Play all audios:
ABSTRACT Class 2 CRISPR–Cas proteins have been widely developed as genome editing and transcriptional regulating tools. Class 1 type I CRISPR–Cas constitutes ~60% of all the CRISPR–Cas
systems. However, only type I–B and I–E systems have been used to control mammalian gene expression and for genome editing. Here we demonstrate the feasibility of using type I–F system to
regulate human gene expression. By fusing transcription activation domain to _Pseudomonas aeruginosa_ type I–F Cas proteins, we activate gene transcription in human cells. In most cases,
type I–F system is more efficient than other CRISPR-based systems. Transcription activation is enhanced by elongating the crRNA. In addition, we achieve multiplexed gene activation with a
crRNA array. Furthermore, type I–F system activates target genes specifically without off-target transcription activation. These data demonstrate the robustness and programmability of type
I–F CRISPR–Cas in human cells. SIMILAR CONTENT BEING VIEWED BY OTHERS HIGHLY EFFICIENT AND SPECIFIC REGULATION OF GENE EXPRESSION USING ENHANCED CRISPR-CAS12F SYSTEM Article 25 June 2024
ENGINEERED MINIMAL TYPE I CRISPR-CAS SYSTEM FOR TRANSCRIPTIONAL ACTIVATION AND BASE EDITING IN HUMAN CELLS Article Open access 23 August 2024 HARNESSING NONCANONICAL CRRNA FOR HIGHLY
EFFICIENT GENOME EDITING Article Open access 07 May 2024 INTRODUCTION Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (_cas_) genes-based defence
systems protect bacteria and archaea against phage and other foreign genetic elements1,2,3. Since the identification of increasing number of _cas_ genes, the CRISPR–Cas systems have been
classified into two Classes (Class 1 and Class 2) and six types (Type I–VI)4 based on the different arrangements of _cas_ genes and the subunits of effector complexes5,6,7. Class 2
CRISPR–Cas systems, the best-studied system with single effector protein (e.g., Cas9, Cas12, or Cas13) for foreign DNA or RNA interference, are subdivided into Type II (Cas9), Type V
(Cas12), and Type VI (Cas13). In the past few years, Class 2 CRISPR–Cas systems have revolutionized both basic and clinical researches, enabling more rapid, precise, and robust genome
editing and modifications in cultured cells and animals8,9,10,11,12,13,14,15,16,17. However, there were only a few applications of Class 1 CRISPR–Cas (Type I, Type III and Type IV) system.
Class 1 type I CRISPR–Cas systems are the most prevalent (~60%) in both bacteria and archaea, whereas class 2 only makes up ~10% of all CRISPR–Cas systems18,19. Differing from the Class 2
CRISPR–Cas systems, the Class 1 type I system relies on Cascade (CRISPR-associated complex for antiviral defense complex) for DNA binding, which further recruits Cas3 to degrade the foreign
DNA20. Cascade, which recognizes and binds specific DNA, is a complex consist of multiple Cas proteins and CRISPR RNA (crRNA). CRISPR–Cas expression involves _cas_ genes expression and
CRISPR transcription, yielding a precursor crRNA (pre-crRNA). The pre-crRNA is processed at the repeat regions by Cse33, Cas621 or Csy422 to generate mature crRNA with different
characteristics. Other Cas proteins then bind onto the crRNA and assemble into a functional Cascade23,24,25,26. Cascade discriminates the self and non-self DNAs by recognizing the PAM
(proto-spacer adjacent motif) sequence27, which triggers a conformational change upon binding28,29. The conformational change finally recruits Cas3 for invasive DNA degradation20,30,31,32.
Compared to the widely used class 2 CRISPR–Cas systems, the multiple-subunit class 1 type I CRISPR–Cas system has distinct properties, for example, generating large fragment deletion in
genome editing with Cas333,34, and multiple subunits for different Cas protein–effector fusion strategies35. These differences between the class 1 and class 2 CRISPR–Cas system may
contribute to the advantages of Class 1 CRISPR–Cas system in some applications. Accroding to recent classification studies, there are seven subtypes (I–A to I–G) in type I CRISPR–Cas
system7,36. In recent years, the type I–A37, I–B38,39, I–E40, and I–F41,42 CRISPR–Cas have been used for prokaryotic gene engineering in _Sulfolobus islandicus_ (I–A)_, Clostridium
pasteurianum_ (I–B)_, Lactobacillus crispatus_ (I–E)_, Zymomonas mobilis_ (I–F), and _Pseudomonas aeruginosa_ (I–F). Besides, type I–B43 and type I–E44,45,46 Cascades can work as
transcription repressor in _Sulfolobus islandicus_ (I–B) _and Escherichia coli_ (I–E). Furthermore, type I–E and I–B CRISPR–Cas systems have been used in human cells33,34,35,47 and plants48
for gene editing and transcription regulation. Therefore, developing tools based on type I CRISPR–Cas system might provide alternative tools for genome editing and gene regulation. Type I–F
CRISPR–Cas system is among the well-studied CRISPR–Cas systems. It has fewer Cascade components than type I–E CRISPR–Cas system (4 vs 5), which will be easier to be controlled and delivered.
The type I–F CRISPR–Cas system was first discovered as CRISPR subtype Ypest from _Yersinia pestis_49,50. The Cascade components of type I–F CRISPR–Cas system were also named as Csy (CRISPR
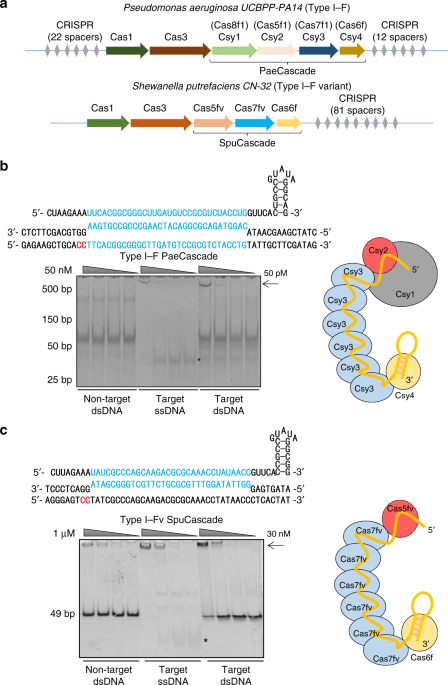
subtype Ypest) subunits, which includes Csy1 (Cas8f1), Csy2 (Cas5f1), Csy3 (Cas7f1), and Csy4 (Cas6f)7,26 (Fig. 1a). In addition, the Cascade of type I–F variant (type I–Fv, or type I–F2)
CRISPR–Cas system, derived from type I–F system, consists of only three subunits: Cas5fv (Cas5f2), Cas6f, and Cas7fv (Cas7f2)4,7 (Fig. 1a). The type I–F and type I–Fv Cascade recognizes
5′-CC PAM on the non-target strand for target binding51,52. Their crRNAs consist of 8-nt 5′ handle for Csy1 and Csy2 binding, 32-nt spacers bound by six copies of Csy3 for target
recognition, and 20-nt 3′ hairpin for Csy4 binding and pre-crRNA processing22. Recently, type I–F CRISPR–Cas system has been used for genome engineering in _Zymomonas mobilis_41 and
_Pseudomonas aeruginosa_42. However, there has not been any report on the exploitation of the type I–F or type I–Fv CRISPR–Cas system for genome manipulation application in human cells yet.
In this study, we explore the possibility of developing programmable type I–F and type I–Fv CRISPR tools for transcription activation in mammalian cells. In contrast to type I–E and I–B,
_Pseudomonas aeruginosa_ type I–F and _Shewanella putrefaciens_ type I–Fv systems require fewer subunits for dsDNA targeting in bacteria53,54. Also, the multiple subunits in type I–F and
type I–Fv might provide different combinations for tagging and increase signal strength when genetic modulators are fused to different subunits. By fusing the VPR (VP64-p65-Rta)
transcription activation domain to the type I–F Cascade subunit Csy3, we achieve both exogenous (e.g., GFP expression) and endogenous (e.g., _HBB, HBG1/2, SOX2, OCT4_, _IL1B_, and _IL1R2_)
gene activation in HEK293T cells. Interestingly, by changing the spacer length of crRNA, we can enhance the activation level of target genes. As is the case for class 2 systems, we can
achieve multiplex gene activation through a customized CRISPR array from a single vector. Finally, the type I–F CRISPR–Cas system can activate target genes specifically without altering the
expression of any predicted off-target genes. These data demonstrate the feasibility of using type I–F CRISPR–Cas system for programmable transcription activation and may have important
implications in their adaptation for genome editing. RESULTS TYPE I–F CRISPR–CAS MAINTAINS ACTIVITY IN HUMAN CELLS Csy1, Csy2, Csy3, and Csy4 constitute the Cascade complex in the
_Pseudomonas aeruginosa_ type I–F CRISPR–Cas system (PaeCascade) (Fig. 1a)26,53,55. Csy1 mediates PAM recognition (5′-CC-3′) at the 5′ end of the protospacer. Csy1 and Csy2 bind to the 5′
handle of the crRNA. Multiple Csy3 binds to the crRNA, serving as the backbone of the complex (Fig. 1b). Each Csy3 binds to 6-nt of the crRNA spacer with the precise number of Csy3 subunits
determined by the length of the crRNA spacer56 (from 14 to 50-nt), resulting in 3–9 copies of Csy357. Csy4 binds to the crRNA 3′ hairpin structure and is responsible for pre-crRNA maturation
(Fig. 1b). In comparison, _Shewanella putrefaciens_ type I–F variant Cascade (SpuCascade) contains only three subunits (Cas5fv, Cas6f, and Cas7fv) (Fig. 1a), leading to its more open
configuration (Supplementary Fig. 1)54. Here, Cas5fv plays an important role in PAM recognition and dsDNA unwinding. Casf7v is involved in crRNA-target ssDNA duplex and non-target ssDNA
binding to stabilize the complex, while Cas6f participates in pre-crRNA processing and crRNA hairpin binding (Fig. 1c). We first expressed and purified PaeCascade and SpuCascade complexes in
_E. coli_ to test their dsDNA binding ability by electrophoretic mobility shift assays (EMSA). As shown in Fig. 1b, c, both PaeCascade and SpuCascade complexes could shift the dsDNA target
probe (crRNA) in vitro. Next, we examined the expression of individual PaeCascade and SpuCascade subunits in 293T cells (Supplementary Fig. 2). While the level of expression differed between
subunits, they could all be readily expressed in mammalian cells. Both Csy4 and Cas6f are involved in crRNA maturation by processing the direct repeat (DR) of pre-crRNA55,57. We, therefore,
tested the activities of ectopically expressed Csy4 and Cas6f using HEK293T cells transiently expressing a DR-GFP fusion sequence (DR-GFP) (Supplementary Fig. 3a). When DR-GFP was
co-expressed with Csy4 or Cas6f, the percentages of GFP positive cells were drastically reduced (Supplementary Fig. 3b), indicating successful cleavage of the DR-GFP fusion mRNA. TARGETED
TRANSCRIPTION ACTIVATION BY TYPE I–F CRISPR–CAS To better examine PaeCascade and SpuCascade, we introduced rtTA (reverse tetracycline-controlled transactivator) expression cassette and eGFP
expression cassette controlled by a minimal CMV promoter plus six copies of the tetracycline-responsive element (TRE) into HEK293T cells by lentiviral vector (TRE-eGFP reporter) (Fig. 1d).
When dCas9-VPR (dCas9 fused to transcription activator VP64-p65-Rta58) was co-transfected with gRNAs targeting the TRE sequence into TRE-eGFP reporter cells, percentages of GFP positive
cells were significantly increased, indicating successful targeting of dCas9-VPR to the promoter and transcriptional activation of eGFP (Supplementary Fig. 4). With the TRE-eGFP reporter
cells, we wanted to test whether PaeCascade and SpuCascade can bind dsDNA and induce transcription activation in mammalian cells. We next fused VPR to each of the codon-optimized PaeCascade
and SpuCascade subunits and generated polycistronic all-in-one expression vectors of the Cascade complexes. To test possible effects due to configuration differences, we generated vectors
with the same subunits in different sequences (Fig. 1e, f and Supplementary Fig. 5). Then we tested their activity in the TRE-eGFP reporter cells together with a TRE-targeting crRNA. Three
configurations of the ectopically expressed PaeCascade complex (1243-VPR, 1234-VPR, and 3241-VPR) were able to activate GFP expression in ~10% of the cells (Fig. 1e). In contrast, despite
having fewer subunits, none of the SpuCascade vectors could activate GFP expression (Fig. 1f). Such differences reaffirm the notion that Cascade complexes have distinct properties from one
another and warrant further mechanistic studies. Given the complicate chromatin structure of eukaryote into consideration (e.g., histone binding, different histone modification, and etc.),
such distinct properties may due to their difference of PAM recognition mechanism (e.g., DNA minor groove vs major groove) and DNA helicase activity54. In the following sections, we will
focus on type I–F PaeCascade and investigate how to use it to effectively and efficiently activate transcription. Given the presence of multiple copies of Csy3 in a functional Cascade
complex, Csy3 may become limiting during complex assembly if all subunits are encoded by a single transcript (Fig. 1e). To address this possibility, we devised a helper-activator strategy
(Fig. 1g). Here, two helper vectors encode the subunits in pairs (Csy1/2 or Csy3/4). Activator vectors encode the subunits in pairs and have one of the subunits fused to VPR, resulting in
four different activators in all (Supplementary Fig. 6). The activator vectors were then co-transfected into the TRE-eGFP reporter cells in combination with a helper vector and TRE-targeting
crRNA (Fig. 1f). Among all the fusion types in the helper-activator 2-vector system, Csy1-VPR, Csy2-VPR, Csy3-VPR, and Csy4-VPR, only the Csy3-VPR fusion 2-vector system have a higher
activating efficiency than the all-in-one 1234-VPR vector system. Further experiments with another two plasmid system, in which Csy1, Csy2, and Csy4 were expressed by P2A fusion in one
plasmid and Csy3-VPR in another, showed highest _HBB_ and _HBG_ activation level in molar ratio = 1:3 (Supplementary Fig. 7a). However, its activation efficiency in _HBG_ was not as good as
Csy3-VPR fusion helper-activator 2-vector system (Supplementary Fig. 7b). Therefore, we decided to use the Csy3-VPR fusion helper-activator 2-vector system for further studies. And in the
hitherto described experiments, Csy3-VPR refers to the Csy3-VPR fusion helper-activator 2-vector system. These data clearly showed that type I–F PaeCascade could be utilized to activate
reporter gene expression. ENDOGENOUS GENE ACTIVATION BY TYPE I–F CRISPR–CAS Unlike most endogenous genes, multiple copies of TREs targeted by Cascade/crRNA existed in the TRE-eGFP reporter
cells. To investigate PaeCascade-mediated transcriptional activation of endogenous genes, we designed a crRNA against ~200 bp upstream of the transcriptional start site (TSS) of the
hemoglobin β protein coding gene (_HBB_). We co-transfected the crRNA expressing vector into HEK293T cells with the Csy3-VPR helper-activator vectors described above. Again, cells expressing
the combination with Csy3-VPR fusion showed the highest transcription activation activity at the HBB locus (~15 fold higher than control cells) (Fig. 2a). For convenience, PaeCascade-VPR
referred to Csy3-VPR in the test below. To determine how PaeCascade VPR fusion complex may differentially activate gene expression at different loci, we picked six genes (_HBB_, _HBG_,
_SOX2_, _OCT4_, _IL1B_, and _IL1R2_) and designed crRNAs targeting different promoter regions (−500 bp to −100 bp upstream TSS) in each locus. In all cases, PaeCascade-VPR was able to
activate endogenous gene expression to varying degrees (Fig. 2b), with the region 100–200 bp upstream of TSS being the best targets (Fig. 2c). And the fold activation of each gene was highly
correlated to their basal expression level, with the weaker expressed genes showed greater fold change (Fig. 2d). These findings indicated that the type I–F PaeCascade complex could
robustly activate endogenous gene transcription. Is PaeCascade-VPR more efficient than gene activation tools based on other CRISPR systems? To answer this question, we compared
PaeCascade-VPR system to the other gene activation tools (dCas9-VPR, dAsCas12a-VPR, and type I–E EcoCascade-VPR). We designed crRNAs or gRNAs of these systems targeting to the same loci of
_HBB_, _HBG_, _SOX2_, and _IL1B_ (Fig. 3a). The results showed that dCas9-VPR had the highest transcription activity for _HBB_ when targeting 170 bp upstream TSS (Fig. 3b). Except for the
_HBB_ -170bp TSS locus, PaeCascade-VPR appeared to outperform dCas9-VPR at activating transcription for gene loci examined (Fig. 3b–e). In all the loci tested, PaeCascade-VPR showed higher
activating efficiency than dAsCas12a-VPR and EcoCascade-VPR (Fig. 3b–e). These data suggested that PaeCascade-VPR may be more efficient than canonical dCas9-VPR and other CRISPR-based
systems, at least at certain gene loci, and represented a worthy addition to molecular tools that could modulate gene expression. ENHANCING TRANSCRIPTION ACTIVATION THROUGH CRRNA ENGINEERING
Since the spacer length of PaeCascade crRNA may be extended (beyond the canonical 32-nt) to accommodate more Csy3 subunits (more Csy3-VPR)53,54, we investigated the effect of spacer length
on PaeCascade-VPR activity at the _HBB_, _HBG_, and _SOX2_ loci (Fig. 4a). Given that the minimal length for Cys3 binding is 6-nt, we varied the length of spacers by multiples of six. In
each case, crRNAs with longer spacers (e.g., 50 and 56-nt) led to more efficient transcriptional activation (Fig. 4a), pointing to a simple yet effective way to regulate and tune endogenous
gene expression through enriching VPR in a certain locus. To test whether Cascade-mediated transcriptional activation could be further manipulated, we co-transfected two crRNAs that target
the same locus with the PaeCascade-VPR complex into cells. Among the six genes tested (_HBB_, _HBG_, _SOX2_, _OCT4_, _IL1B_, and _IL1R2_) (Distances between crRNAs: _HBB_ crRNA1-crRNA2: 27
bp; _HBG_ crRNA1-crRNA2: 55 bp; _SOX2_ crRNA1-crRNA2: 96 bp; _OCT4_ crRNA1-crRNA2: 79 bp; _IL1B_ crRNA1-crRNA2: 71 bp; _IL1R2_ crRNA1-crRNA2: 65 bp), synergistic activation could be observed
at four loci (Fig. 4b), indicating that simultaneous targeting of the PaeCascade-VPR complex to multiple regions of a promoter may enhance its activity. Not surprisingly, the distance
between the two crRNA target regions also had an impact on the extent of transcriptional activation. We designed pairs of crRNAs with different distances and tested their ability to activate
_HBG_ expression (Fig. 4c). A distance about 50–75 bp appeared optimal for the _HBG_ gene in this case. These observations underlined the multiple ways by which PaeCascade-VPR may be
further improved as a robust and efficient tool for gene expression modulation. MULTIPLEXED GENE ACTIVATION BY CUSTOMIZED CRISPR ARRAYS The _Pseudomonas aeruginosa_ CRISPR arrays, which
contain tandem spacers linked by direct repeats (DRs), are transcribed and then processed by Csy4 to generate mature crRNAs that can target different sites22. We, therefore, reasoned that
using customized CRISPR arrays driven by a single Pol. III promoter (e.g., hU6) might allow PaeCascade-VPR to bind multiple regulatory sites and achieve more efficient single gene
activation. To this end, we constructed a vector that should yield a single transcript with spacer 1 and 2 that was subsequently processed by Csy4 into two mature crRNAs (Fig. 5a). Then, we
constructed the CRISPR array expressing vectors to produce two crRNAs that target the gene loci of _HBB_, _HBG_, and _SOX2_ in HEK293T (Fig. 5b). In each case, introducing a single construct
containing the CRISPR array could provide a transcriptional activation level comparable to that using two individual crRNA vectors (Fig. 5b). Furthermore, the same strategy could be used to
produce spacers that target different genes (at least three genes) simultaneously and effectively activate gene transcription (Fig. 5c). The ability of PaeCascade-VPR to activate multiplex
genes simultaneously with a customized CRISPR array in a single construct instead of individual crRNAs in independent constructs enormously simplified the activation system, which increased
the transfection efficiency and makes it not necessary to express and deliver multiplex gRNAs independently in comparison with type II CRISPR system. These data pointed to PaeCascade-VPR as
a powerful and flexible system with much untapped potential for research applications compared to the much better-studied type 2 systems. MISMATCH AND OFF-TARGET ANALYSIS OF PAECASCADE-VPR
SYSTEM Although the DNA-binding property of PaeCascade is crucial for its specificity in mammalian cells, it remains poorly understood. It has been shown that the seed region (first 8-nt of
PAM proximal sequence) within the crRNA is critical for initiating target binding and DNA unwinding26. To further probe the target DNA binding specificity of PaeCascade in mammalian cells,
we generated a series of _HBB_ and _HBG_ targeting crRNA variants with 6-nt mismatches in the 32-nt spacer region (Fig. 6a). Being consistent with previously published data from in vitro
experiments26, mismatches in PAM-proximal regions had the biggest impact on the activity of PaeCascade-VPR, with cells exhibiting the lowest activation levels of _HBB_ and _HBG_ with these
crRNA variants (Fig. 6b). Next, we constructed 32 crRNA variants with single-nucleotide mismatches in the 32-nt spacer to determine the contribution of each position (Fig. 6c). As shown in
Fig. 5d, mismatches at nearly every position reduced the level of gene activation. Again, changes in PAM-distal positions had less impact on _HBB/HBG_ activation than those at PAM-proximal
positions. Intriguingly, mismatches at every 6th position showed far less impact on PaeCascade-VPR activity, regardless of their distance to the PAM (Fig. 6d), consisting with its structure
characteristic53. For type I–F Cascade, the binding of the target strand to crRNA follows a periodic 5 + 1 pattern53. The five consecutive base pairs followed by one base pair gap in which
the unpaired nucleotides of crRNA and target strand kink out in opposite directions53. Therefore, the mismatches in per sixth nucleotide have less impact on target DNA binding and activation
efficiency. These data suggest that target binding by PaeCascade-VPR may be exceptionally sequence specific, with even residues far distal to the PAM playing a role in target DNA binding.
To further investigate the specificity of PaeCascade-VPR system, we searched for the target sites with overlapping target regions of PaeCascade-VPR and dCas9-VPR, which also had potential
off-targets on the TSS of other genes (Fig. 7). To find out the off-target genes, we search for two groups of the potential off-target sites. We searched potential off-target sites with ≤4
mismatches to SpCas9 gRNA as the first group of putative off-targets. Taken the features of PaeCascade crRNA into consideration, mismatches on per 6th bases in PaeCascade crRNA had less
impact on _HBB_ and _HBG_ activation (Fig. 6d), which may be tolerable for target binding. Also, mismatches on 25–32th bases were more tolerable than other bases (Fig. 6b, d). Previously
studies also indicated that PAM-proximal region of type I CRISPR was more important for its binding capacity, and ≥5 mismatches would abolish type I CRISPR interference59,60. So we allowed
mismatches in 6th, 12th, 18th, and 24–32th positions, and found all the possible off-targets with ≤4 mismatches to PaeCascade crRNA as the second group of putative off-targets. Then taking
the two groups together, all the possible off-targets were predicted through sequence similarity, which must also lay on the promoter (≤2 kb upstream or downstream TSS) of a certain gene.
According to the criteria above, we searched for target sites on _HBB_ and _HBG_ promoters. We found three regions with overlapping target sites of PaeCascade and dCas9 for off-target
analysis (Fig. 7). The RNA level of _HBB_ or _HBG_ and their predicted off-target genes were then detected. With the crRNAs or gRNAs targeting to _HBB_ 173 bp upstream TSS, _HBB_ 126 bp
upstream TSS or _HBG_, PaeCascade-VPR and dCas9-VPR can increase the transcription level of _HBB_ and _HBG_ as expected (Fig. 7). For both PaeCascade-VPR and dCas9-VPR, no off-target
activations can be detected in all the putative off-target genes (Fig. 7). These results indicated that the type I–F PaeCascade-VPR system is comparable to dCas9-VPR and may have a high
specificity as a transcription activator in human cells. DISCUSSION In this study, we demonstrated that the type I–F CRISPR–Cas system could be repurposed to activate endogenous gene
expression in human cells. Fusing the Csy3 subunit of type I–F PaeCascade to transcription domain (VPR) led to a crRNA-dependent reporter and endogenous gene activation (Figs. 1, 2). And at
most target genes, PaeCascade-VPR was much efficient than dCas9-VPR, dAsCas12a-VPR, and EcoCascade-VPR (Fig. 3b–e). Besides, having each Csy subunit expressed independently further improved
activation efficiency (Fig. 1g). Moreover, compared to dCas9-VPR, the activation efficiency could be further improved by extending the spacer length of crRNA to recruit more Csy3-VPR protein
to target genes (Fig. 4a). Customized CRISPR arrays enabled efficient multiplex gene activation in human cells (Fig. 5). Saturated mutation of crRNA spacer sequence revealed that target DNA
binding by PaeCascade was sensitive to crRNA-DNA mismatch, suggesting that transcription activation by PaeCascade-VPR might be specific (Fig. 6d). And actually, we did not observe any
off-target effects in the putative off-target genes of PaeCascade-VPR (Fig. 7). Taken together, these data prove that PaeCascade-VPR is a good programmable transcription activator in human
cells. We found that all subunits of PaeCascade (Csy1, Csy2, Csy3, and Csy4) could be fused with VPR without disturbing the formation of functional PaeCascade complex (Fig. 1g), which
provides great flexibility on engineering. It is possible that we can activate gene expression with different kinds of effectors: Cascade-TET1 (Ten-Eleven Translocation dioxygenase1) fusion
for DNA demethylation; Cascade-p300 fusion for histone acetylation; Cascade-VP64 or Cascade-VPR (VP64-p65-Rta) fusion for transcriptional factor recruitment, and achieve stronger and more
persisted gene activation through combining these three methods properly61,62,63,64. So, it might be possible to fuse more transcription regulating domains to the PaeCascade complex to
improve activation efficiency or even achieve long term memory activating of endogenous genes. While our manuscript was under preparation, Adrian et al. reported transcription regulation by
type I–B and type I–E CRISPR–Cas system in human cells65. Although type I–B also used four subunits to activate endogenous genes, type I–B tool was not better than dCas9. Furthermore, type
I–B Cas7 (Csy3 equivalent) failed to induce gene activation when fused to transcription activator65. However, transcription activator fused to Csy3 subunit of type I–F CRISPR system showed
the highest activating level (Fig. 1g). It was even better than dCas9 and other transcription activation systems at most (4/5) tested endogenous sites. In addition to gene activation,
PaeCascade subunits might be fused with transcription repressor to repress gene expression, or nuclease domain to cleave target DNA in human genome65. Previous studies of type I CRISPR have
identified an eight nucleotide PAM-proximal seed region (1–5th, 7th, 8th bases)26,59,60, and the imprecise base-pairing at every sixth position within the 32 nucleotide crRNA
sequence53,54,66, owing to structure feature of every sixth base being flipped out of the RNA–DNA duplex upon target binding. Being consistent with these studies, we found that the
PAM-proximal position is crucial for gene activation of PaeCascade-VPR (Fig. 6a). In contrast, every sixth base had a relatively weak influence on its binding (Fig. 6d). Recent studies that
generating long-range deletions in human embryonic stem cells or HEK293T with EcoCascade-Cas3 revealed no prominent off-target effect either by deep sequencing or by whole genome
sequencing33,34. It had been shown that type I–B and type I–E CRISPR–Cas could induce specific targeted transcription activation in human cells without crRNA-dependent off-target effects35.
According to our research data, we could achieve a high transcription activation level without activation of putative off-target genes by type I–F PaeCascade (Fig. 7). These data indicate
that the specificity of type I system is high in mammalian cells. Transcription activation could be used to upregulate therapeutic gene expression. For example, activating _HBB_ or _HBG_
gene expression might be used to treat β-thalassemia. Further studies are needed to investigate the function and the delivery of PaeCascade-VPR in primary cells (e.g., hematopoietic stem
cell) or in vivo. Other aspects, including the cytotoxicity and immunogenicity of type I–F system, should be studied in detail. Further efforts improving the activation efficiency of
PaeCascade-VPR are also important as well. Only then can type I–F PaeCascade-VPR be a tool for therapeutic gene expression activation. In brief, we found that PaeCascade-VPR can induce
targeted gene activation without off-target effects, indicating that PaeCascade-VPR is a good programmable transcription activator in human cells. Regulating of gene expression by Type I–F
CRISPR system broadens the usage of CRISPR system as a gene regulating tools in mammalian cells. METHODS CELL CULTURE HEK293T cells were obtained from ATCC and cultured in Dulbecco’s
modified Eagle medium (Corning, 10-013-CVR) supplemented with 10% fetal bovine serum at 37 °C and 5% CO2 in humidified incubator, with daily medium change. Cells were split every 2–3 days.
All the cells were mycoplasma negative. Transient transfection of HEK293T cells was performed using PEI (Polysciences, 24765-1). Cells were lysed by Trizol 48 h later for qPCR analysis or
collected 72 h later for flow cytometry analysis. PLASMIDS AND VECTORS Type I–F Cascade (from _Pseudomonas aeruginosa_) _E. coli_ expression plasmids were obtained from Addgene
(pCsy_complex, 89232). Type I–Fv (from _Shewanella putrefaciens_) Cas7fv, Cas5fv, Cas6fv cassettes were cloned into the pET28a vector (Sigma-Aldrich, 69864-3CN) as a polycistronic operon and
include an N-terminal His-tagged Cas7fv fusion (pET28-type I–Fv). The crRNA sequence was cloned into pACYC184 (NEB, X06403) for bacterial expression. Condon-optimized Cas subunits were
sub-cloned into px601 (Addgene, #61591) (replacing the SaCas9 gene) for transfection into mammalian cells. A site for spacer cloning flanked by two Csy4 direct repeats (DR) or Cas6f direct
repeats was ligated into lentiGuide-Puro (addgene #52963) between BsmBI and EcoRI restriction sites to generate pLenti-crRNA-IF or pLenti-crRNA-IFv vectors. Oligos containing spacer
sequences were annealed and ligated into pLenti-crRNA-IF or pLenti-crRNA-IFv for crRNA expression in mammalian cells. For spacer mutant crRNA cloning, oligos with various of mutant spacer
were annealed and ligated into pLenti-crRNA-IF. Sequences are listed in Supplementary Data 1–4. Sequences of plasmids for expression of PaeCascade-VPR, including pCsy1-Csy2, pCsy3-VPR-Csy4,
and pCsy-crRNA-EV, are listed in Supplementary Data 5. PROTEIN EXPRESSION AND PURIFICATION Type I–F and type I–Fv Cascade were expressed and purified using prokaryotic systems. Briefly, BL21
StarTM (DE3) _E. coli_ cells (Thermo Fisher) were transformed with pCsy_complex (or pET28-type I–Fv) together with pACYC184 vector containing corresponding crRNA. When OD600 reached 0.6,
protein expression was induced by 5 mM IPTG and cultured for another 12 h at 25 °C. Cells were harvested and suspended in buffer A (20 mM HEPES-Na pH 8.0, 250 mM NaCl, 20 mM KCl, 20 mM
MgCl2, 40 mM imidazole), disrupted by sonication and purified using Ni Sepharose 6FF column (GE Healthcare). Size exclusion chromatography was performed on a Superdex 200 Tricon 10/300
column (GE Healthcare) in buffer B (20 mM HEPES-Na pH 7.0, 150 mM NaCl, 1 mM DTT, 1 mM EDTA). Fractions containing the target complex were collected. Protein concentration was measured by
BCA protein assay kit (Thermo Fisher, 23225). ELECTROPHORESIS MOBILITY SHIFT ASSAY (EMSA) Target oligonucleotides used were detailed in Supplementary Data 6. Substrate dsDNA was prepared by
annealing two complementary oligos with a molar ratio of 1:1. 200 nM of substrate DNA were incubated with various amount of purified recombinant protein complex at 37 °C for 30 min in
binding buffer (50 mM HEPES-Na pH 7.0, 50 mM NaCl, 1 mM DTT, 1 mM EDTA, 10 IU RNase inhibitor (Thermo Fisher, EO0381)). The products were then separated via non-denaturing TBE-PAGE and
stained by GelredTM (Biotium, 41000). QUANTITATIVE PCR (QPCR) Briefly, total RNA was extracted by TRIZOL (Thermo Fisher) following the manufacture’s instruction and quantified by Nanodrop
1000 (Thermo Fisher). The reverse transcription was carried out using the PrimeScript™RT reagent Kit (TAKARA, RR047Q) following the manufacture’s instruction. Quantitative PCR was carried
out in qTOWER3 system (Analytikjena) using TAKARA TB Green II Real-Time PCR Master Mix following the manufacture’s instruction. Quantitative PCR was performed with indicated primer for
specific genes, and _GAPDH_ served as control. The relative expression level was determined by −ΔΔCt method. qPCR primers are listed in Supplementary Data 7. FLOW CYTOMETRY ANALYSIS Cell was
digested by 0.25% trypsin, and then trypsin digestion was terminated by DMEM containing 10% FBS. Cells were collected and suspended in PBS. The GFP positive cells were detected by CytoFLEX
(Beckman). WESTERN BLOT (WB) Three days post-transfection, cells were lysed in RIPA buffer with protease inhibitor cocktail. Samples were centrifuged at 14,000 × _g_ for 10 min. The
supernatant was harvested and quantified using BCA protein assay kit (Thermo Fisher, 23225) on Victor X5. 25 μg protein was mixed and boiled with 5 × SDS loading buffer. Samples were
separated using SDS-PAGE assay. Protein was transferred to nitrocellulose membranes (Bio-Rad) for 1 hour in transfer buffer at 300 mA. The membranes were blocked at room temperature for 20
min in 5% milk-TBST and incubated with the primary antibody in 3% BSA-TBST at RT for two hours. Then the membranes were washed in TBST and incubated with secondary antibody in 3% BSA-TBST at
RT for one hour and washed in TBST. Blots were visualized using Odyssey finally. The antibodies used for WB were listed below. Rabbit polyclonal anti-GAPDH (Abmart, P30008M) (1:5000
dilution), mouse monoclonal anti-HA antibody (Sigma, H9658) (1:5000 dilution), goat anti-rabbit secondary antibody (Odyssey, 926-32211) (1:5,000 dilution) and the goat anti-mouse secondary
antibody (Odyssey, 926-68070) (1:5,000 dilution). OFF-TARGET PERDITION To predict the putative off-targets for dCas9-VPR, we first searched off-targets with ≤4 mismatches to SpCas9 gRNA. And
for the prediction of PasCascade-VPR, we allowed mismatches in 6th, 12th, 18th, and 24–32nd position, and found all the possible off-targets with ≤4 mismatches to PaeCascade crRNA. Then all
the possible off-target sites were predicted through sequence similarity, which also lay on the promoter (≤2 kb) of a certain gene (UCSC, with Integrated Regulation from ENCODE Tracks and
GeneHancer Regulatory Elements and Gene Interactions). Sequences of all putative off-targets were listed in Supplementary Data 8. SIGNIFICANT ANALYSIS All data were processed and tested
using GraphPad Prism 7.0. For all the data, Gaussian distribution was detected by Shapiro–Wilk normality test. One-way ANOVA (for data having more than two groups) or unpaired _t_ test (for
data having only two groups) was used for data with Gaussian distribution (Normal distribution) and equal SDs. Otherwise, Kruskal–Wallis or Mann–Whitney test was used. Data were displayed as
mean ± S.E.M. Statistical significance level: n.s., not significant; *_P_ < 0.05; **_P_ < 0.01; ***_P_ < 0.001. REPORTING SUMMARY Further information on research design is
available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY All relevant data are available upon request. Sequences of plasmids for expression of
PaeCascade-VPR, including pCsy1-Csy2, pCsy3-VPR-Csy4, and pCsy-crRNA-EV, are listed in Supplementary Data 5. The source data for Figs. 1b, c, e, f, g, 2a, b, d, 3b, c, d, e, 4, 5b, c, 6b, d,
7 and Supplementary Figs. 2, 3b, 4b, and 7 are provided as a Source Data file. CHANGE HISTORY * _ 09 JULY 2020 An amendment to this paper has been published and can be accessed via a link
at the top of the paper. _ REFERENCES * Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. _Science_ 315, 1709–1712 (2007). ADS CAS PubMed Google
Scholar * Pourcel, C., Salvignol, G. & Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for
evolutionary studies. _Microbiology_ 151, 653–663 (2005). CAS PubMed Google Scholar * Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. _Science_ 321,
960–964 (2008). ADS CAS PubMed PubMed Central Google Scholar * Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Classification and nomenclature of CRISPR-Cas systems: where from here?
_CRISPR J._ 1, 325–336 (2018). PubMed PubMed Central Google Scholar * Hille, F. et al. The biology of CRISPR-Cas: backward and forward. _Cell_ 172, 1239–1259 (2018). CAS PubMed Google
Scholar * Koonin, E. V., Makarova, K. S. & Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. _Curr. Opin. Microbiol._ 37, 67–78 (2017). CAS PubMed PubMed
Central Google Scholar * Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. _Nat. Rev. Microbiol._ 18, 67–83 (2020). CAS
PubMed Google Scholar * Chakraborty, S. et al. A CRISPR/Cas9-based system for reprogramming cell lineage specification. _Stem Cell Rep._ 3, 940–947 (2014). CAS Google Scholar * Chen, B.
& Huang, B. Imaging genomic elements in living cells using CRISPR/Cas9. _Methods Enzymol._ 546, 337–354 (2014). CAS PubMed Google Scholar * Doench, J. G. et al. Rational design of
highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. _Nat. Biotechnol._ 32, 1262–1267 (2014). CAS PubMed PubMed Central Google Scholar * Komor, A. C., Kim, Y. B., Packer, M.
S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. _Nature_ 533, 420 (2016). ADS CAS PubMed PubMed Central
Google Scholar * Gaudelli, N. M. et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. _Nature_ 551, 464–471 (2017). ADS CAS PubMed PubMed Central Google
Scholar * Gootenberg, J. S. et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. _Science_ 360, 439–444 (2018). ADS CAS PubMed PubMed Central
Google Scholar * Chen, J. S. et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. _Science_ 360, 436–439 (2018). ADS CAS PubMed PubMed Central
Google Scholar * Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. _Nat. Rev. Mol. Cell Biol._ 17, 5–15
(2016). CAS PubMed Google Scholar * Catarino, R. R. & Stark, A. Assessing sufficiency and necessity of enhancer activities for gene expression and the mechanisms of transcription
activation. _Genes Dev._ 32, 202–223 (2018). CAS PubMed PubMed Central Google Scholar * Hsu, M. N., et al. CRISPR technologies for stem cell engineering and regenerative medicine.
_Biotechnol. Adv._ 37, 107447 (2019). * Hidalgo-Cantabrana, C., Goh, Y. J. & Barrangou, R. Characterization and repurposing of type I and type II CRISPR-Cas systems in bacteria. _J. Mol.
Biol._ 431, 21–33 (2019). CAS PubMed Google Scholar * Burstein, D. et al. New CRISPR-Cas systems from uncultivated microbes. _Nature_ 542, 237–241 (2017). ADS CAS PubMed Google
Scholar * Westra, E. R. et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. _Mol. Cell_ 46, 595–605 (2012).
CAS PubMed PubMed Central Google Scholar * Carte, J., Wang, R., Li, H., Terns, R. M. & Terns, M. P. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in
prokaryotes. _Genes Dev._ 22, 3489–3496 (2008). CAS PubMed PubMed Central Google Scholar * Haurwitz, R. E., Jinek, M., Wiedenheft, B., Zhou, K. & Doudna, J. A. Sequence- and
structure-specific RNA processing by a CRISPR endonuclease. _Science_ 329, 1355–1358 (2010). ADS CAS PubMed PubMed Central Google Scholar * Jore, M. M. et al. Structural basis for
CRISPR RNA-guided DNA recognition by Cascade. _Nat. Struct. Mol. Biol._ 18, 529–536 (2011). CAS PubMed Google Scholar * Mulepati, S., Orr, A. & Bailey, S. Crystal structure of the
largest subunit of a bacterial RNA-guided immune complex and its role in DNA target binding. _J. Biol. Chem._ 287, 22445–22449 (2012). CAS PubMed PubMed Central Google Scholar *
Sashital, D. G., Wiedenheft, B. & Doudna, J. A. Mechanism of foreign DNA selection in a bacterial adaptive immune system. _Mol. cell_ 46, 606–615 (2012). CAS PubMed PubMed Central
Google Scholar * Wiedenheft, B. et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. _Proc. Natl Acad. Sci. USA_ 108,
10092–10097 (2011). ADS CAS PubMed PubMed Central Google Scholar * Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J. & Almendros, C. Short motif sequences determine the
targets of the prokaryotic CRISPR defence system. _Microbiology_ 155, 733–740 (2009). CAS PubMed Google Scholar * Westra, E. R. et al. Type I-E CRISPR-cas systems discriminate target from
non-target DNA through base pairing-independent PAM recognition. _PLoS Genet._ 9, e1003742 (2013). CAS PubMed PubMed Central Google Scholar * Hayes, R. P. et al. Structural basis for
promiscuous PAM recognition in type I-E Cascade from _E. coli_. _Nature_ 530, 499–503 (2016). ADS CAS PubMed PubMed Central Google Scholar * Wiedenheft, B. et al. Structures of the
RNA-guided surveillance complex from a bacterial immune system. _Nature_ 477, 486–489 (2011). ADS CAS PubMed PubMed Central Google Scholar * Mulepati, S. & Bailey, S. In vitro
reconstitution of an Escherichia coli RNA-guided immune system reveals unidirectional, ATP-dependent degradation of DNA target. _J. Biol. Chem._ 288, 22184–22192 (2013). CAS PubMed PubMed
Central Google Scholar * Huo, Y. et al. Structures of CRISPR Cas3 offer mechanistic insights into Cascade-activated DNA unwinding and degradation. _Nat. Struct. Mol. Biol._ 21, 771–777
(2014). CAS PubMed PubMed Central Google Scholar * Dolan, A. E. et al. Introducing a spectrum of long-range genomic deletions in human embryonic stem cells using type I CRISPR-Cas. _Mol.
cell_ 74, 936–950 e935 (2019). CAS PubMed PubMed Central Google Scholar * Morisaka, H. et al. CRISPR-Cas3 induces broad and unidirectional genome editing in human cells. _Nat. Commun._
10, 5302 (2019). * Pickar-Oliver, A. et al. Targeted transcriptional modulation with type I CRISPR-Cas systems in human cells. _Nat. Biotechnol._ 37, 1493–1501 (2019). CAS PubMed PubMed
Central Google Scholar * Makarova, K. S. et al. An updated evolutionary classification of CRISPR-Cas systems. _Nat. Rev. Microbiol._ 13, 722–736 (2015). CAS PubMed PubMed Central Google
Scholar * Li, Y. et al. Harnessing type i and type III CRISPR-Cas systems for genome editing. _Nucleic Acids Res._ 44, e34–e34 (2016). PubMed Google Scholar * Pyne, M. E., Bruder, M. R.,
Moo-Young, M., Chung, D. A. & Chou, C. P. Harnessing heterologous and endogenous CRISPR-Cas machineries for efficient markerless genome editing in Clostridium. _Sci. Rep._ 6,
25666–25666 (2016). ADS CAS PubMed PubMed Central Google Scholar * Cheng, F. et al. Harnessing the native type I-B CRISPR-Cas for genome editing in a polyploid archaeon. _J. Genet
Genomics_ 44, 541–548 (2017). PubMed Google Scholar * Hidalgo-Cantabrana, C., Goh, Y. J., Pan, M., Sanozky-Dawes, R. & Barrangou, R. Genome editing using the endogenous type I
CRISPR-Cas system in _Lactobacillus crispatus_. _Proc. Natl Acad. Sci. USA_ 116, 15774–15783 (2019). CAS PubMed PubMed Central Google Scholar * Zheng, Y. et al. Characterization and
repurposing of the endogenous Type I-F CRISPR-Cas system of Zymomonas mobilis for genome engineering. _Nucleic Acids Res._ 47, 11461–11475 (2019). CAS PubMed PubMed Central Google Scholar
* Xu, Z. et al. Native CRISPR-Cas-mediated genome editing enables dissecting and sensitizing clinical multidrug-resistant _P. aeruginosa_. _Cell Rep._ 29, 1707–1717.e1703 (2019). CAS
PubMed Google Scholar * Stachler, A.-E. & Marchfelder, A. Gene repression in haloarchaea using the CRISPR (clustered regularly interspaced short palindromic repeats)-Cas I-B system.
_J. Biol. Chem._ 291, 15226–15242 (2016). CAS PubMed PubMed Central Google Scholar * Rath, D., Amlinger, L., Hoekzema, M., Devulapally, P. R. & Lundgren, M. Efficient programmable
gene silencing by Cascade. _Nucleic acids Res._ 43, 237–246 (2015). CAS PubMed Google Scholar * Luo, M. L., Mullis, A. S., Leenay, R. T. & Beisel, C. L. Repurposing endogenous type I
CRISPR-Cas systems for programmable gene repression. _Nucleic Acids Res._ 43, 674–681 (2015). CAS PubMed Google Scholar * Chang, Y., Su, T., Qi, Q. & Liang, Q. Easy regulation of
metabolic flux in _Escherichia coli_ using an endogenous type I-E CRISPR-Cas system. _Micro. Cell Fact._ 15, 195–195 (2016). Google Scholar * Cameron, P. et al. Harnessing type I CRISPR-Cas
systems for genome engineering in human cells. _Nat. Biotechnol._ 37, 1471–1477 (2019). CAS PubMed Google Scholar * Young, J. K. et al. The repurposing of type I-E CRISPR-Cascade for
gene activation in plants. _Commun. Biol._ 2, 383 (2019). PubMed PubMed Central Google Scholar * Cady, K. C. et al. Prevalence, conservation and functional analysis of _Yersinia_ and
_Escherichia_ CRISPR regions in clinical Pseudomonas aeruginosa isolates. _Microbiology_ 157, 430–437 (2011). CAS PubMed PubMed Central Google Scholar * Haft, D. H., Selengut, J.,
Mongodin, E. F. & Nelson, K. E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. _PLoS Comput. Biol._ 1, e60–e60
(2005). ADS PubMed PubMed Central Google Scholar * Dwarakanath, S. et al. Interference activity of a minimal Type I CRISPR-Cas system from _Shewanella putrefaciens_. _Nucleic Acids Res._
43, 8913–8923 (2015). CAS PubMed PubMed Central Google Scholar * Rollins, M. F., Schuman, J. T., Paulus, K., Bukhari, H. S. & Wiedenheft, B. Mechanism of foreign DNA recognition by
a CRISPR RNA-guided surveillance complex from Pseudomonas aeruginosa. _Nucleic acids Res._ 43, 2216–2222 (2015). CAS PubMed PubMed Central Google Scholar * Guo, T. W. et al. Cryo-EM
structures reveal mechanism and inhibition of DNA Targeting by a CRISPR-Cas surveillance complex. _Cell_ 171, 414–426 e412 (2017). CAS PubMed PubMed Central Google Scholar * Pausch, P.
et al. Structural variation of type I-F CRISPR RNA guided DNA surveillance. _Mol. cell_ 67, 622–632.e624 (2017). CAS PubMed Google Scholar * Chowdhury, S. et al. Structure reveals
mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. _Cell_ 169, 47–57 e11 (2017). CAS PubMed PubMed Central Google Scholar * Gu, D.-H., Ha, S. C.
& Kim, J.-S. A CRISPR RNA is closely related with the size of the cascade nucleoprotein complex. _Front. Microbiol._ 10, 2458–2458 (2019). PubMed PubMed Central Google Scholar *
Gleditzsch, D. et al. Modulating the Cascade architecture of a minimal Type I-F CRISPR-Cas system. _Nucleic Acids Res,_ 44, 5872–5882 (2016). CAS Google Scholar * Konermann, S. et al.
Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. _Nature_ 517, 583–588 (2015). ADS CAS PubMed Google Scholar * Semenova, E. et al. Interference by clustered
regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. _Proc. Natl Acad. Sci. USA_ 108, 10098–10103 (2011). ADS CAS PubMed PubMed Central Google
Scholar * Fineran, P. C. et al. Degenerate target sites mediate rapid primed CRISPR adaptation. _Proc. Natl Acad. Sci. USA_ 111, E1629–E1638 (2014). CAS PubMed PubMed Central Google
Scholar * Morita, S., Horii, T. & Hatada, I. Editing of DNA methylation using dCas9-Peptide Repeat and scFv-TET1 Catalytic Domain Fusions. _Methods Mol. Biol._ 1767, 419–428 (2018). CAS
PubMed Google Scholar * Liu, X. S. et al. Editing DNA Methylation in the Mammalian Genome. _Cell_ 167, 233–247 e217 (2016). CAS PubMed PubMed Central Google Scholar * Hilton, I. B.
et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. _Nat. Biotechnol._ 33, 510–517 (2015). CAS PubMed PubMed Central Google
Scholar * Chavez, A. et al. Comparison of Cas9 activators in multiple species. _Nat. Methods_ 13, 563–567 (2016). CAS PubMed PubMed Central Google Scholar * Pickar-Oliver, A., et al.
Targeted transcriptional modulation with type I CRISPR-Cas systems in human cells. _Nature Biotechnol._ 37, 1493–1501 (2019). * Mulepati, S., Heroux, A. & Bailey, S. Structural biology.
Crystal structure of a CRISPR RNA-guided surveillance complex bound to a ssDNA target. _Science_ 345, 1479–1484 (2014). ADS CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We would like to thank all the members in Zhou Songyang’s lab for discussions and supports. This work was supported by the National Key R&D Program of China
(2017YFA0102801, 2017YFC1001901, and 2017YFC1001603), the National Natural Science Foundation (91640119, 91019020, 81330055, 31671540, and 31601196), the Guangdong Special Support Program
(2019BT02Y276), the Natural Science Foundation of Guangdong Province (2016A030310206 and 2014A030312011), the Guangzhou Science and Technology Project (201707010085 and 201803010020). AUTHOR
INFORMATION Author notes * These authors contributed equally: Yuxi Chen, Jiaqi Liu. AUTHORS AND AFFILIATIONS * Sun Yat-Sen Memorial Hospital, Sun Yat-sen University; MOE Key Laboratory of
Gene Function and Regulation and Guangzhou Key Laboratory of Healthy Aging Research, School of Life Sciences, Sun Yat-sen University, Guangzhou, 510275, China Yuxi Chen, Jiaqi Liu, Shengyao
Zhi, Qi Zheng, Wenbin Ma, Junjiu Huang, Puping Liang & Zhou Songyang * State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, 510060,
China Yuxi Chen, Yizhi Liu & Zhou Songyang * Key Laboratory of Reproductive Medicine of Guangdong Province, the First Affiliated Hospital and School of Life Sciences, Sun Yat-sen
University, Guangzhou, 510275, China Junjiu Huang * Verna and Marrs Mclean Department of Biochemistry and Molecular Biology, Baylor College of Medicine, One Baylor Plaza, Houston, TX, 77030,
USA Dan Liu & Zhou Songyang * Guangzhou Regenerative Medicine and Health-Guangdong Laboratory (GRMH-GDL), Guangzhou, 510530, China Zhou Songyang Authors * Yuxi Chen View author
publications You can also search for this author inPubMed Google Scholar * Jiaqi Liu View author publications You can also search for this author inPubMed Google Scholar * Shengyao Zhi View
author publications You can also search for this author inPubMed Google Scholar * Qi Zheng View author publications You can also search for this author inPubMed Google Scholar * Wenbin Ma
View author publications You can also search for this author inPubMed Google Scholar * Junjiu Huang View author publications You can also search for this author inPubMed Google Scholar *
Yizhi Liu View author publications You can also search for this author inPubMed Google Scholar * Dan Liu View author publications You can also search for this author inPubMed Google Scholar
* Puping Liang View author publications You can also search for this author inPubMed Google Scholar * Zhou Songyang View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Z.S., P.L., and Y.C. designed the experiments. D.L. helped with the paper. J.L., Y.C., S.Z., and Q.Z. performed the experiments. Z.S. and P.L. supervised the
research. All authors discussed the results and commented on the paper. CORRESPONDING AUTHORS Correspondence to Puping Liang or Zhou Songyang. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s) for their contribution to the peer review of
this work. Peer reviewer reports are available PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY DATA
5 SUPPLEMENTARY DATA 6 SUPPLEMENTARY DATA 7 SUPPLEMENTARY DATA 8 DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, Y., Liu, J., Zhi, S. _et al._ Repurposing type
I–F CRISPR–Cas system as a transcriptional activation tool in human cells. _Nat Commun_ 11, 3136 (2020). https://doi.org/10.1038/s41467-020-16880-8 Download citation * Received: 14 November
2019 * Accepted: 18 May 2020 * Published: 19 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16880-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative