithm implemented in calypso
code47,48. This method is unbiased, not using any known structural information, and has successfully been used to predict various systems under high pressure49,50,51,52,53,54,55,56,57. We
performed structural searches on (NH3)_x_He_y_ (_x_, _y_ = 1, 2, 3) at 0–300 GPa with maximum simulation cells up to four formula units. Each generation of structures was evolved by
selecting the 60% lowest-enthalpy structures in the last step and randomly producing the remaining 40%. The structure searches were considered converged when ~1000 successive structures
were generated without finding a new lowest-enthalpy structure. AB INITIO CALCULATIONS Density functional theory calculations were performed using vasp code58 combined with the generalized
gradient approximation (GGA)59 for the exchange-correlation potential in the form of the Perdew–Burke-–Ernzerhof60 (PBE) functional. The electronic wave functions were expanded in a plane
wave basis set with a cutoff energy of 1000 eV. The electronic interaction was described by means of projector augmented wave 61 pseudopotentials with valence electrons of 1_s_1, 2_s_22_p_3
and 1_s_2 for H, N, and He atoms, respectively. Monkhorst-Pack k-point62 meshes with a grid density of 0.03 Å−1 were chosen to achieve a total energy convergence of better than 1 meV per
atom. The phonon dispersion curves were computed by direct supercell calculation63, as implemented in the phonopy program64. MOLECULAR DYNAMICS The molecular dynamics simulations were also
carried out to explore the superionic property of (NH3)2He compound at high pressures and high temperatures. The simulation supercells contain 32 NH3 molecules and 16 helium atoms and the
Brillouin zone was sampled by Γ point. Each simulation consists of 10,000 time steps with a time step of 0.5 fs. DATA AVAILABILITY The authors declare that the main data supporting the
findings of this study are contained within the paper and its associated Supplementary Information. All other relevant data are available from the corresponding author upon reasonable
request. REFERENCES * Guillot, T. Interiors of giant planets inside and outside the solar system. _Science_ 286, 72–77 (1999). Article ADS CAS PubMed Google Scholar * Hubbard, W. B. _in
Origin and Evolution of Planetary and Satellite Atmospheres_ (eds Atreya, S. K., Pollack, J. B. & Matthews, M. S.) 539–563 (Univ. of Arizona Press, Tucson, AZ, 1989). * Stevenson, D.
Interiors of the giant planets. _Annu. Rev. Earth Planet. Sci._ 10, 257–295 (1982). Article ADS CAS Google Scholar * Hubbard, W. B., M. P., Pearl, J. C. & Stevenson, D. J. _in
Neptune and Triton, D. P. Cruikshank, Ed._ 109–138 (Univ. of Arizona Press, Tucson, AZ, 1995). * Hubbard, W. B. Interiors of the giant planets. _Science_ 214, 145–149 (1981). Article ADS
CAS PubMed Google Scholar * Hubbard, W. B. & MacFarlane, J. J. Structure and evolution of uranus and neptune. _J. Geophys. Res._ 85, 225–234 (1980). Article ADS CAS Google Scholar
* Andrew, W. M., Eric, G. & Megan, A. Spectro-thermometry of m dwarfs and their candidate planets: too hot, too cool, or just right? _Astrophys. J._ 779, 261–268 (2013). Google Scholar
* Bethkenhagen, M. et al. Planetary ices and the linear mixing approximation. _Astrophys. J._ 848, 67 (2017). Article ADS CAS Google Scholar * Transtrum, M. K. et al. Perspective:
Sloppiness and emergent theories in physics, biology, and beyond. _J. Chem. Phys._ 143, 07B201 (2015). Article CAS Google Scholar * Chau, R., Hamel, S. & Nellis, W. J. Chemical
processes in the deep interior of Uranus. _Nat. Commun._ 2, 203 (2011). Article ADS PubMed CAS Google Scholar * Nettelmann, N. et al. Uranus evolution models with simple thermal
boundary layers. _Icarus_ 275, 107–116 (2016). Article ADS CAS Google Scholar * Helled, R., Anderson, J. D., Podolak, M. & Schubert, G. Interior models of Uranus and Neptune.
_Astrophys. J._ 726, 15 (2010). Article ADS Google Scholar * Pickard, C. J. & Needs, R. J. Highly compressed ammonia forms an ionic crystal. _Nat. Mater._ 7, 775–779 (2008). Article
ADS CAS PubMed Google Scholar * Palasyuk, T. et al. Ammonia as a case study for the spontaneous ionization of a simple hydrogen-bonded compound. _Nat. Commun._ 5, 3460 (2014). Article
ADS PubMed CAS Google Scholar * Ninet, S. et al. Experimental and theoretical evidence for an ionic crystal of ammonia at high pressure. _Phys. Rev. B_ 89, 174103 (2014). Article ADS
CAS Google Scholar * Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. _Science_ 283, 44–46 (1999). Article ADS CAS PubMed Google
Scholar * Ancilotto, F., Chiarotti, G. L., Scandolo, S. & Tosatti, E. Dissociation of methane into hydrocarbons at extreme (planetary) pressure and temperature. _Science_ 275, 1288–1290
(1997). Article ADS CAS PubMed Google Scholar * Ninet, S., Datchi, F. & Saitta, A. M. Proton disorder and superionicity in hot dense ammonia ice. _Phys. Rev. Lett._ 108, 165702
(2012). Article ADS CAS PubMed Google Scholar * Laniel, D., Svitlyk, V., Weck, G. & Loubeyre, P. Pressure-induced chemical reactions in the N2 (H2)2 compound: from the N2 and H2
species to ammonia and back down into hydrazine. _Phys. Chem. Chem. Phys._ 20, 4050–4057 (2018). Article CAS PubMed Google Scholar * Petrenko, V. F., Whitworth, R. W. _Physics of ice_
(OUP Oxford, 1999). * Salzmann, C. G., Radaelli, P. G., Mayer, E. & Finney, J. L. Ice XV: a new thermodynamically stable phase of ice. _Phys. Rev. Lett._ 103, 105701 (2009). Article ADS
PubMed CAS Google Scholar * Millot, M. et al. Experimental evidence for superionic water ice using shock compression. _Nat. Phys._ 14, 297 (2018). Article CAS Google Scholar *
Benoit, M., Bernasconi, M., Focher, P. & Parrinello, M. New high-pressure phase of ice. _Phys. Rev. Lett._ 76, 2934 (1996). Article ADS CAS PubMed Google Scholar * Militzer, B.
& Wilson, H. F. New phases of water ice predicted at megabar pressures. _Phys. Rev. Lett._ 105, 195701 (2010). Article ADS PubMed CAS Google Scholar * Wang, Y. et al. High pressure
partially ionic phase of water ice. _Nat. Commun._ 2, 563 (2011). Article ADS PubMed CAS Google Scholar * Pickard, C. J., Martinez-Canales, M. & Needs, R. J. Decomposition and
terapascal phases of water ice. _Phys. Rev. Lett._ 110, 245701 (2013). Article ADS PubMed CAS Google Scholar * Millot, M. et al. Nanosecond x-ray diffraction of shock-compressed
superionic water ice. _Nature_ 569, 251 (2019). Article ADS CAS PubMed Google Scholar * Huang, P. et al. Stability of H3O at extreme conditions and implications for the magnetic fields
of Uranus and Neptune. _Proc. Natl Acad. Sci. USA_ 117, 5638–5643 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Robinson, V. N., Wang, Y., Ma, Y. & Hermann, A.
Stabilization of ammonia-rich hydrate inside icy planets. _Proc. Natl Acad. Sci. USA_ 114, 9003–9008 (2017). Article ADS CAS Google Scholar * Shi, J., Cui, W., Botti, S. & Marques,
M. A. Nitrogen-hydrogen-oxygen ternary phase diagram: New phases at high pressure from structural prediction. _Phys. Rev. Mater._ 2, 023604 (2018). Article CAS Google Scholar * Liu, C. et
al. Topologically frustrated ionisation in a water-ammonia ice mixture. _Nat. Commun._ 8, 1065 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Bethkenhagen, M.,
Cebulla, D., Redmer, R. & Hamel, S. Superionic phases of the 1: 1 water-ammonia mixture. _J. Phys. Chem. A_ 119, 10582–10588 (2015). Article CAS PubMed Google Scholar * Jiang, X.,
Wu, X., Zheng, Z., Huang, Y. & Zhao, J. Ionic and superionic phases in ammonia dihydrate NH3 ⋅ 2H2 O under high pressure. _Phys. Rev. B_ 95, 144104 (2017). Article ADS Google Scholar
* Li, Y. et al. Route to high-energy density polymeric nitrogen t-N via He-N compounds. _Nat. commun._ 9, 722 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Dong, X. et
al. A stable compound of helium and sodium at high pressure. _Nat. Chem._ 9, 440 (2017). Article CAS PubMed Google Scholar * Monserrat, B., Martinez-Canales, M., Needs, R. J. &
Pickard, C. J. Helium-Iron compounds at terapascal pressures. _Phys. Rev. Lett._ 121, 015301 (2018). Article ADS CAS PubMed Google Scholar * Liu, Z. et al. Reactivity of he with ionic
compounds under high pressure. _Nat. Commun._ 9, 951 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Liu, H., Yao, Y. & Klug, D. D. Stable structures of He and H2O
at high pressure. _Phys. Rev. B_ 91, 014102 (2015). Article ADS CAS Google Scholar * Liu, C. et al. Multiple superionic states in helium-water compounds. _Nat. Phys._ 15, 1065–1070
(2019). Article CAS Google Scholar * Zhang, J. et al. Rare helium-bearing compound FeO2 He stabilized at deep-earth conditions. _Phys. Rev. Lett._ 121, 255703 (2018). Article ADS PubMed
Google Scholar * Datchi, F. et al. Solid ammonia at high pressure: a single-crystal x-ray diffraction study to 123 GPa. _Phys. Rev. B_ 73, 174111 (2006). Article ADS CAS Google Scholar
* Loubeyre, P. et al. Equation of state and phase diagram of solid 4He from single-crystal x-ray diffraction over a large P-T domain. _Phys. Rev. Lett._ 71, 2272–2275 (1993). Article ADS
CAS PubMed Google Scholar * Monserrat, B., Drummond, N., Pickard, C. J. & Needs, R. Electron-phonon coupling and the metallization of solid helium at terapascal pressures. _Phys.
Rev. Lett._ 112, 055504 (2014). Article ADS PubMed CAS Google Scholar * Xie, Y., Oganov, A. R. & Ma, Y. Novel high pressure structures and superconductivity of CaLi2. _Phys. Rev.
Lett._ 104, 177005 (2010). Article ADS PubMed CAS Google Scholar * Redmer, R., Mattsson, T. R., Nettelmann, N. & French, M. The phase diagram of water and the magnetic fields of
Uranus and Neptune. _Icarus_ 211, 798–803 (2011). Article ADS CAS Google Scholar * Liu, C. et al. Plastic and superionic helium ammonia compounds under high pressure and high
temperature. _Phys. Rev. X_ 10, 021007 (2020). Google Scholar * Wang, Y., Lv, J., Zhu, L. & Ma, Y. Crystal structure prediction via particle-swarm optimization. _Phys. Rev. B_ 82,
094116 (2010). Article ADS CAS Google Scholar * Wang, Y., Lv, J., Zhu, L. & Ma, Y. Calypso: A method for crystal structure prediction. _Comput. Phys. Commun._ 183, 2063–2070 (2012).
Article ADS CAS Google Scholar * Cui, W. et al. Route to high-_T_ _c_ superconductivity via CH4 -intercalated H3S hydride perovskites. _Phys. Rev. B_ 101, 134504 (2020). Article ADS
CAS Google Scholar * Cui, W. & Li, Y. The role of CALYPSO in the discovery of high-_T_ _c_ hydrogen-rich superconductors. _Chin. Phys. B_ 28, 107104 (2019). Article ADS CAS Google
Scholar * Xu, M. et al. Electrical control of magnetic phase transition in a type-I multiferroic double-metal trihalide monolayer. _Phys. Rev. Lett._ 124, 067602 (2020). Article ADS CAS
PubMed Google Scholar * Lv, J., Wang, Y., Zhu, L. & Ma, Y. Predicted novel high-pressure phases of lithium. _Phys. Rev. Lett._ 106, 015503 (2011). Article ADS PubMed CAS Google
Scholar * Peng, F., Miao, M., Wang, H., Li, Q. & Ma, Y. Predicted lithium-boron compounds under high pressure. _J. Am. Chem. Soc._ 134, 18599–18605 (2012). Article CAS PubMed Google
Scholar * Li, Y., Hao, J., Liu, H., Li, Y. & Ma, Y. The metallization and superconductivity of dense hydrogen sulfide. _J. Chem. Phys._ 140, 174712 (2014). Article ADS PubMed CAS
Google Scholar * Liu, B. et al. Effect of covalent bonding on the superconducting critical temperature of the H-S-Se system. _Phys. Rev. B_ 98, 174101 (2018). Article ADS CAS Google
Scholar * Li, Y., Hao, J., Liu, H., Lu, S. & John, S. T. High-energy density and superhard nitrogen-rich BN compounds. _Phys. Rev. Lett._ 115, 105502 (2015). Article ADS PubMed CAS
Google Scholar * Li, Y. et al. Metallic icosahedron phase of sodium at terapascal pressures. _Phys. Rev. Lett._ 114, 125501 (2015). Article ADS PubMed CAS Google Scholar * Kresse, G.
& Hafner, J. Ab initio molecular dynamics for liquid metals. _Phys. Rev. B_ 47, 558 (1993). Article ADS CAS Google Scholar * Kresse, G. & Joubert, D. From ultrasoft
pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758 (1999). Article ADS CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized
gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865 (1996). Article ADS CAS PubMed Google Scholar * Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the
projector augmented-wave method. _Phys. Rev. B_ 59, 1758 (1999). Article ADS CAS Google Scholar * Monkhorst, H. J. & Pack, J. D. Special points for brillouin-zone integrations.
_Phys. Rev. B_ 13, 5188 (1976). Article ADS MathSciNet Google Scholar * Parlinski, K., Li, Z. Q. & Kawazoe, Y. First-principles determination of the soft mode in cubic ZrO2. _Phys.
Rev. Lett._ 78, 4063–4066 (1997). Article ADS CAS Google Scholar * Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and
CaCl2 -type SiO2 at high pressures. _Phys. Rev. B_ 78, 134106 (2008). Article ADS CAS Google Scholar * Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal,
volumetric and morphology data. _J. Appl. Crystallogr._ 44, 1272–1276 (2011). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors acknowledge funding from the NSFC
under grant No. 11804129, No. 11722433, No. 11804128, No. 11904142 and No. 11674329. Y.L. acknowledges funding from the Six Talent Peaks Project of Jiangsu Province. X.W. acknowledges
project No. TZ2016001 of Science Challenge. All the calculations were performed at the High Performance Computing Center of the School of Physics and Electronic Engineering of Jiangsu Normal
University. Crystal structures were visualized with vesta65. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Quantum Materials Design and Application, School of Physics and
Electronic Engineering, Jiangsu Normal University, Xuzhou, 221116, China Jingming Shi, Wenwen Cui, Jian Hao, Meiling Xu & Yinwei Li * Jiangsu Key Laboratory of Advanced Laser Materials
and Devices, Jiangsu Normal University, Xuzhou, 221116, China Jian Hao * Key Laboratory of Materials Physics, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei, 230031,
China Xianlong Wang Authors * Jingming Shi View author publications You can also search for this author inPubMed Google Scholar * Wenwen Cui View author publications You can also search for
this author inPubMed Google Scholar * Jian Hao View author publications You can also search for this author inPubMed Google Scholar * Meiling Xu View author publications You can also search
for this author inPubMed Google Scholar * Xianlong Wang View author publications You can also search for this author inPubMed Google Scholar * Yinwei Li View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS J.S and Y.L. designed the project. J.S. and W.C performed the calculations. J.S., W.C., X.W., M.X., J.H., and Y.L. analyzed the
data. J.S., W.C., X.W., and Y.L. wrote the paper. CORRESPONDING AUTHORS Correspondence to Xianlong Wang or Yinwei Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer
reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shi, J., Cui, W., Hao, J. _et al._ Formation of ammonia–helium compounds at high pressure. _Nat Commun_ 11, 3164 (2020).
https://doi.org/10.1038/s41467-020-16835-z Download citation * Received: 17 March 2020 * Accepted: 28 May 2020 * Published: 22 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16835-z
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative