Play all audios:
ABSTRACT The precise activation of C–H bonds will eventually provide chemists with transformative methods to access complex molecular architectures. Current approaches to selective C–H
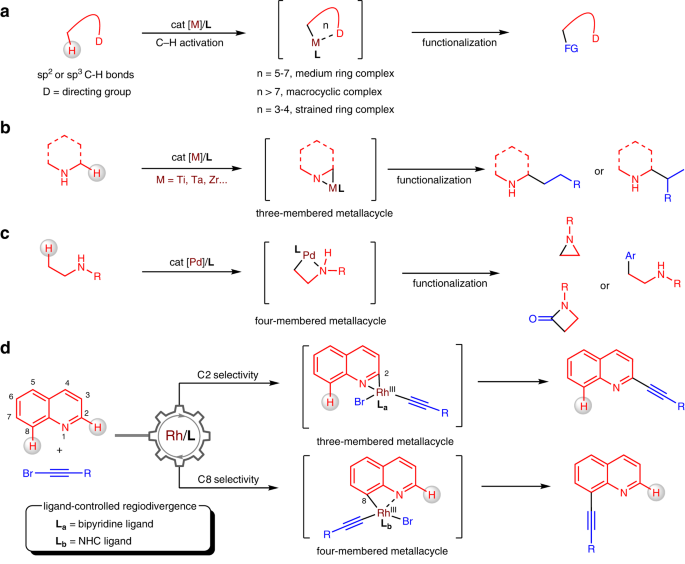
activation relying on directing groups are effective for the generation of five-membered, six-membered and even larger ring metallacycles but show narrow applicability to generate three- and
four-membered rings bearing high ring strain. Furthermore, the identification of distinct small intermediates remains unsolved. Here, we developed a strategy to control the size of strained
metallacycles in the rhodium-catalysed C−H activation of aza-arenes and applied this discovery to tunably incorporate the alkynes into their azine and benzene skeletons. By merging the
rhodium catalyst with a bipyridine-type ligand, a three-membered metallacycle was obtained in the catalytic cycle, while utilizing an NHC ligand favours the generation of the four-membered
metallacycle. The generality of this method was demonstrated with a range of aza-arenes, such as quinoline, benzo[_f_]quinolone, phenanthridine, 4,7-phenanthroline, 1,7-phenanthroline and
acridine. Mechanistic studies revealed the origin of the ligand-controlled regiodivergence in the strained metallacycles. SIMILAR CONTENT BEING VIEWED BY OTHERS SELECTIVE RING EXPANSION AND
C−H FUNCTIONALIZATION OF AZULENES Article Open access 01 December 2023 RING WALKING AS A REGIOSELECTIVITY CONTROL ELEMENT IN PD-CATALYZED C-N CROSS-COUPLING Article Open access 24 May 2022
DIHYDROQUINAZOLINONES AS ADAPTATIVE C(_SP_3) HANDLES IN ARYLATIONS AND ALKYLATIONS VIA DUAL CATALYTIC C–C BOND-FUNCTIONALIZATION Article Open access 03 May 2022 INTRODUCTION Due to the near
universal advantage of C − H bonds in organic molecules, the C − H activation strategy provides an opportunity to functionalize any carbon centre in an atom-economical and streamlined
way1,2,3,4,5,6,7,8,9,10. Because organic molecules typically contain multiple C − H bonds with comparable strengths and steric environments, regiocontrol has been a long-standing challenge
within this type of chemistry11. The differentiation of C − H bonds is traditionally dominated by steric and electronic effects12, and there have been considerable efforts to utilize
directing groups in less constrained molecules (Fig. 1a)13,14,15,16,17. Directed C − H activation is thermodynamically favoured through conformationally rigid five-, six-, and seven-membered
metallacyclic intermediates. Several elegant methods have been exploited to achieve the _meta_- and _para_-selective C − H activation of arenes through chelation-assisted macrocyclic
complexes by directing groups18,19,20,21,22. Despite these advances, the generation of highly strained metallacycles in directed C − H activation remains much desired but more challenging.
Substantial progress has been made regarding the C–H activation of aliphatic amines through strained metallacycles, allowing for site-selective functionalization at the α or β position of
the amino group in one catalytic step23,24. Early transition metals, including titanium, tantalum and zirconium, enable the hydroaminomethylation of alkenes with unprotected N-heterocycles
and amines through metallaaziridine intermediates, affording either linear or branched products (Fig. 1b)25,26,27,28,29,30. A series of C–H functionalizations of aliphatic amines have been
developed by palladium catalysis, showing β-selectivity through a four-membered ring cyclopalladation pathway (Fig. 1c)31,32,33. Compared to small aliphatic metallacycles, the formation of
benzo-fused analogues comes with larger ring strain. We questioned whether such metallacycles could be generated through chelation with inherent nitrogen atoms in aza-arenes. Furthermore,
switching the ring size in a unifying system to differentiate of two C–H bonds would likely have a broad impact on the continued advancement of this field. As a representative example of
bicyclic aza-arenes, quinoline contains seven C − H bonds in the pyridine (C2 to C4) and benzene (C5 to C8) cores that can be functionalized34,35. Structural modification of the pyridine
ring has been achieved by taking advantage of strong electronic and steric bias36,37,38,39,40,41,42,43. An indirect route to access the benzene core involves the oxidation of quinoline to
its _N_-oxide, followed by oxygen-directed C − H functionalization and subsequent reduction to the desired products44,45,46. To provide more flexibility to access the benzene core, metal
clusters and dimers such as Os3(CO)1047, Ru3(CO)1248, and Rh2(OAc)449 have been employed in C − H activation through bridged metal-metal bonds. Enabled by bimetallic palladium catalysis, Yu
and coworkers also designed a class of remote-directing template for differentiation of C − H bonds (C5-C7) at the benzene core of quinolines50,51,52,53. Here, we report the successful
realization of a strategy for regiodivergent C–H activation of aza-arenes enabled by tunably strained metallacycles under monomeric rhodium catalysts (Fig. 1d). The precise differentiation
of two C–H bonds proceeds through a switchable three- or four-membered-ring cyclometallation pathway by just tuning the structure of the ligands. Incorporation of alkyne motifs into
aza-arenes are valuable transformations to build C–C bonds and provide a versatile handle for further modifications. RESULTS REACTION DESIGN As shown in Fig. 2, we first evaluated the
reaction of 3-methylquinoline (1A) with (bromoethynyl)triisopropylsilane (2A). After treatment of [Rh(cod)Cl]2 (5 mol%) with NaO_t_Bu (2.5 equiv) at 120 °C under an Ar atmosphere in toluene,
the desired product 3AA was generated in trace amounts after 12 h (entry 1). After screening a variety of ligands for optimization (for the details of the reaction optimization, see the
Supplementary Information), the optimized conditions for C2-alkynylation were determined, using 10 mol% dtbpy as a ligand. Under these conditions, product 3AA was afforded in 84% yield,
showing 99/1 _r.r_. of the C2 to C8 positions (entry 2). Interestingly, employing NHC ligands54 resulted in a preference for C8 selectivity, and the use of IMes·HCl led to the formation of
product 4AA in 70% yield (C8/C2 = 91/9 _r.r_.) (entry 3). Employment of [Rh(cod)Cl]2 with the optimal ligands resulted in the production of two rhodium complexes, Rh(cod)(dtbpy)Cl and
Rh(cod)(IMes)Cl, both of which were unambiguously assigned by X-ray crystallography. Utilizing the pregenerated Rh(cod)(dtbpy)Cl as the catalyst showed a much lower reactivity than the in
situ generation of the catalyst (entry 4). In contrast, the use of Rh(cod)(IMes)Cl as the catalyst improved the generation of 4AA to 85% yield with 92/8 _r.r_. (entry 5). SCOPE OF THE
METHODOLOGY The scope of the regiodivergent C–H alkynylation was then examined (Fig. 3). In general, a series of commercially available quinolines with varied substituents reacted smoothly
with bromoalkyne 2A, yielding two types of alkynylation products under reaction conditions A and B. The reaction of quinoline (1B) with bromoalkyne 2A underwent C2- and C8-selective C–H
alkynylation, providing products 3BA (98/2 _r.r_.) and 4BA (89/11 _r.r_.) with excellent regioselectivities. The quinolines that incorporated electron-donating groups, including phenyl (1C),
ether (1D-E), silyloxy (1 F), and amino (1G-H) groups, at different positions were readily tolerated with bromoalkyne 2A to produce the corresponding products 3CA-HA and 4CA-HA with
excellent regioselectivity under both reaction conditions. Halide-containing quinolines 1I-P bearing F, Cl, Br, and even I motifs were compatible with the generation of both the C2 and C8
alkynylation products. Among them, substrates 1K-L containing substituents at the C7 position did not sterically block the reaction at the C8 position. Quinoline 1Q bearing a CF3 group was
also compatible with both sets of reaction conditions, affording 3QA and 4QA in 87% and 64% yields, respectively. The use of 6-cyanoquinoline (1R) under condition A became too sluggish to
provide product 3RA, but this substrate did not interfere with productive C–H alkynylation at the C8 position. Quinoline 1 S, containing a phenylethynyl motif at the C3 position inhibited
the reactivity at the C2 position but enabled C8-selective C–H alkynylation to generate product 4SA. As a bidentate nitrogen ligand, 2,2′-biquinoline (1T) could also engage under reaction
conditions B to deliver the expected products 4TA in a good yield. We further explored the scope of alkynyl bromides with quinoline 1A. Compared to reagent 2A, the reaction of TIPS-protected
ethynyl chloride (2A’) showed much lower reactivity in both reaction systems. Alkynylation with propargyl silyl ethers 2B-C afforded the desired products in significantly lower yields, but
maintained the excellent regioselectivity. Alkynyl bromides 2D with a less sterically hindered phenyl group were problematic under both reaction conditions, and the corresponding products
3AD and 4AD were observed in only trace amounts. The poor outcomes are attributed to the lower stability of these bromoalkynes without TIPs group under the reaction conditions55,56,57,58,59.
Other aza-arenes commonly featured in bioactive molecules and functional materials were next targeted. Benzo[_f_]quinoline (5A) was first employed in the catalytic systems and gave desired
products 6AA and 7AA at the C3 and C5 positions, respectively (Fig. 4a). Phenanthridine (5B), the key aromatic unit of many DNA stains, underwent regiodivergent C–H alkynylation at the C6
and C4 positions in high yields with excellent selectivities (Fig. 4b). Treatment of 4,7-phenanthroline (5C) containing two azine motifs under reaction conditions A afforded a promising
mixture of mono- and di-substituted products 6CA and 6CA’ with a high regioselectivity (Fig. 4c). Similarly, the two products 7CA and 7CA’ were also generated under reaction conditions B
with excellent regioselectivity. Notably, the products with one or two alkynyl motifs could be separated by column chromatography on silica. When 1,7-phenanthroline (5D) was treated under
reaction conditions A, C–H alkynylation occurred at the C8 position. However, an unusual C10-functionalized product 7DA’ was obtained under reaction conditions B, indicating that C–H
metalation process preferred to undergo five-membered metallacyclic intermediate (Fig. 4d). Finally, acridine (5E), bearing two C–H bonds (C4 and C5) for activation, gave only the
monosubstituted product 7EA in 85% yield with complete selectivity (Fig. 4e). SYNTHETIC APPLICATIONS To showcase the synthetic utility of this discovery, gram-scale reactions of quinolone 1J
were first performed under both reaction conditions, and products 3JA and 4JA were isolated in 96% and 85% yields without erosion of the regioselectivity (Fig. 5a). Further derivatizations
of 3JA were achieved with synthetically useful intermediate 8, which was obtained in 70% yield by the TBAF-mediated removal of the TIPS group. For instance, hydrogenation of 8 under 1 atm of
hydrogen with a catalytic amount of Pd/BaCO3 yielded alkylation product 9A in high yield. The use of the Wilkinson catalyst for hydrogenation allowed for the chemoselective synthesis of
olefin 9B. The terminal triple bond in 8 could be effectively transformed into an internal bond to provide product 9C in nearly quantitative conversion through the Sonogashira reaction. Ag
catalysis of the reaction of 8 with NBS formed bromination product 9D in excellent yield. In addition, alkyne 8 was easily converted to triazole 9E with BnN3 via a copper-catalysed click
reaction. Based on diverse alkyne conversion, our strategy provides a simple and distinct way to construct a number of pharmaceutically relevant compounds and materials. The reaction of
quinoline 10 under Conditions B furnished C2-alkynylation, and further removal of the TIPS group provided Compound 11 in good yield, acting as a core framework for the tautomerase inhibitor
(Fig. 5b). As a key intermediate for the synthesis of montelukast, Compound 13 was rapidly prepared from quinoline 12 by C2-selective C–H alkynylation with bromoalkyne 2A, further removal of
the TIPS group and reduction with the Wilkinson catalyst under 1 atm of hydrogen (Fig. 5c). Compound 15 is a key intermediate in the synthesis of BN-dibenzo[a,o]picene, and
3,8-dibromo-4,7-phenanthroline (14) must be pregenerated through multistep synthesis from 5C to access this molecule by Sonogashira coupling (Fig. 5d)60. Notably, this molecule can be
prepared in an atom- and step-economic way, further indicating the value of the developed method. MECHANISTIC STUDY In order to gain insights into the reaction pathways and origin of
positional selectivity, density functional theory (DFT) calculations were performed using the reaction of quinoline 1B and alkynyl bromide 2A as the model (Fig. 6). The reaction initially
commences by the formation of 1B and the ligand bound Rh(I) intermediate INT1A, which is set as the relative zero point of the Gibbs free energy. The previous reported Rh(I)-involved C–H
activation by oxidative addition might be a reversible process with an activation energy barrier of 26.2 kcal mol−1 (for the details of the computational data, see the Supplementary
Information)36,37,38. Comparably, INT1A more favorably coordinates with 2A to generate INT2A, which then undergoes C–Br bond oxidative addition to afford Rh(III) species INT3A. In the case
of the dtbpy ligand, the C–Br oxidative addition through transition state TS3A-DTBPY was calculated to have an activation free energy of 27.6 kcal mol−1. This step is the rate-determining
step in the catalytic cycle. The resulting intermediate INT3A-DTBPY occurs C2–H metalation through a _t_butoxide-assisted deprotonation with a 25.8 kcal mol−1 energy
barrier61,62,63,64,65,66. Further reductive elimination and ligand exchange yield the desired products with high selectivity and regenerates INT1A to complete the catalytic cycle. For the
IMes ligand, the C–Br bond oxidative addition to the Rh(I) center from INT2A-IMES has an activation energy of 26.7 kcal mol−1, which shows a comparable energy barrier with the subsequent C–H
activation (26.1 kcal mol−1). In the following C − H metalation, IMes ligand favors C8 selective transition state TS4B-IMES. As shown in Fig. 7, extensive computational studies on different
site selectivity were also carried out. The molecular electrostatic potential map was used to analysis the possible nucleophilic sites of quinoline 1B (Fig. 7a). Quantitatively, the natural
population analysis of 1A shows that C2 has an atomic charge of −0.069e, which is less charged than C8 (−0.192e), illustrating C2 − H is relative electron deficient and more prone to C − H
metallization with electron-rich rhodium catalyst (Supplementary Data 1). Inspecting computed electrostatic potential maps of RH−DTBPY and RH−IMES, the dtbpy ligand exhibits more
electron-donating ability than IMes, allowing INT3A-DTBPY to preferentially react with the C2 position of 1B. The energy barrier of the rhodacycle transition state TS4A-DTBPY that delivers
the three-membered intermediate INT4A-DTBPY is 8.2 kcal mol−1 lower than that of the competing transition state TS4B-DTBPY that leads to the INT4B-DTBPY (Fig. 7b). This energy difference is
consistent with the experimentally excellent site selectivity of 3BA. Without the nitrogen chelation, transition states TS4C-DTBPY and TS4D-DTBPY have activation barriers of 42.0 and 43.9
kcal mol−1, respectively. Such high computed energies are mainly ascribed to the intrinsic inertness of the C − H bonds at the C2 and C8 position of quinoline, indirectly illustrating the
importance of the directing group. The directed C–H metalation transition state through TS4B-IMES has an activation Gibbs free energy of 26.1 kcal mol−1, which is 17.3 kcal mol−1 lower than
the same process through TS4A-IMES (26.1 and 43.4 kcal mol−1) and 11.2 kcal mol−1 lower than the original nucleophilic attack at quinoline C2 position through TS4C-IMES (26.1 and 37.3 kcal
mol−1). In addition, the steric maps around the Rh(III) centre with IMes ligand were analyzed based on the SambVca 2.1 tool (Fig. 7c)65,66. All transition states adopt trigonal bipyramidal
geometries, where Br atom and O_t_Bu group occupy the two vertex positions and the IMes ligand in the NW quadrant of the steric map extends to the NE and SW quadrants. In disfavored
transition state TS4A-IMES, bicyclo 3-4 fused rhodacycle enables the Rh−C bond formation to occur in the SW quadrant, which suffers from significant repulsion with Mes group of the carbene
ligand. The favored transition state TS4B-IMES is a bicyclo 4-4 fused rhodacycle, the expansion of ring size makes the quinoline backbone more horizontally extended and reduce the steric
hindrance between quinoline and TIPS group, thereby effectively lowering the energy barrier of TS4B-IMES. In TS4C-IMES and TS4D-IMES, Rh−N bond distances are elongated to 2.85 and 3.57 Å,
respectively, indicating nitrogen atom in 1B is not anchored around the Rh(III) centre. Taken together, the electronic and steric effects account for the tunability of the strained
metallacycles, achieving different positional selectivity67,68. DISCUSSION In conclusion, the present results demonstrate an important initial advance in C–H activation through a benzo-fused
three- or four-membered ring cyclometallation pathway in a switchable mode. This chemistry provides a unique tool for the functionalization of high-value aza-arenes with divergent site
selectivities controlled by ligands. The switch of the positional selectivity through strained metallacycles fills a major methodological gap in directed C–H activation. We anticipate that
other transformations based on this strategy could be exploited for molecular editing of aza-arene C–H bonds, providing inspiration for the design of new tactics to produce complex bioactive
molecules, natural products and functional materials. METHODS Due to slight variations in experimental protocols for all the processes we present, we refer the reader to the Supplementary
Methods for experimental details. DATA AVAILABILITY Crystallographic data for the structures of Rh(cod)(dtbpy)Cl, Rh(cod)(IMes)Cl, 3IA, 7CA’ and 7DA’ reported in this paper have been
deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2192163, 2192164, 2192165, 2192166 and 2192167 (Supplementary Data 2). Copies of the data can be
obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are
available within the paper and its Supplementary Information, or from the corresponding author upon reasonable request. REFERENCES * Bergman, R. G. C–H activation. _Nature_ 446, 391–393
(2007). Article ADS CAS PubMed Google Scholar * Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. _Chem. Soc. Rev._ 40, 4740–4761
(2011). Article CAS PubMed Google Scholar * Dong, Z. et al. Transition-metal-catalyzed C–H alkylation using alkenes. _Chem. Rev._ 117, 9333–9403 (2017). Article CAS PubMed Google
Scholar * Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. _Chem. Rev._ 117, 8787–8863 (2017). Article CAS PubMed Google Scholar * Wang,
C.-S., Dixneuf, P. H. & Soulé, J.-F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. _Chem. Rev._ 118, 7532–7585 (2018). Article CAS PubMed Google Scholar * Davies,
H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. _Nat. Rev. Chem._ 3, 347–360 (2019). Article CAS PubMed PubMed Central
Google Scholar * Gandeepan, P. et al. 3d Transition metals for C–H activation. _Chem. Rev._ 119, 2192–2452 (2019). Article CAS PubMed Google Scholar * Guillemard, L., Kaplaneris, N.,
Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. _Nat. Rev. Chem._ 5, 522–545 (2021). Article CAS PubMed Google Scholar *
Zhao, B., Prabagar, B. & Shi, Z. Modern strategies for C‒H functionalization of heteroarenes with alternative coupling partners. _Chemistry_ 7, 2585–2634 (2021). Article CAS Google
Scholar * Dalton, T., Faber, T. & Glorius, F. C–H Activation: Toward sustainability and applications. _ACS Cent. Sci._ 7, 245–261 (2021). Article CAS PubMed PubMed Central Google
Scholar * Giri, R. et al. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. _Chem. Soc. Rev._ 38, 3242–3272 (2009). Article CAS PubMed
Google Scholar * Wright, J. S., Scott, P. J. H. & Steel, P. G. Iridium-catalysed C–H borylation of heteroarenes: balancing steric and electronic regiocontrol. _Angew. Chem. Int. Ed._
60, 2796–2821 (2021). Article CAS Google Scholar * Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. _Chem. Rev._ 110, 1147–1169
(2010). Article CAS PubMed PubMed Central Google Scholar * Colby, A. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C−C bond formation via heteroatom-directed C−H bond
activation. _Chem. Rev._ 110, 624–655 (2010). Article CAS PubMed PubMed Central Google Scholar * Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable
or a traceless directing group strategy. _Chem. Soc. Rev._ 43, 6906–6919 (2014). Article CAS PubMed Google Scholar * Sambiagio, C. et al. A comprehensive overview of directing groups
applied in metal-catalysed C–H functionalisation chemistry. _Chem. Soc. Rev._ 47, 6603–6743 (2018). Article CAS PubMed PubMed Central Google Scholar * Liu, B. et al.
Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. _Chem. Rev._ 121, 14957–15074 (2021). Article CAS PubMed PubMed Central Google Scholar
* Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote _meta_-C−H bond assisted by an end-on template. _Nature_ 486, 518–522 (2012). Article ADS CAS PubMed PubMed Central
Google Scholar * Tang, R., Li, G. & Yu, J.-Q. Conformation-induced remote _meta_-C−H activation of amines. _Nature_ 507, 215–220 (2014). Article ADS CAS PubMed PubMed Central
Google Scholar * Xu, H.-J. et al. Rh(III)-catalyzed _meta_-C–H alkenylation with alkynes. _J. Am. Chem. Soc._ 141, 76–79 (2019). Article CAS PubMed Google Scholar * Dutta, U., Maiti,
S., Bhattacharya, T. & Maiti, D. Arene diversification through distal C(sp2)−H functionalization. _Science_ 372, eabd5992 (2021). Article CAS PubMed Google Scholar * Dutta, U. &
Maiti, D. Emergence of pyrimidine-based meta-directing group: Journey from weak to strong coordination in diversifying _meta_-C–H functionalization. _Acc. Chem. Res._ 55, 354–372 (2022).
Article CAS PubMed Google Scholar * He, C., Whitehurst, W. G. & Gaunt, M. J. Palladium-catalyzed C(sp3)–H bond functionalization of aliphatic amines. _Chem_ 5, 1031–1058 (2019).
Article CAS Google Scholar * Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. _Chem. Rev._ 120, 2613–2692
(2020). Article CAS PubMed Google Scholar * Herzon, S. B. & Hartwig, J. F. Direct, Catalytic hydroaminoalkylation of unactivated olefins with N-alkyl arylamines. _J. Am. Chem. Soc._
129, 6690–6691 (2007). Article CAS PubMed PubMed Central Google Scholar * Koperniku, A., Foth, P. J., Sammis, G. M. & Schafer, L. L. Zirconium hydroaminoalkylation. An alternative
disconnection for the catalytic synthesis of α-arylated primary amines. _J. Am. Chem. Soc._ 141, 18944–18948 (2019). Article CAS PubMed Google Scholar * Manßen, M. & Schafer, L. L.
Titanium catalysis for the synthesis of fine chemicals—development and trends. _Chem. Soc. Rev._ 49, 6947–6994 (2020). Article PubMed Google Scholar * DiPucchio, R. C. et al. Direct,
catalytic α-alkylation of N-heterocycles by hydroaminoalkylation: Substrate effects for regiodivergent product formation. _J. Am. Chem. Soc._ 143, 11243–11250 (2021). Article CAS PubMed
Google Scholar * Manßen, M. & Schafer, L. L. Early transition metal-catalyzed hydroaminoalkylation. _Trends Chem._ 3, 428–429 (2021). Article Google Scholar * DiPucchio, R. C., Rosca,
S.-C. & Schafer, L. L. Hydroaminoalkylation for the catalytic addition of amines to alkenes or alkynes: Diverse mechanisms enable diverse substrate scope. _J. Am. Chem. Soc._ 144,
11459–11481 (2022). Article CAS PubMed Google Scholar * McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give
strained nitrogen heterocycles. _Nature_ 510, 129–133 (2014). Article ADS CAS PubMed Google Scholar * He, C. & Gaunt, M. J. Ligand-enabled catalytic C−H arylation of aliphatic
amines by a four-membered ring cyclopalladation pathway. _Angew. Chem. Int. Ed._ 54, 15840–15844 (2015). Article CAS Google Scholar * Willcox, D. et al. A general catalytic β-C–H
carbonylation of aliphatic amines to β-lactams. _Science_ 354, 851–857 (2016). Article ADS CAS PubMed Google Scholar * Stephens, D. E. & Lariono, O. V. Recent advances in the
C–H-functionalization of the distal positions in pyridines and quinolones. _Tetrahedron_ 71, 8683–8716 (2015). Article CAS PubMed PubMed Central Google Scholar * Prabagar, B., Yang, Y.
& Shi, Z. Site-Selective C−H functionalization to access the arene backbone of indoles and quinolines. _Chem. Soc. Rev._ 50, 11249–11269 (2021). Article CAS PubMed Google Scholar *
Lewis, J. C., Bergman, R. G. & Ellman, J. A. Rh(I)-Catalyzed alkylation of quinolines and pyridines _via_ C−H bond activation. _J. Am. Chem. Soc._ 129, 5332–5333 (2007). Article CAS
PubMed PubMed Central Google Scholar * Berman, A. M., Lewis, J. C., Bergman, R. G. & Ellman, J. A. Rh(I)-catalyzed direct arylation of pyridines and quinolines. _J. Am. Chem. Soc._
130, 14926–14927 (2008). Article CAS PubMed PubMed Central Google Scholar * Berman, A. M., Bergman, R. G. & Ellman, J. A. Rh(I)-catalyzed direct arylation of azines. _J. Org. Chem._
75, 7863–7868 (2010). Article CAS PubMed PubMed Central Google Scholar * Ye, M., Gao, G.-L. & Yu, J.-Q. Ligand-promoted C-3 selective C–H olefination of pyridines with Pd
catalysts. _J. Am. Chem. Soc._ 133, 6964–6967 (2011). Article CAS PubMed Google Scholar * Nakao, Y., Yamada, Y., Kashihara, N. & Hiyama, T. Selective C-4 alkylation of pyridine by
nickel/Lewis acid catalysis. _J. Am. Chem. Soc._ 132, 13666–13668 (2010). Article CAS PubMed Google Scholar * Tsai, C.-C. et al. Bimetallic nickel aluminun mediated para-selective
alkenylation of pyridine: direct observation of η2,η1-pyridine Ni(0)-Al(iii) intermediates prior to C–H bond activation. _J. Am. Chem. Soc._ 132, 11887–11889 (2010). Article CAS PubMed
Google Scholar * Kim, J. H. et al. A radical approach for the selective C–H borylation of azines. _Nature_ 595, 677–683 (2021). Article ADS CAS PubMed Google Scholar * Zhang, X. et al.
Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines. _Nature_ 594, 217–222 (2021). Article ADS CAS PubMed Google Scholar * Hwang, H., Kim, J., Jeong, J. &
Chang, S. Regioselective introduction of heteroatoms at the C-8 position of quinoline N-oxides: Remote C–H activation using N-oxide as a stepping stone. _J. Am. Chem. Soc._ 136, 10770–10776
(2014). Article CAS PubMed Google Scholar * Shukla, R. K., Nair, A. M., Khan, S. & Volla, C. M. R. Cobalt-catalyzed C8-dienylationof quinoline-N-oxides. _Angew. Chem. Int. Ed._ 59,
17042–17048 (2020). Article CAS Google Scholar * Stephens, D. E. et al. Palladium-catalyzed C8-selective C−H arylation of quinoline N‑oxides: insights into the electronic, steric, and
solvation effects on the site selectivity by mechanistic and DFT computational studies. _ACS Catal._ 5, 167–175 (2015). Article CAS PubMed Google Scholar * Shapley, J. R., Samkoff, D.
E., Bueno, C. & Churchill, M. R. Reaction of Os3(CO)10(NCCH3)2 with imidazole and related aromatic heterocycles. The crystal and molecular structure of (.mu.-H)Os3(CO)10
(.mu.-3,4-.eta.2-N2C3H3. _Inorg. Chem_. 21, 634–639 (1982). * Fukuyama, T. et al. Ru3(CO)12-Catalyzed site-selective carbonylation reactions at a C−H Bond in aza-heterocycles. _J. Am. Chem.
Soc._ 120, 11522–11523 (1998). Article CAS Google Scholar * Kwak, J., Kim, M. & Chang, S. Rh(NHC)-catalyzed direct and selective arylation of quinolines at the 8-position. _J. Am.
Chem. Soc._ 133, 3780–3783 (2011). Article CAS PubMed Google Scholar * Zhang, Z., Tanaka, K. & Yu, J.-Q. Remote site-selective C–H activation directed by a catalytic bifunctional
template. _Nature_ 543, 538–542 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Ramakrishna, K. et al. Coordination assisted distal C–H alkylation of fused heterocycles.
_Angew. Chem. Int. Ed._ 58, 13808–13812 (2019). Article CAS Google Scholar * Shi, H. et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. _Nat. Chem._
12, 399–404 (2020). Article CAS PubMed PubMed Central Google Scholar * Fan, Z. et al. Molecular editing of aza-arene C–H bonds by distance, geometry and chirality. _Nature_ 610, 87–93
(2022). Article ADS CAS PubMed Google Scholar * Zhao, Q., Meng, G., Nolan, S. P. & Szostak, M. N-Heterocyclic carbene complexes in C–H activation reactions. _Chem. Rev._ 120,
1981–2048 (2020). Article CAS PubMed PubMed Central Google Scholar * Ano, Y., Tobisu, M. & Chatani, N. Palladium-catalyzed direct ethynylation of C(sp3)–H bonds in aliphatic
carboxylic acid derivatives. _J. Am. Chem. Soc._ 133, 12984–12986 (2011). Article CAS PubMed Google Scholar * He, J., Wasa, M., Chan, K. S. L. & Yu, J.-Q. Palladium(0)-catalyzed
alkynylation of C(sp3)−H bonds. _J. Am. Chem. Soc._ 135, 3387–3390 (2013). Article CAS PubMed Google Scholar * Viart, H. M.-F., Bachmann, A., Kayitare, W. & Sarpong, R. β‑Carboline
amides as intrinsic directing groups for C(sp2)−H functionalization. _J. Am. Chem. Soc._ 139, 1325–1329 (2017). Article CAS PubMed Google Scholar * Liao, G. et al. Scalable,
Stereocontrolled formal syntheses of (+)-Isoschizandrin and (+)-Steganone: Development and applications of palladium(II)-catalyzed atroposelective C−H alkynylation. _Angew. Chem. Int. Ed._
57, 3661–3665 (2018). Article CAS Google Scholar * Mondal, A. et al. Sterically controlled late-stage C−H alkynylation of arenes. _J. Am. Chem. Soc._ 141, 18662–18667 (2019). Article CAS
PubMed Google Scholar * Neue, B., Araneda, J. F., Piers, W. E. & Parvez, M. BN-Dibenzo[a,o]picenes: analogues of an unknown polycyclic aromatic hydrocarbon. _Angew. Chem. Int. Ed._
52, 9966–9969 (2013). Article CAS Google Scholar * Qi, X., Li, Y., Bai, R. & Lan, Y. Mechanism of rhodium-catalyzed C−H functionalization: advances in theoretical investigation. _Acc.
Chem. Res._ 50, 2799–2808 (2017). Article CAS PubMed Google Scholar * Davies, D. L., Macgregor, S. A. & McMullin, C. L. Computational studies of carboxylate-assisted C−H activation
and functionalization at group 8−10 transition metal centers. _Chem. Rev._ 117, 8649–8709 (2017). Article CAS PubMed Google Scholar * Lapointe, D. & Fagnou, K. Overview of the
mechanistic work on the concerted metallation-deprotonation pathway. _Chem. Lett._ 39, 1118–1126 (2010). Article Google Scholar * Funes-Ardoiz, I. & Maseras, F. Oxidative coupling
mechanisms: current state of understanding. _ACS Catal._ 8, 1161–1172 (2018). Article CAS Google Scholar * Balcells, D., Clot, E. & Eisenstein, O. C−H bond activation in transition
metal species from a computational perspective. _Chem. Rev._ 110, 749–823 (2010). Article CAS PubMed Google Scholar * Jiang, Y.-Y., Man, X. & Bi, S. Advances in theoretical study on
transition-metal-catalyzed C−H activation. _Sci. China: Chem._ 59, 1448–1466 (2016). Article CAS Google Scholar * Falivene, L. et al. Towards the online computer-aided design of catalytic
pockets. _Nat. Chem._ 11, 872–879 (2019). Article CAS PubMed Google Scholar * Falivene, L. et al. SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps.
_Organometallics_ 35, 2286–2293 (2016). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the National Natural Science Foundation of China (Grants
22025104, 22171134, 21972064, and 21901111), the Fundamental Research Funds for the Central Universities (Grant 020514380254) for their financial support and Tang Scholar. We are also
grateful to the High-Performance Computing Center of Nanjing University for performing the numerical calculations in this paper on its blade cluster system. AUTHOR INFORMATION Author notes *
These authors contributed equally: Longlong Xi, Minyan Wang. AUTHORS AND AFFILIATIONS * State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center
(ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, China Longlong Xi, Minyan Wang, Yong Liang, Yue Zhao & Zhuangzhi Shi Authors * Longlong Xi View
author publications You can also search for this author inPubMed Google Scholar * Minyan Wang View author publications You can also search for this author inPubMed Google Scholar * Yong
Liang View author publications You can also search for this author inPubMed Google Scholar * Yue Zhao View author publications You can also search for this author inPubMed Google Scholar *
Zhuangzhi Shi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.X. developed the catalysts and reactions and performed and analyzed
experiments. M.W. performed the DFT calculation. Y.L. supervised the DFT calculation and commented on the manuscript. Y.Z. performed the crystallographic studies; Z.S. conceived and
supervised the project and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Zhuangzhi Shi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF
ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xi, L., Wang, M., Liang, Y. _et al._ Tunably strained metallacycles enable modular
differentiation of aza-arene C–H bonds. _Nat Commun_ 14, 3986 (2023). https://doi.org/10.1038/s41467-023-39753-2 Download citation * Received: 15 May 2023 * Accepted: 23 June 2023 *
Published: 06 July 2023 * DOI: https://doi.org/10.1038/s41467-023-39753-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative