Play all audios:
ABSTRACT _Fusobacterium nucleatum_ is an oral anaerobe recently found to be prevalent in human colorectal cancer (CRC) where it is associated with poor treatment outcome. In mice,
hematogenous _F. nucleatum_ can colonize CRC tissue using its lectin Fap2, which attaches to tumor-displayed Gal-GalNAc. Here, we show that Gal-GalNAc levels increase as human breast cancer
progresses, and that occurrence of _F. nucleatum_ gDNA in breast cancer samples correlates with high Gal-GalNAc levels. We demonstrate Fap2-dependent binding of the bacterium to breast
cancer samples, which is inhibited by GalNAc. Intravascularly inoculated Fap2-expressing _F. nucleatum_ ATCC 23726 specifically colonize mice mammary tumors, whereas Fap2-deficient bacteria
are impaired in tumor colonization. Inoculation with _F. nucleatum_ suppresses accumulation of tumor infiltrating T cells and promotes tumor growth and metastatic progression, the latter two
of which can be counteracted by antibiotic treatment. Thus, targeting _F. nucleatum_ or Fap2 might be beneficial during treatment of breast cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS
INTRACELLULAR BACTERIA IN CANCER—PROSPECTS AND DEBATES Article Open access 09 October 2023 THE ADHESIN RADD ENHANCES _FUSOBACTERIUM NUCLEATUM_ TUMOUR COLONIZATION AND COLORECTAL
CARCINOGENESIS Article 21 August 2024 KILLING TUMOR-ASSOCIATED BACTERIA WITH A LIPOSOMAL ANTIBIOTIC GENERATES NEOANTIGENS THAT INDUCE ANTI-TUMOR IMMUNE RESPONSES Article 25 September 2023
INTRODUCTION _Fusobacterium nucleatum_ is a common oral non-spore forming gram-negative anaerobe that has long been known to be associated with the development of periodontal disease. More
recently, however, genomic studies provided evidence that _F. nucleatum_ may also be prevalent in colorectal carcinoma1,2. In support of this proposed link with cancer, subsequent studies
demonstrated that _F. nucleatum_ promotes tumorigenesis3,4, affects infiltration of tumor-infiltrating lymphocytes5,6,7, inhibits killing of cancer cells by Natural Killer (NK) cells and
tumor-infiltrating T cells8, and induces resistance to chemotherapy in colon cancer9,10. Moreover, treatment of mice bearing a colon cancer xenograft with antibiotics to eliminate _F.
nucleatum_ concomitantly reduced cancer cell proliferation and overall tumor growth11. Together, these CRC-related fusobacterial effects provide a rational for the observation that high
numbers of _F. nucleatum_ in CRC tissue is inversely correlated with overall survival12 (also reviewed in ref. 13). In addition to CRC, a high burden of _F. nucleatum_ is also associated
with poor prognosis in esophageal cancer14,15. CRC-associated fusobacteria originate from the oral microbial community, and we have previously hypothesized that they reach the colon via the
hematogenous rather than the gastrointestinal route16. In this model, _F. nucleatum_ will enter the bloodstream during transient bacteremia, which is frequent in periodontal disease, and
specifically dock to CRC tissue through its surface-exposed lectin Fap2. Fap2 recognizes Gal-GalNAc, which is a sugar that is abundantly displayed by CRC cells16. We hypothesized that, if
oral _F. nucleatum_ can translocate to colon tumors hematogenously, this bacterium may also reach Gal-GalNAc-displaying tumors in other organs via this route. To address this, we recently
screened different cancer tissue samples for abundant Gal-GalNAc. This screen identified breast cancer as a strong candidate, which was in agreement with previous studies that had detected
Gal-GalNAc in breast cancer17,18,19,20 or observed high accumulation of DNA from the genera _Fusobacterium_ in the microbiome of malignant but not benign breast tissue samples21. Breast
cancer is the most commonly diagnosed cancer and the leading cause of cancer mortality in women22. Therefore, it is important to understand if _F. nucleatum_ has the same profound impact on
the progression and outcome of breast cancer as it has in CRC. Our present study provides several lines of molecular and experimental evidence for this to be the case. We demonstrate that
Gal-GalNAc levels increase along the progression of human breast cancer and that _F. nucleatum_ DNA is overabundant in human breast cancer samples, particularly in those with high Gal-GalNAc
signals. Using two different murine orthotropic models, we show that _F. nucleatum_ colonizes mammary tumors via abundant Gal-GalNAc on tumor cells, with the same Fap2 lectin whereby
recognizes CRC cells. Furthermore, we find that the colonization of mammary tumors by _F. nucleatum_ accelerates breast cancer progression and metastatic development, most likely through
suppression of T cell accumulation in the tumor microenvironment. Importantly, breast tumor exacerbation by _F. nucleatum_ in mice can be counteracted by antibiotic treatment with
metronidazole. RESULTS GAL-GALNAC IS OVERDISPLAYED IN BREAST CANCER We have shown previously that CRC-specific attachment and colonization by _F. nucleatum_ is facilitated by high Gal-GalNAc
levels on CRC cells, and that the Fap2-mediated recognition of this sugar is inhibited with soluble GalNAc or removal of the cell-exposed sugar with O-glycanase16. In addition,
Fap2-deficient _F. nucleatum_ showed impaired attachment to CRC samples, and much reduced CRC colonization in a mouse model16. Since Gal-GalNAc (also known as the Thomsen–Friedenreich
antigen) was known to be displayed in breast cancer17,18,19,20, we hypothesized that _F. nucleatum_ would target breast cancer tissue in a similar manner. To this end, we stained tissue
microarrays containing human breast cancer and matched adjacent non-tumor tissue samples using fluorescein isothiocyanate (FITC)-labeled peanut agglutinin (PNA), a Gal-GalNAc specific
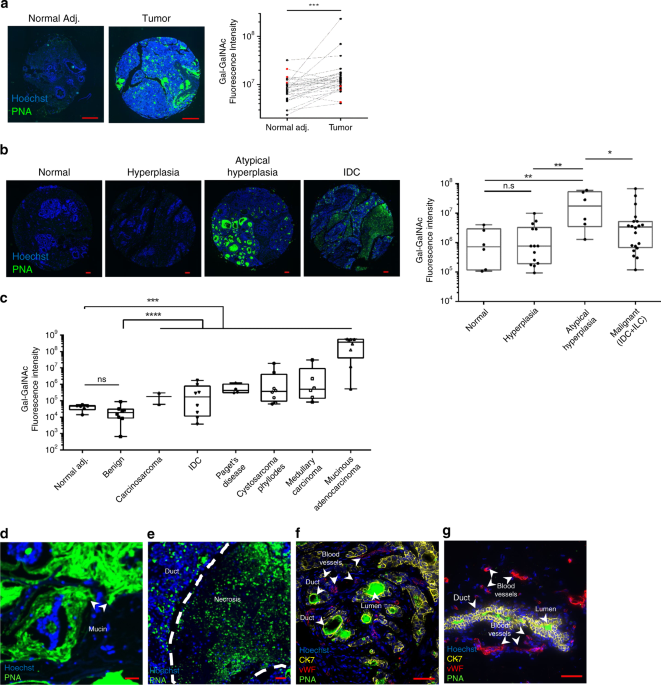
lectin. In 25 of 30 tested paired samples, Gal-GalNAc levels in the tumor samples were higher than those in the matched normal tissue (Fig. 1a). In addition, breast cancer samples showed
significantly higher (_p_ = 0.0003) Gal-GalNAc levels than the matched adjacent non-tumor tissue (Fig. 1a). We then compared Gal-GalNAc levels in samples from normal human breast tissue,
breast tumor tissue including benign tumors, and from different stages along the progression of the disease. Gal-GalNAc signals were lowest in normal [median florescence intensity (MFI) 7.12
× 105] and hyperplasia tissues (MFI 7.55 × 105). Atypical hyperplasia (MFI 1.73 × 107) and malignant (MFI 3.50 × 106) tissue displayed 24- and 4.7-fold, respectively, higher levels of
Gal-GalNAc than did normal tissue (Fig. 1b). Note that the high median of Gal-GalNAc levels in atypical hyperplasia might be also due to the low number of available samples. Interestingly,
benign tumors (MFI 1.89 × 104) showed significantly lower Gal-GalNAc levels than did the different malignant tumors (all with an MFI higher than 1.68 × 105), and overall did not
significantly differ from normal tissue (MFI 4.7 × 104) (Fig. 1c). Comparing by subgroups of breast cancer, mucinous adenocarcinoma displayed at least two orders of magnitude higher amounts
of Gal-GalNAc over the other five cancer subgroups tested (Fig. 1c). Of the latter, mucinous breast cancer showed the highest Gal-GalNAc levels in the mucin layers, whereas in ductal breast
cancer high Gal-GalNAc levels were observed in necrotic regions, ducts, and in the ductal lumen (Fig. 1d–g). _F. NUCLEATUM_ GDNA IS OVERABUNDANT IN BREAST CANCER Next, we tested whether _F.
nucleatum_ occurred in breast cancer, and if so, whether its presence correlates with high Gal-GalNAc levels. For this purpose, we searched for _F. nucleatum_-specific sequences in 50 deep
sequencing libraries of PCR-amplified bacterial 16S rDNA from different breast cancer samples. Since breast tumors have low bacterial biomass, we included as negative controls for possible
signals from contaminating DNA, 50 empty DNA extractions (controls) and 50 no-template PCR controls (NTCs). DNA from 21 CRC samples was included as positive controls. We detected _F.
nucleatum_ in 30% and 57% of the analyzed breast tumors and CRC samples, respectively (Fig. 2a, b). Importantly, _F. nucleatum_ was predominantly found in those breast cancer samples that
displayed high Gal-GalNAc levels (Fig. 2c). These results not only confirm the previously observed prevalence of the _Fusobacterium_ genera in the human breast cancer microbiome21, they also
indicate a significant role of Fap2-Gal-GalNAc interactions in fusobacterial colonization of breast cancer tissue. FAP2 BINDS THROUGH GAL-GALNAC DISPLAYED ON BREAST CANCER In our previous
work establishing the Fap2 outer membrane protein as a major bacterial Gal-GalNAc lectin, we generated two Fap2-deficient mutants, K50 and D22, of _F. nucleatum_ ATCC 2372616,23. Here, we
used these strains to assay whether _F. nucleatum_ through Fap2 specifically attaches also to breast cancer cells. Using microarrays containing samples of malignant and non-cancer (normal
and benign) human breast tissue, we found that the Fap2-proficient wild-type strain (WT) ATCC 23726 exhibited significantly higher attachment to malignant breast cancer sections (median
21.96 Fn/mm2, Fig. 3a) than to normal (median 7.26 Fn/mm2) or benign breast tumor (median 8.59 Fn/mm2) sections. By contrast, the Fap2-deficient K50 mutant showed significantly impaired
attachment to malignant breast cancer tissue (median 12.03 Fn/mm2), compared with the wild-type parental strain (median 21.96 Fn/mm2), while its attachment to normal breast sections (median
6.20 Fn/mm2) or benign tissue (median 5.41 Fn/mm2) was similar to WT (Fig. 3a). Of note, while the Fap2-deficient K50 mutant still attached better to breast cancer tissue, as compared with
normal or benign sections, this tumor-specific attachment was not statistically significant. We speculate that other _F. nucleatum_ tumor-binding factors such as FadA3, or the
CEACAM1-binding CbpF outer-surface protein24 may enable _F. nucleatum_ to recognize breast cancer cells even in the absence of Fap2. Next, we used flow cytometry to compare attachment of WT
versus Fap2-deficient _F. nucleatum_ to several breast cancer cell lines, i.e., murine AT3 and 4T1, and human MCF-7. Mouse melanoma cell line, B16, served as negative control. Based on PNA
binding, all three tested breast cancer cell lines displayed higher Gal-GalNAc levels than the B16 melanoma cell line (Fig. 3b, Supplementary Fig. 4). Similarly, _F. nucleatum_ was found to
attach only to the breast cancer cell lines. Supporting the predicted Fap2-mediated recognition of Gal-GalNAc, wild-type _F. nucleatum_ ATCC 23726 showed significantly higher attachment than
the K50 and D22 mutants (Fig. 3b). Addition of soluble GalNAc suppressed attachment, most likely by saturation of the Fap2 lectin (Fig. 3b). Interestingly, both wild-type and Fap2-deficient
bacteria invariably attached better to AT3 cells than to MCF-7 or 4T1 cells, suggesting that the AT3 cell line possesses additional (surface) factors for fusobacterial attachment. In
conclusion, the above results strongly argue _F. nucleatum_ primarily uses its Fap2 lectin to home to breast cancer tissue with high levels of Gal-GalNAc. _F. NUCLEATUM_ PREFERENTIALLY
COLONIZES BREAST CANCER Transient bacteremia is common in periodontal disease13,25,26, and provides an opportunity for oral fusobacteria to enter the circulatory system not only during
dental treatment but also during daily routine. Once in the bloodstream, previously oral _F. nucleatum_ might translocate to breast cancers hematogenously. To test this, we simulated
transient bacteremia by intravascular injection of _F. nucleatum_ in the 4T1 orthotropic BALB/c mouse model of mammary cancer. In this model, 4T1 cells are transplanted into the mammary fat
pad of female BALB/c mice where they are highly tumorigenic and invasive. As illustrated in Fig. 4a, we transplanted 1 × 106 4T1 cells into the mammary fat pad, and when tumors reached a
size of 500 mm3, mice were inoculated once with 5 × 107 _F. nucleatum_ ATCC 23726 by tail vein injection. Analysis of tissue 24 h post inoculation revealed that, consistent with our human
breast cancer samples (see above), Gal-GalNAc levels (measured using FITC-labeled PNA) in the mouse cancer sections were higher (6.8-fold when comparing the medians) than in sections of
matching normal tissues (Fig. 4b, right panel). We observed an at least two orders of magnitude higher median abundance of the bacteria, using _F. nucleatum_ gDNA as a proxy, in the tumor
sections than in the matching normal tissue (Fig. 4c). By contrast, we failed to detect bacteria in the mammary of control tumor-free mice (No tumor, Fig. 4c) treated the same way,
indicating that blood-borne _F. nucleatum_ require the presence of a tumor to localize to breast tissue. FAP2 MEDIATES BREAST CANCER COLONIZATION BY _F. NUCLEATUM_ To validate the observed
mammary cancer colonization by _F. nucleatum_ in a different animal model and evaluate the role of Fap2 in the process, we used the AT3 orthotropic mammary cancer model in C57BL/6 mice. Mice
with tumors were generated as described above for the 4T1 model, yet this time injected with either wild-type _F. nucleatum_ ATCC 23726, Fap2-deficient _F. nucleatum_ K50, or the
Gram-negative, anaerobic periodontal bacterium _Porphyromonas gingivalis_ (ATCC 33277) as a control. Note that the latter species has been reported to be overabundant in oral squamous cell
carcinoma27,28,29,30. Tumors and unaffected tissue from an adjacent mammary were analyzed 24 h post inoculation (Fig. 4d). As in the BALB/c model above, _F. nucleatum_ ATCC 23726 was
enriched in the tumor compared with adjacent normal tissue, as determined by both, colony counting and qPCR (Fig. 4e, left and right panels respectively). Fap2 deficiency (mutant K50)
resulted in an at least a three orders of magnitude drop in mammary tumor colonization, as compared with WT bacteria (Fig. 4e). Interestingly, these results closely match those obtained with
a CRC mouse model16. They imply that while neoplastic tissue certainly plays a critical role in enriching _F. nucleatum_ in tumors, breast cancer-specific enrichment by _F. nucleatum_ is
crucially driven by Fap2. Also in agreement with CRC colonization by _F. nucleatum_20, breast tumors are not colonized by just any oral anaerobic bacterium associated with periodontitis.
This is to say that in mice inoculated with _P. gingivalis_, bacterial numbers in tumors were below the detection limit of culturing (∼2 CFU/g tissue) or qPCR (Fig. 4e). Thus, _F. nucleatum_
likely possess distinctive features for tumor colonization, such as Fap2 (the focus of this work), FadA3 or CbpF24,31. Finally, in order to directly visualize fusobacterial breast cancer
colonization, we tracked FITC-labeled _F. nucleatum_ ATCC 23726 in animals by multiphoton microscopy. To this end, we inoculated mammary tumor-bearing C57BL/6 (AT3 orthotropic mammary cancer
model, see above) with 5 × 107 FITC-labeled bacteria or injected PBS (the vehicle used to inject the bacteria) as control. Twenty-four hours post inoculation, we detected signals of
fusobacteria in all tumor samples, whereas there was no signal in any of the adjacent normal mammary samples, nor in samples harvested from the sham-inoculated mice (Fig. 4f). ANTIBIOTICS
INHIBIT _F. NUCLEATUM_-INDUCED TUMOR EXACERBATION Given that colonization by _F. nucleatum_ accelerates the formation of colonic tumor3,4,11, we used the AT3 orthotropic mammary cancer model
to test whether it affects tumor progression in breast cancer as well, following the scheme in Fig. 5a. When tumors reached 500 mm3 in size (day 1), mice were randomly divided into five
treatment (intravascular injection or inoculation) groups: PBS vehicle (V), wild-type _F. nucleatum_ ATCC 23726 (726) or a Fap2 mutant (K50), _F. nucleatum_ ATCC 23726 and additional
treatment with metronidazole (726+MTZ.), or metronidazole alone (MTZ.). Starting with metronidazole (MTZ.) or PBS (V), bacteria were inoculated 30 min later. Treatment with metronidazole or
PBS vehicle was repeated after 8 h, twice a day (morning and evening) in the following 2 days (days 2–3), and daily for 4 more days. On day 8, mice were sacrificed, and tumor and lungs were
harvested to weigh breast cancer tissue and count lung metastases, respectively. As shown in Fig. 5b, mice inoculated with _F. nucleatum_ ATCC 23726, but not with Fap2-deficient K50
bacteria, displayed significantly larger tumors than those in the control group. Metronidazole treatment alone did not affect tumor size (Fig. 5b), but did prevent tumor enlargement in mice
inoculated with fusobacteria (Fig. 5b, c). The median number of lung metastases in mice inoculated with _F. nucleatum_ ATCC 23726 was at least two orders of magnitude higher than in the
sham-inoculated mice (Fig. 5e). The _F. nucleatum_-driven increase in metastases numbers and size was also readily visible in mice bearing GFP-expressing AT3 tumors (Fig. 5f). By contrast,
Fap2-deficient bacteria (K50), while promoting some increase in the number of metastases, did not cause a statistical significant higher burden of metastases. As with tumor size,
metronidazole treatment prevented the pro-metastasis effect of _F. nucleatum_ (Fig. 5e). _F. NUCLEATUM_ MODULATES IMMUNITY AGAINST MAMMARY TUMORS We considered several different mechanisms
whereby _F. nucleatum_ induces an increase in breast tumor size: (i) enhancing proliferation of the cancer cells; (ii) inhibiting apoptosis of the cancer cells; or (iii) manipulating
anti-tumor immunity. Immunohistochemistry staining for the Ki67 proliferation marker or the apoptosis marker CC3 cleaved caspase 3 revealed no differences between _F. nucleatum_-infected and
sham-infected tumors (Supplementary Fig. 1). Similarly, we found no indication for increased proliferation or reduced apoptosis in an RNA-seq based comparison of AT3 cells incubated for 24
h with or without _F. nucleatum_ ATCC 23726 (Supplementary Fig. 3, Supplementary Data 1 and 2). These results suggested that breast tumor acceleration by _F. nucleatum_ in the AT3 mouse
model might be immune-mediated. We previously discovered that _F. nucleatum_ can inhibit anti-tumor immunity by activating two human (though not the mouse homologs of) immune-suppression
checkpoint receptors, TIGIT and CEACAM18,31. In addition, tissue load of _F. nucleatum_ was previously found to be inversely correlated with density of tumor-infiltrating lymphocytes in
colorectal carcinoma tumors5,6,7. Therefore, to determine whether immune cell accumulation might be affected by _F. nucleatum_, we used flow cytometry to compare levels of NK cells, CD4+ T
cells and CD8+ T cells between AT3 mammary tumors from _F. nucleatum_-infected and uninfected mice. This analysis showed that inoculation by _F. nucleatum_ resulted in fewer CD4+ and CD8+ T
cells (Fig. 6a, Supplementary Fig. 5a–c). To evaluate the involvement of T, B, and NK cells in fusobacterial acceleration of the progression of the AT3 tumor, we repeated our _F. nucleatum_
inoculation scheme in SCID-beige mice, which lack T, B, and NK cells. Unlike in C57BL/6 mice, AT3 tumors grown in uninfected SCID-beige mice were not smaller than those in the infected mice
(Fig. 6b). We interpret this finding to suggest that in the immune-competent C57BL/6 mice, growth of AT3 breast tumor is restricted by T, B, or NK cells, and that _F. nucleatum_ directly or
indirectly dampens this immune-mediated tumor control. Searching the above-mentioned RNA-seq data for additional potential mechanisms whereby the presence of _F. nucleatum_ might accelerate
development of breast tumors, we noticed an overexpression of matrix metalloproteinase 9 in the AT3 cells incubated with _F. nucleatum_ (Supplementary Fig. 3). Proteases of the matrix
metalloproteinase (MMPs) family play vital roles in many biological processes that involve matrix remodeling. In particular, MMP-9 activity has been related to cancer pathology including
invasion, angiogenesis, and metastasis32. While many MMPs expressed in breast cancer are produced by stromal cells, MMP-9 is produced mainly by the tumor cells themselves32. Echoing this
general patterns, a gel zymography assay with gelatin as substrate showed unaltered secretion of MMP-2 by cell lines AT3 (murine mammary tumor) or MDA-MB-231 (human breast cancer) incubated
with _F. nucleatum_ ATCC 23726 or with _F. nucleatum_ ATCC 25586; by contrast, the secretion of MMP-9 was significantly and invariably increased when both cell lines where incubated with
each of the two _F. nucleatum_ strains (Fig. 6c). Therefore, in addition to immune modulation as the putative major mechanism of _F. nucleatum_ in the AT3 mouse model in C57BL/6 mice,
induction of MMP might be another mechanism whereby _F. nucleatum_ accelerates breast tumor progression. DISCUSSION The involvement of viruses in tumor development has been investigated for
over a century, whereas the interest in potentially similar roles of bacteria in cancer biology has been rather recent, with the primary focus being the carcinogenic role of _Helicobacter
pylori_ in gastric cancer33,34. Periodontitis is a common bacterial-induced inflammatory disease. Existing data provide support for positive association between periodontitis and risk of
cancer35,36, particularly oral, lung, and pancreatic cancers37. A recent meta-analysis also predicted that periodontal disease increased the risk of breast cancer by 1.22-fold38. Of the many
different bacteria presently linked to cancer development, _F. nucleatum_ has so far been associated only with colorectal and esophageal cancers. Being an oral microbe, it was plausible
that _F. nucleatum_ reaches CRC tissue by descending through the digestive tract4. However, our recent finding that oral _F. nucleatum_ might probably colonize CRC via the hematogenous
route, and its selectivity for tumors that display Gal-GalNAc16,20, suggested that additional tumors might be colonized by this bacterium. As to a specific candidate cancer, we have
demonstrated here that Gal-GalNAc levels increase as breast cancer progresses (Fig. 1a, b), supporting earlier reports of Gal-GalNAc detection in breast cancer samples17,18,19,20. Breast
cancer represents a sequence of events, starting with non-neoplastic epithelium and subsequent progress through the stages of hyperplasia, atypical hyperplasia, carcinoma in situ and
invasive adenocarcinoma. It has been hypothesized that the conversion between benign hyperplasia and carcinoma in situ (the stage preceding invasive carcinoma) is made at the transition from
hyperplasia to atypical ductal hyperplasia39. Interestingly, we observed the sharpest rise in Gal-GalNAc levels in this very transition phase (Fig. 1b). In addition, Gal-GalNAc levels in
breast cancer were 4.7-fold higher than in normal tissue (Fig. 1b), but only 1.4-fold higher than in matched normal adjacent tissue (Fig. 1a). These results suggest that the proximal tumor
also affects the adjacent non-tumor tissue, causing aberrant Gal-GalNAc levels in the latter. These results are in line with previous ones demonstrating that normal tumor adjacent tissue
represents an intermediate state between healthy and tumor40. The sum of our in vitro and in vivo results strongly support a model whereby _F. nucleatum_ colonizes not only colorectal
cancer16, but also breast cancer through recognition of Gal-GalNAc by Fap2. They also imply, that as in CRC3,4,16, colonization of breast cancer by _F. nucleatum_ is secondary to tumor
initiation: in the absence of tumors, no _F. nucleatum_ bacteria were detectable in the mouse mammary glands. Colonization likely occurs as blood supply is attracted to the tumor, enabling
sufficient bacterial trafficking from the mouth, and after tumor Gal-GalNAc levels raised. Importantly and in agreement with its role in CRC3,4, colonization by _F. nucleatum_ accelerated
the progression of mammary tumor, yet strictly in a Fap2-dependent manner (Fig. 5b, c). That is, tumors were not enlarged in mice inoculated with Fap2-deficient bacteria. Since _F.
nucleatum_ only activates human TIGIT and CEACAM1 and not their murine homologs8,31, tumor acceleration in the mouse models used here cannot involve impaired T cell and NK cell
killing-activity via the activation of these important checkpoints. Nonetheless, our observation of reduced CD4+ and CD8+ T cell levels in _F._ _nucleatum_-infected tumors (Fig. 6a), and the
lack of acceleration of AT3 tumors by _F. nucleatum_ in SCID-beige mice (Fig. 6b), both indicate a role of immunity in fusobacterial tumor acceleration in the AT3 mouse model. Thus, it
appears that in the immune-competent C57BL/6 mice, growth of AT3 breast tumor is normally restricted by NK, B, or T cells, but in the presence of _F. nucleatum_ T cells become reduced,
resulting in tumor growth acceleration. The reduction of immune cells might involve apoptosis. Apoptosis was shown to be induced by _F. nucleatum_ in lymphocytes, through its lectin Fap241.
Importantly, we expect the pro-tumorigenic effect of _F. nucleatum_ to be much stronger in humans because the activity of the NK and few T cells present in the tumor will be further weakened
by inhibitory interactions of Fap2 with TIGIT and of CbpF with CEACAM1. There are several important similarities of our results with previous observations with CRC. First, lower numbers of
T cells have also been reported in CRC samples containing _F. nucleatum_5,6,7. Second, metronidazole treatment to target _F. nucleatum_ reduces tumor growth not only in CRC11, but also
prevents fusobacteria-associated acceleration of breast cancer (Fig. 5b, c). Since the metronidazole treatment had no effect on tumor development in the absence of _F. nucleatum_, it must be
the tissue sterilization by the antibiotic that nullifies the fusobacterial-mediated tumor acceleration (Fig. 5b). Metastasis causes as much as 90% of cancer-associated mortality in
general, and is also the main cause of death by breast cancer. Whereas patients with localized breast cancer have a 5-year survival rate of 98%, this drops to 26% in patients with metastatic
breast cancer42. In our mouse model, we observed not only an enlargement of primary AT3 mammary tumors by _F. nucleatum_, but also a significant increase in the number of lung metastases
(Fig. 5e, f). The fusobacterial-driven increase in metastasis was clearly dependent on Fap2, i.e., it was not statistically significant with the Fap2-deficient K50 mutant (Fig. 5e). Further
work is needed to delineate the pro-metastasis mechanisms of _F. nucleatum_, for example, whether the increase in metastasis load is due to _F. nucleatum_ increasing the size of the primary
tumors. However, if we normalize metastases number by tumor weight, we see a higher metastasis burden in the lungs of mice with _F. nucleatum_ than in those without bacterial inoculation
(Supplementary Fig. 2). In other words, there are more lung metastases in the presence of _F. nucleatum_, even if the primary tumors are of the same size as in non-inoculated mice. In
addition, 8 days post _F. nucleatum_ infection may be too short to detect new metastasis with H&E staining used here. We therefore conclude for now that it was the _F. nucleatum_-driven
growth acceleration of pre-infection metastases that led us to observe more metastases at the end of the experiment. This hypothesis is also supported by the visualization of lungs
metastases in mice implanted with GFP-expressing AT3 cells, where the metastases in mice infected with _F. nucleatum_ are clearly larger than those in the sham-infected mice (Fig. 5f).
Finally, our current study supports the previous important observation of overabundant DNA of the genus _Fusobacterium_ in the human breast cancer microbiome21. Moreover, we show here that
_F. nucleatum_ DNA signals in human breast cancer correlate with the extent of Gal-GalNAc displayed by the tumors (Fig. 2c). Together, these results provide strong evidence for a model
whereby _F. nucleatum_ generally reaches tumors via the hematogenous route and specifically attaches to them via a bacterial lectin-host sugar (Fap2-Gal-GalNAc) interaction. This new
knowledge, combined with our demonstration that metronidazole treatment counteracted acceleration of development and metastatic progression in mammary tumors (Fig. 5), suggests that
targeting _F. nucleatum_ might benefit treatment not only of CRC11 but also of breast cancer. METHODS HUMAN SAMPLE ACQUISITION AND DNA EXTRACTION Tumor samples were collected and analyzed
according to IRB-approved protocols by the Sheba Medical Center and the Maccabi Health Care Services. Fifty formalin-fixed paraffin-embedded (FFPE) samples from breast tumors and 21 fresh
frozen colon tumor samples were collected after informed consent was obtained. DNA was extracted with the UltraClean Tissue & Cells DNA Isolation kit (MoBio) according to the
manufacturer’s instructions with a 10 min bead beating step (Vortex adapter –Qiagen) before proteinase K digestion. Extraction of DNA from FFPE samples was done as described above with some
adaptations. Briefly: samples were deparaffinized prior to digestion with proteinase K. Lysates were bead beated with 0.1 mm zirconia/silica beads (Biospec) for 10 min. Fifty empty tubes
were added to the DNA extraction and were subjected to 16S sequencing together with sample DNA in order to detect and monitor contaminating bacterial DNA. 16S SEQUENCING AND DATA ANALYSIS
16S-rDNA amplification and sequencing were performed using a novel method that allows the processing of DNA from FFPE samples and increases the resolution of the assay because it amplifies
five regions on the 16S rRNA gene. The method is described in detail in ref. 43. Fifty no-template controls (NTC) were added to the PCR amplification and further processed into libraries to
differentiate (together with the extraction controls) contaminating bacterial DNA from true signal in the samples. Five regions of the 16S rRNA gene were amplified using 100 ng DNA as an
input and a set of 10 multiplexed primers (Supplementary Table 1). Sequencing libraries were loaded together with 20% of PhiX on an Illumina Hi-seq or Mi-seq system. Reads were demultiplexed
per sample, filtered and aligned to each of the five amplified regions based on the primers’ sequences. The SMURF package was applied to combine read counts from the five regions into a
coherent profiling results solving a maximum likelihood problem44. The Greengenes database (May 2013 version) was used as a reference. BACTERIAL STRAINS AND GROWTH CONDITIONS _F. nucleatum_
strains ATCC 25586, ATCC 23726, the isogenic Fap2-inactivated mutants K50, D22 and _P. gingivalis_ ATCC 33277 were grown in Wilkins Chalgren broth (Oxoid, UK) or on Columbia agar plates
(Oxoid, UK) supplemented with 5% defibrinated sheep blood (Novamed, Israel). The bacteria were grown in an anaerobic chamber (Bactron I–II Shellab, USA) in an atmosphere of 90% N2, 5% CO2,
and 5% H2 at 37 °C. Thiamphenicol (2.5 µg/ml) was added for K50 and D22. TISSUE MICROARRAY ANALYSIS Breast cancer tissue array BR1006, array BR1003a, and HBre-Duc060CS-01 (US Biomax) were
used in these studies. Details about the cases for each core on the array are available on the US Biomax Web site (https://www.biomax.us/). FLUORESCENCE MICROSCOPY AND SECTION PREPARATION
TMA slides were stained with H&E or processed for immunofluorescence microscopy. TMA slides went through deparaffinization as follows: xylene twice 5 min, xylene 1 min, xylene/ethanol
(50%/50%) 1 min, ethanol twice 10 min, 90% ethanol 2 min, 80% ethanol 2 min, 70% ethanol 2 min and then washed in DDW. Slides went under heat-induced epitope retrieval using sodium citrate
10 mM pH 6.0 in a microwave for 20 min, then 40 min in room temperature and washed 5 min in PBS. For PNA (Sigma-Aldrich), anti-CK-7 (Abcam) and anti-von willebrand factor (Dako) binding,
sections were blocked with PBS supplemented with 10% BSA, 10% FBS, and 5% Triton for 2 h at room temperature followed by incubation with FITC-labeled PNA (50 µg/ml in PBS), anti-CK-7
(1:200), or anti-von willebrand factor (1:500) overnight at 4 °C. The slides were then washed three times with PBS for 15 min and for anti-CK-7 and anti-von willebrand factor a second
antibody Cy5 and Cy3 was added, respectively (Jackson, 1:100), for 2 h, washed three times with PBS for 15 min and then incubated with Hoechst 33258 diluted 1:5000 for 15 min at room
temperature. Fluorescence intensity FITC-labeled PNA was evaluated using the ImagePro Analyzer 7.0 software (Cybernetics, USA). For _F. nucleatum_ binding, bacteria were labeled with Cy5 or
Cy3 (PA25001 Life Sciences GE) solution diluted 0.1 mg/ml in PBS. Sections were blocked with TBS (0.05 M Tris-HCl [pH 7.8], 0.1 M NaCl) supplemented with 20% BSA, 20% FBS, and 5% Triton for
6 h at room temperature, followed by incubation with the labeled bacteria (3 × 107 bacteria/ml blocking solution) for 6 h at room temperature and then overnight at 4 °C. The slides were then
washed once with PBS + TWEEN 0.5% followed by two washes with PBS for 15 min each, and then incubated with Hoechst 33258 diluted 1:5000 for 15 min at room temperature. IMMUNOHISTOCHEMISTRY
Two slides 10-µm thick and 10 µm apart were prepared from the center of each formalin-fixed paraffin-embedded tumor. Slides were stained with Hematoxylin and one for ki67 (ɑ
ki67—ThermoScientific (SP6) RM—9106-SO, diluted x 200) and the other for CC3 (ɑ CC3—Cell Signaling—9661S, diluted x 300). 10 randomly areas from each slide were captured in ×20 lens. Binary
area (µm2) of ten areas were averaged for both DAB (+) and Hematoxylin (+). Each symbol represents the ratio between the average of DAB (+) to Hematoxylin (+) in the tumor. CELL LINES AND
TISSUE CULTURE The human breast cancer cell line MCF-7 and MDA-MB-231, mouse BALB/c breast cancer model cell line 4T1, mouse C57BL/6 breast cancer model cell line AT3, and mouse C57BL/6
melanoma model cell line B16, were cultured with DMEM with 10% fetal bovine serum, 1% L-Glutamine, penicillin–streptomycin and amino acid (Biological Industries). FLOW CYTOMETRY _F.
nucleatum_ strains ATCC 23726 and the isogenic Fap2-inactivated mutants K50, D22 (109 CFU/ml) were labeled with fluorescein isothiocyanate (FITC, 0.1 mg/ml in PBS; Sigma-Aldrich) for 30 min
at room temperature and washed three times in PBS. FITC-labeled bacteria were used at a multiplicity of infection of 3. Bacteria were incubated with cells in 96-well plates, for 30 min at
room temperature and washed twice prior to flow cytometry (LSRFortessa Analyzer, BD, USA). Analysis was performed using FlowJo 10.0.8 software (Tree Star, Ashland, OR, USA). FITC-labeled PNA
lectin (Sigma-Aldrich) was incubated at a final concentration of 140 nM per 2.5 × 105 cells per well. For competition experiments, bacteria were incubated with GalNAc (concentration range:
0, 25, 100, and 400 mM) for 30 min prior to incubation with cells. ANIMAL MODELS All mouse experiments were carried out under protocol MD-17-15239-5 approved by the Hebrew University of
Jerusalem Ethics Committee and signed by the chairman Prof. Sara Eyal. Six- to seven-week-old female Balb/C, C57BL/c, and SCID-beige mice were purchased from Envigo (Israel). Mice were kept
at a relative humidity 40–70%, 20–24 °C, and in 12 h dark/light cycles (07:00–19:00 light). IN VIVO BREAST CANCER COLONIZATION Female BALB/c mice were orthotopically (mammary fat pad)
injected with 1 × 106 4T1 tumor cells. When tumor reached 500 mm3 size, mice were injected intravenously with 5 × 107 _F. nucleatum_ ATCC 23726. Female C57BL/c mice were orthotopically
(mammary fat pad) injected with 1 × 106 AT3 tumor cells. At tumor size of 500 mm3, mice were randomly divided to three groups and injected intravenously with 5 × 107 _F. nucleatum_ ATCC
23726, _F. nucleatum_ MUT K50 and _P. gingivalis_ ATCC 33277. After 24 h, breast tumor and normal tissue from an adjacent mammary were harvested from each mice and homogenized under sterile
conditions. QUANTIFICATION OF BACTERIA USING PLATING AND QPCR Tumor and non-tumor adjacent tissue samples were homogenized in 500 µl of PBS for 45 s at 4.5 m/s using a FastPrep (MP
Biomedicals, USA) and plated on Columbia agar plates supplemented with 0.15% (final concentration) crystal violet and 5% (final concentration) defibrinated sheep blood45. Colonies were
enumerated after 7 days of incubation under anaerobic conditions. DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to manufacturer’s instructions. A
custom TaqMan primer/probe set was used to amplify _F. nucleatum_ and _P. gingivalis_ DNA. The cycle threshold (Ct) values were normalized to the amount of murine gDNA in each reaction by
using a primer/probe set for the reference gene (Gapdh). Each reaction contained 1 ng of DNA and was assayed in triplicate in 20 µL reactions containing 2× qPCRBIO Lo-ROX Probe Mix as
appropriate for individual qPCR machines. Reaction conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 s at 95 °C, and 1 min at 60 °C16. The sequences of all
primers can be found in Supplementary Table 1. MULTIPHOTON MICROSCOPY OF FITC-LABELED _F. NUCLEATUM_ AT3 breast cancer cell line was injected to the mammary fat pad of 6–7-week-old female
C57BL/6 mice. At tumor size of 500 mm3, mice were randomly divided to two groups; one group injected intravenous with 5 × 107 FITC-labeled _F. nucleatum_ ATCC 23726 (_n_ = 5) and the second
control group, with PBS vehicle (_n_ = 3). After 24 h, breast tumor tissue was harvested from each mice. A slice from the fresh tissue was cut and placed on a slide with a drop of water and
covered with a cover slip. Image sections were taken using a Nikon Multiphoton A1MP set to 740 nm wavelength using ×25 objectives. EFFECT OF _F. NUCLEATUM_ ON THE PROGRESSION OF MAMMARY
TUMOR AT3 breast cancer cell line was injected to the mammary fat pad of 6–7-week-old female C57BL/6 mice. When tumor reached 500 mm3 size (day 1), mice were randomly divided into five IV
injected treatment groups: PBS (V), 5 × 107 _F. nucleatum_ ATCC 23726 and treated with metronidazole (726+MTZ.), metronidazole only (MTZ.), 5 × 107 _F. nucleatum_ ATCC 23726 treated with PBS
vehicle (726), or with 5 × 107 Fap2-deficient _F. nucleatum_ K50 treated with PBS (K50). For metronidazole or vehicle treatment, mice were injected with metronidazole or PBS 30 min before
IV inoculation with _F. nucleatum_. Treatment with metronidazole or vehicle was repeated after 8 h, twice a day (morning and evening) in the following 2 days (days 2–3), and daily for 4 more
days. On day 8 mice were sacrificed, and tumors and lungs were harvested. Breast cancer tissue was weighed and metastases were counted by histological examination (experimental scheme can
be seen Fig. 4a). When the GFP-expressing AT3 breast cancer cell line was implanted in C57BL/6 mice, relatively few mice developed cancer presumably due to anti-GFP immunity. In mice in
which breast tumor was developed, metastases were visualized using Nikon SMZ25 fluorescent binocular in an ×0.5 objectives. CHARACTERIZATION OF AT3 TUMOR IMMUNE INFILTRATE Lymphocytes were
purified from homogenized primary tumors by centrifugation on Lymphoprep (STEMCELL Technologies, Cat number 07851). Lymphocytes were characterized using anti-mouse CD3-allophyco-cyanin clone
145-2C11 (Cat. number 100312, BioLegend) for T cells together with either anti-mouse CD4 PE clone GK1.5 (Cat. number 100408, BioLegend) or anti-mouse CD8 PE clone 53-6.7 (Cat. number
100708, BioLegend). For the characterization of NK cells we used anti-mouse NKp46 (CD335, NCR1) APC clone 29A1.4 (Cat. number 137608, BioLegend). RNA-SEQ Infection of AT3 cells was carried
out as describe before46 with the following modifications. Two days prior infection 2 × 105 AT3 cells were seeded in six-well plates and 2 ml complete DMEM. The plates were transferred to an
incubator with O2-control set to 1% O2 and the media exchanged after 24 h with complete DMEM without antibiotics. A day before infection, _F. nucleatum_ ATCC 23726 was inoculated in
Columbia broth under anaerobic conditions. On the next day, the culture was diluted 1:50 in fresh Columbia broth and grown to an OD600 of 0.2. Bacterial cells were harvested (5 min at 4400 ×
_g_ in room temperature) resuspended in fresh, reduced complete DMEM (lacking antibiotics) and added to the AT3 cells at a multiplicity of infection (m.o.i.) of 10. The plates were
centrifuged for 5 min at 250 × _g_ at room temperature and placed back into the O2-controlled incubator. Twenty-four hours after infection, the cells were washed once with PBS before
extracting total RNA using the mirVana kit (Ambion) following the manufacturer’s instructions. The infection was performed in three biological replicates. cDNA libraries for Illumina
sequencing were generated by the Core Unit SysMed (University Würzburg). Five hundred nanograms of total RNA were used for the library generation using oligo-dT capture beads for poly-A-mRNA
enrichment via the TruSeq Stranded mRNA Library Preparation Kit (Illumina) according to manufacturer’s instructions. The success of the library preparation was controlled by electrophoresis
on Agilent High Sensitivity Bioanalyzer microfluidic chips prior to sequencing of pooled libraries (including 1% PhiX control library). For sequencing the single-end mode on the NextSeq 500
platform (Illumina) with the High-Output Kit v2.5 (75 cycles) was used. The generated RNA-seq data was deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series
accession number GSE144143. FASTQ files were generated and quality filtered using the local run manager software from Illumina (v2.2.0) and FastQC (v.0.11.7;
https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Mapping was performed using the READemption47 tool (v0.4.3) using the _Mus muculus_ reference sequence provided by ENSEMBL
(GRCm38.p6). After mapping, strand-specific gene-wise quantification was performed using READemption based upon the annotation provided by ENSEMBL (GRCm38.p6). Only uniquely mapped reads
were used as input for differential expression analysis via the edgeR package (v3.24.3)48,49 using an upper-quartile normalization and a prior count of 1. Only gene showing at least 10
uniquely mapped reads were considered for the analysis and all genes with an adjusted _p-_value of 0.05 and passing the log2 fold change threshold (−2 ≤ log2FC ≥ 2) were considered
significant differentially expressed. In order to assess enrichment of genes involved in different processes a gene set enrichment analysis (GSEA50,51 v4.0.3) was performed using the curated
Molecular Signatures Database (MSigDB 7.0) for GO terms (biological processes) available via GSEA. The log2 fold changes reported by the edgeR analysis were used as input for the GSEA which
was run in ranked list mode (statistic setting: classic) and limited to gene sets with 15–500 genes. An overview of all GO terms reported with an FDR-corrected _p_-value ≤0.05 are reported
in Supplementary Data 1 and the top 15 based upon the normalized enrichment score are shown in Supplementary Fig. 3. MMPS DETECTION USING GEL ZYMOGRAPY AT3 and MDA-MB-231 cells were cultured
on 6-well plate at 37 °C in DMEM medium supplemented with 10% serum and 1% L-glutamine for 24 h to reach subconfluent density under microaerobic conditions using the Oxoid CampyGen
atmosphere generating system. _F. nucleatum_ strains ATCC 25586 and ATCC 23726 were brought to OD600 = 1 and added to the cell cultures at cell to bacterial ratio of 1:100. The cultures were
then grown for 24 h in the absence of serum. Growth media were collected and conditioned by passing through 0.22 µm filter to remove bacteria and cancer cells, and concentrated 10X using 50
kDa membrane filters (Amicon Ultra 50k). For zymogram analysis52, 20 µg of conditioned media were dissolved in sample buffer (192 mM Tris-HCl [pH 6.8], 30% glycerol, 9% SDS) without
β-mercaptoethanol and without boiling, and subjected to SDS-PAGE using 7.5% gels containing 1 mg/ml Gelatin from porcine skin (Sigma-Aldrich, Germany). Following electrophoresis, the gels
were washed twice for 30 min at room temperature with washing buffer (50 mM Tris-HCl [pH 8.0], 5 mM CaCl2, 1 µM ZnCl2, 0.016% NaN3), containing 2.5% Triton X-100, then incubated for 10 min
in incubation buffer (washing buffer containing 1% Triton X-100) and incubated overnight at 37 °C with fresh incubation buffer. Proteolytic activity was visualized as a clear band against a
blue background after staining with Coomassie brilliant blue R-250. STATISTICAL ANALYSIS The various groups were compared against each other in pairs: the exact Wilcoxon test was used for
pairs that were matched, the exact Mann–Whitney test for independent samples (Nonparametric Tests, IBM SPSS Statistics for Windows, Version 25, 2017, Armonk, NY: IBM Corp.). When the same
hypothesis was tested on more than one pair, and the individual pairs were mutually independent, the corresponding _p_-values were combined by Fisher’s method53. Wherever the pairs were not
independent, the multiple comparisons were adjusted for using the Holm modification54 of the Bonferroni correction. In all presented boxplots, whiskers represent extrema, box bounds
represent upper and lower quartiles, and center-line represents the median value. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting
Summary linked to this article. DATA AVAILABILITY The raw 16S sequencing data have been deposited in the NCBI BioProject database- accession # PRJNA624822. The RNA-seq data that support the
findings of this study has been deposited in GEO with the accession code GSE144143. The source data underlying Figs. 1a–c; 2a–c; 3a, b; 4b, c, e; 5b, e; 6a–c; Supplementary Figs. 1 and 2 are
provided as a Source data file. Remaining data is available in the Article, Supplementary Information files or available from the authors upon request. REFERENCES * Castellarin, M. et al.
_Fusobacterium nucleatum_ infection is prevalent in human colorectal carcinoma. _Genome Res._ 22, 299–306 (2012). Article CAS PubMed PubMed Central Google Scholar * Kostic, A. D. et al.
Genomic analysis identifies association of _Fusobacterium_ with colorectal carcinoma. _Genome Res._ 22, 292–298 (2012). Article CAS PubMed PubMed Central Google Scholar * Rubinstein,
MaraÂ. R. et al. _Fusobacterium nucleatum_ promotes colorectal carcinogenesis by modulating E-Cadherin/β-catenin signaling via its FadA adhesin. _Cell Host Microbe_ 14, 195–206 (2013).
Article CAS PubMed PubMed Central Google Scholar * Kostic, A. D. et al. _Fusobacterium nucleatum_ potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment.
_Cell Host Microbe_ 14, 207–215 (2013). Article CAS PubMed PubMed Central Google Scholar * Mima, K. et al. _Fusobacterium nucleatum_ and T cells in colorectal carcinoma. _JAMA Oncol._
1, 653–661 (2015). Article PubMed PubMed Central Google Scholar * Hamada, T. et al. _Fusobacterium nucleatum_ in colorectal cancer relates to immune response differentially by tumor
microsatellite instability status. _Cancer Immunol. Res._ 6, 1327–1336 (2018). Article CAS PubMed PubMed Central Google Scholar * Chen, T. et al. TOX expression decreases with
progression of colorectal cancers and is associated with CD4 T-cell density and _Fusobacterium nucleatum_ infection. _Hum. Pathol._ 79, 93–101 (2018). Article CAS PubMed Google Scholar *
Gur, C. et al. Binding of the Fap2 protein of _Fusobacterium nucleatum_ to human inhibitory receptor TIGIT protects tumors from immune cell attack. _Immunity_ 42, 344–355 (2015). Article
CAS PubMed PubMed Central Google Scholar * Yu, T. et al. _Fusobacterium nucleatum_ promotes chemoresistance to colorectal cancer by modulating autophagy. _Cell_ 170, 548–563 e516 (2017).
Article CAS PubMed PubMed Central Google Scholar * Zhang, S. et al. _Fusobacterium nucleatum_ promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in
colorectal cancer. _J. Exp. Clin. Cancer Res._ 38, 14 (2019). Article PubMed PubMed Central Google Scholar * Bullman, S. et al. Analysis of _Fusobacterium_ persistence and antibiotic
response in colorectal cancer. _Science_ 358, 1443–1448 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Mima, K. et al. _Fusobacterium nucleatum_ in colorectal carcinoma
tissue and patient prognosis. _Gut_ 65, 1973–1980 (2016). Article CAS PubMed Google Scholar * Brennan, C. A. & Garrett, W.S. _Fusobacterium nucleatum_—symbiont, opportunist and
oncobacterium. _Nat. Rev. Microbiol._ 17, 156–166 (2019). Article CAS PubMed PubMed Central Google Scholar * Yamamura, K. et al. Human microbiome _Fusobacterium nucleatum_ in esophageal
cancer tissue is associated with prognosis. _Clin. Cancer Res._ 22, 5574–5581 (2016). Article CAS PubMed Google Scholar * Yamamura, K. et al. Intratumoral _Fusobacterium nucleatum_
levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. _Clin. Cancer Res._ 25, 6170–6179 (2019). Article CAS PubMed PubMed Central Google
Scholar * Abed, J. et al. Fap2 mediates _Fusobacterium nucleatum_ colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. _Cell Host Microbe_ 20, 215–225 (2016).
Article CAS PubMed PubMed Central Google Scholar * Springer, G. F., Desai, P. R. & Scanlon, E. F. Blood group MN precursors as human breast carcinoma-associated antigens and
“naturally” occurring human cytotoxins against them. _Cancer_ 37, 169–176 (1976). Article CAS PubMed Google Scholar * Patil, S. A. et al. Overexpression of alpha2,3sialyl T-antigen in
breast cancer determined by miniaturized glycosyltransferase assays and confirmed using tissue microarray immunohistochemical analysis. _Glycoconj. J._ 31, 509–521 (2014). Article CAS
PubMed PubMed Central Google Scholar * Kolbl, A. C., Jeschke, U., Friese, K. & Andergassen, U. The role of TF- and Tn-antigens in breast cancer metastasis. _Histol. Histopathol._ 31,
613–621 (2016). PubMed Google Scholar * Abed, J. et al. Tumor targeting by _Fusobacterium nucleatum_: a pilot study and future perspectives. _Front Cell Infect. Microbiol._ 7, 295 (2017).
Article PubMed PubMed Central CAS Google Scholar * Hieken, T. J. et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. _Sci. Rep._ 6, 30751
(2016). Article ADS CAS PubMed PubMed Central Google Scholar * Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries. _CA Cancer J. Clin._ 68, 394–424 (2018). Article PubMed Google Scholar * Coppenhagen-Glazer, S. et al. Fap2 of _Fusobacterium nucleatum_ is a galactose-inhibitable adhesin
involved in coaggregation, cell adhesion, and preterm birth. _Infect. Immun._ 83, 1104–1113 (2015). Article CAS PubMed PubMed Central Google Scholar * Brewer, M. L. et al.
_Fusobacterium_ spp. target human CEACAM1 via the trimeric autotransporter adhesin CbpF. _J. Oral. Microbiol._ 11, 1565043 (2019). Article CAS PubMed PubMed Central Google Scholar *
Dayer, M. J. et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. _Lancet_ 385, 1219–1228 (2015). Article PubMed Google
Scholar * Thornhill, M. H. et al. Antibiotic prophylaxis and incidence of endocarditis before and after the 2007 AHA recommendations. _J. Am. Coll. Cardiol._ 72, 2443–2454 (2018). Article
PubMed Google Scholar * Binder Gallimidi, A. et al. Periodontal pathogens _Porphyromonas gingivalis_ and _Fusobacterium nucleatum_ promote tumor progression in an oral-specific chemical
carcinogenesis model. _Oncotarget_ 6, 22613–22623 (2015). Article PubMed Google Scholar * Gao, S. et al. Presence of _Porphyromonas gingivalis_ in esophagus and its association with the
clinicopathological characteristics and survival in patients with esophageal cancer. _Infect. Agent Cancer_ 11, 3 (2016). Article PubMed PubMed Central Google Scholar * Katz, J., Onate,
M. D., Pauley, K. M., Bhattacharyya, I. & Cha, S. Presence of _Porphyromonas gingivalis_ in gingival squamous cell carcinoma. _Int J. Oral. Sci._ 3, 209–215 (2011). Article PubMed
PubMed Central Google Scholar * Peters, B. A. et al. Oral microbiome composition reflects prospective risk for esophageal cancers. _Cancer Res._ 77, 6777–6787 (2017). Article CAS PubMed
PubMed Central Google Scholar * Gur, C. et al. _Fusobacterium nucleatum_ supresses anti-tumor immunity by activating CEACAM1. _Oncoimmunology_ 8, e1581531 (2019). Article PubMed PubMed
Central Google Scholar * Mehner, C. et al. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer.
_Oncotarget_ 5, 2736–2749 (2014). Article PubMed PubMed Central Google Scholar * Moss, S. F. The clinical evidence linking _Helicobacter pylori_ to gastric cancer. _Cell Mol.
Gastroenterol. Hepatol._ 3, 183–191 (2017). Article PubMed Google Scholar * Salama, N. R., Hartung, M. L. & Muller, A. Life in the human stomach: persistence strategies of the
bacterial pathogen _Helicobacter pylori_. _Nat. Rev. Microbiol._ 11, 385–399 (2013). Article CAS PubMed PubMed Central Google Scholar * Corbella, S. et al. Is periodontitis a risk
indicator for cancer? A meta-analysis. _PLoS ONE_ 13, e0195683 (2018). Article PubMed PubMed Central CAS Google Scholar * Nwizu, N. N. et al. Periodontal disease and incident cancer
risk among postmenopausal women: results from the women’s health initiative observational cohort. _Cancer Epidemiol. Biomark. Prev._ 26, 1255–1265 (2017). Article Google Scholar * Michaud,
D. S., Fu, Z., Shi, J. & Chung, M. Periodontal disease, tooth loss, and cancer risk. _Epidemiol. Rev._ 39, 49–58 (2017). Article PubMed PubMed Central Google Scholar * Shao, J. et
al. Periodontal disease and breast cancer: a meta-analysis of 1,73,162 participants. _Front Oncol._ 8, 601 (2018). Article PubMed PubMed Central ADS Google Scholar * Pinder, S. E. &
Ellis, I. O. The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)–current definitions and classification.
_Breast Cancer Res._ 5, 254–257 (2003). Article PubMed PubMed Central Google Scholar * Aran, D. et al. Comprehensive analysis of normal adjacent to tumor transcriptomes. _Nat. Commun._
8, 1077 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Kaplan, C. W. et al. _Fusobacterium nucleatum_ apoptosis-inducing outer membrane protein. _J. Dent. Res._ 84,
700–704 (2005). Article ADS CAS PubMed Google Scholar * Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. _CA Cancer J. Clin._ 66, 7–30 (2016). Article PubMed
Google Scholar * Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. _Science_ 368, 973–980 (2020). Article CAS PubMed PubMed Central
Google Scholar * Fuks, G. et al. Combining 16S rRNA gene variable regions enables high-resolution microbial community profiling. _Microbiome_ 6, 17 (2018). Article PubMed PubMed Central
Google Scholar * Van Tassell, J. A. et al. Evaluation of various selective media for the detection of _Pseudomonas_ species in pasteurized milk. _J. Dairy Sci._ 95, 1568–1574 (2012).
Article PubMed CAS Google Scholar * Schulte, L. N., Eulalio, A., Mollenkopf, H. J., Reinhardt, R. & Vogel, J. Analysis of the host microRNA response to _Salmonella_ uncovers the
control of major cytokines by the let-7 family. _EMBO J._ 30, 1977–1989 (2011). Article CAS PubMed PubMed Central Google Scholar * Forstner, K. U., Vogel, J. & Sharma, C. M.
READemption-a tool for the computational analysis of deep-sequencing-based transcriptome data. _Bioinformatics_ 30, 3421–3423 (2014). Article PubMed CAS Google Scholar * Robinson, M. D.,
McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. _Bioinformatics_ 26, 139–140 (2010). CAS PubMed
Google Scholar * McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. _Nucleic Acids Res._
40, 4288–4297 (2012). Article CAS PubMed PubMed Central Google Scholar * Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide
expression profiles. _Proc. Natl Acad. Sci. USA_ 102, 15545–15550 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Mootha, V. K. et al. PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately downregulated in human diabetes. _Nat. Genet._ 34, 267–273 (2003). Article CAS PubMed Google Scholar * Doron, L. et al.
Identification and characterization of fusolisin, the _Fusobacterium nucleatum_ autotransporter serine protease. _PLoS ONE_ 9, e111329 (2014). Article ADS PubMed PubMed Central CAS
Google Scholar * Fisher, R. A. _Statistical Methods for Research Workers_, 5 edn, 103 (Oliver & Boyd, London, 1934). * Holm, S. A. A simple sequentially rejective multiple test
procedure. _Scand. J. Stat._ 6, 65–70 (1979). MathSciNet MATH Google Scholar Download references ACKNOWLEDGEMENTS We thank Professor Norman Grover for his valuable assistance in
statistics, Dr. Zakhariya Manevitch and Dr. Yael Feinstein-Rotkopf for their valuable help in microcopy, Ms. Noam Koren and Dr. Oded Heyman for their useful discussion. This work was
supported by the Israel Cancer Research Fund Project grant, the Israel Science Foundation Moked grant and the Israel Ministry of Science and Technology Personalized Medicine grant. We thank
the Vogel Stiftung Dr. Eckernkamp for supporting F.P. with a Dr. Eckernkamp Fellowship, and the Core Unit SysMed and IZKF (project Z-6), University of Würzburg, for RNA-seq data generation.
AUTHOR INFORMATION Author notes * These authors contributed equally: Lishay Parhi, Tamar Alon-Maimon. AUTHORS AND AFFILIATIONS * The Institute of Dental Sciences, The Hebrew
University-Hadassah School of Dental Medicine, Jerusalem, Israel Lishay Parhi, Tamar Alon-Maimon, Asaf Sol, Amjad Shhadeh, Jawad Abed, Naseem Maalouf & Gilad Bachrach * Department of
Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel Deborah Nejman & Ravid Straussman * Department of Developmental Biology and Cancer Research, Institute for Medical
Research Israel Canada (IMRIC), Hebrew University-Hadassah Medical School, Jerusalem, Israel Tanya Fainsod-Levi, Olga Yajuk & Zvi Granot * Department of Immunology and Cancer Research,
Institute for Medical Research Israel Canada (IMRIC), Hebrew University-Hadassah Medical School, Jerusalem, Israel Batya Isaacson & Ofer Mandelboim * Department of General and
Oncological Surgery-Surgery C, The Chaim Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel Aviram Nissan * The Pathology Institute, Maccabi Healthcare Services, Rehovot, Israel Judith
Sandbank & Einav Yehuda-Shnaidman * Helmholtz Institute for RNA-based Infection Research (HIRI), Helmholtz Center for Infection Research (HZI), Würzburg, Germany Falk Ponath & Jörg
Vogel * Institute for Molecular Infection Biology, Medical Faculty, University of Würzburg, Würzburg, Germany Jörg Vogel Authors * Lishay Parhi View author publications You can also search
for this author inPubMed Google Scholar * Tamar Alon-Maimon View author publications You can also search for this author inPubMed Google Scholar * Asaf Sol View author publications You can
also search for this author inPubMed Google Scholar * Deborah Nejman View author publications You can also search for this author inPubMed Google Scholar * Amjad Shhadeh View author
publications You can also search for this author inPubMed Google Scholar * Tanya Fainsod-Levi View author publications You can also search for this author inPubMed Google Scholar * Olga
Yajuk View author publications You can also search for this author inPubMed Google Scholar * Batya Isaacson View author publications You can also search for this author inPubMed Google
Scholar * Jawad Abed View author publications You can also search for this author inPubMed Google Scholar * Naseem Maalouf View author publications You can also search for this author
inPubMed Google Scholar * Aviram Nissan View author publications You can also search for this author inPubMed Google Scholar * Judith Sandbank View author publications You can also search
for this author inPubMed Google Scholar * Einav Yehuda-Shnaidman View author publications You can also search for this author inPubMed Google Scholar * Falk Ponath View author publications
You can also search for this author inPubMed Google Scholar * Jörg Vogel View author publications You can also search for this author inPubMed Google Scholar * Ofer Mandelboim View author
publications You can also search for this author inPubMed Google Scholar * Zvi Granot View author publications You can also search for this author inPubMed Google Scholar * Ravid Straussman
View author publications You can also search for this author inPubMed Google Scholar * Gilad Bachrach View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS G.B, L.P., T.A.M., and Z.G. conceived and designed the study. L.P. and T.A.M. performed cell culture experiments, microscopy work, and animal experiments. A.S. performed data
analysis. R.S. conceived and designed the collection of clinical samples by A.N., J.S., and E.Y.S., and D.N. supervised the processing of samples and performed the 16S-rDNA amplification and
analysis. A.S. performed gel zonography. J.A. and N.M. handled bacterial cultures and performed part of the fluorescence microscopy. T.F.L. and O.Y. assisted in the animal experiments and
performed mouse histology and immunohistochemistry. J.V. designed RNA-seq experiments and analysis and supervised F.P. who performed them. O.M. contributed to the study design and analysis
and supervised B.I. who profiled tumor associated immune cells. G.B. wrote the paper together with L.P., T.A.M., and J.V. CORRESPONDING AUTHOR Correspondence to Gilad Bachrach. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their
contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Parhi, L.,
Alon-Maimon, T., Sol, A. _et al._ Breast cancer colonization by _Fusobacterium nucleatum_ accelerates tumor growth and metastatic progression. _Nat Commun_ 11, 3259 (2020).
https://doi.org/10.1038/s41467-020-16967-2 Download citation * Received: 05 May 2019 * Accepted: 02 June 2020 * Published: 26 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16967-2
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative