Play all audios:
ABSTRACT Low muscle strength is an important heritable indicator of poor health linked to morbidity and mortality in older people. In a genome-wide association study meta-analysis of 256,523
Europeans aged 60 years and over from 22 cohorts we identify 15 loci associated with muscle weakness (European Working Group on Sarcopenia in Older People definition: _n_ = 48,596 cases,
18.9% of total), including 12 loci not implicated in previous analyses of continuous measures of grip strength. Loci include genes reportedly involved in autoimmune disease (_HLA-DQA1_ _p_ =
4 × 10−17), arthritis (_GDF5_ _p_ = 4 × 10−13), cell cycle control and cancer protection, regulation of transcription, and others involved in the development and maintenance of the
musculoskeletal system. Using Mendelian randomization we report possible overlapping causal pathways, including diabetes susceptibility, haematological parameters, and the immune system. We
conclude that muscle weakness in older adults has distinct mechanisms from continuous strength, including several pathways considered to be hallmarks of ageing. SIMILAR CONTENT BEING VIEWED
BY OTHERS RARE GENETIC VARIANTS IMPACT MUSCLE STRENGTH Article Open access 10 June 2023 A GENOME-WIDE ASSOCIATION STUDY IDENTIFIES A LOCUS ASSOCIATED WITH KNEE EXTENSION STRENGTH IN OLDER
JAPANESE INDIVIDUALS Article Open access 20 May 2024 CAUSAL ROLE OF IMMUNE CELLS IN MUSCLE ATROPHY: MENDELIAN RANDOMIZATION STUDY Article Open access 29 October 2024 INTRODUCTION
Age-associated loss of muscle strength (termed dynapenia)1 is one of the characteristic changes occurring with advancing age, and muscle weakness is considered a fundamental component of
frailty and sarcopenia2. Individuals over 70 years old typically demonstrate up to 20% lost muscle mass compared with individual in their twenties3. Although definitions of reduced muscle
function in older people have focused on loss of muscle mass (sarcopenia) evidence now shows that muscle weakness itself is often more predictive of negative health outcomes4. Muscle
weakness causes difficulties in daily functioning (i.e., disability) and low muscle strength (measured as hand grip strength, considered a biomarker of general dynapenia) is predictive of
future morbidity and mortality3 over the long term5. Despite intensive research, causes of and contributors to muscle weakness in later life remain to be fully elucidated6. Importantly,
muscle strength is heritable (48–55% in 1757 male twin pairs aged 45–96)7, and can thus be used for genetic investigations. Previously a genome-wide association study (GWAS) by the CHARGE
(Cohorts for Heart and Aging Research in Genomic Epidemiology) consortium identified two loci associated with maximum hand grip strength (as a quantitative trait) in 27,581 Europeans aged 65
and over8. Another study on maximum hand grip strength (divided by weight) in mostly middle-aged UK Biobank participants (334,925 people aged 40–70, mean aged 56) identified and replicated
64 loci, many of which are known to have a role in determining anthropometric measures of body size9,10. These previous studies that considered grip strength as a continuous phenotype across
young and old individuals may not provide insights into the age related loss of muscle strength that leads to a magnitude of weakness sufficient to call it a disease. Given the limited data
on genetic contributions to a clinically meaningful level of muscle weakness in older adults, we aimed to determine the genetic variants and investigate causal pathways associated with low
measured grip strength. In this work, we report a GWAS of weakness in 256,523 older adults (aged 60+ years) of European ancestries from the CHARGE consortium. The primary analysis was based
on the established 2010 European Working Group on Sarcopenia in Older People (EWGSOP) definition of low grip strength, and results were compared to an analysis of the alternative Foundations
of the National Institutes of Health (FNIH) definition based on its association with functional outcomes, with additional analyses stratified by sex. The associated loci and subsequent
pathway and Mendelian randomization analysis reveal causal pathways to weakness at older ages distinct from overall strength during the life course, highlighting specific diseases (such as
osteoarthritis) and link to hallmark aging mechanisms such as cell cycle control. RESULTS STUDY DESCRIPTION The meta-analysis comprised 256,523 individuals of European descent aged 60 years
or older at assessment from 22 independent cohorts with maximum hand grip strength recorded—including the UK Biobank, the US Health and Retirement Study, the Framingham Heart Study, and
others. In total, 46,596 (18.9%) of all participants had muscle weakness (dynapenia) based on hand grip strength (EWGSOP definition: grip strength <30 kg Male; <20 kg Female).
Individual study characteristics are described in the Supplementary Information and in Supplementary Table 1. Our primary analysis of EWGSOP definition low grip strength will be described
first, with subsequent additional analyses described in later sections: these include analysis of males and females separately and use of the alternative low grip strength criteria provided
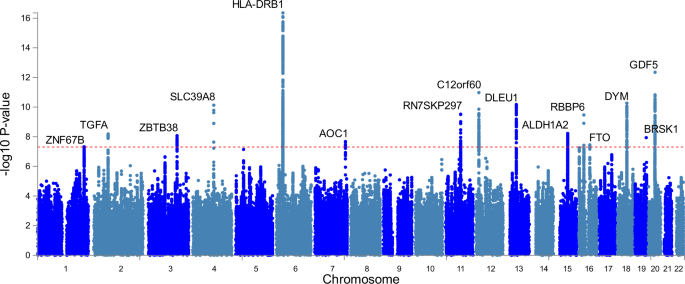
by the FNIH data. GWAS OF LOW MUSCLE STRENGTH IDENTIFIES 15 LOCI We found 15 genomic risk loci to be associated (_p_ < 5 × 10−8; 8 loci _p_ < 5 × 10−9) with EWGSOP definition low hand
grip strength in our GWAS meta-analysis of 22 cohorts (_n_ = 256,523, _n_ = 48,596 cases), adjusted for age, sex, and technical covariates (Fig. 1, Table 1; Supplementary Data 1). The
strongest associations were with variants close to _HLA-DQA1_ (rs34415150, beta/log-OR per G allele = 0.0833, _p_ = 4.4 × 10−17), _GDF5_ (rs143384, beta per A allele=0.0545, _p_ = 4.5 ×
10−13) and _DYM_ (rs62102286, beta per T allele=0.0487, _p_ = 5.5 × 10−11). Twelve of the fifteen lead SNPs from the GWAS have not previously been identified in studies of grip strength
analyzed on a continuous scale across all ages (Supplementary Data 2 & 3) and only 3 of the 64 loci associated with overall muscle strength11 are significant in our analysis of low
strength (Supplementary Data 4). This included the three most strongly associated variants near _HLA-DQA1_ (previously implicated in rheumatoid arthritis: see Supplementary Data 2), _GDF5_
(‘Growth differentiation factor 5’: previously implicated in height, waist hip ratio, muscle mass, and osteoarthritis) and _DYM_ (‘Dymeclin’: implicated in in height). Six other variants
were previously linked to height and four to osteoarthritis. None were significantly (_p_ < 5 × 10−8) associated with lean muscle mass, although rs10952289 near AOC1 is nominally
associated with appendicular lean muscle mass (_p_ = 6 × 10−4)12. The test of cohort heterogeneity in METAL for all 15 lead SNPs was not statistically significant (nominal het _p_ >
0.05). Full summary statistics for the meta-analysis are available for download (visit the Musculoskeletal Knowledge Portal http://www.mskkp.org/ or the GWAS catalogue
https://www.ebi.ac.uk/gwas/). Overall, two of the fifteen identified lead variants (or proxies) have not previously been implicated in anthropometric or musculoskeletal phenotypes in the
GWAS catalogue (see Supplementary Data 2). This included _ALDH1A2_ (‘Aldehyde Dehydrogenase 1 Family Member A2’: involved in the synthesis of retinoic acid), and a variant near _FTO_ (‘FTO
Alpha-Ketoglutarate Dependent Dioxygenase’: involved in the oxidative demethylation of different forms of RNA). Although the lead _ALDH1A2_ SNP itself has not been identified in previous
GWAS, other independent variants (_R_2 < 0.6) at the same locus (e.g., rs3204689) have been found to be associated with osteoarthritis (Supplementary Data 2). The Lambda GC (genomic
control, λGC) value was high (1.13; see Supplementary Fig. 1 for QQ plot), however the intercept from Linkage Disequilibrium Score Regression (LDSC) analysis was close to 1 (0.97, SE 0.007),
indicating that the inflation in test statistics is primarily due to polygenicity (many variants with small effects on low grip strength), rather than bias due to population
stratification13. An intercept below 1 is not unusual for analyses adjusted with genomic control. The single nucleotide polymorphism (SNP) based heritability (_h__2_) of low grip strength
was 0.044 (SE 0.0027), i.e., 4.4%, by LD Score Regression. In sex-stratified analysis there were eight significant genomic risk loci associated with EWGSOP low grip strength in females only
(total _n_ = 132,443 with _n_ = 33,548 cases, 25.3%; see Supplementary Table 2). Seven of the eight loci were either present in the main analysis or were correlated with corresponding
variants, however rs7185040 (chr16: 2145787), mapped to gene _PKD1_, was only significant in the analysis of females, although the association is borderline in the analysis of males and
females together (females _p_ = 3 × 10−8; combined analysis _p_ = 5.5 × 10−8). The analysis of males only (total _N_ = 118,371 with 13,327 cases, 11.3%) identified three genomic loci
associated with the EWGSOP low grip strength definition. Two of these variants appeared to be distinct signals from the overall analysis and were not associated with low grip in females (see
Supplementary Table 3 for details): rs774787160 mapped to gene _DSCAM_ (males _p_ = 1 × 10−8; females _p_ = 0.9) and rs145933237 mapped to mir-466, which was only nominally associated in
females (males _p_ = 2 × 10−8; females _p_ = 0.01). In the analysis of 116 mitochondrial genetic variants (MAF >0.01) available in the UK Biobank directly genotyped microarray data, no
variants reached “genome-wide” significance (_p_ > 5 × 10−8). Two were associated with EWGSOP-defined low hand grip strength at nominal significance (_p_ < 0.00043, i.e.,
Bonferroni-adjustment for mitochondrial variants). rs41518645 is a missense variant (p.Asp171Asn) in _MT-CYB_, identified in Plink logistic regression analysis (_p_ = 0.0003). rs201950015 is
intronic, located between genes _CO1_ and _ATP6/8_ (_p_ = 0.00042). These findings need further scrutiny in studies assessing the influence of mitochondrial dysfunction on muscle function
and metabolism. See Supplementary Data 5. GWAS OF LOW GRIP STRENGTH BASED ON FNIH CRITERIA In secondary analysis we performed GWAS using the low grip strength definition published by the
FNIH14. This criterion uses lower grip strength cut-offs (<26 kg for males and <16 kg for females) than the EWGSOP definition15, resulting in fewer cases (_n_ = 19,345, 7.6% of total).
Five loci were significant in the analysis (_p_ < 5 × 10−8), only one of which was not identified in the EWGSOP low grip strength analysis described previously (see Supplementary Table
4, either the same SNP or in high LD with the EWGSOP lead SNP at that loci, for example rs3771501 and rs958685 _R_2 = 0.90). This single base-pair deletion (rs1403785912–chr9:4284961:T:-)
mapped to _GLIS3_ (“GLIS family zinc finger 3”—a repressor and activator of transcription), and may be specifically associated with more strict definitions of weakness (EWGSOP _p_ value =
1.2 × 10−3). GENE EXPRESSION AND PATHWAYS We used data from the Genotype-Tissue Expression project (GTEx) v8 to identify whether the variants associated with low grip strength affect
expression of genes (Table 1; Supplementary Data 6). Of the top 15 EWGSOP-associated variants 12 are eQTLs for at least one gene. For 8 of these, the nearest gene to the variant by
chromosomal location is known to have altered expression, but other genes in the locus may also be affected. This is consistent with a recent study showing that the “nearest gene” is often a
good candidate for being a causal pathway16. For the top two loci (_HLA-DQA1_ and _GDF5_) the variants are eQTLs for these nearest genes, however for the _SLC39A8_ locus the lead SNP
(rs13107325) is not an eQTL for _SLC39A8_, but is an eQTL for _UBE2D3_ (‘Ubiquitin Conjugating Enzyme E2 D3’) in the aorta. In MAGMA analysis we found 80 GO processes enriched in low grip
strength-associated genes (see Supplementary Data 7 for details), mainly involved in the immune system and antigen presentation. Using MetaXcan17 we identified 24 genes with expression in
skeletal muscle significantly enriched in the low grip strength GWAS, after Benjamini-Hochberg adjustment for multiple testing (Supplementary Data 8). We used the International Mouse
Phenotyping Consortium database (www.mousephenotype.org) to investigate the possible phenotypes associated with the genes highlighted by MetaXcan, with many having clear effects on relevant
phenotypes such as “growth”, “lean mass”, “body weight”, “angiogenesis”, “thymus involution”, and “lipid metabolism” (Supplementary Data 9). We used LDSC-SEG to determine tissue-specific
gene expression and chromatin modification enrichment in the low grip strength GWAS results18. We found no significant enrichment for the genetic determinants of low grip strength in
expression profiles and epigenetic changes after adjustment for multiple testing (Benjamini-Hochberg-adjusted false discovery rate >0.05). See Supplementary Data 10 for details. GENETIC
CORRELATIONS AND MENDELIAN RANDOMIZATION We assessed ten common age-related diseases for their genetic correlation with low grip strength (Fig. 2) using published genome-wide summary
statistics and LDSC13. The largest genetic overlap was with osteoarthritis (29.7% genetic correlation, SE 6.3%), but also strong positive correlations with coronary artery disease (19.5%, SE
3.0%), type-2 diabetes (15.8%, SE 3.1%) and rheumatoid arthritis (12.7%, SE 7.9%). We observed no significant genetic correlation after multiple-testing correction with the remaining
diseases examined (osteoporotic fracture risk, Alzheimer’s disease, stroke, chronic kidney disease, breast cancer and colorectal cancer). We also determined genetic correlations with five
anthropometric traits (Fig. 2), and found significant positive correlations with waist:hip ratio (13.0%, SE 2.7%) and BMI (9.1%, SE 2.5%), i.e. greater adiposity correlated with weakness in
60+ year olds. Significant negative correlations were observed with lean muscle mass (whole body: −30.9%, SE 6.1%; and appendicular: −26.5%, SE 5.8%) and with height (−37.4%, SE 2.3%). See
Supplementary Table 5 for details. We examined 83 traits in Mendelian randomization analysis to find evidence for shared causal pathways with weakness (low grip EWGSOP) at older ages:
primary results presented are betas from inverse variance-weighted regression using the “TwoSampleMR” R package19 (Fig. 3; Supplementary Data 11). We found significantly increased likelihood
of weakness (multiple testing-adjusted _p_ values < 0.05) with genetically predicted rheumatoid arthritis (Odds ratio = 1.03, Benjamini-Hochberg adjusted _p_ value = 5.3 × 10−4),
presence of type-2 diabetes (OR = 1.05, BH _p_ = 2.5 × 10−3), or asthma and allergic disease (OR = 1.07, BH _p_ = 9.4 × 10-3) (Supplementary Data 11). Genetic predisposition to greater age
of menarche (OR = 0.92, BH _p_ = 5.3 × 10-4), birth weight (OR = 0.80, BH _p_ = 5.3 × 10−4) and waist-hip ratio (WHR) adjusted for BMI in women only (OR = 0.85, BH _p_ = 5.3 × 10−4) were
protective of low grip strength as defined by the EWGSOP definition in both sexes (after adjustment for multiple testing). For each significant analysis we also examined the results from the
weighted-median and MR-Egger tests to check consistency and for horizontal pleiotropy. Only birth weight had an MR-Egger beta that was inconsistent with the main effect (−0.03 compared to
−0.2), although the intercept did not significantly deviate from 0 (_p_ = 0.1) and the MR-Egger confidence intervals overlap the IVW effect (95% CIs −0.33 to 0.27). In addition, the WHR
association should be interpreted with caution, as the analysis of WHR variants associated with both sexes were not statistically significant (nominal IVW _p_ = 0.01). The analysis of
females only also identified depression (OR = 1.21, BH _p_ = 2.75 × 10−2), and colorectal cancer (OR = 1.06, BH _p_ = 2.75 × 10−2) (Supplementary Data 12). Although the MR-Egger intercept
for depression was not statistically different from 0, there is potential for horizontal pleiotropy confounding this result (seen on Supplementary Fig. 3). In the males, type-2 diabetes
significantly increased odds of EWGSOP low grip (OR = 1.08, BH _p_ = 3.0 × 10−3) whereas greater plateletcrit (the volume occupied by platelets in the blood as a percentage) (OR = 0.91, _p_
= 3.0 × 10−3) and other haematological parameters appear to be protective (Supplementary Data 13). To explore the effect of genetic predisposition to low grip strength at older ages we
created an unweighted genetic risk score (GRS) in the UK Biobank European sample by summing the number of low grip strength-associated alleles (15 genetic variants so 30 alleles, mean number
of alleles = 12.6, SD = 2.3). We opted to use an unweighted score as use of weights from analyses including the discovery sample can bias associations and lead to overestimated effects
(so-called “winner’s curse”)20. We first confirmed the association with low grip strength in UK Biobank participants (OR per allele 1.036: 95% CIs 1.030 to 1.041, _p_ = 6 × 10−40). The low
grip GRS was also associated with increased Frailty Index21 (increase in points per allele = 0.013: 0.006 to 0.021, _p_ = 4 × 10−4). LOW GRIP STRENGTH LOCI INDEPENDENCE FROM MUSCULOSKELETAL
TRAITS AND DISEASES To determine whether the genetic variants associated with low grip strength identified in the GWAS were independent of anthropometric traits or prevalent musculoskeletal
comorbidities we performed regression analyses in the UK Biobank cohort with adjustment for the following covariates: height, weight, skeletal muscle mass (determined using bioimpedance
analysis), osteoarthritis, Rheumatoid arthritis, osteoporosis, Dupuytren’s contracture (one or more fingers permanently bent), and rhizarthrosis (arthritis of the thumb). Disease diagnoses
were either self-reported, hospital diagnosed, or inferred from relevant surgical procedures (for example Palmar Fasciectomy to treat Dupuytren’s contracture), and hip or knee replacements
resulting from osteoarthritis: UK Biobank hospital episode statistics diagnosis and operations data available up to March 2017. See Supplementary Table 6 for diagnostic and surgical codes
used. The association between 8 of the 15 EWGSOP loci and low grip strength was attenuated after adjusting for height, including rs143384 (initial UKB _p_ = 3.7 × 10−11; adjusted UKB _p_ =
3.8 × 10−2) and rs7624084 (initial UKB p = 9.3 × 10−7; adjusted UKB _p_ = 4.9 × 10−1). Adjustment for weight or BMI did not substantially attenuate any of the associations. Overall, the
associations were not attenuated by adjustment for osteoarthritis, Rheumatoid arthritis, osteoporosis, Dupuytren’s, or rhizarthrosis. See Supplementary Data 14 for detailed results.
DISCUSSION In this study of 256,523 Europeans aged 60 years and over we found that 15 genetic loci were associated with the EWGSOP definition of low grip strength (dynapenia), plus two
additional loci for the FNIH definition that used a more strict definition for muscle weakness. Only three of these are known to be associated with continuous strength measures in GWAS, and
only 3 of the 64 known overall strength loci are associated with clinically low grip strength used in our study. These suggest that the genetic causes of clinically meaningful weakness at
older ages are partly distinct. Two of the low grip strength-associated genetic signals identified have not been reported by GWAS prior to the time of analysis (March 2020), further
demonstrating that low strength in older people may have distinct genetic underpinnings. We did also find prominent overlaps with osteoarthritis and Rheumatoid arthritis, and also with
cardiovascular disease and type-2 diabetes. Additional links to asthma and allergy were also found. The pathways implicated appear to include hallmark mechanisms of ageing22, for example
cell cycle control related to the cancer control retinoblastoma pathway. However other ageing pathways such as telomere length23, and many lifespan-associated loci including _APOE_24, were
not associated. The strongest association found was rs34415150, near the _HLA-DQA1_ gene. Genetic variants at this locus have been implicated in a wide range of conditions, including
autoimmune diseases such as Rheumatoid arthritis25, and continuous grip strength9. HLA haplotypes _HLA-DQA1_*03:01 and _HLA-DRB1_*04:01, have been previously linked to sarcopenia in older UK
Biobank participants26. HLA-DQA1 is associated with chronic inflammation in muscle of untreated children with juvenile dermatomyositis (inflammatory myopathies in children, which one of the
characteristics is muscle weakness)27. In addition, in a multi-trait analysis of age-related diseases _HLA-DQA1_ was identified and it may therefore contribute to underlying ageing
mechanisms as a “geroscience locus”28. Overall 6 of the 15 genomic risk loci for EWGSOP low grip strength have been previously associated (or are in LD) with osteoarthritis (rs143384 –
_GDF5_, rs13107325 – _SLC39A8_, rs34464763 – _C12orf60_, rs2899611 - _ALDH1A2_, rs958685 – _TGFA_ and rs79723785 - _BRSK1_), of which two are also linked to adiposity, a known risk factor
for osteoarthritis29. We found that rs143384 in the 5’ untranslated region of growth/differentiation factor 5 (_GDF5_) was the second most strongly associated variant with low grip strength.
_GDF5_ is a protein in the transforming growth factor beta (TGF-β) family, with key roles in bone and joint development30,31. It was the first locus identified for osteoarthritis32, with a
reported odds ratio of 1.79, as well as one of the first identified for height33. GDF5 is known to reduce expression of cartilage extracellular matrix-degrading enzymes in human primary
chondrocytes34, thereby may be an potential intervention target for avoiding weakness at older ages, although work is needed to determine when intervention would be most effective. In
follow-up analyses we found that the association between rs143384 and low grip strength was independent of prevalent osteoarthritis in UK Biobank participants, although we cannot rule out an
effect of sub-clinical osteoarthritis. However, the association was completely attenuated after adjustment for standing height, suggesting the effect of the variant is mediated by
developmental traits such as bone length. Mice with loss of _Gdf5_ function exhibited severely impaired knee development, and the regulatory region pinpointed to mediate this effect in
humans includes osteoarthritis-associated genetic variants35. Although the observed association between variants mapping to _GDF5_ and low strength might be mediated by hand osteoarthritis
pain compromising grip strength, there is evidence of a direct effect of GDF5 on muscle36. The locus on Chr 18 was near to the DYM gene. Loss of function mutations in this gene are
associated with Dyggve-Melchior-Clausen syndrome and Smith-McCort dysplasia respectively. Mice lacking this gene present with chondrodysplasia resulting from impaired endochondral bone
formation and abnormalities of the growth plate that begin to manifest shortly birth37. In homozygous mutant mice, and in patients with loss of function mutations in this gene, both the
axial and the appendicular skeleton are affected. As is noted in Supplementary Data 2, this gene is also associated with height in the GWAS catalogue. Interestingly, this gene is highly
expressed in skeletal muscle in humans38, however its function in muscle is not completely understood. _SLC39A8_ encodes for the metal ion transporter ZIP8 which has been shown to be
upregulated in chondrocytes present in osteoarthritic cartilage39. The lead SNP rs13107325 is a missense variant within _SLC39A8_ which has been previously associated with osteoarthritis40.
On chromosome 12 genetic variants known to affect expression of _MGP_ (Matrix Gla Protein) in the tibial nerve (among other tissues) are associated with osteoarthritis of the hand but not of
the hip or knee41. MGP is an inhibitor of arterial and soft tissue calcifications, with links to atherosclerosis42. Consistent with this, older women with severe abdominal aortic
calcification have greater decline in grip strength over 5 years43. More recently a study suggested MGP may also regulate muscle development and atrophy44. We also identified variants known
to affect Transforming Growth Factor Alpha (_TGFA_) expression (increased in the testis, decreased in the brain), which is implicated in cell proliferation, differentiation and development.
This locus has previously been identified for overall strength10, and suggestive evidence from a study of 1,323 participants linked rs2862851, a variant in linkage disequilibrium with the
lead SNP rs958685 (_R_2 = 0.90, D’ = 1.0), with increased risk of osteoarthritis in the knee (OR = 1.4, _p_ = 3.1 × 10−4)45. The low grip strength locus on chromosome 15 is near _ALDH1A2_,
which has a key role in the pathogenesis of osteoarthritis46. Low grip strength-associated variants at this locus have previously been identified for severe osteoarthritis of the hand, and
may explain why this locus is associated with low grip strength measured by hand dynamometer46. Knocking out this gene in mice is perinatally lethal, however at embryonic day 18.5 mice
lacking this gene do present with numerous cartilage gene defects47. We also identified variants that affect expression of _CHRDL2_ (Chordin Like 2) in the thyroid, known to interact with
mouse Gdf5, which is upregulated in human osteoarthritic joint cartilage cell line48. In addition, down-regulation of _CHRDL2_ expression has been linked to the progression of severe
osteoarthritis in the knee joint49, suggesting a role for CHRDL2 in cartilage repair. Two of the identified loci (_RBBP6_ ‘RB Binding Protein 6, Ubiquitin Ligase’ and _ZBTB38_ ‘Zinc Finger
And BTB Domain Containing 38’) form an axis involved in DNA replication and chromosomal stability50. RBBP6 ubiquitinates the transcriptional repressor ZBTB38, destabilizing it and reducing
its action on the replication factor MCM10. In mice, _Zbtb38_ is highly expressed in skeletal muscle, loss of this methyl-CpG-binding protein (which is also known as _Cibz_), promotes
myogenic differentiation. Conversely, expression of _Zbtb38_ is decreased in satellite cells during muscle regeneration51, suggesting that like other members of this gene family, this gene
is involved in cellular differentiation. Variants associated with low grip strength in our analysis are known to decrease ZBTB38 expression in whole blood and skeletal muscle (among others)
and increase expression in the skin. Mitochondrial dysfunction is a hallmark of aging, yet we found no variants associated with low strength at genome-wide significance levels in a
sub-analysis of UK Biobank only. Variants in _MT-CYB_ were nominally significant (p = 0.0003): MT-CYB (mitochondrial cytochrome b) is part of the mitochondrial respiratory chain, and
essential for Complex III formation. Monogenic diseases associated with _MT-CYB_ include exercise intolerance and “Additional features include lactic acidosis, muscle weakness and/or
myoglobinuria” (https://www.uniprot.org/uniprot/P00156). Recent work by Cohen et al. have highlighted the importance of mitochondrial peptides such as humanin in many age-related diseases52,
however we found no variants in these genes associated with low grip strength passing multiple testing correction. In comparison to a previous study that analysed grip strength as a
continuous measure in the UK Biobank cohort9 that included all aged (40–70 years), we found that only three of the 64 identified variants were significantly (_p_ < 5 × 10−8) associated
with EWGSOP low grip strength (Supplementary Data 2). The top association was rs13107325 (_SLC39A8_ linear grip _p_ = 4.4 × 10−23, low grip strength meta-analysis _p_ = 7.4 × 10−11), then
rs2430740 (_C12orf60_ linear grip _p_ = 6 × 10−12, low grip strength meta-analysis _p_ = 2.6 × 10−9) and finally rs11236203 (_POLD3_, linear grip _p_ = 8.4 × 10−10, low grip strength
meta-analysis _p_ = 2.8 × 10−8). Two less-significant associations (<1 × 10−6) were seen with rs3821269 (_TGFA_ linear grip _p_ = 3.5E × 10−15, low grip strength meta-analysis _p_ = 8.0 ×
10−8) and rs1556659 (_ENSG00000232985_ linear grip _p_ = 1.1 × 10−11, low grip strength meta-analysis _p_ = 3.5 × 10−7). A previous GWAS by the CHARGE consortium identified two loci
associated with maximum hand grip strength recorded in 27,581 Europeans aged 65 or older8. We found that neither locus was associated with low grip strength in our analysis (rs752045 EWGSOP
_p_ = 0.67, rs3121278 EWGSOP _p_ = 0.15). We observed minimal overlap between loci associated with low grip strength and general anthropometric traits such as height and continuous measures
of strength. The SNP-based heritability estimate for EWGSOP low grip strength in older adults was 4.4% (SE 0.3%). This was somewhat lower than the 13% (SE 0.4%) SNP-based heritability for
continuous grip strength reported in UK Biobank participants aged 40–709. This may be partly explained by our study using a binary cut-off for low grip compared to the quantitative analysis
of grip strength. These results emphasize that the genetics of muscle weakness and overall strength are distinct. The SNP-based heritability estimates observed here are lower than those from
studies of twins, for example a study of 1,757 male twin pairs aged 45–96 found the heritability of continuous strength to be 48–55%7, however other studies have shown that heritability
declines significantly as age advances as environmental factors explain more of the variance3, though still up to 22% has been reported for muscle strength. In contrast to the twin studies
our estimates of heritability are restricted to common SNPs with MAF ≥ 1%, and thus represent a lower bound of the overall genetic variance of low strength in older people. Despite seeing
little overlap at the individual locus level between low grip strength at older ages and other diseases we did observe significant genetic correlations, especially with osteoarthritis (30%
overlap). However in Mendelian Randomization analysis we did not observe a causal relationship between osteoarthritis and low grip strength: taken together, this suggests that osteoarthritis
shares causal risk factors and biological pathways with low grip strength at older ages, such as obesity, but may not cause it. In addition, osteoarthritis has diverse loci associated with
different joints, for instance osteoarthritis in the hand appears to have a distinct genetic signal: the low grip strength locus associated with _MGP_ expression has been previously linked
to osteoarthritis in the fingers and hand, but not the hip or knee41. Although our results were robust to adjustment for osteoarthritis (including rhizarthrosis—arthritis of the thumb) this
may suggest that arthritis in the hand needs to be accounted for in measures of muscle weakness, as hand grip strength may not always reflect muscle strength elsewhere, e.g., lower-extremity
strength. Our Mendelian Randomization analyses highlighted specific traits and diseases which may share causal pathways with weakness at older ages. This included growth and development
traits such as birth weight, waist:hip ratio, and pubertal timing (age at menarche) in women (highly genetically correlated – 75% – with age at voice breaking in men53), where greater values
were protective. Although puberty timing is highly polygenic, it is strongly genetically correlated with BMI (−35%)53, with complex interactions: i.e., being thinner in childhood is
associated with delayed menarche54, but later menarche results in taller adult height55. These results are consistent with the observation that growth and development traits are associated
with strength trajectories in later life56. We chose to not adjust for body size in our primary analysis in case interesting or novel effects were masked, but in follow-up analysis
determined that all except two of the loci identified were predominantly independent of participant height and weight. Raised red blood cell parameters - especially plateletcrit, the
proportion of blood occupied by platelets - appear to be protective in males but associations were attenuated or nonsignificant in females. A number of studies have recently reported a link
between raised platelet counts, inflammation, and sarcopenia cross-sectionally57. However our results suggest that plateletcrit across the lifecourse (rather than after sarcopenia onset) may
be different. Lastly, only four of the conditions we investigated (which included coronary artery disease, and some common cancers) appear to causally increase risk of weakness in older
people: depression, asthma/allergic diseases, Rheumatoid arthritis, and type-2 diabetes. These are diverse conditions and further underlines the multifactorial causes of weakness in older
people. There are a number of limitations to our analyses. The data included are predominantly from subjects at the younger end of the age 60 plus demographic, and is not enriched for frail
individuals. The sample size for sex-specific analyses is limited, especially for men, likely contributing to the fewer significant associations observed. The results from sex-stratified
analyses need to be interpreted with caution, as a recent pre-print on bioRxiv has shown that some variants are spuriously associated with sex in cohorts such as the UK Biobank, likely due
to their effect on differential participation58. Our analysis was limited to relatively common variants (prevalence >1%) in subjects with European ancestries only. The analyses of the
FNIH strength cutpoints also have limited power, given the low prevalence of the phenotype, although studying the extremes of a continuous trait can provide increased power if stronger
associations are uncovered. We did not include additional analysis of the revised EWGSOP2 low grip cut-points6 as these are almost identical to the FNIH criteria, which many cohorts had
already analyzed; future analyses should include this. Analysis of rare and structural variants, and analyses in other ancestral groups will give a more complete picture of the genetic
landscape for low grip strength or dynapenia. Some Mendelian Randomization analyses have limited power due to the lack of strong instruments, and therefore null results for these analyses
should be interpreted with caution. To conclude, genetic variation in 15 loci are related to muscle weakness in people aged 60 plus, of European descent, with limited overlap with loci
associated with the full range of muscle strength in 40–70 year olds. The loci implicated may be involved in hallmark pathways of ageing including cell cycle control and inflammation, along
with loci implicated in arthritis and pathways involved in the development and maintenance of the musculoskeletal system. METHODS GWAS OF LOW GRIP STRENGTH IN OLDER PEOPLE We conducted a
GWAS meta-analysis of low grip strength in participants aged 60 years or older of European ancestry from 22 studies yielding a combined sample of 254,894 individuals. Individual studies used
different genotyping platforms and imputation was predominantly performed using the Haplotype reference consortium (HRC) v1.1 panel. See Supplementary Information for details on individual
study methods. Two definitions of low muscle hand grip strength were utilized at the time of analysis. The primary analysis was of the 2010 EWGSOP criteria for sarcopenic grip strength (Grip
strength <30 kg Male; <20 kg Female). In secondary analysis we considered a more data-driven definition with more strict thresholds by the FNIH sarcopenia project 2014 (Grip strength
<26 kg Male; <16 kg Female) for comparison. Given the known differences in strength between males and females (on average) we also performed sex stratified analyses. GWAS was performed
by each cohort individually (see Supplementary Methods) using regression models, adjusted for age, sex (except in sex-specific models), and population substructure, accounting for
relatedness and technical covariates as required by the individual study. No adjustment for anthropometric measures was made in the primary analysis, but the effects were explored in
sensitivity analyses (see below). Fixed-effects inverse variance weighted meta-analysis was performed using METAL59 using the GWAS summary statistics generated by each cohort, with genomic
control for population structure (see Supplementary Methods for details). The following quality control filters were applied: minor allele frequency (MAF) > 0.01, imputation info score of
> 0.4, and the variant present in at least two studies (UK Biobank – the largest included cohort—plus at least one other). The final analysis therefore included 9,678,524 genetic
variants. Associations that achieved a _p_ < 5 × 10−8 were considered statistically significant, with those reaching the more stringent threshold of _p_ < 5 × 10−9 highlighted.
Distinct loci were initially defined as two significant variants separated by >500 kb. To identify independent signals at each locus we used FUMA (Functional Mapping and Annotation of
Genome-Wide Association Studies)60, which uses Linkage Disequilibrium (LD) information to determine independence (_r_2 threshold = 0.1 for independent significant SNP). We used Linkage
Disequilibrium Score Regression (LDSC, v1.0.0) to estimate the level of bias (i.e., from population stratification and cryptic relatedness) in the GWAS, and the SNP-based heritability of low
grip strength13. LOCUS OVERLAP WITH DISEASES AND ANTHROPOMETRIC TRAITS The GWAS catalogue of published loci-trait associations11 was searched to identify whether low grip
strength-associated loci determined from our meta-analysis are known to influence other traits or diseases. In addition, we performed sensitivity analyses in the UK Biobank sample to
determine whether associations between variants and low grip strength identified in the meta-analysis were robust to adjustment for the following traits or diseases: height, weight, body
mass index, osteoarthritis, rheumatoid arthritis, and osteoporosis (prevalent diseases were from the self-reported data at baseline or in the Hospital Episode Statistics). Analyses were
performed in STATA (v 15) using logistic regression models adjusted for age, sex, principal components 1 to 10, assessment centre, and genotyping array (the UK Biobank using two different
Affymetrix microarrays that shared >95% of sites – see Supplementary Methods). GENE ONTOLOGY PATHWAYS, TISSUE ENRICHMENT, AND EQTL ANALYSES We utilized FUMA to perform functional
interpretation of the GWAS results60. In particular, FUMA performs gene-set analysis (using Multi-marker Analysis of GenoMic Annotation (MAGMA)61) to identify pathways enriched amongst the
significant genes (weighted by the SNP-associations in proximity to them), in addition to searching eQTL databases to identify SNPs that significantly alter the expression of genes in
various tissues. We used MetaXcan to determine whether gene-level transcriptomic associations in GTEx v7 skeletal muscle data were enriched in the GWAS summary statistics for low grip
strength17. The analysis included 7512 genes with measured expression in the dataset; we applied Benjamini-Hochberg multiple-testing correction, with adjusted _p_ values < 0.05 deemed to
be significant. LD Score Regression applied to specifically expressed genes (LDSC-SEG) allows the identification of enriched tissue activity associated with GWAS results18. We applied
LDSC-SEG (v1.0.0) to the GWAS summary statistics using the datasets ‘Multi_tissue_gene_expr‘ and ‘Multi_tissue_chromatin‘ provided by the authors. We applied Benjamini-Hochberg
multiple-testing correction for the 703 tests (n gene expression = 205, n chromatin = 498), with adjusted _p_ values < 0.05 deemed to be significant. GENETIC CORRELATIONS AND MENDELIAN
RANDOMIZATION We investigated the genetic correlation between the low grip strength trait and 10 diseases - chosen because they are common, chronic diseases of aging - using LDSC (v1.0.0)13
and published GWAS summary statistics for the following diseases: Alzheimer’s disease62, breast cancer63, chronic kidney disease64, coronary artery disease65, osteoporotic fracture risk66,
osteoarthritis67, prostate cancer68, rheumatoid arthritis69, stroke70, and type-2 diabetes71. We also calculated genetic correlations with the following anthropometric traits: height72, body
mass index (BMI)72, waist:hip ratio (WHR)73, whole-body lean mass12, and appendicular lean mass12. We also undertook a phenotype-wide Mendelian randomization (MR) association study to
examine the causal effect of 83 traits on low hand grip strength. We used the “TwoSampleMR” (v0.4.23) package in R19 to perform the analysis of genetic instruments from the 83 traits, which
including those traits with clear biological rationale (for example, adiposity) and others that are more exploratory for hypothesis generation (for example, puberty timing). Follow up
sensitivity analysis of the identified traits was by the MR-Egger and using weighted median estimation methods provided in the package. REPORTING SUMMARY Further information on research
design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The GWAS summary statistics and supporting information on low grip strength in older
people are available on the Musculoskeletal Knowledge Portal (www.mskkp.org) and the GWAS catalogue (www.ebi.ac.uk/gwas accession numbers GCST90007526, GCST90007527, GCST90007528,
GCST90007529, GCST90007530 and GCST90007531). The International Mouse Phenotyping Consortium database is located (https://www.mousephenotype.org/). eQTL data is available from
(https://gtexportal.org/). Catalogue of GWAS associations is available (https://www.ebi.ac.uk/gwas/). All relevant additional data is available on request from the authors. Information on
the 22 individual cohorts is included in the Supplementary Information file. CODE AVAILABILITY See our associated GitHub repository for example scripts used for the main analyses
(https://github.com/pasted/gw_meta_analysis_low_muscle_strength). All relevant additional code is available on request from the authors. REFERENCES * Clark, B. C. & Manini, T. M. What is
dynapenia? _Nutrition_ 28, 495–503 (2012). Article PubMed PubMed Central Google Scholar * Manini, T. M. & Clark, B. C. Dynapenia and aging: an update. _J. Gerontol. Ser. A_ 67A,
28–40 (2012). Article Google Scholar * Mitchell, W. K. et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review.
_Front. Physiol._ 3, 260 (2012). Article PubMed PubMed Central Google Scholar * Cawthon, P. M. et al. Establishing the link between lean mass and grip strength cut points with mobility
disability and other health outcomes: proceedings of the sarcopenia definition and outcomes consortium conference. _J. Gerontol. Ser. A_ https://doi.org/10.1093/gerona/glz081 (2019). Article
Google Scholar * Rantanen, T. et al. Midlife hand grip strength as a predictor of old age disability. _J. Am. Med. Assoc._ 281, 558–560 (1999). Article CAS Google Scholar *
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. _Age Ageing_ 48, 16–31 (2019). Article PubMed Google Scholar * Frederiksen, H. et al. Hand
grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. _Genet. Epidemiol._ 23, 110–122 (2002). Article PubMed Google
Scholar * Matteini, A. M. et al. GWAS analysis of handgrip and lower body strength in older adults in the CHARGE consortium. _Aging Cell_ 15, 792–800 (2016). Article CAS PubMed PubMed
Central Google Scholar * Tikkanen, E. et al. Biological insights into muscular strength: genetic findings in the UK biobank. _Sci. Rep._ 8, 6451 (2018). Article ADS PubMed PubMed
Central CAS Google Scholar * Willems, S. M. et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. _Nat. Commun._ 8,
16015 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Buniello, A. et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and
summary statistics 2019. _Nucleic Acids Res_ 47, D1005–D1012 (2019). Article CAS PubMed Google Scholar * Zillikens, M. C. et al. Large meta-analysis of genome-wide association studies
identifies five loci for lean body mass. _Nat. Commun._ 8, 80 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Bulik-Sullivan, B. K. et al. LD Score regression
distinguishes confounding from polygenicity in genome-wide association studies. _Nat. Genet._, 47, 291–295 (2015). Article CAS PubMed PubMed Central Google Scholar * Studenski, S. A. et
al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. _J. Gerontol. A. Biol. Sci. Med. Sci._ 69, 547–558 (2014). Article PubMed
PubMed Central Google Scholar * Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People.
_Age Ageing_ 39, 412–423 (2010). Article PubMed PubMed Central Google Scholar * Stacey, D. et al. ProGeM: a framework for the prioritization of candidate causal genes at molecular
quantitative trait loci. _Nucleic Acids Res._ 47, e3–e3 (2019). Article CAS PubMed Google Scholar * Barbeira, A. N. et al. Exploring the phenotypic consequences of tissue specific gene
expression variation inferred from GWAS summary statistics. _Nat. Commun._ 9, 1–20 (2018). Article CAS Google Scholar * Finucane, H. K. et al. Heritability enrichment of specifically
expressed genes identifies disease-relevant tissues and cell types. _Nat. Genet._ 50, 621–629 (2018). Article CAS PubMed PubMed Central Google Scholar * Hemani, G. et al. The MR-base
platform supports systematic causal inference across the human phenome. _Elife_ 7, e34408 (2018). Article PubMed PubMed Central Google Scholar * Burgess, S. & Thompson, S. G. Use of
allele scores as instrumental variables for Mendelian randomization. _Int. J. Epidemiol._ 42, 1134–1144 (2013). Article PubMed PubMed Central Google Scholar * Williams, D. M., Jylhava,
J., Pedersen, N. L. & Hagg, S. A frailty index for UK Biobank participants. _J Gerontol Med. Sci._ 74, 582–587 (2019). Article Google Scholar * López-Otín, C., Blasco, M. A.,
Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. _Cell_ 153, 1194–1217 (2013). Article PubMed PubMed Central CAS Google Scholar * Kuo, C.-L., Pilling, L. C., Kuchel,
G. A., Ferrucci, L. & Melzer, D. Telomere length and aging-related outcomes in humans: A Mendelian randomization study in 261,000 older participants. Aging Cell e13017 (2019)
https://doi.org/10.1111/acel.13017. * Timmers, P. R. et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. _Elife_
8, 1–71 (2019). Article Google Scholar * Cortes, A., Albers, P. K., Dendrou, C. A., Fugger, L. & McVean, G. Identifying cross-disease components of genetic risk across hospital data in
the UK Biobank. Nat. Genet. (2019) https://doi.org/10.1038/s41588-019-0550-4. * Jones, G. et al. Sarcopenia and variation in the Human Leukocyte Antigen complex. J. Gerontol. A. Biol. Sci.
Med. Sci. (2019) https://doi.org/10.1093/gerona/glz042. * Chen, Y.-W. et al. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile
dermatomyositis. _BMC Immunol._ 9, 43 (2008). Article PubMed PubMed Central CAS Google Scholar * Melzer, D., Pilling, L. C. & Ferrucci, L. The genetics of human ageing. Nat. Rev.
Genet. (2019) https://doi.org/10.1038/s41576-019-0183-6. * Murphy, L. et al. Lifetime risk of symptomatic knee osteoarthritis. _Arthritis Care Res_ 59, 1207–1213 (2008). Article Google
Scholar * Francis-West, P. H. et al. Mechanisms of GDF-5 action during skeletal development. _Development_ 126, 1305–1315 (1999). Article CAS PubMed Google Scholar * Capellini, T. D. et
al. Ancient selection for derived alleles at a GDF5 enhancer influencing human growth and osteoarthritis risk. _Nat. Genet._ 49, 1202–1210 (2017). Article CAS PubMed PubMed Central
Google Scholar * Miyamoto, Y. et al. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. _Nat. Genet._ 39, 529–533 (2007). Article CAS
PubMed Google Scholar * Sanna, S. et al. Common variants in the GDF5-UQCC region are associated with variation in human height. _Nat. Genet._ 40, 198–203 (2008). Article CAS PubMed
PubMed Central Google Scholar * Uhalte, E. C., Wilkinson, J. M., Southam, L. & Zeggini, E. Pathways to understanding the genomic aetiology of osteoarthritis. _Hum. Mol. Genet_ 26,
R193–R201 (2017). Article CAS Google Scholar * Pregizer, S. K. et al. Impact of broad regulatory regions on Gdf5 expression and function in knee development and susceptibility to
osteoarthritis. _Ann. Rheum. Dis._ 77, 450 (2018). Article CAS PubMed Google Scholar * Traoré, M. et al. An embryonic CaVβ1 isoform promotes muscle mass maintenance via GDF5 signaling in
adult mouse. _Sci. Transl. Med._ 11, eaaw1131 (2019). * Osipovich, A. B., Jennings, J. L., Lin, Q., Link, A. J. & Ruley, H. E. Dyggve-Melchior-Clausen syndrome: chondrodysplasia
resulting from defects in intracellular vesicle traffic. _Proc. Natl Acad. Sci. USA_ 105, 16171–16176 (2008). Article ADS CAS PubMed PubMed Central Google Scholar * Paupe, V. et al.
Recent advances in Dyggve-Melchior-Clausen syndrome. _Mol. Genet. Metab._ 83, 51–59 (2004). Article CAS PubMed Google Scholar * Kim, J.-H. et al. Regulation of the catabolic cascade in
osteoarthritis by the zinc-ZIP8-MTF1 axis. _Cell_ 156, 730–743 (2014). Article CAS PubMed Google Scholar * Tachmazidou, I. et al. Identification of new therapeutic targets for
osteoarthritis through genome-wide analyses of UK Biobank data. _Nat. Genet._ 51, 230–236 (2019). Article CAS PubMed PubMed Central Google Scholar * den Hollander, W. et al. Genome-wide
association and functional studies identify a role for matrix Gla protein in osteoarthritis of the hand. _Ann. Rheum. Dis._ 76, 2046–2053 (2017). Article CAS Google Scholar * Herrmann,
S. M. et al. Polymorphisms of the human matrix Gla protein (MGP) gene, vascular calcification, and myocardial infarction. _Arterioscler. Thromb. Vasc. Biol._ 20, 2386–2393 (2000). Article
CAS PubMed Google Scholar * Rodríguez, A. J. et al. Aortic calcification is associated with five-year decline in handgrip strength in older women. _Calcif. Tissue Int._ 103, 589–598
(2018). Article PubMed CAS Google Scholar * Ahmad, S., Jan, A. T., Baig, M. H., Lee, E. J. & Choi, I. Matrix gla protein: an extracellular matrix protein regulates myostatin
expression in the muscle developmental program. _Life Sci._ 172, 55–63 (2017). Article CAS PubMed Google Scholar * Cui, G. et al. Association of common variants in TGFA with increased
risk of knee osteoarthritis susceptibility. _Genet. Test. Mol. Biomark._ 21, 586–591 (2017). Article CAS Google Scholar * Styrkarsdottir, U. et al. Severe osteoarthritis of the hand
associates with common variants within the ALDH1A2 gene and with rare variants at 1p31. _Nat. Genet._ 46, 498–502 (2014). Article CAS PubMed Google Scholar * Vermot, J., Niederreither,
K., Garnier, J.-M., Chambon, P. & Dollé, P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. _Proc. Natl Acad. Sci. USA_ 100,
1763–1768 (2003). Article ADS CAS PubMed PubMed Central Google Scholar * Nakayama, N. et al. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of
developing cartilage and osteoarthritic joint cartilage. _Development_ 131, 229–240 (2004). Article CAS PubMed Google Scholar * Chou, C.-H. et al. Insights into osteoarthritis
progression revealed by analyses of both knee tibiofemoral compartments. _Osteoarthr. Cartil._ 23, 571–580 (2015). Article Google Scholar * Miotto, B. et al. The RBBP6/ZBTB38/MCM10 axis
regulates DNA replication and common fragile site stability. _Cell Rep._ 7, 575–587 (2014). Article CAS PubMed Google Scholar * Oikawa, Y. et al. The methyl-CpG-binding protein CIBZ
suppresses myogenic differentiation by directly inhibiting myogenin expression. _Cell Res_ 21, 1578–1590 (2011). Article CAS PubMed PubMed Central Google Scholar * Yen, K., Lee, C.,
Mehta, H. & Cohen, P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. _J. Mol. Endocrinol._ 50, R11–R19 (2013). Article CAS PubMed PubMed Central
Google Scholar * Day, F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. _Nat. Genet._ 49,
834–841 (2017). Article CAS PubMed PubMed Central Google Scholar * Ruth, K. S. et al. Events in early life are associated with female reproductive ageing: a UK Biobank study. _Sci.
Rep._ 6, 1–9 (2016). Article CAS Google Scholar * Day, F. R., Perry, J. R. B. & Ong, K. K. Genetic regulation of puberty timing in humans. _Neuroendocrinology_ 102, 247–255 (2015).
Article CAS PubMed Google Scholar * Kuh, D., Hardy, R., Blodgett, J. M. & Cooper, R. Developmental factors associated with decline in grip strength from midlife to old age: a British
birth cohort study. _BMJ Open_ 9, 1–12 (2019). Article Google Scholar * Liaw, F. Y. et al. Higher platelet-to-lymphocyte ratio increased the risk of sarcopenia in the community-dwelling
older adults. _Sci. Rep._ 7, 16609 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Pirastu, N. et al. Genetic analyses identify widespread sex-differential participation
bias. bioRxiv (unpublished Prepr (2020). https://doi.org/10.1101/2020.03.22.001453. * Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide
association scans. _Bioinformatics_ 26, 2190–2191 (2010). Article CAS PubMed PubMed Central Google Scholar * Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional
mapping and annotation of genetic associations with FUMA. _Nat. Commun._ 8, 1826 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * de Leeuw, C. A., Mooij, J. M., Heskes,
T. & Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. _PLoS Comput. Biol._ 11, e1004219 (2015). Article PubMed PubMed Central CAS Google Scholar * Jansen, I. E. et
al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 258533 (2019) https://doi.org/10.1038/s41588-018-0311-9. *
Michailidou, K. et al. Association analysis identifies 65 new breast cancer risk loci. _Nature_ 551, 92–94 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Pattaro, C. et
al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. _Nat. Commun._ 7, 10023 (2016). Article ADS CAS PubMed PubMed Central
Google Scholar * van der Harst, P. & Verweij, N. identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. _Circ. Res._
122, 433–443 (2018). Article PubMed PubMed Central CAS Google Scholar * Trajanoska, K. et al. Assessment of the genetic and clinical determinants of fracture risk: genome wide
association and mendelian randomisation study. _BMJ_ 362, k3225 (2018). Article PubMed PubMed Central Google Scholar * Zengini, E. et al. Genome-wide analyses using UK Biobank data
provide insights into the genetic architecture of osteoarthritis. _Nat. Genet._ 50, 549–558 (2018). Article CAS PubMed PubMed Central Google Scholar * Schumacher, F. R. et al.
Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. _Nat. Genet._ 50, 928–936 (2018). Article CAS PubMed PubMed Central Google Scholar *
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. _Nature_ 506, 376–381 (2014). Article ADS CAS PubMed Google Scholar * Malik, R. et al.
Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. _Nat. Genet._ 50, 524–537 (2018). Article CAS PubMed PubMed
Central Google Scholar * Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet.
(2018) https://doi.org/10.1038/s41588-018-0241-6. * Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European
ancestry. _Hum. Mol. Genet_ 27, 3641–3649 (2018). Article CAS PubMed PubMed Central Google Scholar * Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat
distribution in 694 649 individuals of European ancestry. _Hum. Mol. Genet_ 28, 166–174 (2019). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was part
funded by an award to DM by the UK Medical Research Council (MR/M023095/1). A full list of acknowledgements and grant support can be found in Supplementary Note 1 (in the Supplementary
Information). We would like to acknowledge the use of the University of Exeter High-Performance Computing (HPC) facility in carrying out this work. AUTHOR INFORMATION Author notes * These
authors contributed equally: Garan Jones, Katerina Trajanoska, Adam J. Santanasto, Najada Stringa, Chia-Ling Kuo, Janice L. Atkins. * These authors jointly supervised this work: George A.
Kuchel, Luigi Ferrucci, David Karasik, Fernando Rivadeneira, Douglas P. Kiel, Luke C. Pilling. AUTHORS AND AFFILIATIONS * Epidemiology and Public Health Group, Institute of Biomedical and
Clinical Science, University of Exeter Medical School, Exeter, UK Garan Jones, Janice L. Atkins, David Melzer & Luke C. Pilling * Department of Internal Medicine, Erasmus Medical Center,
Rotterdam, The Netherlands Katerina Trajanoska, M. Carola Zillikens, Andre G. Uitterlinden & Fernando Rivadeneira * Department of Epidemiology Medicine, Erasmus Medical Center,
Rotterdam, The Netherlands Katerina Trajanoska & Fernando Rivadeneira * University of Pittsburgh, Department of Epidemiology, Pittsburgh, PA, USA Adam J. Santanasto, Ryan Cvejkus &
Joseph Zmuda * Department of Epidemiology and Biostatistics, Amsterdam UMC- Vrije Universiteit, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands Najada Stringa, Natasja
M. van Schoor & Martijn Huisman * Biostatistics Center, Connecticut Convergence Institute for Translation in Regenerative Engineering, UConn Health, Farmington, CT, USA Chia-Ling Kuo *
School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia Joshua R. Lewis * School fo Public Health University of Sydney, Sydney, NSW, Australia Joshua R. Lewis
* Medical School, University of Western Australia, Crawley, WA, Australia Joshua R. Lewis * McKusick-Nathans Institute, Department of Genetic Medicine, Johns Hopkins University School of
Medicine, Baltimore, MD, USA ThuyVy Duong & Dan E. Arking * Lübeck Interdisciplinary Plattform for Genome Analytics, Institutes of Neurogenetics and Cardiogenetics, University of Lübeck,
Lübeck, Germany Shengjun Hong & Lars Bertram * Cardiovascular Health Research Unit, Department of Medicine, and Department of Biostatistics, University of Washington, Seattle, WA, USA
Mary L. Biggs * MRC Epidemiology Unit, Institute of Metabolic Science, University of Cambridge School of Clinical Medicine, Cambridge, CB2 0QQ, UK Jian’an Luan, Claudia Langenberg &
Nicholas J. Wareham * Biostatistics Department, Boston University School of Public Health, Boston, MA, USA Chloe Sarnowski & Kathryn L. Lunetta * Longitudinal Study Section,
Translational Gerontology branch, National Institute on Aging, Baltimore, MD, USA Toshiko Tanaka * Department of Genetics, Washington University School of Medicine, St. Louis, MO, USA Mary
K. Wojczynski * Centre for Bone and Arthritis Research, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg,
Gothenburg, Sweden Maria Nethander, Dan Mellström & Claes Ohlsson * Bioinformatics Core Facility, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden Maria Nethander *
Department of Psychiatry and Psychotherapy, University Medicine Greifswald, Greifswald, Germany Sahar Ghasemi * Institute for Community Medicine, University Medicine Greifswald, Greifswald,
Germany Sahar Ghasemi & Alexander Teumer * Rush Alzheimer’s Disease Center & Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, USA Jingyun Yang, David
A. Bennett & Aron S. Buchman * Department of Medicine and Public Health, Rey Juan Carlos University, Madrid, Spain Stefan Walter * CIBER of Frailty and Healthy Aging (CIBERFES), Madrid,
Spain Stefan Walter, Leocadio Rodriguéz-Mañas, Francisco J. García & José A. Carnicero * Center for Demography of Health and Aging, University of Wisconsin-Madison, Madison, WI, USA
Kamil Sicinski * School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, UK Erika Kague * Department of Orthopedics, University of Colorado, Aurora, CO, USA
Cheryl L. Ackert-Bicknell * Department of Medicine/Geriatrics, University of Mississippi School of Medicine, Jackson, MS, USA B. Gwen Windham * Human Genetics Center, Department of
Epidemiology, Human Genetics, and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, USA Eric Boerwinkle & Megan L.
Grove * Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX, USA Eric Boerwinkle * Department of Epidemiology, University of North Carolina, Chapel Hill, NC, 27516, USA
Misa Graff * Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin, Germany Dominik Spira & Ilja Demuth * Berlin
Institute of Health, Department of Endocrinology and Metabolism, Berlin, Germany Dominik Spira & Ilja Demuth * Charité - Universitätsmedizin Berlin, BCRT - Berlin Institute of Health
Center for Regenerative Therapies, Berlin, Germany Ilja Demuth * Department of Internal Medicine, Section of Geriatric Medicine, Academic Medical Center, University of Amsterdam, Amsterdam,
The Netherlands Nathalie van der Velde * Wageningen University, Division of Human Nutrition, PO-box 17, 6700 AA, Wageningen, The Netherlands Lisette C. P. G. M. de Groot * Cardiovascular
Health Research Unit, Departments of Medicine, Epidemiology, and Health services, University of Washington, Seattle, WA, USA Bruce M. Psaty * Kaiser Permanente Washington Health Research
Institute, Seattle, WA, USA Bruce M. Psaty * Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA Michelle C. Odden * Department of Epidemiology and
Institute of Public Genetics, University of Washington, Seattle, WA, USA Alison E. Fohner * Geriatric Unit, Azienda Sanitaria Firenze (ASF), Florence, Italy Stefania Bandinelli *
Epidemiology and Biostatistics, Department of Public Health, Faculty of Health Science, University of Southern Denmark, Odense, Denmark Qihua Tan * Geriatric Medicine, Institute of Medicine,
Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden Dan Mellström * Clinical and Molecular Osteoporosis Research Unit, Department of Orthopedics and Clinical Sciences, Lund
University, Skåne University Hospital, Malmö, Sweden Magnus Karlsson * Center for Translational and Systems Neuroimmunology, Department of Neurology, Columbia University Medical Center, New
York, NY, USA Philip L. De Jager * Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA Philip L. De Jager * Interfaculty Institute for Genetics and Functional
Genomics, University Medicine Greifswald, Greifswald, Germany Uwe Völker * Department of Restorative Dentistry, Periodontology, Endodontology, and Preventive and Pediatric Dentistry,
University Medicine Greifswald, Greifswald, Germany Thomas Kocher * Department of Geriatrics, Getafe University Hospital, Getafe, Spain Leocadio Rodriguéz-Mañas * Department of Geriatrics,
Hospital Virgen del Valle, Complejo Hospitalario de Toledo, Toledo, Spain Francisco J. García * Professor of Public Policy, Georgetown University, Washington, DC, USA Pamela Herd *
Sahlgrenska University Hospital, Department of Drug Treatment, Gothenburg, Sweden Claes Ohlsson * Section of General Internal Medicine, Boston University School of Medicine, Boston, MA, USA
Joanne M. Murabito * Center on Aging, University of Connecticut Health, 263 Farmington Avenue, Farmington, CT, 06030, USA George A. Kuchel * National Institute on Aging, Baltimore, MD, USA
Luigi Ferrucci * Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA David Karasik * Azrieli Faculty of Medicine, Bar Ilan University, Safed, Israel David Karasik *
Marcus Institute for Aging Research, Hebrew SeniorLife and Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Broad Institute of MIT & Harvard,
Boston, MA, USA Douglas P. Kiel Authors * Garan Jones View author publications You can also search for this author inPubMed Google Scholar * Katerina Trajanoska View author publications You
can also search for this author inPubMed Google Scholar * Adam J. Santanasto View author publications You can also search for this author inPubMed Google Scholar * Najada Stringa View author
publications You can also search for this author inPubMed Google Scholar * Chia-Ling Kuo View author publications You can also search for this author inPubMed Google Scholar * Janice L.
Atkins View author publications You can also search for this author inPubMed Google Scholar * Joshua R. Lewis View author publications You can also search for this author inPubMed Google
Scholar * ThuyVy Duong View author publications You can also search for this author inPubMed Google Scholar * Shengjun Hong View author publications You can also search for this author
inPubMed Google Scholar * Mary L. Biggs View author publications You can also search for this author inPubMed Google Scholar * Jian’an Luan View author publications You can also search for
this author inPubMed Google Scholar * Chloe Sarnowski View author publications You can also search for this author inPubMed Google Scholar * Kathryn L. Lunetta View author publications You
can also search for this author inPubMed Google Scholar * Toshiko Tanaka View author publications You can also search for this author inPubMed Google Scholar * Mary K. Wojczynski View author
publications You can also search for this author inPubMed Google Scholar * Ryan Cvejkus View author publications You can also search for this author inPubMed Google Scholar * Maria
Nethander View author publications You can also search for this author inPubMed Google Scholar * Sahar Ghasemi View author publications You can also search for this author inPubMed Google
Scholar * Jingyun Yang View author publications You can also search for this author inPubMed Google Scholar * M. Carola Zillikens View author publications You can also search for this author
inPubMed Google Scholar * Stefan Walter View author publications You can also search for this author inPubMed Google Scholar * Kamil Sicinski View author publications You can also search
for this author inPubMed Google Scholar * Erika Kague View author publications You can also search for this author inPubMed Google Scholar * Cheryl L. Ackert-Bicknell View author
publications You can also search for this author inPubMed Google Scholar * Dan E. Arking View author publications You can also search for this author inPubMed Google Scholar * B. Gwen
Windham View author publications You can also search for this author inPubMed Google Scholar * Eric Boerwinkle View author publications You can also search for this author inPubMed Google
Scholar * Megan L. Grove View author publications You can also search for this author inPubMed Google Scholar * Misa Graff View author publications You can also search for this author
inPubMed Google Scholar * Dominik Spira View author publications You can also search for this author inPubMed Google Scholar * Ilja Demuth View author publications You can also search for
this author inPubMed Google Scholar * Nathalie van der Velde View author publications You can also search for this author inPubMed Google Scholar * Lisette C. P. G. M. de Groot View author
publications You can also search for this author inPubMed Google Scholar * Bruce M. Psaty View author publications You can also search for this author inPubMed Google Scholar * Michelle C.
Odden View author publications You can also search for this author inPubMed Google Scholar * Alison E. Fohner View author publications You can also search for this author inPubMed Google
Scholar * Claudia Langenberg View author publications You can also search for this author inPubMed Google Scholar * Nicholas J. Wareham View author publications You can also search for this
author inPubMed Google Scholar * Stefania Bandinelli View author publications You can also search for this author inPubMed Google Scholar * Natasja M. van Schoor View author publications You
can also search for this author inPubMed Google Scholar * Martijn Huisman View author publications You can also search for this author inPubMed Google Scholar * Qihua Tan View author
publications You can also search for this author inPubMed Google Scholar * Joseph Zmuda View author publications You can also search for this author inPubMed Google Scholar * Dan Mellström
View author publications You can also search for this author inPubMed Google Scholar * Magnus Karlsson View author publications You can also search for this author inPubMed Google Scholar *
David A. Bennett View author publications You can also search for this author inPubMed Google Scholar * Aron S. Buchman View author publications You can also search for this author inPubMed
Google Scholar * Philip L. De Jager View author publications You can also search for this author inPubMed Google Scholar * Andre G. Uitterlinden View author publications You can also search
for this author inPubMed Google Scholar * Uwe Völker View author publications You can also search for this author inPubMed Google Scholar * Thomas Kocher View author publications You can
also search for this author inPubMed Google Scholar * Alexander Teumer View author publications You can also search for this author inPubMed Google Scholar * Leocadio Rodriguéz-Mañas View
author publications You can also search for this author inPubMed Google Scholar * Francisco J. García View author publications You can also search for this author inPubMed Google Scholar *
José A. Carnicero View author publications You can also search for this author inPubMed Google Scholar * Pamela Herd View author publications You can also search for this author inPubMed
Google Scholar * Lars Bertram View author publications You can also search for this author inPubMed Google Scholar * Claes Ohlsson View author publications You can also search for this
author inPubMed Google Scholar * Joanne M. Murabito View author publications You can also search for this author inPubMed Google Scholar * David Melzer View author publications You can also
search for this author inPubMed Google Scholar * George A. Kuchel View author publications You can also search for this author inPubMed Google Scholar * Luigi Ferrucci View author
publications You can also search for this author inPubMed Google Scholar * David Karasik View author publications You can also search for this author inPubMed Google Scholar * Fernando
Rivadeneira View author publications You can also search for this author inPubMed Google Scholar * Douglas P. Kiel View author publications You can also search for this author inPubMed
Google Scholar * Luke C. Pilling View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS G.J., K.T., A.J.S., N.S., C-LK., and J.L.A. contributed
equally to this work. G.A.K., L.F., D.K., F.R., D.K.K., and L.C.P. jointly supervised this work. D.E.A., B.G.W., E.B., M.L.G., M.G., I.D., N.v.d.V, L.C.P.G.M.d.G, B.M.P., C.L., N.J.W., S.B.,
N.M.v.S, M.H, J.M.Z., D.M., M.K., D.A.B., A.S.B., P.L.D.J., A.G.U., A.T., L.R.M., F.G.G., J.C., P.H., L.B., C.O., J.M.M, L.F., D.K., F.R., and D.P.K. designed and managed individual
studies. B.G.W., N.M.v.S, M.H, D.A.B., A.S.B., P.L.D.J., L.R.M., F.G.G., J.C., and P.H. collected data. G.J., K.T., A.J.S., N.S, J.R.L, J.M.M, G.A.K, L.F., D.M., D.K., F.R., D.P.K. and
L.C.P. reviewed the analysis plan. G.J., K.T., A.J.S., N.S, C.K., T.V.D., S.H., M.L.B., J.L., C.S., K. L. L., T.T, M.K.W., R.C., M.N., S.G., J.Y., M.C., S.W., K.S., A.T., and L.C.P. analyzed
the data. G.J. and L.C.P. performed the meta-analysis. G.J., C.A-B and L.C.P. performed the pathway and other analyses. J.M.M, G.A.K, L.F., D.M., D.K., F.R., D.P.K., and L.C.P. supervised
the overall study design. G.J., K.T., A.J.S., N.S, C.K., J.L.A., J.M.M, G.A.K, L.F., D.M., D.K., F.R., D.P.K. and L.C.P. wrote the manuscript. G.J., K.T., A.J.S., N.S, C.K., J.L.A., J.R.L,
T.V.D., S.H., M.L.B., J.L., C.S., K. L. L., T.T, M.K.W., R.C., M.N., S.G., J.Y., M.C., S.W., K.S., E.K., C.A-B., D.E.A., B.G.W., E.B., M.L.G., M.G., D.S., I.D., N.v.d.V, L.C.P.G.M.d.G,
B.M.P., M.C.O., A.E.F., C.L., N.J.W., S.B., N.M.v.S, M.H, Q.T., J.M.Z., D.M., M.K., D.A.B., A.S.B., P.L.D.J., A.G.U., U.V., T.K., A.T., L.R.M., F.G.G., J.C., P.H., L.B., C.O., J.M.M, G.A.K,
L.F., D.M., D.K., F.R., D.P.K. and L.C.P. reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Luke C. Pilling. ETHICS DECLARATIONS COMPETING INTERESTS D.P.K. is a consultant for
Solarea Bio, received institutional grants from Amgen and Radius Health, and received royalties for publication Wolters Kluwer. M.C.O. serves as a consultant for Cricket Health, a kidney
care company. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. All other authors declare no competing interests. ADDITIONAL
INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Dominic Furniss and the other, anonymous reviewer for their contribution to the peer review of this work. Peer reviewer
reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1-14 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jones, G., Trajanoska, K., Santanasto, A.J. _et al._
Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. _Nat Commun_ 12, 654 (2021). https://doi.org/10.1038/s41467-021-20918-w Download
citation * Received: 13 May 2020 * Accepted: 22 December 2020 * Published: 28 January 2021 * DOI: https://doi.org/10.1038/s41467-021-20918-w SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative