Play all audios:
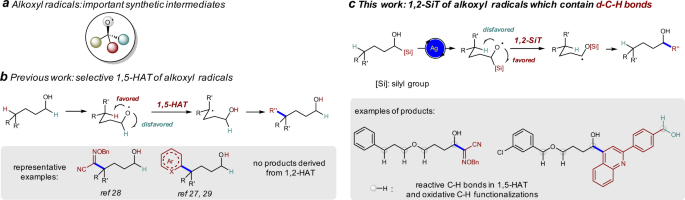
ABSTRACT Controlling the reactivity of reactive intermediates is essential to achieve selective transformations. Due to the facile 1,5-hydrogen atom transfer (HAT), alkoxyl radicals have
been proven to be important synthetic intermediates for the δ-functionalization of alcohols. Herein, we disclose a strategy to inhibit 1,5-HAT by introducing a silyl group into the
α-position of alkoxyl radicals. The efficient radical 1,2-silyl transfer (SiT) allows us to make various α-functionalized products from alcohol substrates. Compared with the direct
generation of α-carbon radicals from oxidation of α-C-H bond of alcohols, the 1,2-SiT strategy distinguishes itself by the generation of alkoxyl radicals, the tolerance of many functional
groups, such as intramolecular hydroxyl groups and C-H bonds next to oxygen atoms, and the use of silyl alcohols as limiting reagents. SIMILAR CONTENT BEING VIEWED BY OTHERS ALKENE
1,1-DIFUNCTIONALIZATIONS VIA ORGANOMETALLIC-RADICAL RELAY Article 28 September 2023 ENANTIOSELECTIVE RADICAL C–H AMINATION FOR THE SYNTHESIS OF Β-AMINO ALCOHOLS Article 22 June 2020
STEREOSPECIFIC _SYN_-DIHALOGENATIONS AND REGIODIVERGENT _SYN_-INTERHALOGENATION OF ALKENES VIA VICINAL DOUBLE ELECTROPHILIC ACTIVATION STRATEGY Article Open access 02 May 2024 INTRODUCTION
Radicals, anions, cations, carbenes, and others are key reactive intermediates in synthesis1. These intermediates usually show different reactivity, facilitating the development of
complementary methodologies for the synthesis of molecules that are important in material and/or life-related field2,3,4. Among various radicals, alkoxyl radicals have gained increasing
attention (Fig. 1a)5,6,7,8,9. Although the previous studies to generate alkoxyl radicals usually need pre-activated alcohols or corresponding precursors10,11,12,13,14,15,16,17,18,19,20,21,
recent work on the direct activation of alcohols with photocatalysis and/or transition-metal catalysis largely broaden their synthetic utility22,23,24,25,26,27,28,29. When δ-C–H bonds are
present, the intramolecular 1,5-hydrogen atom transfer (HAT) from the δ-position via a low-energy six-membered ring transition state is usually favored over the transfer of hydrogen atoms at
other positions, thus alkoxyl radical-mediated δ-C–H functionalization is widely studied (Fig. 1b)6. For example, the synthesis of δ-alkoxylimino alcohols and intermolecular
δ-heteroarylation of alcohols through 1,5-HAT of alkoxyl radicals have been achieved (Fig. 1b)27,28,29. However, alkoxyl radical-mediated α-functionalization of alcohols have not been
reported30,31. It is also known that excess amount of alcohols are usually required in oxidative C–H functionalization reactions, and it is challenging to control the selectivity when
multiple oxidizable C–H bonds are present in the substrate30,31. Silicon possesses empty _d_ orbitals and C–Si bond is longer than C–H bond. We envisioned that 1,2-silyl transfer (SiT) of
alkoxyl radical via three-membered ring transition state (also known as radical Brook rearrangement, RBR) might be easier than the corresponding 1,2-HAT and might be favored over 1,5-HAT
process (Fig. 1c). RBR was initially proposed to explain the cyclopropanation product of the photoreaction between acylsilanes and electron-poor olefins in 198132,33,34,35. However, the
synthetic application of RBR was nearly ignored in the following decades35,36,37,38. Until 2017, Smith and group found that benzylic radicals can be generated from the oxidation of
hypervalent silicate intermediate by a photo-excited Ir complex37. In 2020, our group revealed that the Mn-catalyzed RBR is superior to anion Brook rearrangement in the direct
trifluoroethanol transfer reactions38. Herein, we show that radical 1,2-SiT is favored over 1,5-HAT under Ag-catalysis conditions, and selective α-C–C bond formation reactions are achieved
without any δ-functionalization product, in which the use of alcohols as limiting reagents in the reaction of oxime ethers and the tolerance of various C–H bonds and benzyl alcohols
demonstrate the synthetic potential of our methodology (Fig. 1c)30,31. RESULTS RADICAL 1,2-SIT IN THE SYNTHESIS OF Α-HYDROXYL OXIME ETHERS Oximes and oxime ethers are important synthetic
building blocks, and they have also been found to be core structural motifs of multiple bioactive molecules28,39,40,41. In 2018, Jiao and co-workers reported the synthesis of δ-alkoxylimino
alcohols through 1,5-HAT of alkoxyl radicals, but no α-alkoxylimino alcohols were isolated28. Previous methods to prepare α-hydroxyl oxime ethers mainly rely on the reduction of
alkoxyliminyl substituted ketones, which themselves need multistep synthesis41. To the best of our knowledge, there is no report on radical-mediated synthesis of α-alkoxylimino alcohols.
Therefore, we choose to investigate the reaction between α-silyl alcohol 1A and sulfonyl oxime ether 2 to check whether α-functionalization product or δ-functionalization product can be
obtained. OPTIMIZATION OF THE REACTION CONDITIONS FOR THE SYNTHESIS OF Α-HYDROXYL OXIME ETHERS Previously, we found the Mn(II)/Mn(III)-catalyzed metal alkoxide (M-OR) homo-cleavage strategy
was an efficient way to generate alkoxyl radicals for the direct transfer of trifluoroethanol and difluoroethanol units38. Therefore, we focused on the investigation of various
transition-metal salts for M-OR homo-cleavage. After extensive investigations, we found that AgNO3 was a better pre-catalyst than CuCl2, FeCl3, NiBr2, Mn(OAc)3, and AgI (Fig. 2, entries
1–6). When the reaction was carried out in MeCN/H2O (v/v = 1:1) at 80 °C for 12 h with K2S2O8 as an oxidant, a yield of 47% was afforded for compound 3A, without any detection of
δ-functionalization product (entry 6). When the solvent was changed to acetone/H2O (v/v = 1:1), the yield of α-functionalization product 3A increased to 51% (entry 7). Increasing the
concentration of the reaction resulted in an improved yield of compound 3A (71%, entry 8). Other oxidants such as Na2S2O8, (NH4)2S2O8, Dess–Martin periodinane, and _tert_-butyl
peroxybenzoate afforded lower efficiency of the reaction (entries 9–12). Lowering the reaction temperature also resulted in a decreased yield of compound 3A (entries 13 and 14). The control
experiment showed that, without AgNO3, only 20% yield of compound 3A was observed by proton nuclear magnetic resonance, although a large amount of decomposition of compounds 1A and 2 (entry
15) were found. However, no 3A was generated without K2S2O8, and the conversions of compounds 1A and 2 were also low, indicating that AgNO3 alone cannot catalyze the reaction (entry 16).
INFLUENCE OF SILYL GROUPS ON THE EFFICIENCY OF THE REACTION Encouraged by the favored α-functionalization over δ-functionalization in the reaction between compounds 1A and 2, we then
investigated the influence of the silyl substituent on the efficiency of the desired α-functionalization reaction. It was found that both electronic property and steric hindrance of the
silyl group showed a significant effect on our reaction. The electron-withdrawing effect of the phenyl group on the silicon atom appears to play a positive role in this reaction (Fig. 3).
However, the steric hindrance on the silicon atom shows a negative effect in this reaction (1A vs 1AD and 1AE; 1AB vs 1AC). In all cases, aldehyde derived from compound 1 was formed as
by-product. The substituents might not only affect the transfer ability of the silyl group but also affect the stability and reactivity of the radical intermediate III (see below). Moreover,
the different substituents of the silyl groups also affect the C–Si bond length and bond dissociation energy, which might also be important factors in 1,2-SiT. Again, none of the reactions
afforded δ-functionalization product. MECHANISM STUDY After identification of a suitable silyl group to promote the efficient synthesis of α-alkoxylimino alcohol 3A, we set to investigate
whether the reaction proceeded through radical 1,2-SiT or not. Firstly, our study reveals that the OH group is important for the success of the reaction. The use of compound 1A-1 as starting
material resulted in no anticipated product 3A-1 (Fig. 4a). Protected α-silyl alcohol 1A-2 only gave 5% yield of compound 3A-2 (Fig. 4b), indicating that the generation of carbon radical
via direct oxidative cleavage of C–Si bond is less likely to be the major pathway in the reaction with 1A42,43,44,45. This result was consistent to the similar oxidation potential of α-silyl
alcohol and the methyl-protected counterpart46. When silyl ether compound 1A-3 was applied in the reaction with compound 2, free diol 3A-3 was obtained in 44% yield (Fig. 4c), suggesting
that silyl ether can be hydrolyzed under aqueous reaction condition. Further study of the reaction of triphenylsilyl-substituted alcohol 1A-4 with compound 2 under no H2O condition showed
that compound 3A-4 could be synthesized in 10% yield with 2% yield of desilylated compound 3A (Fig. 4d). The lower yield of 3A-4 and 3A might be explained by the low solubility under the
non-aqueous conditions (Fig. 4d). Jiao and co-workers have shown that alcohols can participate in δ-functionalization via radical 1,5-HAT under Ag catalysis28. The reaction of non-silylated
alcohol 1A-5 indeed afforded 1,5-HAT product 3A-5 in 40% yield without the formation of ɑ-functionalization product 3A (Fig. 4e). Interestingly, when a silyl alcohol 1A-6, which contains
another C–OH bond, was tested in the reaction with compound 2, the major product is C–Si bond functionalization product 3A-3 (38% yield; Fig. 4f), further indicating radical 1,2-SiT is
favored over 1,5-HAT. The tolerance of free alcohol is an advantage of our method, since diols are challenging substrates for the oxidative C–H bond functionalization chemistry30,31.
Subsequently, we investigated the energy barrier of 1,2-SiT and 1,5-HAT of alkoxyl radical intermediate A using density functional theory (DFT) calculation employing the method M06-2X (for
details, see the Supplementary information 3i–n)47,48,49. As shown in Fig. 5, alkoxyl radical A was set as the starting point for the free-energy profiles. 1,2-SiT via transition state B-TS
to generate radical intermediate D is quite easy with an energy barrier of only 1.3 kcal/mol, and this process is exothermic (26.6 kcal/mol). However, 1,5-HAT via transition state C-ts to
generate radical intermediate E is an endothermic reaction (4.9 kcal/mol) with an energy barrier of 14.3 kcal/mol. The calculation results show that 1,2-SiT process of radical A is both
dynamically and thermodynamically favored over the corresponding 1,5-HAT. Based on our experimental DFT calculation results and previous reports28, a plausible mechanism involving 1,2-SiT
was proposed in Fig. 6. Oxidation of Ag(I) by K2S2O8 might afford Ag(II)50, which would undergo ligand exchange with alcohol 1A and results in the generation of intermediate I. Homolysis of
intermediate I could produce alkoxyl radical II and Ag(I). Carbon radical III would be generated through 1,2-SiT, which is favored over 1,5-HAT. Intermediate IV might be generated from the
addition–elimination process between carbon radical III and compound 2, but we cannot rule out the possibility of its formation through trapping III with iminyl radical generated from
homolysis of compound 2 (for details, see Supplementary information). PhSO2 radical might be converted to PhSO3H under the oxidation condition in the aqueous solution15. PhSO3− was detected
by high-resolution mass spectrometry analysis of the reaction mixture, which supports this proposal (for details, see Supplementary information). The alcohol product 3 would be produced
after desilylation under aqueous condition. SCOPE OF THE REACTION BETWEEN Α-SILYL ALCOHOL 1A AND SULFONYL OXIME ETHER 2 Subsequently, we investigated the scope of the radical substitution
reaction between α-silyl alcohol 1 and sulfonyl oxime ether 2. The reaction showed broad substrate scope, and various α-silyl alcohols could participate in the reaction, affording
corresponding α-alkoxylimino alcohols in 48–70% yields. When 1 g of 1A was employed, product 3A could be isolated in 60% yield. The reaction can tolerate many functional groups, such as
C(sp3)-Br, C(sp3)-N3, C(sp2)-F, C(sp2)-Cl, C(sp2)-Br, C(sp2)-CN, C(sp2)-OCF3, and an ester group. These functional groups can be used for further transformations. When the δ-C–H bond is next
to an oxygen atom, the 1,5-HAT of the alkoxyl radicals could be more favored, because the new radical can be stabilized by the oxygen through hyper-conjugation interaction30,31. However,
under our reaction conditions, not only the normal δ-C–H bond can be tolerated, but the δ-C–H bond next to an oxygen atom can also be tolerated (Fig. 7, 3G, 3I–3O, 3S). Moreover, benzylic,
α-oxy, and α-benzoyloxy C–H groups, which are usually reactive in oxidative C–H bond cleavage reactions, are maintained under our reaction conditions (Fig. 7, 3G–3X)30,31. In addition, our
reaction can be applied in the synthesis of alcohols, which contain a β-substituent. Compound 3Y was synthesized in 65% yield, which is in sharp contrast to the failure to synthesize
alcohols in previous Ag-catalyzed reaction28. The relative lower yield was found for the assembly of tertiary alcohol 3Z (20% yield), 3AA (31% yield), and 3AB (41% yield). In all cases, the
alcohols 3 were obtained directly after the reaction, without the extra deprotection step of anticipated silyl ether intermediate IV. In all cases, the alcohol substrates were used as
limiting reagents, which further highlight the synthetic potential of the current method since the oxidative α-C–H functionalization of alcohols usually need excess amount of alcohols, and
in many cases, alcohols must be used as a solvent to achieve synthetic useful yield30,31. SYNTHETIC TRANSFORMATIONS OF COMPOUND 3A Compound 3A was easily transformed to methylated product
1A-2 in 57% yield, while the imine group was untouched (Fig. 8). The CN group could be hydrolyzed in the presence of H2O2 and K2CO3, affording amide 3A-8 in 72% yield (Fig. 8). RADICAL
1,2-SIT IN THE CATALYTIC MINISCI REACTION FOR THE SYNTHESIS OF Α-HETEROARYL ALCOHOLS Heteroaryl groups are important structural motifs and Minisci reaction is one of the most atom-economic
ways to introduce heteroaryl groups into organic molecules, by cleaving the C(sp2)–H bond51,52. The 1,5-HAT process of alkoxyl radicals was used by Zhu’s group and Chen’s group in the
hypervalent iodine-mediated radical Minisci reaction and various δ-heteroaryl-substituted alcohols have been made27,29. To the best of our knowledge, there has been no report on Minisci type
α-heteroarylation through 1,2-HAT of alkoxyl radicals. Although direct radical α-heteroarylation of alcohols was achieved by the intermolecular H abstraction, they usually need excess
amount of alcohols as reagents31,51,52. In most cases, alcohols are used as the solvent, which limits the application of those methods, especially when complex alcohols are needed and/or the
alcohols are solids. Encouraged by the success of the application of radical 1,2-SiT in the direct synthesis of alkoxylimino alcohols, we probed the applicability of this strategy in the
catalytic Minisci reaction for the synthesis of secondary alkyl heteroaryl alcohols. After a quick optimization of reaction conditions (for details, see Supplementary information), we found
that similar Ag-catalysis conditions could be applied in the reactions between α-silyl alcohol 1 and heteroarenes 4 (Fig. 9). Quinolines with methyl and aryl substituents are competent
reaction partners, delivering the desired α-heteroarylation products 5A–K in 53–79% yields. The F, Cl, Br, CN, OMe, and Me groups on the aryl substituents are tolerated. Isoquinoline
derivatives such as Cl-, Br-, MeO-, and BnO-substituted isoquinolines performed well, affording products 5M–R in 53–74% yields. An α-silyl alcohol containing a long alkyl chain also works,
affording compound 5R in 64% yield and 5T in 60% yield. Moreover, phenanthridine can participate in the reaction, giving corresponding alkyl heteroaryl alcohol 5S in 51% yield. One of the
disadvantages of the previous direct α-heteroarylation of alcohol is the need to use excess amount of alcohol, which is formidable when the complex is applied. We found that only two
equivalent of α-silyl alcohol was required in the current radical Minisci reaction, and the relatively complex alcohols 5V–Z were prepared in 50–62% yields. It is worth noting that even
benzyl alcohol can be tolerated (5AA, 50%; 5AB, 51%). These two compounds would be challenging to synthesize via the oxidative C–H bond functionalization methodology because the benzyl
alcohol would result in trouble30,31. Moreover, the synthesis of alcohols, which contain a β-substituent, was successful and compound 5AC was synthesized in 62% yield, which is in sharp
contrast to the failure to synthesize alcohols in previous Ag-catalyzed reaction28. Again, no δ-heteroarylation products were isolated in all cases shown in Fig. 9. DISCUSSION In this work,
we found that the introduction of a silyl group to the α-position of alcohols can effectively inhibit 1,5-HAT of the corresponding alkoxyl radicals. The substituents on the silicon are found
to be important to achieve efficient 1,2-SiT. The carbon radicals derived from 1,2-SiT are applied in the radical substitution reactions of sulfonyl oxime ether and heteroarenes to prepare
α-alkoxylimino alcohols and alkyl heteroaryl alcohols. Compared with the direct generation of α-carbon radicals from the oxidation of α-C–H bond of alcohols, the 1,2-SiT strategy
distinguished itself by the generation of alkoxyl radicals, the tolerance of many functional groups such as intramolecular hydroxyl groups and C–H bonds next to oxygen atoms, and the use of
silyl alcohols as limiting reagents. Our experimental finding broadens the synthetic application of alkoxyl radicals. Further application of the 1,2-SiT of alkoxyl radicals is underway in
our laboratory. METHODS TYPICAL PROCEDURE 1 (3A) In an Ar-protected glove box, AgNO3 (6.8 mg, 0.04 mmol, 20 mol%), 2 (90.0 mg, 0.30 mmol, 1.5 equiv.), and 1A (50.0 mg, 0.20 mmol) were added
into a reaction tube. After that, the tube was taken out of the box, acetone/H2O (0.75 mL/0.75 mL) and K2S2O8 (108.0 mg, 0.40 mmol, 2.0 equiv.) were added under N2. The tube was then sealed,
and the resulting mixture was kept stirring at 80 °C in a heating block for 12 h. The reaction mixture was quenched with water (5 mL), extracted with ethyl acetate (3 × 10 mL), and the
organic phase was combined and washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified with column chromatography on silica
gel (200–300 mesh) with petroleum ether/ethyl acetate (PE/EA) (8/1, v/v) as eluent to afford 38.0 mg of the title compound as a faint yellow oil (69% yield). TYPICAL PROCEDURE 2 (5A) Under
N2 atmosphere, AgNO3 (6.8 mg, 0.04 mmol, 20 mol%), CH3CN/H2O (1.67 mL/0.33 mL), 4A (28.6 mg, 0.20 mmol), CF3COOH (22.8 mg, 0.2 mmol, 1.0 equiv.), 1A (100 mg, 0.40 mmol, 2.0 equiv.), and
K2S2O8 (118.8 mg, 0.44 mmol, 2.2 equiv.) were added into a reaction tube. The tube was then sealed, and the resulting mixture was kept stirring at 80 °C in a heating block for 12 h. The
reaction mixture was quenched with saturated NaHCO3 aqueous solution (10 mL), extracted with ethyl acetate (3 × 10 mL), and the organic phase was combined and washed with brine, dried over
anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified with column chromatography on silica gel (200–300 mesh) with PE/EA (10/1, v/v) as eluent to afford
27.0 mg of the title compound as a faint yellow oil (53% yield). DATA AVAILABILITY The authors declare that all other data supporting the findings of this study are available within the
article and Supplementary information files, and also are available from the corresponding author on reasonable request. REFERENCES * Smith, M. B. _March’s Advanced Organic Chemistry:
Reactions, Mechanisms, and Structure_ 7th edn (Wiley, 2013). * Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes.
_ACS Cent. Sci._ 3, 692–700 (2017). Article CAS PubMed PubMed Central Google Scholar * Lo, J. C. et al. Fe-catalyzed C-C bond construction from olefins via radicals. _J. Am. Chem. Soc._
139, 2484–2503 (2017). Article CAS PubMed PubMed Central Google Scholar * Yi, H. et al. Recent advances in radical C-H activation/radical cross-coupling. _Chem. Rev._ 117, 9016–9085
(2017). Article CAS PubMed Google Scholar * Renaud, P. & Sibi, M. P. (eds). _Radicals in Organic Synthesis_ (Wiley-VCH, 2001). * Čeković, Ž. Reactions of δ-carbon radicals generated
by 1,5-hydrogen transfer to alkoxyl radicals. _Tetrahedron_ 59, 8073–8090 (2003). Article CAS Google Scholar * Jia, K. & Chen, Y. Visible-light-induced alkoxyl radical generation for
inert chemical bond cleavage/functionalization. _Chem. Commun._ 54, 6105–6112 (2018). Article CAS Google Scholar * Guo, J.-J., Hu, A. & Zuo, Z. Photocatalytic alkoxy radical-mediated
transformations. _Tetrahedron Lett._ 59, 2103–2111 (2018). Article CAS Google Scholar * Stateman, L. M., Nakafuku, K. M. & Nagib, D. A. Remote C-H functionalization via selective
hydrogen atom transfer. _Synthesis_ 50, 1569–1586 (2018). Article CAS PubMed PubMed Central Google Scholar * Barton, D. H. R., Beaton, J. M., Geller, L. E. & Pechet, M. M. A new
photochemical reaction. _J. Am. Chem. Soc._ 82, 2640–2641 (1960). Article CAS Google Scholar * Čekovió, Ž., Dimttruević, L., Djokić, G. & Srnić, T. Remote functionalisation by ferrous
ion-cupric ion induced decomposition of alkyl hydroperoxides. _Tetrahedron_ 35, 2021–2026 (1979). Article Google Scholar * Kundu, R. & Ball, Z. T. Copper-catalyzed remote sp3 C-H
chlorination of alkyl hydroperoxides. _Org. Lett._ 12, 2460–2463 (2010). Article CAS PubMed Google Scholar * Petrović, G. & Čeković, Ž. Alkylation of remote non-activated δ-carbon
atoms: addition of δ-carbon radicals, generated by 1,5-hydrogen transfer in alkoxy radical intermediates, to activated olefins. _Tetrahedron_ 55, 1377–1390 (1999). Article Google Scholar *
Heusler, K. & Kalvoda, J. Intramolecular free-radical reactions. _Angew. Chem. Int. Ed._ 3, 525–538 (1964). Article Google Scholar * Walling, C. R. & Positive, A. Padwa Halogen
compounds. VII. Intramolecular chlorinations with long chain hypochlorites. _J. Am. Chem. Soc._ 85, 1597–1601 (1963). Article CAS Google Scholar * Concepción, J. I., Francisco, C. G.,
Hernández, R., Salazar, J. A. & Suárez, E. Intramolecular hydrogen abstraction. iodosobenzene diacetate, an efficient and convenient reagent for alkoxy radical generation. _Tetrahedron
Lett._ 25, 1953–1956 (1984). Article Google Scholar * Beckwith, A. L. J. & Hay, B. P. Generation of alkoxy radicals from TV-alkoxypyridinethiones. _J. Am. Chem. Soc._ 110, 4415–4416
(1988). Article CAS Google Scholar * Kim, S., Lee, T. A. & Song, Y. Facile generation of alkoxy radicals from N-alkoxyphthalimides. _Synlett_ 1998, 471–472 (1998). Article Google
Scholar * Zhang, J., Li, Y., Zhang, F., Hu, C. & Chen, Y. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)-H functionalization under mild reaction
conditions. _Angew. Chem. Int. Ed._ 55, 1872–1875 (2016). Article CAS Google Scholar * Wang, C., Harms, K. & Meggers, E. Catalytic asymmetric Csp3-H functionalization under photoredox
conditions by radical translocation and stereocontrolled alkene addition. _Angew. Chem. Int. Ed._ 55, 13495–13498 (2016). Article CAS Google Scholar * Zhang, J. et al.
Visible-light-induced alkoxyl radicals enable alpha-C(sp3)-H bond allylation. _iScience_ 23, 100755 (2020). Article ADS CAS PubMed Google Scholar * Yayla, H. G., Wang, H., Tarantino, K.
T., Orbe, H. S. & Knowles, R. R. Catalytic ring-opening of cyclic alcohols enabled by PCET activation of strong O-H bonds. _J. Am. Chem. Soc._ 138, 10794–10797 (2016). Article CAS
PubMed PubMed Central Google Scholar * Zhao, K. et al. Catalytic ring expansions of cyclic alcohols enabled by proton-coupled electron transfer. _J. Am. Chem. Soc._ 141, 8752–8757 (2019).
Article CAS PubMed PubMed Central Google Scholar * Jia, K., Zhang, F., Huang, H. & Chen, Y. Visible-light-induced alkoxyl radical generation enables selective C(sp3)-C(sp3) bond
cleavage and functionalizations. _J. Am. Chem. Soc._ 138, 1514–1517 (2016). Article CAS PubMed Google Scholar * Hu, A., Guo, J. J., Pan, H. & Zuo, Z. Selective functionalization of
methane, ethane, and higher alkanes by cerium photocatalysis. _Science_ 361, 668–672 (2018). Article ADS CAS PubMed Google Scholar * Wu, X. et al. Tertiary-alcohol-directed
functionalization of remote C(sp3)-H bonds by sequential hydrogen atom and heteroaryl migrations. _Angew. Chem. Int. Ed._ 57, 1640–1644 (2018). Article CAS Google Scholar * Wu, X. et al.
Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)-H bonds. _Nat. Commun._ 9, 3343 (2018). Article ADS PubMed CAS PubMed Central Google Scholar *
Zhu, Y. et al. Silver-catalyzed remote Csp3-H functionalization of aliphatic alcohols. _Nat. Commun._ 9, 2625 (2018). Article ADS PubMed CAS PubMed Central Google Scholar * Li, G. X.,
Hu, X., He, G. & Chen, G. Photoredox-mediated remote C(sp3)-H heteroarylation of free alcohols. _Chem. Sci._ 10, 688–693 (2019). Article CAS PubMed Google Scholar * Guo, S.-r, Kumar,
P. S. & Yang, M. Recent advances of oxidative radical cross-coupling reactions: direct α-C(sp3)-H bond functionalization of ethers and alcohols. _Adv. Syn. Catal._ 359, 2–25 (2017).
Article CAS Google Scholar * Niu, L., Liu, J., Liang, X. A., Wang, S. & Lei, A. Visible light-induced direct alpha C-H functionalization of alcohols. _Nat. Commun._ 10, 467 (2019).
Article ADS PubMed CAS PubMed Central Google Scholar * Brook, A. G. Isomerism of some α-hydroxysilanes to silyl ethers. _J. Am. Chem. Soc._ 80, 1886–1889 (1958). Article CAS Google
Scholar * Moser, W. H. The Brook rearrangement in tandem bond formation strategies. _Tetrahedron_ 57, 2065–2084 (2001). Article CAS Google Scholar * Smith, A. B. III & Wuest, W. M.
Evolution of multi-component anion relay chemistry (ARC): construction of architecturally complex natural and unnatural products. _Chem. Commun_. 2008, 5883–5895 (2008). * Dalton, J. C.
& Bourque, R. A. Mechanistic photochemistry of acylsilanes. 2. Reaction with electron-poor olefins. _J. Am. Chem. Soc._ 103, 699–700 (1981). Article CAS Google Scholar * Huang, C. H.,
Chang, S. Y., Wang, N. S. & Tsai, Y. M. The application of intramolecular radical cyclizations of acylsilanes in the regiospecific formation of cyclic silyl enol ethers. _J. Org. Chem._
66, 8983–8991 (2001). Article CAS PubMed Google Scholar * Deng, Y., Liu, Q. & Smith, A. B. III Oxidative [1,2]-Brook rearrangements exploiting single-electron transfer:
photoredox-catalyzed alkylations and arylations. _J. Am. Chem. Soc._ 139, 9487–9490 (2017). Article CAS PubMed PubMed Central Google Scholar * Chen, X. et al. Direct transfer of tri-
and di-fluoroethanol units enabled by radical activation of organosilicon reagents. _Nat. Commun._ 11, 2756 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Kobayashi, S.
& Ishitani, H. Catalytic enantioselective addition to imines. _Chem. Rev._ 99, 1069–1094 (1999). Article CAS PubMed Google Scholar * Gaspar, B. & Carreira, E. M. Cobalt
catalyzed functionalization of unactivated alkenes: regioselective reductive C-C bond forming reactions. _J. Am. Chem. Soc._ 131, 13214–13215 (2009). Article CAS PubMed Google Scholar *
Mo, K. et al. Chemo‐ and stereoselective reduction of β‐keto‐α‐oximino nitriles by using Baker’s yeast. _Eur. J. Org. Chem_. 2015, 1137–1143 (2015). * Gutenberger, G., Steckhan, E. &
Blechert, S. α-Silyl ethers as hydroxymethyl anion equivalents in photoinduced radical electron transfer additions. _Angew. Chem. Int. Ed._ 37, 660–662 (1998). Article CAS Google Scholar
* Jackl, M. K., Legnani, L., Morandi, B. & Bode, J. W. Continuous flow synthesis of morpholines and oxazepanes with silicon amine protocol (slap) reagents and lewis acid facilitated
photoredox catalysis. _Org. Lett._ 19, 4696–4699 (2017). Article CAS PubMed Google Scholar * Khatun, N., Kim, M. J. & Woo, S. K. Visible-light photoredox-catalyzed
hydroalkoxymethylation of activated alkenes using α-silyl ethers as alkoxymethyl radical equivalents. _Org. Lett._ 20, 6239–6243 (2018). Article CAS PubMed Google Scholar * Nam, S. B.,
Khatun, N., Kang, Y. W., Park, B. Y. & Woo, S. K. Controllable one-pot synthesis for scaffold diversity via visiblelight photoredox-catalyzed giese reaction and further transformation.
_Chem. Commun._ 56, 2873–2876 (2020). Article CAS Google Scholar * Yoshida, J., Maekawa, T., Murata, T., Matsunaga, S. & Isoe, S. The origin of β-silicon effect in electron-transfer
reactions of silicon-substituted heteroatom compounds. electrochemical and theoretical studies. _J. Am. Chem. Soc._ 112, 1962–1970 (1990). Article CAS Google Scholar * Short, M. A.,
Shehata, M. F., Sanders, M. A. & Roizen, J. L. Sulfamides direct radical-mediated chlorination of aliphatic C–H bonds. _Chem. Sci._ 11, 217 (2020). Article CAS Google Scholar * Houk,
K. N. et al. Distortion-controlled reactivity and molecular dynamics of dehydro-Diels–Alder reactions. _J. Am. Chem. Soc._ 138, 8247–8252 (2016). Article PubMed CAS Google Scholar * Xu,
X. F. & Truhlar, D. G. Accuracy of effective core potentials and basis sets for density functional calculations, including relativistic effects, as illustrated by calculations on arsenic
compounds. _J. Chem. Theory Comput._ 7, 2766–2779 (2011). Article CAS PubMed Google Scholar * Walling, C. & Camaioni, D. M. Role of silver(II) in silver-catalyzed oxidations by
peroxydisulfate. _J. Org. Chem._ 43, 3266–3271 (1978). Article CAS Google Scholar * Duncton, M. A. J. Minisci reactions: versatile CH-functionalizations for medicinal chemists.
_MedChemComm_ 2, 1135 (2011). Article CAS Google Scholar * Proctor, R. S. J. & Phipps, R. J. Recent advances in Minisci-type reactions. _Angew. Chem. Int. Ed._ 58, 13666–13699 (2019).
Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to NSFC (21901191, 21822303), Fundamental Research Funds for the Central Universities (2042018kf0023,
2042019kf0006), State Key Laboratory of Bioorganic & Natural Products Chemistry (BNPC18237), and Wuhan University for financial support. We are thankful to Prof. Aiwen Lei and Prof. Xumu
Zhang at Wuhan University for the generous provision of the basic instruments. AUTHOR INFORMATION Author notes * These authors contributed equally: Zhaoliang Yang, Yunhong Niu. AUTHORS AND
AFFILIATIONS * The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds and Materials, Ministry of Education, Wuhan University, Wuhan, People’s Republic of
China Zhaoliang Yang, Yunhong Niu, Suo Chen, Shanshan Liu, Zhengyu Li, Xiang Chen, Yunxiao Zhang & Xiao Shen * School of Chemistry and Chemical Engineering, Chongqing Key Laboratory of
Theoretical and Computational Chemistry, Chongqing University, Chongqing, People’s Republic of China Xiaoqian He & Yu Lan * College of Chemistry and Molecular Engineering, Zhengzhou
University, Zhengzhou, People’s Republic of China Yu Lan Authors * Zhaoliang Yang View author publications You can also search for this author inPubMed Google Scholar * Yunhong Niu View
author publications You can also search for this author inPubMed Google Scholar * Xiaoqian He View author publications You can also search for this author inPubMed Google Scholar * Suo Chen
View author publications You can also search for this author inPubMed Google Scholar * Shanshan Liu View author publications You can also search for this author inPubMed Google Scholar *
Zhengyu Li View author publications You can also search for this author inPubMed Google Scholar * Xiang Chen View author publications You can also search for this author inPubMed Google
Scholar * Yunxiao Zhang View author publications You can also search for this author inPubMed Google Scholar * Yu Lan View author publications You can also search for this author inPubMed
Google Scholar * Xiao Shen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.S. designed and directed the investigations and composed the
manuscript with revisions provided by the other authors. Z.Y. and Y.N. developed the catalytic method. Z.Y., Y.N., and S.C. studied the substrate scope. X.H. and Y.L. conducted the
calculations. Z.Y., Y.N., X.H., S.C., S.L., Z.L., X.C., Y.Z., Y.L., and X.S. were involved in the analysis of results and discussions of the project. CORRESPONDING AUTHORS Correspondence to
Yu Lan or Xiao Shen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the
anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, Z., Niu, Y., He, X. _et al._ Tuning the
reactivity of alkoxyl radicals from 1,5-hydrogen atom transfer to 1,2-silyl transfer. _Nat Commun_ 12, 2131 (2021). https://doi.org/10.1038/s41467-021-22382-y Download citation * Received:
26 October 2020 * Accepted: 04 March 2021 * Published: 09 April 2021 * DOI: https://doi.org/10.1038/s41467-021-22382-y SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative