Play all audios:
ABSTRACT Sophisticated mechanically interlocked molecules (MIMs) with interesting structures, properties and applications have attracted great interest in the field of supramolecular
chemistry. We herein report a highly efficient self-assembly of heterometallic triangular necklace 1 containing Cu and Pt metals with strong antibacterial activity. Single-crystal X-ray
analysis shows that the finely arranged triangular necklace 1 has two racemic enantiomers in its solid state with intriguing packing motif. The superior antibacterial activity of necklace 1
against both standard and clinically drug-resistant pathogens implies that the presence of Cu(I) center and platinum(II) significantly enhance the bacterium-binding/damaging activity, which
is mainly attributed to the highly positively charged nature, the possible synergistic effect of heterometals in the necklace, and the improved stability in culture media. This work clearly
discloses the structure-property relationships that the existence of two different metal centers not only facilitates successful construction of heterometallic triangular necklace but also
endows it with superior nuclease properties and antibacterial activities. SIMILAR CONTENT BEING VIEWED BY OTHERS DESIGNING NARCISSISTIC SELF-SORTING TERPYRIDINE MOIETIES WITH HIGH
COORDINATION SELECTIVITY FOR COMPLEX METALLO-SUPRAMOLECULES Article Open access 24 September 2021 STABLE PEPTIDE-ASSEMBLED NANOZYME MIMICKING DUAL ANTIFUNGAL ACTIONS Article Open access 05
July 2024 TAILORING TOPOLOGY AND BIO-INTERACTIONS OF TRIAZINE FRAMEWORKS Article Open access 26 June 2024 INTRODUCTION Mechanically-interlocked molecules (MIMs) such as catenanes1,2,3,4,5,6,
rotaxanes7,8,9,10,11,12,13,14,15,16,17,18, molecular necklaces19,20, and molecular knots21,22 have been a main research focus in supramolecular chemistry due to their innately topologically
nontrivial architectures, thus always challenging the imagination and skills of synthetic chemists23,24,25,26,27. Molecular necklaces, as important members in MIMs family, are derived from
catenanes, in which three or more side rings as molecular “beads” are threaded onto a central ring as molecular “chains”28. The early research of molecular necklace could be traced back to
the work reported by Sauvage et al., who generated a mixture of molecular necklaces by accident in a very low yield and characterized their structures by using electrospray ionization mass
spectrometry (ESI-MS)29. Moreover, Stoddart et al.30,31,32, Chiu et al.33, Grubbs et al.34, and Wu et al.35 also reported a series of molecular necklaces through covalent synthesis assisted
by weak donor–acceptor π–π interactions or dynamic covalent chemistry, but the yield was also relatively low. Notably, Kim and coworkers realized the highly efficient assembly of molecular
necklaces by taking advantage of coordination bonds and cucurbituril-based host–guest chemistry, which is definitely a big breakthrough36,37,38. Recently, Li, Stang, and co-workers have
presented the highly efficient construction of the largest molecular necklace so far through hierarchical self-assembly involving coordination interactions and the subsequent host–guest
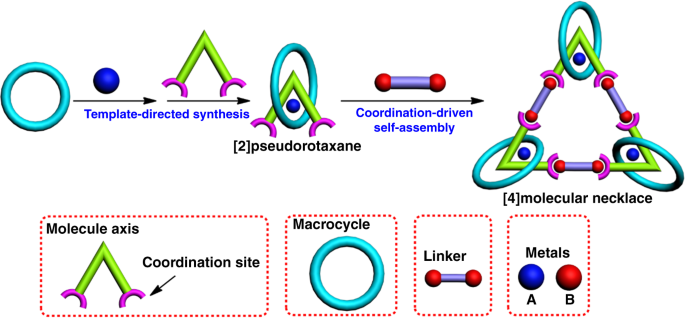
chemistry39,40. Generally, a well-established molecular necklace is self-assembled from three main components: molecular axis as chain, macrocycle as bead and molecular linker that joints
them together (Fig. 1). The prevalent strategy to design molecular necklaces is mainly based on threading and ring-closing two processes, basically driven by noncovalent interactions such as
host–guest interactions, coordination bonds, dynamic covalent chemistry, etc. Obviously, metals, as the “gems” of molecular necklaces, are of great importance to the efficient synthesis of
metallic necklaces41. In particular, metals in molecular necklace would not only facilitate the threading process due to their template effect but also participate in the ring-closing
process via coordination-driven self-assembly42,43. Moreover, the existence of metals will bring some interesting properties and applications of the resultant necklace. However, most of the
documented metallic necklaces only involve one kind metal in their architectures, and no heterometallic molecular necklace has been reported so far. Therefore, there is a high demand for the
design of functionalized molecular necklaces that possess heterometal centers and intriguing properties for applications. It’s worth-noting that, though a major breakthrough in molecular
necklaces regarding to their impressive structures as well as well-developed synthetic strategy has been achieved, the study of their properties and potential applications have persistently
lagged behind expectations. To the best of our knowledge, the focus of the chemistry of MIMs, especially for molecular necklaces, has still stayed on molecular design and synthesis stage.
Studies involving their applications are hardly seen at present in this area. The intrinsic nature of coordination-driven self-assemblies including their high dynamic metal–ligand
coordination bonds, diverse metal ions, positively charged molecular nature, together with their highly tunable coordination geometries endows them with great merits in biomedical
applications44. Indeed, the biomedical applications of coordination-driven self-assembly of metallacycles and metallacages have been systematically investigated in last decade, and they can
serve well as anticancer agents, drug delivery systems, biosensors, DNA intercalators, antibacterial agents and so on45,46. It is anticipated that molecular necklaces, the distinct type of
metal coordination-driven self-assemblies, may also be developed for biomedical applications, especially when their biomolecule-interaction efficiency and stability are highly improved. The
emerging drug-resistant bacterial pathogens have been becoming great threats to human health, and thus provoke the development of advanced antibacterial agents47,48. The bacterial DNA, cell
wall, and plasma membrane are critical components of bacterial cells essential for survival and growth49. The negatively charged property of DNA, bacterial cell wall (displaying negatively
charged lipopolysaccharides or teichoic acid), and plasma membrane (exposing negatively charged phospholipids)50 indicates that they are realistic targets of the positively charged metal
coordinating complexes. The coordination-driven self-assembly of the positively charged metallosupramolecular complexes just provides a powerful platform to enhance the electric charges for
severely disruption of the targeted DNA/cell wall/plasma membrane, and to improve the stability for prolonging the interaction time between the necklaces and the bacterial cells. Inspired by
the recent development of biomedical and biochemical applications of coordination-driven self-assembly, we envision that molecular necklaces possessing multiple metals as well as the
enhanced electric charges and stability may potentially serve as efficient DNA intercalators and bacterial cell wall/plasma membrane-disrupting agents, thus opening a promising window to
their biomedical applications. In this study, we report the construction of a heterometallic triangular necklace 1 containing both Cu and Pt metals with strong antibacterial activity through
a highly efficient “threading-followed-by-ring-closing” approach driven by metal ligand coordination (Fig. 1). The elegant structure of necklace 1 is successfully determined by X-ray
crystallographic analysis, revealing that the finely arranged triangular necklace 1 has two racemic enantiomers in its solid state with intriguing packing motif. The existence of two
different metal centers not only facilitates the successful construction of necklace 1 but also endows it with superior nuclease properties and activities. Our studies further show that the
excellent antibacterial activity might be mainly attributed to the synergistic effect of heterometals in the necklace, which enhances its bacterium-binding and cell wall/plasma
membrane-disrupting capacity for killing the bacterial cells. RESULTS SYNTHESIS The major challenge for the synthesis of heterometallic molecular necklace in this study is the compatibility
of the selected two coordination motifs. Therefore, two orthogonal 2,9-disubstituted Cu(I)-bis(phenanthroline)s ([Cu(phen)2]+) and platinum(II)–pyridine coordination motifs are particularly
selected as they are mutually compatible, i.e., 2,9-disubstituted [Cu(phen)2]+ complex will not affect platinum(II)–pyridine coordination process51,52, probably duo to the steric
hindrance53. The synthetic route to molecular necklace 1 is depicted in Fig. 2. Pseudorotaxane D1 consisting of two functional ligands of pyridine and phenanthroline was synthesized from the
starting materials M1 and D2 according to a literature method54 with minor modifications (Supplementary Fig. 1). Then heterometallic triangular necklace 1 was synthesized through the
straightforward coordination-driven self-assembly between pyridine donor D1 and diplatinum (II) acceptor unit A1 in nearly quantitative yield. Notably, pseudorotaxane D1 could generated in
situ without the further purification. Therefore, the heterometallic triangular necklace 1 was achieved in quantitative one-pot synthesis simply by mixing M1 and D2 with A1 (Supplementary
Fig. 2). More impressively, the necklace 1 could be isolated in pure form, which enables us to further study its properties and potential applications. In order to elaborate the challenge of
the synthesis of heterometallic triangular necklace, some additional controlled experiments were carried out. For instance, another molecular necklace 7 through coordination-driven
self-assembly between 3,8-disubstituted [Cu(phen)2]+ D5 and the corresponding diplatinum (II) acceptors A3 was proposed (Supplementary Figs. 8 and 9). However, the complicated 1H and 31P
nuclear magnetic resonance (NMR) spectrum indicated the unsuccessful self-assembly process (Supplementary Fig. 20). This indicates that not all the ligands are applicable to self-assemble to
form the designed necklace, thus proving the challenge to synthesize such heterometallic molecular necklace. It indicates that 3,8-disubstituted [Cu(phen)2]+ and platinum(II)–pyridine these
two coordination motifs are not compatible, i.e., the controlled experiment demonstrated that 3,8-disubstituted [Cu(phen)2]+ is likely to interfere the platinum(II)–pyridine coordination
process because of the potential competing coordination reaction between platinum(II) and nitrogen in 3,8-disubstituted [Cu(phen)2]+ (Supplementary Figs. 10 and 20). For comparison,
[3]catenane 2 as the analog of 1 with two [Cu(phen)2]+ units was also synthesized by using the same protocol (Supplementary Fig. 3). In order to systematically investigate the subsequent
biological activity of necklace, we further synthesized a series of metallacycles featuring only platinum(II)–pyridine coordination moieties without copper metal (i.e., 3, 4, 5, and 6) for
comparison (Supplementary Figs. 4–7, 21–24). 1 and 2 were well characterized with one-dimensional (1-D) NMR, two-dimensional correlation spectroscopy, diffusion-ordered NMR spectroscopy
(DOSY), nuclear overhauser effect spectroscopy, and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) (Supplementary Figs. 11–19). The structure of pseudorotaxane D1 and
necklace 1 were also unambiguously confirmed by X-ray crystallographic analysis. NMR AND MASS CHARACTERIZATIONS Multinuclear NMR (1H and 31P) spectroscopy was applied to characterize the
structural information of the necklace 1 by comparing with the corresponding ligands. The 31P NMR spectrum of the necklace 1 displayed a sharp singlet peak at _δ_ 14.24 ppm, which
significantly shifted upfield from the starting platinum acceptor A1 by approximately 5.27 ppm (Fig. 3a). This change, as well as the decrease of 31P-195Pt coupling constant (_ca_. Δ_J_ =
−212.5 Hz) is consistent with the π-back donation from the platinum atoms, indicating the successful coordination between D1 and A1. Additionally, the necklace 1 exhibited a well-resolved 1H
NMR spectrum (Fig. 3b), where the protons of the pyridine rings exhibited downfield shifts (_H_α: 8.27–8.75 ppm; _H_β: 6.43–6.88 ppm) resulting from the loss of electron density upon
coordination of the pyridine N atom with the Pt(II) metal center. Moreover, the 2D-DOSY analysis of the necklace 1 in acetone-_d_6 revealed a single band at log_D_ ≈ −8.8 (Fig. 3c). Besides,
the ESI-MS-TOF mass spectrometry provided further evidence for the formation of the target interlocked assembly. In the mass spectrum of the necklace 1 (Supplementary Fig. 13), peaks at
_m_/_z_ = 1644.80 and 1287.21 were observed, corresponding to [M-4PF6]4+ and [M-5PF6]5+ moieties, respectively, where _M_ represents the intact assemblies. The NMR and mass analysis of
[3]catenane 2 experienced a phenomenon similar to necklace 1. Therefore, the NMR and mass results both preliminarily proved the successful construction of the necklace 1 and [3]catenane 2,
verifying the rationality of the developed synthetic strategy. CRYSTALLOGRAPHIC ANALYSIS Single crystal suitable for X-ray crystallographic analysis was obtained for pseudorotaxane D1 by
slow evaporation of its acetone solution (Fig. 4a). A racemic mixture of enantiomers was found in the single crystal of D1, which is attributed to the intrinsically chiral nature of
[Cu(phen)2]+ unit55. In its packing structure, multiple intramolecular and intermolecular interactions were observed. Specifically, one pyridyl face-to-face packed with intramolecular
phenanthroline with a centroid-to-centroid distance of 3.813 Å, while the other pyridyl stacked with phenoxy groups of the neighboring enantiomer with centroid-to-centroid distance of 4.183
Å. Intramolecular π–π stacking between phenoxy group and phenanthroline was also observed with a centroid-to-centroid distance of 3.533 Å. Consequently, two enantiomers alternatively packed
together into a column along the _a_ axis. Typically, it is very difficult to obtain good single crystals of large cationic macrocycles based on Pt(II)-N(pyridine) coordinative bonds, not to
mention such complicated molecular necklace. Fortunately, single crystal of heterometallic triangular necklace with good quality was obtained by slow diffusion of ether into acetone
solution (Fig. 4b). The compound crystallizes in P-1 space group with the asymmetric unit is the whole triangular necklace 1 molecule. The large void inside the triangular necklace is filled
with disordered solvents, which are removed using SQUEEZE routine of the Platon program during the crystal structure refinement56. To the best of our knowledge, this is the first time to
present a quality crystal of heterometallic molecular necklace based on [3 + 3] Pt(II)-N cationic metallacycle. As shown in Fig. 4b, three polyether phenanthroline macrocycles were ideally
threaded onto a [3 + 3] Pt(II)-N cationic metallacycle, thus resulting in a finely arranged triangular necklace surrounded by nine hexafluorophosphate anions. Two enantiomers were also found
in the crystal of 1 on account to the orientation of [Cu(phen)2]+ unit. The size of the necklace is also very impressive with the exterior length of approximately 37 Å and internal length
of approximately 15 Å, leading to a cavity with diameters of ~8 Å. Regarding to the packing motif, three [Cu(phen)2]+ units exhibited strong π–π stacking with the adjacent molecules with a
centroid-to-centroid distance of 4.427 Å. As a result, every triangular molecule in solid state was arranged very closely to each other and stacked in a stagger packing mode, thereinto two
enantiomers in each layer was alternatively aligned. The casual discovery of the chirality in molecular necklace would enrich the recent development of chiral chemistry in MIMs such as
rotaxanes and catenanes57. The above results also highlight that the stoichiometry and position of Cu(I)-contained donor and the Pt(II)-contained acceptor unit can be precisely integrated
into the well-defined molecular necklace in such coordination-driven self-assembly process, which is of high importance to the following biomedical applications. DNA-CLEAVING AND
ANTIBACTERIAL ACTIVITIES DNA, a double helix carrying the genetic instructions, is of high importance for most of organisms. DNA is the primary target for most of anticancer drugs and
anti-infection agents58. Investigations on DNA cleavage and developments of efficient chemical nucleases have attracted wide interest owing to their potential applications as promising
therapeutic agents as well as diagnostic structural probes to analyze DNA information. Among all the chemical nucleases reported so far, transition metal complexes have obvious advantages
because metal complexes with natural cationic character particularly favor the redox, hydrolysis and other photoreactions, thus leading to strong affinity to DNA59,60. For example,
[Cu(phen)2]+ units have been widely studied as chemical nucleases for highly efficient DNA cleavage since the redox properties of the metal could not only promote the reactive oxygen species
(ROS) generation but also have strong noncovalent interactions with DNA61. Recent studies have demonstrated that ditopic coordination compound containing copper and platinum centers
displayed some intriguing nuclease properties with the enhanced DNA cleavage efficiency62,63,64,65. However, very few mechanically-interlocked molecules, especially molecular necklaces, have
demonstrated their application prospects in this area66,67. On this basis, the study of the nuclease properties and application in DNA cleavage of the designed heterometallic molecular
necklace 1 is of great interest. The DNA-cleavage of necklace 1 was then first systematically studied and compared with the above designed [3]catenane 2, metallacycles 3–6, pseudorotaxane D1
as well as di-Pt(II) acceptors A1 and A2. Indeed, DNA cleavage assay revealed that both the molecular necklace 1 and [3]catenane 2 had much higher DNA cleavage activity than the donor D1,
the acceptors A1 and A2, and the metallacycles 3, 4, 5 and 6 (Supplementary Fig. 25). Especially, necklace 1 led to thorough DNA degradation in 10 min when their concentrations reached up to
6 μM, indicating the higher cleavage activity than [3]catenane 2 (Supplementary Fig. 26). The DNA cleavage activity of necklace 1 was also observed in the 1-treated bacterial cells, which
showed remarkable intracellular DNA fragmentation as compared to the control cells (Supplementary Fig. 27). These results implied that necklace 1 might have the highest biological activity
among the tested molecules. Meanwhile, the Cu(I)-containing molecule D1, rather than the Cu(I)-free molecules (i.e., A1, A2, 3, 4, 5, and 6), also led to obvious DNA degradation, but its DNA
cleavage efficiency was much lower than the necklace (Supplementary Fig. 25). This observation indicated that Cu(I) plays a critical role in DNA cleavage by the necklace, and its efficiency
was remarkably enhanced by the Pt(II)-containing acceptor. Strong DNA cleavage activity of the coordination complexes commonly indicates high antibacterial ability68,69,70. Moreover, recent
studies have further unveiled some interesting findings that metallacycles and metallacages exhibited strong cell wall (together with plasma membrane)-intercalating ability and
antibacterial activity44,45,46,71. Considering the fact that molecular necklace 1 has combinational positive charges, π–π stacking ability, platinum(II)–pyridine coordination and
[Cu(phen)2]+ units, we speculate that it may possess strong antibacterial activity. The positively charged molecular necklace 1 might possess antibacterial activity owing to its binding with
the negatively charged components of the cell wall/plasma membrane, e.g., liposaccharides (LPS) and phospholipids. The binding ability of the molecular necklace to the bacterial cells was
then investigated by both glass-adhering test and dynamic light scattering (DLS) analysis using the well-known bacterial pathogen _Pseudomonas aeruginosa_. Confocal observation of the
4′,6-diamidino-2-phenylindole (DAPI)-stained cells on the glass surfaces revealed that the glass surface coated with the necklace 1 could bind abundant bacterial cells, while the surfaces
coated with [3]catenane 2 or the metallacycles 3–6 only had weak bacterial binding ability (Fig. 5a). Fluorescence intensity quantification further indicated that 1 could bind 1-fold more
bacterial cells than [3]catenane 2, and 2–3-fold more cells than the metallacycles without copper metals (Supplementary Fig. 28). DLS analysis of the molecule-bacteria interaction suspension
further showed that necklace 1 led to the higher size distribution of the bacterial groups (from 620–1980 nm to 3760–4400 nm) than that of [3]catenane 2 (to 750–3548 nm), indicating much
more severe cell aggregation caused by the binding of 1 (Supplementary Fig. 29a). In contrast, the metallacycles 3–6 without copper metals had no remarkable effect on the size distribution
of the bacterial groups (Supplementary Fig. 29b), confirming the important role of the molecular necklace on cell binding ability. Moreover, 1-coated glass could adsorb much higher levels of
LPS than other complexes (Fig. 5b). These observations suggested that the heterometallic necklace 1 featured strong bacterial binding ability, which is most likely because the necklace 1
has more positive charges and consequently stronger interaction with the cell wall LPS. The strong binding of the heterometallic necklace with the bacterial cells might result in severe
plasma membrane damage and consequent cell death. We then used the 5(6)-carboxyl fluorescein (CF)-leakage model to evaluate the ability of the molecular necklace to damage the plasma
membrane72,73,74. Expectedly, while [3]catenane 2 and the metallacycles 3–6 only led to weak and slow CF release (<30% even after 90 min) from the 1,2-dioleoyl-sn-glycero-3-phosphocholine
(DOPC) liposomes, the heterometallic necklace 1 caused drastic CF release (~70% in 15 min, and >80% after 90 min) (Fig. 5c), indicating the strong plasma membrane-damaging ability of 1.
Owing to the strong bacterium-binding and cell membrane-damaging ability of necklace 1, we expected that it may severely kill the bacterial cells. Colony forming unit assays showed that,
while [3]catenane 2 and the metallacycles 3–6, together with their donor D1, the Cu+ precursor Cu(MeCN)4PF6, and the acceptors (A1 and A2), only caused a decrease in cell viability to
30–65%, the heterometallic necklace 1 could kill almost all of the bacterial cells (Fig. 5d, e, Supplementary Fig. 30). Statistical analysis also revealed that necklace 1 had much lower IC50
against the bacterial cells than that of the other complexes (Supplementary Table 1). More importantly, heterometallic necklace 1, as compared to the control complexes, also showed
excellent antibacterial activity to the clinically isolated drug-resistant pathogens, such as the ciprofloxacin/penicillin double-resistant _P. aeruginosa_ strain (IC50 = 3.98 μM), the
penicillin/tetracycline _Escherichia coli_ strain (IC50 = 1.84 μM), and the multidrug-resistant _Staphylococcus aureus_ (MRSA) strain (IC50 = 5.16 μM), indicating that it may be a superior
candidate of antibacterial agents for fighting against multidrug-resistant pathogens. An interesting observation is that all of the Cu(I)-containing molecules (i.e., 1, 2, D1, and
Cu(MeCN)4PF6) had higher antibacterial activity than the Cu(I)-free molecules (i.e., 3, 4, 5, 6, A1, and A2) (Fig. 5d, Supplementary Fig. 30, Table S1). In addition, we also found that all
of the Cu(I)-containing molecules have remarkably stronger LPS-binding and membrane-damaging ability than the other molecules (Supplementary Fig. 31). These results indicated that Cu(I)
plays an important role in killing bacterial cells by enhancing the interaction between 1 and the cell membrane components. Notably, only the cooperation of the Cu(I)-contained donor and the
Pt(II)-contained acceptor in the necklace 1 generated the highest cell wall/plasma membrane-binding/damaging and bacterial killing ability since the Cu(I)-free matallacycle 4 and the
Pt(II)-free donor D1 exhibited much lower antibacterial capacity than necklace 1 (Fig. 5, Supplementary Fig. 30). Coordination-driven formation of the necklace 1 framework is also critical
for efficiently killing the pathogens. To prove this contribution, a control experiment was carried out. The antibacterial activities of the ligand mixtures of D1 + A1′ or D1 + A2′ have been
surveyed. Notably, the physical blends of D1 + A1′ or D1 + A2′ could not self-assemble into the necklace since Pt was protected by iodide or bromide moieties. It was found that, compared to
the necklace 1, the mixtures of D1 + A1′ or D1 + A2′ exhibited much weaker bacterium-killing efficiency (Supplementary Fig. 32). These results further implied the significance of necklace
formation in bacterial killing. From the above discussion, we may conclude that stoichiometric Cu(I)-contained donor and the Pt(II)-contained acceptor can be precisely integrated into the
platform of necklace 1, which is highly conducive to its outstanding bacterial killing ability. The strong antibacterial activity of the heterometallic necklace 1 was related to severe
bacterial cell death. Specifically, confocal observation of the DAPI/propidium iodide (PI) double stained cells revealed that the necklace 1 induced severe cell aggregation and caused almost
thorough cell death (indicated by the PI-positive cells with red fluorescence) (Fig. 5f). In contrast, [3]catenane 2 and the metallacycles 3–6 did not cause obvious cell aggregation and
only led to partial cell death (Fig. 5f). Similarly, SEM observation showed that heterometallic necklace 1 resulted in much more severe corruption of the cell structures than other complexes
(Fig. 5g), confirming drastic cell death induced by necklace 1. Interestingly, the cells of each group shared the similar levels of intracellular reactive oxygen species (ROS)
(Supplementary Fig. 33), excluding the possible contribution of ROS accumulation to the difference in the antibacterial ability of all tested species. The contribution of cell wall/plasma
membrane disruption in bactericidal activity of the necklace 1 was further confirmed by cell surface distribution of the necklace 1 and ultrathin section observations of the bacterial cells
(Fig. 6a, b). Energy dispersive spectroscopy mapping showed that both Cu and Pt were abundantly accumulated on the cell surface and co-localized with the cellular elements (e.g., O, N, and
P) (Fig. 6a), indicating the direct contact between the necklace and the cell surface. TEM observations of the bacterial ultrathin sections further revealed that the control cells had intact
cell wall and plasma membrane embracing the cytoplasm (Fig. 6b). In contrast, most of the necklace-treated cells experienced severely cell wall/plasma membrane disruption and consequent
cytoplasm leakage from the disrupted sites (Fig. 6b), emphasizing the critical role of direct contact between the necklace 1 and the bacterial cell wall/plasma membrane components in
bacterial death. In addition, severe DNA fragmentation was observed in necklace 1-treated cells (Supplementary Fig. 27), implying that DNA cleavage caused by internalized necklace 1 was also
involved in its antibacterial performance. Taken together, these results revealed that the heterometallic necklace 1 possessing more positive charges and two metal centers featured much
stronger bacterium-binding activity and superior cell wall/plasma membrane/DNA-disrupting capacity than the control complexes, which might be mainly attributed to the synergistic combination
of heterometals in the necklace (Fig. 6c). The stability of necklace 1 and other assemblies in bacterial culture media is certainly worth considering. We then studied their stabilities
under a variety of conditions by in situ 1H NMR and 31P NMR spectroscopy. The preliminary results demonstrated that all assemblies including necklace 1 are relatively stable towards
different pH and tryptone, which is used as a nitrogen source in culture media, as indicated by the unchanged 1H NMR and 31P NMR spectra after adding N,N-Diisopropylethylamine, triflic acid
(TfOH), or tryptone (Supplementary Figs. 37–40). However, all assemblies would be disassembled in Luria–Bertani (LB) medium because their 31P NMR spectra became complicated when adding LB
medium after 1 h (Supplementary Fig. 41). For comparison, the time dependent 31P NMR spectra of necklace 1 and metallacycle 4 were recorded and their degradation curves are shown in
Supplementary Fig. 42. The results revealed that necklace 1 had a higher resistance to LB medium compared to metallacycle 4, e.g., on the one hand, the intact metallacycle 4 remained less
than 10% of its initial content after 3 min while necklace 1 still kept about 50%; on the other hand, metallacycle 4 was thoroughly destructed within 9 min while a much longer time (~45 min)
was needed for necklace 1 to be completely degraded. The enhancement of the stability towards LB medium for necklace 1 was probably due to its aggregation, which decreases its exposed
molecular surface area and protects it from LB medium. The stability of coordination-driven self-assembly is essentially related to the intrinsic dynamic nature of metal-ligand coordination
chemistry75. Based on large amounts of investigation and research on stimuli-responsive metal–ligand assemblies76,77, we believe that the degradation of necklace 1 probably stems from the
ions such as chloride ion in LB medium (Supplementary Figs. 43–50). Obviously, coordination-driven formation of necklace 1 architecture rendered the molecule more stable in biological
systems than other metallacycles, and hence prolonged the time of necklace 1-bacterium interaction to sufficiently kill bacterial cells. Together, these results indicate that Cu(I) and
Pt(II) in necklace 1 in combination play essential roles in bacterial killing: (1) Cu(I) functions in bacterial binding to the cell wall/plasma membrane and disrupts their ultra-structures,
together with in promoting DNA cleavage; (2) Pt(II) enhances the biomolecule-interaction capacity of Cu(I) to more efficiently disrupt targeted cell components, e.g., lipopolysaccharides,
phospholipids, and DNA; (3) Stoichiometric Cu(I)-contained donor and the Pt(II)-contained acceptor can be precisely integrated into the architecture of necklace 1, which thus enhances the
bacterial binding/damaging capacity and stability of the necklace. DISCUSSION In summary, a heterometallic triangular necklace 1 was successfully synthesized through a
“threading-followed-by-ring-closing” approach driven by coordination interaction. The crystal structure of 1 disclosed an elegant triangular necklace architecture containing a large [3 + 3]
Pt(II)-N cationic metallacycle interlocked with three polyether phenanthroline macrocycles. The existence of two different metal centers not only facilitated the successful construction of
necklace 1 but also endowed it with superior nuclease properties and antibacterial activities. Our studies revealed that the self-assembly of heterometallic necklace would significantly
enhance its bactericidal activity. This enhancement might be mainly attributed to the synergistic effect of heterometals in the necklace, endowing it superior bacterium-binding and cell
wall/plasma membrane-disrupting capacity for killing the bacterial cells. “Threading-followed-by-ring-closing” approach combining with coordination-driven self-assembly would allow us to
further construct more complicated molecular necklaces in the future. And the promising DNA cleavage and antibacterial activities results obtained herein would also attract broad interests
and provide directions for future chemical design in this field. METHODS The synthesis and characterization of new compounds present in this work, and the experimental details and additional
data of DNA cleavage, bacterium-binding and antimicrobial tests are described in the Supplementary Information. DATA AVAILABILITY The data that support the findings of this study are
available from the authors on reasonable request, see author contributions for specific data sets. The X-ray crystallographic coordinates for structures reported in this study have been
deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 1889123 (D1) and 1889124 (1). These data can be obtained free of charge from The Cambridge
Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. REFERENCES * Niu, Z. & Gibson, H. W. Polycatenanes. _Chem. Rev._ 109, 6024–6046 (2009). Article CAS PubMed Google
Scholar * Evans, N. H. & Beer, P. D. Progress in the synthesis and exploitation of catenanes since the millennium. _Chem. Soc. Rev._ 43, 4658–4683 (2014). Article CAS PubMed Google
Scholar * Gil-Ramírez, G., Leigh, D. A. & Stephens, A. J. Catenanes: fifty years of molecular links. _Angew. Chem. Int. Ed._ 54, 6110–6150 (2015). Article CAS Google Scholar * Li, S.
H., Zhang, H. Y., Xu, X. & Liu, Y. Mechanically selflocked chiral gemini-catenanes. _Nat. Commun._ 6, 7590 (2015). Article ADS PubMed PubMed Central CAS Google Scholar * Wu, Q. et
al. Poly[n]catenanes: synthesis of molecular interlocked chains. _Science_ 358, 1434–1439 (2017). Article ADS CAS PubMed Google Scholar * Sauvage, J. P. From chemical topology to
molecular machines (Nobel Lecture). _Angew. Chem. Int. Ed._ 56, 11080–11093 (2017). Article CAS Google Scholar * Nepogodiev, S. A. & Stoddart, J. F. Cyclodextrin-based catenanes and
rotaxanes. _Chem. Rev._ 98, 1959–1976 (1998). Article CAS PubMed Google Scholar * Bruns, C. J. & Stoddart, J. F. Rotaxane-based molecular muscles. _Acc. Chem. Res._ 47, 2186–2199
(2014). Article CAS PubMed Google Scholar * Durola, F. et al. Cyclic [4]rotaxanes containing two parallel porphyrinic plates: toward switchable molecular receptors and compressors. _Acc.
Chem. Res._ 47, 633–645 (2014). Article CAS PubMed Google Scholar * Xue, M., Yang, Y., Chi, X., Yan, X. & Huang, F. Development of Pseudorotaxanes and rotaxanes: from synthesis to
stimuli-responsive motions to applications. _Chem. Rev._ 115, 7398–7501 (2015). Article CAS PubMed Google Scholar * Wang, W. et al. Organometallic rotaxane dendrimers with
fourth-generation mechanically interlocked branches. _Proc. Natl Acad. Sci. USA_ 112, 5597–5601 (2015). Article ADS CAS PubMed Google Scholar * Tian, J. et al. Supramolecular
metal-organic frameworks that display high homogeneous and heterogeneous photocatalytic activity for H2 production. _Nat. Commun._ 7, 11580 (2016). Article ADS CAS PubMed PubMed Central
Google Scholar * Meng, Z., Xiang, J. F. & Chen, C. F. Directional molecular transportation based on a catalytic stopper-leaving rotaxane system. _J. Am. Chem. Soc._ 138, 5652–5658
(2016). Article CAS PubMed Google Scholar * Altmann, P. J. & Pöthig, A. Pillarplexes: a metal-organic class of supramolecular hosts. _J. Am. Chem. Soc._ 138, 13171–13174 (2016).
Article CAS PubMed Google Scholar * Zhu, K., Baggi, G. & Loeb, S. J. Ring-through-ring molecular shuttling in a saturated [3]rotaxane. _Nat. Chem._ 10, 625–630 (2018). Article CAS
PubMed Google Scholar * Wang, X. Q. et al. Dual stimuli-responsive rotaxane-branched dendrimers with reversible dimension modulation. _Nat. Commun._ 9, 3190 (2018). Article ADS PubMed
PubMed Central CAS Google Scholar * Zhang, Q. et al. Muscle-like artificial molecular actuators for nanoparticles. _Chem_ 4, 2670–2684 (2018). Article CAS Google Scholar * Ke, H. et
al. Shear-induced assembly of a transient yet highly stretchable hydrogel based on pseudopolyrotaxanes. _Nat. Chem._ 11, 470–477 (2019). Article CAS PubMed Google Scholar * Kim, K.
Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies. _Chem. Soc. Rev._ 31, 96–107 (2002). Article CAS PubMed Google Scholar * Erbas-Cakmak,
S. et al. Rotary and linear molecular motors driven by pulses of a chemical fuel. _Science_ 358, 340–343 (2017). Article ADS CAS PubMed Google Scholar * Fielden, S. D. P., Leigh, D. A.
& Woltering, S. L. Molecular knots. _Angew. Chem. Int. Ed._ 56, 11166–11194 (2017). Article CAS Google Scholar * Zhang, L. et al. Stereoselective synthesis of a composite knot with
nine crossings. _Nat. Chem._ 10, 1083–1088 (2018). Article CAS PubMed Google Scholar * Sauvage, J.-P. & Dietrich-Buchecker, C. _Molecular Catenanes, Rotaxanes and Knots. A Journey
through the World of Molecular Topology_ (Wiley, Hoboken, 2007). * Beves, J. E., Blight, B. A., Campbell, C. J., Leigh, D. A. & McBurney, R. T. Strategies and tactics for the
metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. _Angew. Chem. Int. Ed._ 50, 9260–9327 (2011). Article CAS Google Scholar * Bruns, C. J. & Stoddart, J.
F. _The Nature of the Mechanical Bond: From Molecules to Machines_ (Wiley, Hoboken, 2016). * Denis, M. & Goldup, S. M. The active template approach to interlocked molecules. _Nat. Rev.
Chem._ 1, 1–18 (2017). Article CAS Google Scholar * Sluysmans, D. & Stoddart, J. F. The burgeoning of mechanically interlocked molecules in chemistry. _Trends Chem._ 1, 185–197
(2019). Article Google Scholar * Whang, D., Park, K. M., Heo, J., Ashton, P. & Kim, K. Molecular necklace: quantitative self-assembly of a cyclic oligorotaxane from nine molecules. _J.
Am. Chem. Soc._ 120, 4899–4900 (1998). Article CAS Google Scholar * Bitsch, F., Dietrich-Buchecker, C. O., Khémiss, A. K., Sauvage, J. P. & Van Dorsselaer, A. Multiring interlocked
systems: structure elucidation by electrospray mass spectrometry. _J. Am. Chem. Soc._ 113, 4023–4025 (1991). Article CAS Google Scholar * Amabilino, D. B., Ashton, P. R., Stoddart, J. F.,
White, A. J. P. & Williams, D. J. Kinetic and thermodynamic effects in the self-assembly of [3]catenanes in the solution and solid states. _Chemistry_ 4, 460–468 (1998). Article CAS
Google Scholar * Chiu, S. H. et al. Making molecular-necklaces from rotaxanes. _Tetrahedron_ 58, 807–814 (2002). Article CAS Google Scholar * Nguyen, M. T., Ferris, D. P., Pezzato, C.,
Wang, Y. & Stoddart, J. F. Densely charged dodecacationic [3]- and tetracosacationic radial [5]catenanes. _Chem_ 4, 2329–2344 (2018). Article CAS Google Scholar * Chang, C. F. et al.
Using host-guest complexation to fold a flexible linear organic string: kinetically controlled syntheses of [3]catenanes and a five-membered molecular necklace. _Angew. Chem. Int. Ed._ 51,
10094–10098 (2012). Article ADS CAS Google Scholar * Clark, P. G., Guidry, E. N., Chan, W. Y., Steinmetz, W. E. & Grubbs, R. H. Synthesis of a molecular charm bracelet via click
cyclization and olefin metathesis clipping. _J. Am. Chem. Soc._ 132, 3405–3412 (2010). Article CAS PubMed Google Scholar * Dasgupta, S. & Wu, J. Template-directed synthesis of
kinetically and thermodynamically stable molecular necklace using ring closing metathesis. _Org. Biomol. Chem._ 9, 3504–3515 (2011). Article CAS PubMed Google Scholar * Roh, S.-G. et al.
Synthesis of a five-membered molecular necklace: a 2+2 approach. _Angew. Chem. Int. Ed._ 38, 637–641 (1999). Article Google Scholar * Park, K. M. et al. Designed self-assembly of
molecular necklaces. _J. Am. Chem. Soc._ 124, 2140–2147 (2002). Article CAS PubMed Google Scholar * Ko, Y. H. et al. Designed self-assembly of molecular necklaces using host-stabilized
charge-transfer interactions. _J. Am. Chem. Soc._ 126, 1932–1933 (2004). Article CAS PubMed Google Scholar * Li, S. et al. Self-assembly of triangular and hexagonal molecular necklaces.
_J. Am. Chem. Soc._ 136, 5908–5911 (2014). Article CAS PubMed Google Scholar * Ye, Y. et al. Self-assembly of [3]catenanes and a [4]molecular necklace based on a cryptand/paraquat
recognition motif. _Org. Lett._ 17, 2804–2807 (2015). Article CAS PubMed Google Scholar * Lewis, J. E. M., Beer, P. D., Loeb, S. J. & Goldup, S. M. Metal ions in the synthesis of
interlocked molecules and materials. _Chem. Soc. Rev._ 46, 2577–2591 (2017). Article CAS PubMed Google Scholar * Cook, T. R. & Stang, P. J. Recent developments in the preparation and
chemistry of metallacycles and metallacages via coordination. _Chem. Rev._ 115, 7001–7045 (2015). Article CAS PubMed Google Scholar * Chen, L. J. & Yang, H. B. Construction of
stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. _Acc. Chem. Res._ 51, 2699–2710 (2018). Article CAS PubMed Google Scholar *
Cook, T. R., Vajpayee, V., Lee, M. H., Stang, P. J. & Chi, K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. _Acc. Chem. Res._ 46, 2464–2474
(2013). Article CAS PubMed Google Scholar * Sepehrpour, H., Fu, W., Sun, Y. & Stang, P. J. Biomedically relevant self-assembled metallacycles and metallacages. _J. Am. Chem. Soc._
141, 14005–14020 (2019). Article CAS PubMed Google Scholar * Gao, S. et al. Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated
virus coat protein. _Proc. Natl Acad. Sci. USA_ 116, 23437–23443 (2019). Article CAS PubMed Google Scholar * Willyard, C. The drug-resistant bacteria that pose the greatest health
threats. _Nature_ 543, 15 (2017). Article ADS CAS PubMed Google Scholar * Brown, E. D. & Wright, G. D. Antibacterial drug discovery in the resistance era. _Nature_ 529, 336–343
(2016). Article ADS CAS PubMed Google Scholar * Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria,
mycobacteria and fungi. _Nat. Rev. Microbiol._ 13, 620–630 (2015). Article CAS PubMed PubMed Central Google Scholar * Egan, A. J., Cleverley, R. M., Peters, K., Lewis, R. J. &
Vollmer, W. Regulation of bacterial cell wall growth. _FEBS J._ 284, 851–867 (2017). Article CAS PubMed Google Scholar * Saha, M. L., De, S., Pramanik, S. & Schmittel, M.
Orthogonality in discrete self-assembly – survey of current concepts. _Chem. Soc. Rev._ 42, 6860–6909 (2013). Article CAS PubMed Google Scholar * Wu, G. Y. et al. Supramolecular polymer
cross-linked by discrete tris-[2]pseudorotaxane metallacycles and its redox-responsive behavior. _Inorg. Chem._ 57, 15414–15420 (2018). Article CAS PubMed Google Scholar * Schmittel, M.
et al. Cap for copper(I) ions! Metallosupramolecular solid and solution state structures on the basis of the dynamic tetrahedral [Cu(phenAr2)(py)2]+ motif. _Inorg. Chem._ 48, 8192–8200
(2009). Article CAS PubMed Google Scholar * Alvariño, C., Simond, D., Lorente, P. M., Besnard, C. & Williams, A. F. Chains, necklaces and weaving chain-link grids from self-assembly
reactions. _Chemistry_ 21, 8851–8858 (2015). Article PubMed CAS Google Scholar * Cetin, M. M. et al. Characterization and photocatalytic behavior of 2,9-di(aryl)−1,10-phenanthroline
copper(I) complexes. _Dalton Trans._ 46, 6553–6569 (2017). Article CAS PubMed Google Scholar * Sheldrick, G. M. Crystal structure refinement with SHELXL. _Acta Cryst._ C71, 3–8 (2015).
MATH Google Scholar * Jamieson, E. M. G., Modicom, F. & Goldup, S. M. Chirality in rotaxanes and catenanes. _Chem. Soc. Rev._ 47, 5266–5311 (2018). Article CAS PubMed PubMed Central
Google Scholar * Hurley, L. H. DNA and its associated processes as targets for cancer therapy. _Nat. Rev. Cancer_ 2, 188–200 (2002). Article CAS PubMed Google Scholar * Metcalfe, C.
& Thomas, J. A. Kinetically inert transition metal complexes that reversibly bind to DNA. _Chem. Soc. Rev._ 32, 215–224 (2003). Article CAS PubMed Google Scholar * Suntharalingam, K.
& Vilar, R. Interaction of metal complexes with nucleic acids. _Annu. Rep. Prog. Chem. Sect. A_ 107, 339–358 (2011). Article CAS Google Scholar * Sigman, D. S., Mazumder, A. &
Perrin, D. M. Chemical nucleases. _Chem. Rev._ 93, 2295–2316 (1993). Article CAS Google Scholar * De Hoog, P. et al. New approach for the preparation of efficient DNA cleaving agents:
ditopic copper-platinum complexes based on 3-clip-phen and cisplatin. _J. Med. Chem._ 50, 3148–3152 (2007). Article PubMed CAS Google Scholar * Dong, X. et al. Promotive effect of the
platinum moiety on the DNA cleavage activity of copper-based artificial nucleases. _Inorg. Chem._ 49, 2541–2549 (2010). Article CAS PubMed Google Scholar * Ng, N. S. et al. The
antimicrobial properties of some copper(ii) and platinum(ii) 1,10-phenanthroline complexes. _Dalt. Trans._ 42, 3196–3209 (2013). Article CAS Google Scholar * Liu, C., Wang, M., Zhang, T.
& Sun, H. DNA hydrolysis promoted by di- and multi-nuclear metal complexes. _Coord. Chem. Rev._ 248, 147–168 (2004). Article CAS Google Scholar * Mishra, A. et al. DNA binding and
unwinding by self-assembled supramolecular heterobimetallacycles. _Organometallics_ 30, 6343–6346 (2011). Article CAS PubMed PubMed Central Google Scholar * Casini, A., Woods, B. &
Wenzel, M. The promise of self-assembled 3D supramolecular coordination complexes for biomedical applications. _Inorg. Chem._ 56, 14715–14729 (2017). Article CAS PubMed Google Scholar *
Liu, H. K. & Sadler, P. J. Metal complexes as DNA intercalators. _Acc. Chem. Res._ 44, 349–359 (2011). Article CAS PubMed Google Scholar * Kumaravel, G., Ponya Utthra, P. &
Raman, N. Exploiting the biological efficacy of benzimidazole based Schiff base complexes with L-Histidine as a co-ligand: combined molecular docking, DNA interaction, antimicrobial and
cytotoxic studies. _Bioorg. Chem._ 77, 269–279 (2018). Article CAS PubMed Google Scholar * Kalaiarasi, G. et al. New binuclear Ni(ii) metallates containing ONS chelators: synthesis,
characterisation, DNA binding, DNA cleavage, protein binding, antioxidant activity, antimicrobial and in vitro cytotoxicity. _N. J. Chem._ 41, 2543–2560 (2017). Article CAS Google Scholar
* Wang, H. et al. Supramolecular Kandinsky circles with high antibacterial activity. _Nat. Commun._ 9, 1815 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Gao, J.,
Zhang, O., Ren, J., Wu, C. & Zhao, Y. Aromaticity/bulkiness of surface ligands to promote the interaction of anionic amphiphilic gold nanoparticles with lipid bilayers. _Langmuir_ 32,
1601–1610 (2016). Article CAS PubMed Google Scholar * Foster, B., Larios, M. & Smith, V. Investigation of the effects of titanium dioxide and cerium oxide nanoparticles on liposomes
using fluorescent dye leakage. _FASEB J._ 30, lb74 (2016). Google Scholar * Liu, Y. & Liu, J. Zn2+ induced irreversible aggregation, stacking, and leakage of choline phosphate
liposomes. _Langmuir_ 33, 14472–14479 (2017). Article CAS PubMed Google Scholar * Lehn, J. M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive
chemistry. _Chem. Soc. Rev._ 36, 151–160 (2007). Article CAS PubMed Google Scholar * McConnell, A. J., Wood, C. S., Neelakandan, P. P. & Nitschke, J. R. Stimuli-responsive
metal–ligand assemblies. _Chem. Rev._ 115, 7729–7793 (2015). Article CAS PubMed Google Scholar * Li, Z.-Y. et al. Cross-linked supramolecular polymer gels constructed from discrete
multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. _J. Am. Chem. Soc._ 136, 8577–8589 (2014). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS H.-B.Y. thanks NSFC/China (Nos. 21572066 and 21625202), Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-05-E00012), and Program for
Changjiang Scholars and Innovative Research Team in University for financial support. X.S. acknowledges the financial supports sponsored by Shanghai Sailing Program (19YF1412900) and the
Fundamental Research Funds for the Central Universities. Q.Y. thanks NSFC/China (Nos. 31870139). We thank Dr. Yiwen Wang and Dr. Bing Ni for the TEM study. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N. Zhongshan Road,
Shanghai, 200062, P. R. China Gui-Yuan Wu, Xueliang Shi, Yi-Xiong Hu, Guang-Qiang Yin, Xiao-Li Zhao, Lin Xu & Hai-Bo Yang * Vinh University, 182 LeDuan Street, Vinh, Vietnam Hoa Phan *
State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM) and College of Chemistry and Chemical Engineering,
Xiamen University, Xiamen, 361005, China Hang Qu * Department of Chemistry, University of South Florida, Tampa, FL, 33620, USA Xiaopeng Li * Key Laboratory of Molecular Microbiology and
Technology, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, 300071, P. R. China Qilin Yu Authors * Gui-Yuan Wu View author publications You can also search for
this author inPubMed Google Scholar * Xueliang Shi View author publications You can also search for this author inPubMed Google Scholar * Hoa Phan View author publications You can also
search for this author inPubMed Google Scholar * Hang Qu View author publications You can also search for this author inPubMed Google Scholar * Yi-Xiong Hu View author publications You can
also search for this author inPubMed Google Scholar * Guang-Qiang Yin View author publications You can also search for this author inPubMed Google Scholar * Xiao-Li Zhao View author
publications You can also search for this author inPubMed Google Scholar * Xiaopeng Li View author publications You can also search for this author inPubMed Google Scholar * Lin Xu View
author publications You can also search for this author inPubMed Google Scholar * Qilin Yu View author publications You can also search for this author inPubMed Google Scholar * Hai-Bo Yang
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.-B.Y., X.S., Q.Y., and G.-Y.W. conceived the project, analyzed the data, and wrote the
manuscript. G.-Y.W. performed the most of experiments. H.P., H.Q., and X.-L.Z. conducted single crystal analyses. Y.-X.Hu., G.-Q.Y., X.L., and L.X. helped in experiments and data analyses.
CORRESPONDING AUTHORS Correspondence to Xueliang Shi, Qilin Yu or Hai-Bo Yang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER
REVIEW INFORMATION _Nature Communication_ thanks the anonymous reviewer for their contributions to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, GY., Shi, X.,
Phan, H. _et al._ Efficient self-assembly of heterometallic triangular necklace with strong antibacterial activity. _Nat Commun_ 11, 3178 (2020). https://doi.org/10.1038/s41467-020-16940-z
Download citation * Received: 25 September 2019 * Accepted: 21 May 2020 * Published: 23 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16940-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative