Play all audios:
ABSTRACT DNA ligase I (LIG1) catalyzes the ligation of the nick repair intermediate after gap filling by DNA polymerase (pol) β during downstream steps of the base excision repair (BER)
pathway. However, how LIG1 discriminates against the mutagenic 3′-mismatches incorporated by polβ at atomic resolution remains undefined. Here, we determine the X-ray structures of LIG1/nick
DNA complexes with G:T and A:C mismatches and uncover the ligase strategies that favor or deter the ligation of base substitution errors. Our structures reveal that the LIG1 active site can
accommodate a G:T mismatch in the wobble conformation, where an adenylate (AMP) is transferred to the 5′-phosphate of a nick (DNA-AMP), while it stays in the LIG1-AMP intermediate during
the initial step of the ligation reaction in the presence of an A:C mismatch at the 3′-strand. Moreover, we show mutagenic ligation and aberrant nick sealing of dG:T and dA:C mismatches,
respectively. Finally, we demonstrate that AP-endonuclease 1 (APE1), as a compensatory proofreading enzyme, removes the mismatched bases and interacts with LIG1 at the final BER steps. Our
overall findings provide the features of accurate versus mutagenic outcomes coordinated by a multiprotein complex including polβ, LIG1, and APE1 to maintain efficient repair. SIMILAR CONTENT
BEING VIEWED BY OTHERS LESION RECOGNITION BY XPC, TFIIH AND XPA IN DNA EXCISION REPAIR Article 19 April 2023 MISPAIR-BOUND HUMAN MUTS–MUTL COMPLEX TRIGGERS DNA INCISIONS AND ACTIVATES
MISMATCH REPAIR Article Open access 28 January 2021 HUMAN POLYMERASE Θ HELICASE POSITIONS DNA MICROHOMOLOGIES FOR DOUBLE-STRAND BREAK REPAIR Article 28 February 2025 INTRODUCTION Human DNA
ligases catalyze phosphodiester bond formation between 5′-phosphate (P) and 3′-hydroxyl (OH) termini on the ends of broken DNA strands using a high-energy cofactor ATP and Mg2+ in three
chemical and sequential steps: (i) nucleophilic attack on ATP by the ligase and formation of a covalent intermediate in which an adenylate (AMP) is linked to an active site lysine (LIG-AMP),
(ii) transfer of the AMP to the 5′-phosphate-terminated DNA strand to form a DNA-AMP intermediate, and (iii) ligase-catalyzed attack by 3′-OH of the nick on DNA-AMP to join adjacent 3′-OH
and 5′-P ends and liberate AMP1,2,3,4,5. The accuracy of the nick sealing reaction relies on the formation of a Watson–Crick base pair between the 3′-OH and 5′-P ends that requires high
fidelity DNA synthesis by DNA polymerase6. Mismatched nucleotides inserted by DNA polymerases can cause base substitutions, and in the case of no proofreading, this could lead to genomic
instability and human diseases7,8,9. In the presence of 3′-damaged or modified ends, DNA ligases can fail, resulting in the formation of 5′-adenylated-DNA (5′-AMP), also referred to as an
abortive ligation product10. However, despite the importance of nick sealing at the end of almost all DNA repair pathways as well as DNA replication, the mechanism of mismatch discrimination
by which a human DNA ligase can encounter mutagenic DNA ends during the three steps of the ligation reaction remains unknown. Because nicks are potentially deleterious DNA lesions that may
lead to the formation of lethal double-strand breaks, a thorough understanding of the nick sealing mechanism is crucial for a comprehensive understanding of genome maintenance3,5. Base
excision repair (BER) requires tight coordination that entails the substrate-product channeling of DNA intermediates between repair proteins so that the release and accumulation of toxic and
mutagenic single-strand break intermediates are minimized in cells11,12,13. The downstream steps of the BER pathway involve DNA synthesis by DNA polymerase (pol) β and subsequent nick
sealing by DNA ligase I (LIG1) or IIIα to complete the repair of small single-base DNA lesions10. Polβ, an error-prone polymerase without 3′−5′ proofreading activity, can incorporate
mismatch nucleotides at a frequency of 1 in ~5000 during template-directed DNA synthesis14. Furthermore, several of the cancer-associated polβ variants possess aberrant repair functions,
such as reduced fidelity stemming from impaired discrimination against incorrect nucleotide incorporation, and expression of these variants in cells induces cellular transformation and
genomic instability15,16. In our studies, we have found that polβ mismatch or oxidized nucleotide 7,8-dihydro-8′-oxo-dGTP (8-oxodGTP) insertion products can generate a problematic nick
repair intermediate for the subsequent ligation step in the BER pathway6,10,17,18,19,20,21,22,23,24,25,26,27,28. These findings contribute to understanding the important molecular
determinants that ensure accurate BER pathway coordination or result in the impaired handoff from polβ to DNA ligase at the downstream steps. Nevertheless, the extent to which discrimination
by a DNA ligase counteracts mutagenic repair products during the final nick sealing step of the BER pathway remains unknown. Particularly, it is unclear how LIG1 dictates accurate versus
mutagenic outcomes while engaging with polβ-mediated base substitution errors at atomic resolution. In the present study, we defined the molecular basis of the human LIG1 mismatch
discrimination mechanism via moderate resolution structures of LIG1/nick DNA duplexes containing A:C and G:T mismatches at the 3′-strand. Our structures revealed that the LIG1 active site
can accommodate G:T mismatch in the wobble conformation, where the ligase validates mutagenic G:T ligation during the adenyl transfer step of the ligation reaction (DNA-AMP). We also
captured LIG1 in complex with nick DNA harboring Watson–Crick A:T base pair at this second step. However, we found that the ligase active site lysine (K568) stays adenylated (LIG1-AMP) while
engaging with a A:C mismatch, which refers to the first ligation step. Furthermore, we showed mutagenic and defective nick sealing of 3′-G:T and 3′-A:C mismatches, respectively. Finally,
our results demonstrate that AP-endonuclease 1 (APE1), a complementary DNA-end processing enzyme, removes a mismatched base (3′-dG or 3′-dA) from the nick DNA substrates via its exonuclease
activity and coordinates with LIG1 for mismatch removal coupled to DNA ligation at the final steps of the BER pathway. Our overall results demonstrate the strategies by which LIG1 engages
with the base substitution errors incorporated by polβ and reveal the requirement of a multiprotein assembly (polβ, LIG1, and APE1) to maintain repair efficiency. RESULTS STRUCTURES OF
LIG1/NICK REPAIR INTERMEDIATES WITH MISMATCHED DNA ENDS To elucidate the ligase strategies that deter or favor the ligation of repair intermediates that mimic polβ mismatch insertion
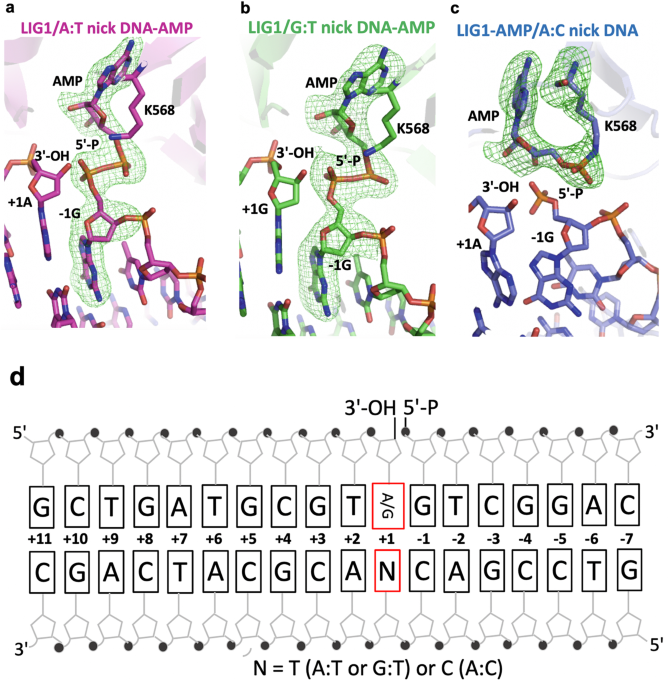
products at atomic resolution, we determined the structures of LIG1/nick DNA duplexes containing G:T and A:C mismatches as well as cognate A:T base pair at the 3′-strand (Table 1 and Fig.
1). In the structure of LIG1 bound to a nick DNA duplex containing a cognate A:T base pair, the 5′-terminus was adenylated, and a DNA-adenylate (DNA-AMP) intermediate was observed (Fig. 1a).
Similarly, we showed that the ligase active site could accommodate a G:T mismatch during step 2 of the ligation reaction when AMP is transferred to the 5′-P of nick DNA (Fig. 1b). The
superimposition of LIG1 structures with G:T mismatch and A:T base pair revealed no significant differences, with a superimposed Cα root mean square deviation of 0.609 Å. In both structures,
the LIG1/nick conformation was poised for nick sealing during step 3 of the ligation reaction. However, in the structure of the LIG1/nick DNA duplex containing an A:C mismatch, we showed
that the ligase active site exhibited the LIG1-adenylate conformation, where the active side lysine residue (K568) was covalently bound to the AMP phosphate (Fig. 1c). This refers to step 1
of the ligation reaction. Our results indicate that LIG1 stays in its initial adenylated state and cannot move forward with subsequent adenyl group transfer to the 5′-P on the downstream
strand to activate the ligase for attack by the upstream 3′-OH of the nick DNA (Fig. 1d). These observations suggest that the A:C base pair imparts the active site conformation that
suppresses the further chemical steps of catalysis. Overall, our LIG1/mismatch structures demonstrate that the ligase is trapped as the adenylated-DNA intermediate (AMP-DNA), which favors
the mutagenic ligation of the G:T mismatch, and the active site remains in an inactive conformation (AMP-LIG1), which deters nick sealing of the A:C mismatch. We observed a Watson–Crick
conformation with two hydrogen bonds for cognate A:T base pairs, while G:T and A:C mismatches exhibited the wobble conformation (Fig. 2a). The superimposition of the LIG1 A:T, G:T, and A:C
structures showed differences in the positions of the mismatched termini at the 3′-OH end of nick DNA (Fig. 2b, c). Furthermore, our LIG1/mismatch structures revealed that the ribose adopted
a different sugar pucker depending on the identity of the 3′-OH base pair, cognate versus mismatched. We observed the C3′-endo pucker in the LIG1/A:T structure while the LIG1/G:T and
LIG1/A:C mismatch structures exhibited the C4′-exo sugar pucker (Supplementary Fig. 1). In addition, similar to the previously reported LIG1 structures29,30,31,32, LIG1 containing the DNA
binding (DBD), adenylation (AdD), and oligonucleotide binding (OBD) domains completely encircles the nick DNA containing the cognate or mismatched ends (Supplementary Figs. 2 and 3). LIG1
ACTIVE SITE SHOWS DISTINCT DNA CONFORMATIONS DEPENDING ON THE IDENTITY OF THE MISMATCH Our LIG1/mismatch structures demonstrate that the ligase active site exhibits distinct
mismatch-specific conformations and shows significant differences in the positions of the 5′-P and 3′-OH strands at the nick around the upstream and downstream DNA (Fig. 3a). In the G:T and
A:T structures showing the formation of the 5′-5′ phosphoanhydride AMP-DNA intermediate, we observed that 5′-P is closer to the 3′-OH strand of the nick to ensure proper positioning and to
seal the phosphodiester backbone (Fig. 3b). The distances between the ends of the nick DNA for A:T and G:T were 2.1 Å and 2.8 Å, respectively. In the LIG1/A:C structure, the 3′-OH of the
nick DNA was rotated 50° from that of LIG1/A:T. The overlay of both structures demonstrated significant differences in the conformations of the 5′-strand due to clear shifts in the positions
of the −1G, −2T, and −3C nucleotides relative to the upstream DNA (Fig. 3c). Moreover, the position of phenylalanine (Phe) at 872 (F872), which is located upstream of the nick and
positioned close to the deoxyribose moiety of the nucleotide at the 5′-end, shows an important difference between the LIG1/A:T and A:C structures (Fig. 4). The overlay of both LIG1
structures demonstrated that the F872 distorts the alignment upstream of the A:T nick, where −1G and +1 A nucleotides are in parallel between the 3′ and 5′ strands (Fig. 4a). Notably,
superimposition of the structures in placement of the wobble A:C mismatch and the cognate A:T base pair at the active site of LIG1 demonstrated the shift in the position of F872 (Fig. 4b).
As reported in the previously solved LIG1 structures29,30,31,32, F635 and F872, which are located on the AdD and OBD domains of LIG1, respectively, are forced into the minor groove and play
important roles in the catalysis33. However, in the LIG1/A:T and G:T structures, we did not observe a difference in the position of F635, which was located near the upstream end, where it
was positioned near the sugar moiety of the 3′-end (Supplementary Fig. 4). The interaction interface between R874 and the −2T nucleotide of the downstream DNA showed a change in the LIG1/A:T
versus A:C structures (Fig. 5a). In both LIG1/mismatch structures, we found conformational differences at the active site residues Arg(R)589 and Leu(L)544, which were positioned close to
the 5′-P end of the nick (Fig. 5b). Similarly, the distance between the R589 and L544 side chains was shifted because of the differences in the position of AMP, forming DNA-AMP and K568-AMP,
as shown in the overlay of the LIG1/A:T and A:C structures, respectively (Fig. 5b). EFFICIENCY OF DOWNSTREAM BER STEPS INVOLVING MISMATCH-CONTAINING REPAIR INTERMEDIATES To understand the
efficiency of the downstream steps of the BER pathway and to investigate the substrate discrimination of LIG1 against nick DNA substrates in the presence of G:T and A:C mismatches, we
performed BER assays in vitro (Supplementary Fig. 5). We first evaluated the efficiency of the LIG1 wild-type or EE/AA mutant using the nick DNA substrates containing 3′-preinserted dG:T and
dA:C mismatches (Fig. 6 and Supplementary Fig. 6). For both proteins, we observed efficient ligation of the 3′-dG:T mismatch (Fig. 6a, b; lanes 9−14). This was very similar to the ligation
products of 3′-dA:T nick DNA in the control reactions (Fig. 6a, b; lanes 2–7). However, the end-joining efficiency of LIG1 was diminished in the presence of dA:C mismatch at the 3′-end (Fig.
6a, b; lanes 16–21). There was ~30- to 90-fold difference in the amount of ligation products between dG:T and dA:C mismatches with wild-type (Fig. 6c) and EE/AA (Fig. 6d) mutant of LIG1
(Supplementary Fig. 7). For both proteins, we observed the DNA intermediates with 5′-adenylate (AMP) in the presence of the 3′-mismatches (Supplementary Fig. 8). We then evaluated the
efficiency of polβ gap filling and subsequent ligation steps in the coupled assays. For this purpose, we tested polβ dGTP:T and dATP:C mismatch nucleotide insertion coupled to DNA ligation
at the same time points in reactions including both polβ and LIG1 (wild-type or EE/AA mutant). In these assays, we used one nucleotide gap DNA substrates with a template C or T (Fig. 7 and
Supplementary Fig. 9). Our results showed that the repair products after polβ dGTP:T insertion were ligated efficiently by wild-type LIG1 (Fig. 7a; lanes 7−10). These products were similar
to the ligation products of the nick repair intermediate after polβ dGTP:C insertion in the control reactions (Fig. 7a; lanes 2–5). The amounts of ligation products were relatively lower for
polβ dGTP:T mismatch insertion than for dGTP:C correct nucleotide insertion (Fig. 7b). However, we did not observe ligation products in the reaction of polβ dATP mismatch insertion opposite
C (Fig. 7a; lanes 12−15). This could have been due to inefficient A:C mismatch insertion and reduced base substitution fidelity of polβ, as reported for all possible incorrect base
pairings34,35,36. For the LIG1 EE/AA mutant, we obtained similar results showing the efficient ligation of polβ dGTP:C and dGTP:T insertion products (Fig. 7c; lanes 2–5 and 7-10,
respectively). However, in the presence of the low-fidelity LIG1, the products of self-ligation (i.e., end joining of one nucleotide gap DNA itself) appeared simultaneously with the complete
ligation of nicked polβ insertion products (Fig. 7c, compare lines 5 and 10), as we have reported previously24,27. The amounts of ligation products after both polβ insertions were also
found to be similar to those with the wild-type enzyme (Fig. 7d and Supplementary Fig. 10). INTERPLAY BETWEEN APE1 AND LIG1 DURING PROCESSING OF THE NICK REPAIR INTERMEDIATES WITH MISMATCHED
ENDS DNA repair intermediates with a damaged or mismatched base at the 3′-end can block pathway coordination and become persistent DNA strand breaks if not repaired37. It has been reported
that APE1, the BER protein involved in the initial steps of the repair pathway, can act as a proofreader of polβ errors and remove a mismatched or damaged base from the nick repair
intermediates38. To investigate the processing of mutagenic nick repair products with mismatched bases at the 3′-end, we examined the role of APE1 as a compensatory DNA end-processing
enzyme. For this purpose, we first evaluated the 3′-5′ exonuclease activity of APE1 in the reaction mixture that included nick DNA substrates containing 3′-preinserted dG:T and dA:C
mismatches. Furthermore, we investigated the processing of the nick DNA substrates with 3′-dG:T and 3′-dA:C mismatches in the coupled reactions, including both APE1 and LIG1, to test the
efficiency of mismatch removal and ligation simultaneously (Supplementary Fig. 11). We did not observe a significant difference in the mismatch base removal efficiency of APE1 between
3′-dG:T and 3′-dA:C mismatches (Fig. 8a). Our results demonstrated that APE1 could remove 3′-dG and 3′-dA bases from nick DNA substrates with template bases of T and C, respectively
(Supplementary Fig. 12). In the coupled assays, APE1 mismatch removal products accumulated along with the ligation products for the nick DNA substrates with 3′-dG:T mismatch (Fig. 8b;
compare lanes 2 and 3–7). However, we mainly observed the products of 3′-dA mismatch removal by APE1 from the nick DNA substrate with 3′-dA:C (Fig. 8b; compare lanes 9 and 11–14,
Supplementary Fig. 13). Finally, we quantitatively monitored the real-time kinetics of the protein-protein interaction between APE1 and LIG1 by surface plasmon resonance (SPR) assay. In this
assay, the interacting protein partner of APE1 was immobilized on CM5 biosensors onto which the LIG1 protein was passed as an analyte. Our results showed protein-protein interaction with an
equilibrium binding constant (K_D_) of 117 nM between APE1 and LIG1 (Fig. 8c). In previously published studies, physical interactions for APE1 have been reported for BER proteins such as
DNA glycosylase, polβ, and XRCC133,39. Thermodynamic and domain mapping studies have also shown that polβ interacts with the N-terminal noncatalytic region of LIG140. Overall, our results
indicate that the multiprotein repair complex containing polβ, APE1, and LIG1 can determine the efficiency of BER at the downstream steps when a nick repair intermediate with an incompatible
end is generated due to polymerase-mediated mutagenic mismatch insertions. DISCUSSION Spontaneous mutagenesis from DNA replication errors has been a prominent source of base substitution
errors in the tumor suppressor genes in different types of cancer41. The intrinsic formation of Watson–Crick like mismatches has been reported as an important determinant of the base
substitution mutagenesis such as mismatches G:T and A:C that sit well within the dimensions of the DNA double helix to maintain the geometry of a Watson–Crick base pair42. The effects of the
A:C mismatch on helix stability and dynamics differ from those of the G:T base pair43. The G:T is the most common mismatch arising from the deamination of 5-methylcytosine to thymine and
the conformation and dynamics of G:T mismatch (wobble versus Watson–Crick) are the best studied base pair by X-ray crystallography and NMR relaxation dispersion studies in DNA and RNA
duplexes44,45,46. Our study represents the LIG1 structures for these particular G:T and A:C mismatches at the ligase active site. DNA polymerases employ a series of pre-chemistry
conformational checkpoints to exclude the incorporation of mismatches47. The mechanisms of mismatch discrimination by DNA polymerases from diverse families and organisms have been
extensively reported through structural studies with a series of mismatches at the polymerase active site (Supplementary Table 1). In particular, G:T, T:G, A:C, and C:A mismatches are able
to adopt a multitude of distinct conformations with exquisite sensitivity to factors such as their location, electrostatic environment of the polymerase active site, the identity of the
catalytic metal ion (Mg2+ or Mn2+), correct downstream dNTP binding and strand specificity48,49,50,51,52,53,54,55,56,57,58,59,60,61. They have been hypothesized to avoid discrimination by
DNA polymerases due to a Watson–Crick-like (WC-like) base pairing conformations caused by ionization or tautomerization of the nucleobases, which leads to a base pair with the proper
geometry and base stacking for catalysis48,49,50,51,52,53,54,55,56,57. The first structural evidence of a WC-like conformation was shown in the crystal structure of human X family polλ
between an incoming nonhydrolyzable analog of dGTP and template T or in a wobble conformation when a dG:T mismatch is located at the primer terminus48. Similarly, the _Bacillus_ DNA
polymerase I large fragment active site accommodates a dC:A mismatch in a WC-like or wobble conformation depending on which catalytic metal ion is present49,50. For polβ, the largest
distortion has been reported for A:C mismatch, where O3′ of the primer terminus sugar is positioned away from the active site via preclusion of direct template base
interactions51,52,53,54,55,56. Furthermore, the conformation of G:T mismatch at the primer terminus of the polβ active site is changed between the binary complex and the ternary complex with
a correct incoming dNTP. In the binary complex, the mismatch assumes a wobble conformation; however, when the correct incoming dNTP is added, the mismatch changes to a weak Hoogsteen base
pair53,54,55. In addition, it has been shown in the structures of polμ that a G:T mismatch adopts a WC-like base pair and that mutations at the polymerase active site cause a change in
conformation57. Despite the evidence regarding these extensive DNA polymerase structures with a variety of mismatches48,49,50,51,52,53,54,55,56,57,58,59,60,61, how human DNA ligases engage
with various toxic DNA repair intermediates that mimic the polymerase-mediated mutagenic mismatch insertion products at the final ligation step of almost all DNA repair pathways is still
unclear. In the present study, our results revealed that LIG1 counteracts the polβ-promoted mutagenesis products distinctly depending on the architecture of the nick repair intermediate with
mismatched ends (Supplementary Fig. 14). Our LIG1/nick DNA complex structures demonstrate that the ligase active site can accommodate a G:T mismatch in the wobble conformation and that the
enzyme can fulfill the mutagenic ligation of polβ G:T misinsertion product. On the other hand, LIG1 discriminates against A:C mismatch with a large distortion of 3′-OH and 5′-P ends at the
nick. In this case, the nick repair intermediate with the A:C mismatch could serve as a fidelity checkpoint for the removal of the 3′-mismatched base by a proofreading enzyme such as APE1.
Accordingly, it has been shown in APE1 structural studies that the nick DNA and instability of mismatched bases facilitate 3′-end cleaning, where a mismatched end is stabilized by protein
contacts38. Metal ion plays important roles in the ligation reaction during step 1 (deprotonates the lysine nucleophile and activate it to attack on the ATP α-phosphate) and step 3
(activates 3′-OH nucleophilic attack on the 5′-P) of the ligation reaction62. DNA ligase/ATP and ligase/ATP/Mg2+ complexes for ATP-dependent ligases from other sources, such as
_Saccharomyces cerevisiae_, _Pyrococcus furiosus_, _Mycobacterium tuberculosis_ LigD, and DNA ligase in a noncovalent complex with AMP, highlight the requirement of metal ions for ligase
adenylation63,64,65,66,67,68,69,70,71,72. The previously solved crystal structures of LIG1 reveal a Mg2+-dependent high-fidelity (MgHiFi) site that is coordinated by the two conserved
glutamate residues at the junction between the adenylation (Glu[E]346) and DNA-binding (Glu[E]592) domains of the ligase in direct interaction with DNA30. In our study, we used the LIG1
mutant (E346A/E592A or EE/AA) with mutagenesis at the MgHiFi site for crystallization of LIG1/nick with G:T and A:C mismatches in the absence of Mg2+. We compared the RMSD values among our
LIG1 EE/AA structures and these previously solved LIG1 structures for cognate C:G and damaged 8-oxoG:A base pairs (Supplementary Table 2). There was no significant difference in either
cognate base pair (A:T and C:G) in the presence or absence of the metal ion under crystal conditions for either wild-type or EE/AA mutant of LIG1 (Supplementary Fig. 3a). Furthermore, the
superimposition of the LIG1/8-oxoG:A structure with our LIG1/G:T structure showed structural similarity in both structures for the formation of the DNA-AMP intermediate, referring to step 2
of the ligation reaction, in the absence of Mg2+ (Supplementary Fig. 15). The present study represents the human LIG1 structure with G:T and A:C mismatches, particularly at the ligase active
site bound to ATP (LIG1-AMP) in step 1 of the ligation reaction, and the importance of the F872 side chain that exhibits a clear shift for positioning the enzyme active site to deter the
nick sealing of potentially toxic repair intermediates. As shown for a myriad of DNA polymerases48,49,50,51,52,53,54,55,56,57,58,59,60,61, our LIG1/G:T mismatch structure reveals the
molecular mechanism by which a human ligase can escape mismatch discrimination, leading to the formation of premutagenic repair products. Further structure/function studies with both BER DNA
ligases (I and IIIα) are required for all other possible noncanonical base pairs at the 3′-end of the nick DNA to comprehensively understand how human DNA ligases discriminate against the
mutagenic repair intermediates containing mismatched or damaged 3′-end which could be formed due to the aberrant gap filling by polβ, which has been reported to be increased in the
cancer-associated variants with reduced fidelity15,16. METHODS PREPARATION OF DNA SUBSTRATES FOR CRYSTALLIZATION AND BER ASSAYS Oligodeoxyribonucleotides with and without a
6-carboxyfluorescein (FAM) label were obtained from Integrated DNA Technologies (IDT). The nick DNA substrates containing 3′-preinserted correct (dA:T) or mismatches (dG:T and dA:C) with a
FAM label at the 3′-end were used for DNA ligation assays in the reaction mixture including LIG1 (wild-type or EE/AA mutant) alone (Supplementary Table 3). The one nucleotide gap DNA
substrates containing FAM labels at both 3′- and 5′-ends were used in the coupled assays to observe the ligation of polβ correct or mismatch nucleotide insertion products by LIG1 (wild-type
or EE/AA mutant) in the reaction mixture including both polβ and LIG1 (Supplementary Table 4). The nick DNA substrates containing 3′-preinserted correct (dA:T) or mismatches (dG:T and dA:C)
with a FAM label at the 5′-end were used for APE1 exonuclease assays in the reaction mixture including APE1 alone (Supplementary Table 5). The nick mismatch containing DNA substrates with
FAM labels at both 3′- and 5′-ends were used in the coupled assays to observe APE1 mismatch removal and ligation in the reaction mixture including both APE1 and LIG1 (Supplementary Table 6).
The nick DNA substrates containing cognate A:T and mismatches G:T and A:C base pairs were used in the LIG1 X-ray crystallography studies (Supplementary Table 7 and Fig. 1d). All
double-stranded DNA substrates were prepared by annealing upstream, downstream, and template primers17,18,19,20,21,22,23,24,25,26,27,28. PROTEIN PURIFICATIONS Human his-tag full-length
(1-918) wild-type LIG1 and C-terminal (Δ261) E346A/E592A (EE/AA) LIG1 mutant were overexpressed in Rosetta (DE3) pLysS _E. coli_ cells (Millipore Sigma) and grown in Terrific Broth (TB)
media with kanamycin (50 μg ml−1) and chloramphenicol (34 μg ml−1) at 37 °C17,18,19,20,21,22,23,24,25,26,27,28. Once the OD was reached 1.0, the cells were induced with 0.5 mM isopropyl
β-D-thiogalactoside (IPTG) and the overexpression was continued overnight at 28 °C. After the centrifugation, the cells were lysed in the lysis buffer containing 50 mM Tris-HCl (pH 7.0), 500
mM NaCl, 20 mM imidazole, 10% glycerol, 1 mM PMSF, an EDTA-free protease inhibitor cocktail tablet by sonication at 4 °C. The lysate was pelleted at 31,000 x g for 1 h at 4 °C. The cell
lysis solution was filter clarified and then loaded onto the HisTrap HP column (GE Health Sciences) that was previously equilibrated with the binding buffer including 50 mM Tris-HCl (pH
7.0), 500 mM NaCl, 20 mM imidazole, and 10% glycerol. The column was washed with the binding buffer and then followed by washing buffer containing 50 mM Tris-HCl (pH 7.0), 500 mM NaCl, 35 mM
imidazole, and 10% glycerol. The protein was finally eluted with an increasing imidazole gradient 0–500 mM at 4 °C. The collected fractions were then subsequently loaded onto HiTrap Heparin
(GE Health Sciences) column that was equilibrated with the binding buffer containing 50 mM Tris-HCl (pH 7.0), 50 mM NaCl, 1 mM EDTA, 5% glycerol, and the protein was eluted with a linear
gradient of NaCl up to 1 M. LIG1 protein was further purified by Resource Q and finally by Superdex 200 10/300 (GE Health Sciences) columns in the buffer containing 20 mM Tris-HCl (pH 7.0),
200 mM NaCl, and 1 mM DTT. Human his-tag full-length APE1 was overexpressed in BL21(DE3) _E. coli_ cells (Invitrogen) in Lysogeny Broth (LB) media at 37 °C for 8 h, induced with 0.5 mM IPTG
and the overexpression was continued for overnight at 28 °C. After the cells were harvested, lysed at 4 °C, and then clarified as described above, the supernatant was loaded onto HisTrap HP
column (GE Health Sciences) and purified with an increasing imidazole gradient (0-300 mM) elution at 4 °C. The collected fractions were then subsequently loaded onto HiTrap Heparin column
(GE Health Sciences) with a linear gradient of NaCl up to 1 M. APE1 protein was then further purified by Superdex 200 increase 10/300 chromatography (GE Healthcare) in the buffer containing
20 mM Tris-HCl (pH 7.0), 200 mM NaCl, and 1 mM DTT. Human GST-tag full-length polβ was overexpressed in One Shot BL21(DE3) _E. coli_ cells (Invitrogen) in LB media at 37 °C for 8 h, induced
with 0.5 mM IPTG, and the overexpression was continued for overnight at 28 °C17,18,19,20,21,22,23,24,25,26,27,28. After cell lysis at 4 °C by sonication in the lysis buffer containing 1X PBS
(pH 7.3), 200 mM NaCl, 1 mM DTT, cOmplete Protease Inhibitor Cocktail (Roche), the lysate was pelleted at 31,000 x g for 1 h and then clarified by centrifugation and filtration. The
supernatant was loaded onto GSTrap HP column (GE Health Sciences) and purified with the elution buffer containing 50 mM Tris-HCl (pH 8.0) and 10 mM reduced glutathione. To cleave a GST-tag,
the recombinant protein was incubated with PreScission Protease (GE Health Sciences) for 16 h at 4 °C in the buffer containing 1X PBS (pH 7.3), 200 mM NaCl, and 1 mM DTT. After the cleavage,
the polβ protein was subsequently passed through a GSTrap HP column, and the protein without GST-tag was then further purified by loading onto Superdex 200 gel filtration column (GE Health
Sciences) in the buffer containing 50 mM Tris-HCl (pH 7.5) and 400 mM NaCl. All proteins purified in this study were dialyzed against the storage buffer including 25 mM Tris-HCl (pH 7.0),
200 mM NaCl, concentrated, frozen in liquid nitrogen, and stored at −80 °C. Protein quality was evaluated onto 10% SDS-PAGE, and the protein concentration was measured using absorbance at
280 nm. CRYSTALLIZATION AND STRUCTURE DETERMINATION LIG1 C-terminal (Δ261) EE/AA mutant was used for crystals production. All LIG1-DNA complex crystals were grown at 20 °C using the hanging
drop method. LIG1 (at 26 mgml−1)/DNA complex solution was prepared in 20 mM Tris-HCl (pH 7.0), 200 mM NaCl, 1 mM DTT, 1 mM EDTA and 1 mM ATP at 1.5:1 DNA:protein molar ratio and then mixed
with 1 μl reservoir solution containing 100 mM MES (pH 6.6), 100 mM lithium acetate, and 20% (w/v) polyethylene glycol PEG3350. All crystals grew in 1–2 days. They were then washed in the
reservoir solution with 20% glycerol and flash cooled in liquid nitrogen for data collection. The crystals were maintained at 100 K during X-ray diffraction data collection using the
beamline 7B2 at Cornell High Energy Synchrotron Source (CHESS). The diffraction images were indexed and integrated using HKL2000. All structures were solved by the molecular replacement
method using PHASER using PDB entry 6P0D as a search model73,74. Iterative rounds of model building in COOT and refinement with PHENIX or REFMAC5 were used to produce the final
models75,76,77. 3D program was used for sugar pucker analysis78. All structural images were drawn using PyMOL (The PyMOL Molecular Graphics System, V0.99, Schrödinger, LLC). Detailed
crystallographic statistics are provided in Table 1. DNA LIGATION ASSAYS The ligation assays using the nick DNA substrates containing 3′-preinserted correct dA:T, mismatches dG:T and dA:C
were performed to test the ligation efficiency of wild-type and EE/AA mutant of LIG1 (Supplementary Fig. 5a)17,18,19,20,21,22,23,24,25,26,27,28. Briefly, the ligation assays were performed
in the mixture containing 50 mM Tris-HCl (pH 7.5), 100 mM KCl, 10 mM MgCl2, 1 mM ATP, 1 mM DTT, 100 µgml−1 BSA, 10% glycerol, and 500 nM DNA substrate in a final volume of 10 µl. The
reactions were initiated by the addition of 100 nM LIG1 (wild-type or EE/AA mutant), incubated at 37 °C, and stopped at the time points indicated in the figure legends. The reaction products
were then quenched with an equal amount of gel loading buffer containing 95% formamide, 20 mM ethylenediaminetetraacetic acid, 0.02% bromophenol blue and 0.02% xylene cyanol. After
incubation at 95 °C for 3 min, the reaction products were separated by electrophoresis on an 18% denaturing polyacrylamide gel. The gels were scanned with a Typhoon PhosphorImager (Amersham
Typhoon RGB), and the data were analyzed using ImageQuant software. BER ASSAYS TO MEASURE DNA LIGATION OF POLΒ NUCLEOTIDE INSERTION PRODUCTS The coupled assays using the one nucleotide gap
DNA substrates with template A or C were performed to test the ligation of polβ nucleotide insertion (correct or mismatch) in the reaction mixture including both polβ and LIG1 (Supplementary
Fig. 5b)17,18,19,20,21,22,23,24,25,26,27,28. Briefly, the coupled assays were performed in a mixture containing 50 mM Tris-HCl (pH 7.5), 100 mM KCl, 10 mM MgCl2, 1 mM ATP, 1 mM DTT, 100
µgml−1 BSA, 10% glycerol, 100 µM dNTP, and 500 nM DNA substrate in a final volume of 10 µl. The reactions were initiated by the addition of pre-incubated enzyme mixture of polβ/LIG1 (100 nM)
and incubated at 37 °C for the time points as indicated in the figure legends. The reaction products were then mixed with an equal amount of gel loading buffer, separated by electrophoresis
on an 18% denaturing polyacrylamide gel, and analyzed as described above. BER ASSAYS TO MEASURE APE1 EXONUCLEASE ACTIVITY AND LIG1 LIGATION APE1 exonuclease assays using the nick DNA
substrates with 3′-preinserted mismatches dG:T and dA:C were performed to examine APE1 proofreading role for removing a mismatched base (Supplementary Fig. 11a). Briefly, APE1 activity
assays were performed in the reaction mixture containing 50 mM HEPES (pH 7.4), 100 mM KCl, 3 mM MgCl2, 0.1 mg ml−1 BSA, and 500 nM DNA substrate in a final volume of 10 µl. The reactions
were initiated by the addition of 50 nM APE1, incubated at 37 °C for the time points as indicated in the figure legends, quenched by mixing with 100 mM EDTA, and then mixed with an equal
amount of gel loading buffer. The nick DNA substrates including 3′-preinserted mismatches dG:T and dA:C were used for repair assays to test APE1 exonuclease and DNA ligation activities in
the same reaction mixture (Supplementary Fig. 11b). Briefly, the repair assays were performed in a mixture containing 50 mM HEPES (pH 7.4), 100 mM KCl, 5 mM MgCl2, 1 mM ATP, 0.1 mg ml−1 BSA,
and 500 nM DNA substrate in a final volume of 10 µl. The reactions were initiated by the addition of pre-incubated enzyme mixture including APE1/LIG1 (100 nM), incubated at 37 °C for the
time points as indicated in the figure legends. The reaction products were quenched by mixing with 100 mM EDTA and then mixed with an equal amount of gel loading buffer. The reaction
products were separated by electrophoresis on an 18% denaturing polyacrylamide gel and analyzed as described above. APE1 AND LIG1 PROTEIN-PROTEIN INTERACTION ASSAY The protein-protein
interaction between APE1 and LIG1 was measured by surface plasmon resonance (SPR) in real time using Biacore X-100 (GE Healthcare)28. Briefly, one flow cell of the CM5 sensor chip was
activated at 25 °C with 1:1 mixture of 0.2 M EDC and 0.05 M NHS in water, and then APE1 protein was injected over the flow cell in 10 mM sodium acetate at pH 5.0 at a flow rate of 10 µl/min.
The binding sites were blocked using 1 M ethanolamine. LIG1 (at the concentration range of 0–1.6 µM) was then injected for 3 min at a flow rate of 30 µl/min in the binding buffer containing
20 mM HEPES pH 7.4, 150 mM NaCl, 3 mM EDTA and 0.005% (v/v) Surfactant P20. After a dissociation phase for 3–4 min, 0.2% SDS was injected for 30 sec to regenerate the chip surface.
Non-specific binding to a blank flow cell was subtracted to obtain corrected sensorgrams. All data were analyzed using BIAevaluation software version 2.0.1 and fitted to a 1:1 (Langmuir)
binding model to obtain equilibrium constant (K_D_). REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article.
DATA AVAILABILITY The data that support this study are available from the corresponding author upon request. Atomic coordinates and structure factors for the reported crystal structures have
been deposited in the RCSB Protein Data Bank under accession numbers 7SUM, 7SXE, and 7SX5. Source data are provided with this paper. REFERENCES * Timson, D. J., Singleton, M. R. &
Wigley, D. B. DNA ligases in the repair and replication of DNA. _Mutat. Res._ 460, 301–318 (2000). Article CAS PubMed Google Scholar * Shuman, S. DNA ligases: progress and prospects. _J.
Biol. Chem._ 284, 17365–17369 (2009). Article CAS PubMed PubMed Central Google Scholar * Tomkinson, A. E., Vijayakumar, S., Pascal, J. M. & Ellenberger, T. DNA ligases: structure,
reaction mechanism, and function. _Chem. Rev._ 106, 687–699 (2006). Article CAS PubMed Google Scholar * Doherty, A. J. & Suh, S. W. Structural and mechanistic conservation in DNA
ligases. _Nucleic Acids Res._ 28, 4051–4058 (2000). Article CAS PubMed PubMed Central Google Scholar * Ellenberger, T. & Tomkinson, A. E. Eukaryotic DNA ligases: structural and
functional insights. _Annu. Rev. Biochem._ 77, 313–338 (2008). Article CAS PubMed PubMed Central Google Scholar * Çağlayan, M. Interplay between DNA polymerases and DNA ligases:
Influence on substrate channeling and the fidelity of DNA ligation. _J. Mol. Biol._ 431, 2068–2081 (2019). Article PubMed PubMed Central CAS Google Scholar * Arana, E. M. & Kunkel,
T. A mutator phenotypes due to DNA replication infidelity. _Semin. Cancer Biol._ 20, 304–311 (2010). Article CAS PubMed PubMed Central Google Scholar * Modrich, P. DNA mismatch
correction. _Annu. Rev. Biochem._ 56, 435–466 (1987). Article CAS PubMed Google Scholar * Topal, M. D. & Fresco, J. R. Complementary base pairing and the origin of substitution
mutations. _Nature_ 263, 285–289 (1976). Article ADS CAS PubMed Google Scholar * Çağlayan, M. & Wilson, S. H. Oxidant and environmental toxicant-induced effects compromise DNA
ligation during base excision DNA repair. _DNA Repair_ 35, 85–89 (2015). Article PubMed PubMed Central CAS Google Scholar * Beard, W. A. et al. Eukaryotic base excision repair: new
approaches shine light on mechanism. _Ann. Rev. Biochem._ 88, 137–162 (2019). Article CAS PubMed Google Scholar * Prasad, R., Shock, D. D., Beard, W. A. & Wilson, S. H. Substrate
channeling in mammalian base excision repair pathways: passing the baton. _J. Biol. Chem._ 285, 40479–40488 (2010). Article CAS PubMed PubMed Central Google Scholar * Wilson, S. H.
& Kunkel, T. A. Passing the baton in base excision repair. _Nat. Struct. Biol._ 7, 176–178 (2000). Article CAS PubMed Google Scholar * Beard, W. A. et al. Efficiency of correct
nucleotide insertion governs DNA polymerase fidelity. _J. Biol. Chem._ 277, 47393–47398 (2002). Article CAS PubMed Google Scholar * Donigan, K. A. et al. Human polymerase β is mutated in
high percentage of colorectal tumors. _J. Biol. Chem._ 287, 23830–23839 (2012). Article CAS PubMed PubMed Central Google Scholar * Sweasy, J. B. et al. Expression of DNA polymerase β
cancer-associated variants in mouse cells results in cellular transformation. _Proc. Natl Acad. Sci. USA_ 102, 14350–14355 (2005). Article ADS CAS PubMed PubMed Central Google Scholar
* Çağlayan, M. et al. Role of polymerase β in complementing aprataxin deficiency during abasic-site base excision repair. _Nat. Struct. Mol. Biol._ 21, 497–499 (2014). Article PubMed
PubMed Central CAS Google Scholar * Çağlayan, M. et al. Complementation of aprataxin deficiency by base excision repair enzymes. _Nucleic Acids Res._ 43, 2271–2281 (2015). Article PubMed
PubMed Central CAS Google Scholar * Çağlayan, M. et al. Complementation of aprataxin deficiency by base excision repair enzymes in mitochondrial extracts. _Nucleic Acids Res._ 45,
10079–10088 (2017). Article PubMed PubMed Central CAS Google Scholar * Çağlayan, M. et al. Oxidized nucleotide insertion by pol β confounds ligation during base excision repair. _Nat.
Commun._ 8, 14045 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Çağlayan, M. & Wilson, S. H. Role of DNA polymerase β oxidized nucleotide insertion in DNA ligation
failure. _J. Radiat. Res._ 58, 603–607 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Çağlayan, M. & Wilson, S. H. Pol μ dGTP mismatch insertion opposite T coupled
with ligation reveals a promutagenic DNA intermediate during double strand break repair. _Nat. Commun._ 9, 4213 (2018). Article ADS PubMed PubMed Central CAS Google Scholar *
Çağlayan, M. Pol μ ribonucleotide insertion opposite 8-oxodG facilitates the ligation of premutagenic DNA repair intermediate. _Sci. Rep._ 10, 940 (2020). Article ADS MathSciNet PubMed
PubMed Central CAS Google Scholar * Çağlayan, M. The ligation of pol β mismatch insertion products governs the formation of promutagenic base excision DNA repair intermediates. _Nucleic
Acids Res._ 48, 3708–3721 (2020). Article PubMed PubMed Central CAS Google Scholar * Çağlayan, M. Pol β gap filling, DNA ligation and substrate-product channeling during base excision
repair opposite oxidized 5-methylcytosine modifications. _DNA Repair_ 95, 102945 (2020). Article PubMed PubMed Central CAS Google Scholar * Tang, Q., Kamble, P. & Çağlayan, M. DNA
ligase I variants fail in the ligation of mutagenic repair intermediates with mismatches and oxidative DNA damage. _Mutagenesis_ 35, 391–404 (2020). Article CAS PubMed PubMed Central
Google Scholar * Kamble, P., Hall, K., Chandak, M., Tang, Q. & Çağlayan, M. DNA ligase I fidelity the mutagenic ligation of pol β oxidized and mismatch nucleotide insertion products in
base excision repair. _J. Biol. Chem._ 296, 100427 (2021). Article CAS PubMed PubMed Central Google Scholar * Tang, Q. & Çağlayan, M. The scaffold protein XRCC1 stabilizes the
formation of polβ/gap DNA and ligase IIIα/nick DNA complexes in base excision repair. _J. Biol. Chem._ 297, 101025 (2021). Article CAS PubMed PubMed Central Google Scholar * Pascal, J.
M. et al. Human DNA ligase I completely encircles and partially unwinds nicked DNA. _Nature_ 432, 473–478 (2004). Article ADS CAS PubMed Google Scholar * Tumbale, P. P. et al.
Two-tiered enforcement of high-fidelity DNA ligation. _Nat. Commun._ 10, 5431 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Williams, J. S. et al. High-fidelity DNA
ligation enforces accurate Okazaki fragment maturation during DNA replication. _Nat. Commun._ 12, 482 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Jurkiw, T. J. et
al. LIG1 syndrome mutations remodel a cooperative network of ligand binding interactions to compromise ligation efficiency. _Nucleic Acids Res._ 49, 1619–1630 (2021). Article CAS PubMed
PubMed Central Google Scholar * Moor, N. A. & Lavrik, O. I. Protein-protein interactions in DNA base excision repair. _Biochemistry_ 83, 411–422 (2018). CAS PubMed Google Scholar *
Beard, W. A. et al. Enzyme-DNA interactions required for efficient nucleotide incorporation and discrimination in human DNA polymerase β. _J. Biol. Chem._ 271, 12141–12144 (1996). Article
CAS PubMed Google Scholar * Beard, W. A., Shock, D. D., Yang, X. P., DeLauder, S. F. & Wilson, S. H. Loss of DNA polymerase β stacking interactions with templating purines, but not
pyrimidines, alters catalytic efficiency and fidelity. _J. Biol. Chem._ 277, 8235–8242 (2002). Article CAS PubMed Google Scholar * Ahn, J., Kraynov, V. S., Zhong, X., Werneburg, B. G.
& Tsai, M. D. DNA polymerase β: effects of gapped DNA substrates on dNTP specificity, fidelity, processivity, and conformational changes. _Biochem. J._ 331, 79–87 (1998). Article CAS
PubMed PubMed Central Google Scholar * Andres, S. N. et al. Recognition and repair of chemically heterogeneous structures at DNA ends. _Environ. Mol. Mutagen._ 56, 1–21 (2015). Article
ADS CAS PubMed Google Scholar * Whitaker, A. M., Flynn, T. S. & Freudenthal, B. D. Molecular snapshots of APE1 proofreading mismatches and removing DNA damage. _Nat. Commun._ 9, 399
(2019). Article ADS CAS Google Scholar * Moor, N. A. et al. Quantitative characterization of protein-protein complexes involved in base excision DNA repair. _Nucleic Acids Res._ 43,
6009–6022 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Prasad, R. et al. Specific interaction of DNA polymerase β and DNA ligase I in a multiprotein base excision
repair complex from bovine testis. _J. Biol. Chem._ 271, 16000–16007 (1996). Article CAS PubMed Google Scholar * Bacolla, A., Cooper, D. N. & Vasquez, K. M. Mechanisms of base
substitution mutagenesis in cancer genomes. _Genes_ 5, 108–146 (2014). Article PubMed PubMed Central CAS Google Scholar * Watson, J. D. & Crick, F. H. Genetical implications of the
structure of deoxyribonucleic acid. _Nature_ 171, 964–967 (1953). Article ADS CAS PubMed Google Scholar * Rossetti, G. et al. The structural impact of DNA mismatches. _Nucleic Acids
Res._ 43, 4309–4321 (2015). Article CAS PubMed PubMed Central Google Scholar * Bellacosa, A. & Drohat, A. C. Role of base excision repair in maintaining the genetic and epigenetic
integrity of CpG sites. _DNA Repair_ 32, 33–42 (1015). * Kimsey, I. J. et al. Visualizing transient Watson-Crick-like mispairs in DNA and RNA duplexes. _Nature_ 519, 315–320 (2015). Article
ADS CAS PubMed PubMed Central Google Scholar * Szymanski, E. S. et al. Direct NMR evidence that transient tautomeric and anionic states in dG.dT form Watson-Crick like base pairs. _J.
Am. Chem. Soc._ 139, 4326–4329 (2017). Article CAS PubMed PubMed Central Google Scholar * Joyce, C. M. & Benkovic, S. J. DNA polymerase fidelity: Kinetics, structure, and
checkpoints. _Biochemistry_ 43, 14317–14324 (2004). Article CAS PubMed Google Scholar * Bebenek, K., Pedersen, L. C. & Kunkel, T. A. Replication infidelity via a mismatch with
Watson-Crick geometry. _Proc. Natl Acad. Sci. USA_ 108, 1862–1867 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, W., Hellinga, H. W. & Beese, L. S. Structural
evidence for the rare tautomer hypothesis of spontaneous mutagenesis. _Proc. Natl Acad. Sci. USA_ 108, 17644–17648 (2011). Article ADS CAS PubMed PubMed Central Google Scholar *
Johnson, S. J. & Beese, L. S. Structures of mismatch replication errors observed in a DNA polymerase. _Cell_ 116, 803–816 (2004). Article CAS PubMed Google Scholar * Krahn, J. M.,
Beard, W. A. & Wilson, S. H. Structural insights into DNA polymerase β deterrents for misincorporation support an induced-fit mechanism for fidelity. _Structure_ 12, 1823–1832 (2004).
Article CAS PubMed Google Scholar * Beard, W. A. & Wilson, S. H. Structural insights into the origins of DNA polymerase fidelity. _Structure_ 11, 489–496 (2003). Article CAS PubMed
Google Scholar * Batra, V. K., Beard, W. A., Shock, D. D., Pedersen, L. C. & Wilson, S. H. Nucleotide-induced DNA polymerase active site motions accommodating a mutagenic DNA
intermediate. _Structure_ 13, 1225–1233 (2005). Article CAS PubMed Google Scholar * Batra, V. K., Beard, W. A., Pedersen, L. C. & Wilson, S. H. Structures of DNA polymerase mispaired
DNA termini transitioning to pre-catalytic complexes support an induced-fit fidelity mechanism. _Structure_ 24, 1863–1875 (2016). Article CAS PubMed PubMed Central Google Scholar *
Batra, V. K., Beard, W. A., Shock, D. D., Pedersen, L. C. & Wilson, S. H. Structures of DNA polymerase β with active-site mismatches suggest a transient abasic site intermediate during
misincorporation. _Mol. Cell._ 30, 315–324 (2008). Article CAS PubMed PubMed Central Google Scholar * Koag, M. C., Nam, K. & Lee, S. The spontaneous replication error and the
mismatch discrimination mechanisms of human DNA polymerase β. _Nucleic Acids Res._ 42, 11233–11245 (2014). Article CAS PubMed PubMed Central Google Scholar * Guo, M. et al. Mechanism of
genome instability mediated by human DNA polymerase mu misincorporation. _Nat. Commun._ 12, 3759 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Trincao, J. et al. DPO4
is hindered in extending a G·T mismatch by a reverse wobble. _Nat. Struc. Mol. Biol._ 11, 457–462 (2004). Article CAS Google Scholar * Vaisman, A., Ling, H., Woodgate, R. & Yang, W.
Fidelity of Dpo4: effect of metal ions, nucleotide selection and pyrophosphorolysis. _EMBO J._ 24, 2957–2967 (2005). Article CAS PubMed PubMed Central Google Scholar * Xia, S. &
Konigsberg, W. H. Mispairs with Watson-Crick base-pair geometry observed in ternary complexes of an RB69 DNA polymerase variant. _Protein Sci._ 23, 508–513 (2014). Article CAS PubMed
PubMed Central Google Scholar * Zhao, Y. et al. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase η. _Proc. Natl Acad. Sci. USA_ 110, 8146–8151 (2013). Article
ADS CAS PubMed PubMed Central Google Scholar * Unciuleac, M., Goldgur, Y. & Shuman, S. Two-metal versus one-metal mechanisms of lysine adenylylation by ATP-dependent and
NAR+-dependent polynucleotide ligases. _Proc. Natl Acad. Sci. USA_ 114, 2592–2597 (2017). Article CAS PubMed PubMed Central Google Scholar * Tomkinson, A. E., Tappe, N. J. &
Friedberg, E. C. DNA Ligase I from _Saccharomyces cerevisiae_: Physical and biochemical characterization of the CDC9 gene product. _Biochemistry_ 31, 11762–11771 (1992). Article CAS PubMed
Google Scholar * Nishida, H., Kiyonari, S., Ishino, Y. & Morikawa, K. The closed structure of an archaeal DNA ligase from _Pyrococcus furiosus_. _J. Mol. Biol._ 360, 956–967 (2006).
Article CAS PubMed Google Scholar * Chen, Y. et al. Structure of the error-prone DNA ligase of African swine fever virus identifies critical active site residues. _Nat. Commun._ 10, 387
(2019). Article ADS CAS PubMed PubMed Central Google Scholar * Unciuleac, M., Goldgur, Y. & Shuman, S. Structures of ATP-bound DNA ligase D in a closed domain conformation reveal a
network of amino acid and metal contacts to the ATP phosphates. _J. Biol. Chem._ 294, 5094–5104 (2019). Article CAS PubMed PubMed Central Google Scholar * Shuman, S. & Lima, C. D.
The polynucleotide ligase and RNA capping enzyme superfamily of covalent nucleotidyltransferases. _Curr. Opin. Struct. Biol._ 14, 757–764 (2004). Article CAS PubMed Google Scholar *
Odell, M., Sriskanda, V., Shuman, S. & Nikolov, D. Crystalstructure of eukaryotic DNA ligase–adenylate illuminates the mechanism of nick sensing and strand joining. _Mol. Cell_ 6,
1183–1193 (2000). Article CAS PubMed Google Scholar * Nair, P. A. et al. Structural basis for nick recognition by a minimal pluripotent DNA ligase. _Nat. Struct. Mol. Biol._ 14, 770–778
(2007). Article CAS PubMed Google Scholar * Gong, C., Martins, A., Bongiorno, P., Glickman, M. & Shuman, S. Biochemical and genetic analysis of the four DNA ligases of mycobacteria.
_J. Biol. Chem._ 279, 20594–20606 (2004). Article CAS PubMed Google Scholar * Gong, C. et al. Mechanism of non-homologous end joining in mycobacteria: a low-fidelity repair system driven
by Ku, ligase D and ligase C. _Nat. Struct. Mol. Biol._ 12, 304–312 (2005). Article CAS PubMed Google Scholar * Akey, D. et al. Crystal structure and nonhomologous end joining function
of the ligase component of _Mycobacterium_ DNA ligase D. _J. Biol. Chem._ 281, 13412–13423 (2006). Article CAS PubMed Google Scholar * Otwinowski, Z. & Minor, W. Processing of X-ray
diffraction data collected in oscillation mode. _Methods Enzymol._ 276, 307–326 (1997). Article CAS PubMed Google Scholar * McCoy, A. J. et al. Phaser crystallographic software. _J.
Appl. Crystallogr._ 40, 658–674 (2007). Article CAS PubMed PubMed Central Google Scholar * Emsley, P. et al. Features and development of Coot. _Acta Crystallogr. D. Biol. Crystallogr._
66, 486–501 (2010). Article CAS PubMed PubMed Central Google Scholar * Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta
Crystallogr. D. Biol. Crystallogr._ 66, 213–221 (2010). Article CAS PubMed PubMed Central Google Scholar * Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal
structures. _Acta Crystallogr. D. Biol. Crystallogr._ 67, 355–367 (2011). Article CAS PubMed PubMed Central Google Scholar * Li, S., Olson, W. K. & Lu, X. J. Web 3DNA 2.0 for the
analysis, visualization, and modeling of 3D nucleic acid structures. _Nucleic Acids Res._ 47, W26–W34 (2019). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS This work is based upon research conducted at the Center for High Energy X-ray Sciences (CHEXS), which is supported by the National Science Foundation under award
DMR−1829070, and the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award 1-P30-GM124166-01A1 from the National Institute of General Medical Sciences,
National Institutes of Health, and by New York State’s Empire State Development Corporation (NYSTAR). The authors thank Jacob E. Combs (McKenna Lab, University of Florida) for his assistance
with crystal shipment and data collection. This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences Grant 4R00ES026191 and the
University of Florida Thomas H. Maren Junior Investigator Fund P0158597 to M.Ç. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry and Molecular Biology, University of
Florida, Gainesville, FL, 32610, USA Qun Tang, Mitchell Gulkis, Robert McKenna & Melike Çağlayan Authors * Qun Tang View author publications You can also search for this author inPubMed
Google Scholar * Mitchell Gulkis View author publications You can also search for this author inPubMed Google Scholar * Robert McKenna View author publications You can also search for this
author inPubMed Google Scholar * Melike Çağlayan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization M.Ç., methodology and
investigation M.Ç., M.G., T.Q.; writing-original draft, M.Ç., T.Q.; writing-reviewing, editing original draft and revision, M.Ç., T.Q., M.G., and R.M.; funding acquisition M.Ç. CORRESPONDING
AUTHOR Correspondence to Melike Çağlayan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_
thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE
DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Tang, Q., Gulkis, M., McKenna, R. _et al._ Structures of LIG1 that engage with mutagenic mismatches inserted by polβ in base excision repair. _Nat Commun_ 13, 3860 (2022).
https://doi.org/10.1038/s41467-022-31585-w Download citation * Received: 08 December 2021 * Accepted: 23 June 2022 * Published: 05 July 2022 * DOI: https://doi.org/10.1038/s41467-022-31585-w
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative