Play all audios:
ABSTRACT Receptor-mediated transport of soluble proteins is nature’s key to empowering eukaryotic cells to access a plethora of macromolecules, either by direct accumulation or as products
from resulting biochemical pathways. The transport efficiency of these mechanisms results from the receptor’s capability to capture, transport, and release ligands on the one hand and the
cycling ability that allows for performing multiple rounds of ligand transport on the other. However, the plant VACUOLAR SORTING RECEPTOR (VSR) protein family is diverse, and their
ligand-specificity and bidirectional trafficking routes and transport mechanisms remain highly controversial. Here we employ nanobody-epitope interaction-based molecular tools to assess the
function of the VSR 7 in vivo. We demonstrate the specificity of the VSR7 for sequence-specific vacuolar sorting signals, and we trace its anterograde transport and retrograde recycling
route. VSR7 localizes at the _cis_-Golgi apparatus at steady state conditions and transports ligands downstream to release them in the _trans-_Golgi network/early endosome (TGN/EE) before
undergoing clathrin-dependent recycling from the TGN/EE back to the _cis_-Golgi. SIMILAR CONTENT BEING VIEWED BY OTHERS HOPS, CORVET AND NEWLY-IDENTIFIED HYBRID TETHERING COMPLEXES
CONTRIBUTE DIFFERENTIALLY TOWARDS MULTIPLE MODES OF ENDOCYTOSIS Article Open access 31 October 2023 CARGO SORTING ZONES IN THE _TRANS_-GOLGI NETWORK VISUALIZED BY SUPER-RESOLUTION CONFOCAL
LIVE IMAGING MICROSCOPY IN PLANTS Article Open access 26 March 2021 ARF1 COMPARTMENTS DIRECT CARGO FLOW VIA MATURATION INTO RECYCLING ENDOSOMES Article Open access 04 October 2024
INTRODUCTION A main distinction between the sorting of vacuolar proteins in plants and lysosomal proteins in mammals is the nature of the vacuolar/lysosomal sorting signal. In this regard,
mammals utilize mannose-6-phosphate residues on _N_-linked oligosaccharyl chains that emerge upon posttranslational glycan modification in the _trans_-Golgi network1, the last fenestrated
cisternae of the Golgi apparatus. These signals are recognized by mannose-6-phosphate receptors2 that operate between the TGN and the early endosome (EE) and the plasma membrane (PM) and the
EE, respectively. In sharp contrast, plant vacuolar sorting signals are not based on glycosylation but are encoded in short amino acid sequences and are thus recognizable immediately upon
protein synthesis and folding in the endoplasmic reticulum (ER)3. Albeit sequence-specific in their context, plant vacuolar sorting signals are diverse. Some sequence-specific vacuolar
sorting signals (ssVSS) like the asparagine-proline-isoleucine-arginine-leucine (NPIRL) motif of the thiol protease aleurain from barley (_Hordeum vulgare_)4 or the storage protein sporamin
from sweet potato (_Ipomoea batatas_)5 locate in N-terminal propeptide of the respective protein and remain functional even if transplanted to the C-terminus6. However, the C-terminal
vacuolar sorting signals (ctVSS) like the tetrapeptide alanine-phenylalanine-valine-tyrosine (AFVY) of the bean (_Phaseolus vulgaris_) storage protein phaseolin7 strictly depends on their
location of the utmost C-terminus8. Vacuolar targeting in plants is complicated because plant cells possess functionally different types of vacuoles for lysis (LV) and protein storage (PSV)
that might originate independently9,10 and may coexist11 or transform into each other, dependent on tissue and physiological condition12,13. Plant vacuolar sorting receptors were discovered
almost 30 years ago14,15. Soon after that, it became clear that two large gene families encode vacuolar sorting receptors in higher plants, the VACUOLAR SORTING RECEPTOR (VSR) family, which
consists of seven members, termed VSR1-7 in _Arabidopsis_16,17, and the RECEPTOR HOMOLOGY-TRANSMEMBRANE-RING-H2 (RMR) family, that consists of 6 family members in _Arabidopsis_18,19,20. In
this regard, RMR proteins seem to facilitate protein transport to the PSV20, while VSR sort to LVs and PSVs21,22The VSR family is grouped phylogenetically into three distinct classes: class
I: VSR1 and VSR2; class II: VSR3 and VSR4; class III VSR5, VSR6, and VSR7)16,17,23, (Supplementary Fig. 1). Most of our knowledge regarding VSR function results only from the analysis of the
genetically redundant class I VSR1 and the class II VSRs 3 and 4, while information regarding the function of the class III VSRs 5, 6 and the most distant member, the VSR7, are scarce. In
this study, we have analyzed the role of VSR7 in direct comparison to the VSR4 as a reference. We used fusion proteins of GFP- and α-synuclein-binding variable domains of heavy-chain
antibodies from camelids termed nanobodies and epitope-tagged proteins as molecular tools to generate VSR7 sensors for assessing receptor-ligand interaction and compartment-specific tracing
of its transport route. Our results identify the VSR7 as a _cis_-Golgi localizing VSR that transports ligands downstream to the _trans-_Golgi network/early endosome (TGN/EE) and recycles
back to the _cis_-Golgi in a clathrin-dependent way. RESULTS THE VSR7 POSSESSES LIGAND-BINDING ABILITY We have recently developed a strategy for assessing compartment-specific
receptor-ligand interactions based on the nanobody-epitope interaction-triggered intermolecular assembly of sensor proteins in living cells24. To assess the function of the genetically most
distant member of the VSR protein family, the VSR7 (Supplementary Fig. 1), we have employed a VSR7 sensor system that self-assembles from the GFP-binding nanobody (NbG)-tagged N-terminal
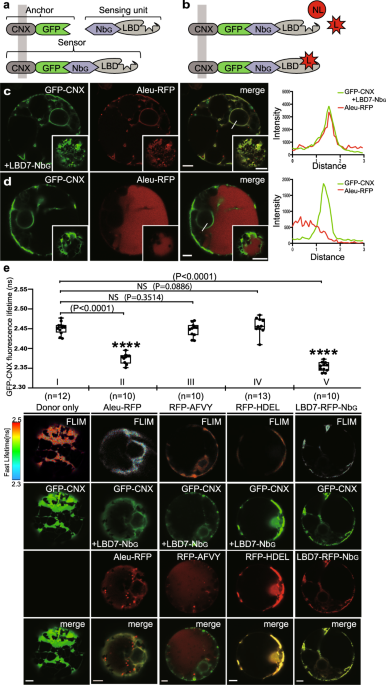
luminal binding domain (LBD) of the VSR7, LBD7-NbG as the sensing unit, and the GFP epitope-tagged, ER-localizing type I transmembrane anchor protein GFP-CNX in the lumen of the ER (Fig. 1a)
for testing the interaction with a putative red fluorescent protein (RFP)-tagged ligand (Fig. 1b). Confocal laser-scanning microscopy (CLSM)-based analysis revealed that the coexpression of
the ER-targeted VSR7 sensor GFP-CNX/LBD7-NbG with the ssVSS-carrying reporter Aleu-RFP prevented the vacuolar delivery of the reporter and caused it’s colocalization with the VSR7 sensor in
the ER, instead (Fig. 1c). In control cells that lacked the NbG-tagged LBD7, Aleu-RFP did not accumulate in the ER but showed the typical vacuolar pattern of the reporter (Fig. 1d),
suggesting that the observed ER accumulation occurred due to an interaction with the sensor in the ER. To obtain further direct evidence for VSR7 sensor-ligand interaction, we combined
fluorescence lifetime imaging microscopy (FLIM) with conventional CLSM-based colocalization analysis. In this approach, FLIM allows the detection of occurring interactions between the
GFP-based sensor and an RFP-based reporter protein as a quantifiable Förster resonance energy transfer (FRET)-induced reduction of the fluorescence lifetime of the energy-donating GFP. The
resulting FLIM images, therefore, reveal the location-specific fluorescence lifetime of the GFP as a false color-encoded image, while the conventional CLSM 3-channel imaging shows the
localization of the fluorescent proteins in the respective cell (Fig. 1e). We assessed ligand binding by recording the fluorescence lifetime of the GFP-based VSR7 sensor in the presence of
the two commonly used vacuolar reporter proteins Aleu-RFP and RFP-AFVY as putative VSR7 ligands and the ER resident reporter protein RFP-HDEL as a non-ligand (Fig. 1e, Supplementary Fig. 2).
The expression of Aleu-RFP caused a severe reduction of the GFP’s fluorescence lifetime compared to the “donor only” control, which records the location-specific fluorescence lifetime of
the GFP as a reference. The Aleu-RFP-caused reduction of fluorescence lifetime was almost as strong as the reduction that occurred due to the direct attachment of a red fluorescent fusion
protein LBD7-RFP-NbG by nanobody-epitope interaction, which served as a positive control to retrieve the strongest obtainable fluorescence lifetime reduction. Surprisingly, the
ctVSS-carrying vacuolar reporter RFP-AFVY did not colocalize with the sensor in the ER, but exhibited the typical vacuolar pattern. The FLIM analysis revealed, that the expression of the
RFP-AFVY did not significantly change the VSR7 sensor’s fluorescence lifetime, and the recorded values were comparable to those obtained when the ER-localizing non-ligand RFP-HDEL was
expressed, instead. Together, this suggests that the LBD7 sensor specifically binds to the ssVSS-carrying vacuolar reporter Aleu-RFP but does not interact with the ctVSS of the vacuolar
reporter RFP-AFVY in vivo. The key to receptor-mediated sorting processes is binding, and release of ligands when sorting is complete. Ligand binding and release depend on the biochemical
properties of a given compartment. Having shown that the lumen of the ER provides ligand binding conditions for the LBD of the VSR7, we assessed next the Golgi and the TGN/EE as the major
waystations of the vacuolar transport route, whether they promote ligand binding or release. To achieve a Golgi-specific LBD7-ligand interaction analysis, we targeted the VSR7 sensing unit
via nanobody-epitope interaction to the established GFP-epitope-tagged type II _cis_-Golgi-specific membrane protein anchor α-mannosidase 1, Man1-GFP as previously reported24 (Fig. 2).
Coexpression of the VSR7 sensor components with the ligand Aleu-RFP and a neutral Golgi marker Man1-cyan fluorescent protein (CFP) triggered an almost perfect colocalization of the ligand
Aleu-RFP with the VSR7 sensor in the Golgi (Fig. 2a). This colocalization strictly depends on the coexpression of the receptor domain and is not seen in controls lacking a luminal binding
domain24 (Fig. 2b), in which the Aleu-RFP colocalises with the MVB/LE marker CFP-BP80, a fluorescent LBD-deprived derivative of the binding protein of 80 kDa (BP80), instead (Supplementary
Fig. 3). In sharp contrast, TGN/EE targeting of the VSR7 sensor via the type II TGN/EE-specific fluorescent membrane anchor syntaxin of plants 61, SYP61-GFP, did not cause an evident
colocalization of Aleu-RFP neither with the VSR7 sensor, or the Golgi marker (Fig. 2c), and appeared to be identical to controls lacking the LBD7-NbG (Fig. 2d), indicating that the TGN/EE
could be the release compartment of the VSR7, as was previously reported for the VSR424. However, due to the strong vacuolar background signals of the Aleu-RFP, it is difficult to
quantitatively judge whether or not small amounts of the ligand interacted with the LBD7 sensor in this crucial compartment. To overcome this obstacle, we envisaged performing a quantitative
FLIM analysis in which the readout is not perturbed by even strong fluorescent signals from compartments, other than the sensor location. We targeted the LBD7-NbG sensing unit via the
compartment-specific membrane anchor SYP61-GFP to assemble VSR7 sensors in the TGN/EE, and assessed the fluorescence lifetime changes of the GFP-based VSR7 sensor in the coexpression with
either Aleu-RFP or RFP-AFVY (Fig. 2e). Althoug expressed to high levels, neither the ligand Aleu-RFP, nor RFP-AFVY altered significantly the fluorescence life time of the sensor compared to
the dual-color sensor SYP61-GFP/LBD7-RFP-NbG as positive control. This shows, that the in the early secretory pathway observed interaction between the VSR7 sensor and Aleu-RFP (Fig. 1e) did
not occur in the TGN/EE, and thus identifies the TGN/EE as the first non-binding compartment of the vacuolar transport route. We, therefore, speculated, that the TGN/EE could be the release
compartment of the VSR7-mediated transport route. VSR-ligand interaction is pH dependent, with ligand binding at neutral pH and release of ligands at acidic pH14. Since the TGN/EE is the
most acidic compartment of the vacuolar route25, we speculated that the lack of sensor-ligand interaction could be due to the low pH of the TGN/EE. To test for this, we employed the drug
concanamycin A (Conc A), a specific inhibitor of vacuolar-type H+-ATPases (V-ATPases) that was shown to affect the TGN/EE-localizing vacuolar-type H+-ATPase subunit a1 (VHA-a1)26 thereby
inhibiting the acidification of the TGN/EE25, and performed a FLIM-based ligand interaction analysis (Fig. 2e). The drug strongly reduced the vacuolar pattern of Aleu-RFP and AFVY in the
localization analysis and increased the fluorescence lifetime of the donor-only control to values similar to those recorded in the FLIM analysis with the ER-targeted GFP-CNX-based sensor
before (compare Fig. 2e (V) to Fig. 1e (I)), suggesting the change of the compartmental pH perturbed the vacuolar transport. At these conditions, Aleu-RFP strongly reduced the fluorescence
lifetime of the VSR7 sensor to levels comparable to the dual-color sensor SYP61-GFP/LBD7-RFP-NbG, but RFP-AFVY had no significant influence on the fluorescence lifetime of the VSR7 sensor.
Together, this suggests that the VSR7-ligand interaction is pH dependent, with ligand binding at neutral pH in the early secretory pathway and release at acidic pH in the TGN/EE. THE VSR7 IS
A _CIS_-GOLGI-LOCALIZING VSR THAT CYCLES BETWEEN THE _CIS_-GOLGI AND THE TGN/EE To elucidate VSR7 function, we performed a localization analysis using a fluorescent, HA-tagged full-length
receptor, GFP-VSR7 (Fig. 3), in coexpression with the TGN/EE-localizing fluorescent full-length VSR4, RFP-VSR4 (Supplementary Fig. 4) and fluorescent markers for the TGN/EE (SYP61-RFP), the
MVB/LE (RFP-BP80), the _trans-_Golgi (sialyl transferase (ST)-RFP), and the _cis-_Golgi (Man1-RFP) (Fig. 3a–e). Surprisingly, at steady state conditions, GFP-VSR7 colocalized only with the
_cis-_Golgi marker Man1-RFP, and no overlap of signals was recorded in case of coexpression with any of the other compartmental markers or the VSR4 (see Supplementary Fig. 5 for
quantification). This suggests that the VSR7 is a _cis-_Golgi-localizing vacuolar sorting receptor. Since our VSR7 sensor-based ligand binding analysis identified the _cis_-Golgi as a
compartment that promotes ligand-binding, we tested next the _cis_-Golgi-localizing full-length VSR7 for its ligand-specificity regarding the two types of VSS (Fig. 3f–i). Coexpression of
GFP-VSR7 together with the ssVSS-carrying ligand Aleu-RFP or the ctVSS-carrying non-ligand RFP-AFVY together with _cis_-Golgi or TGN/EE marker, respectively, revealed an overlap of signals
from GFP-VSR7 and Aleu-RFP (Fig. 3f, g) but not RFP-AFVY (Fig. 3h, i), demonstrating that the full-length VSR binds specifically to the ssVSS but not to the ctVSS in vivo. Interestingly,
irrespective of the coexpression with the ligand Aleu-RFP or the non-ligand RFP-AFVY, all GFP-VSR7 signals were found to localize only in the _cis_-Golgi, the identified ligand binding
compartment (Fig. 3f, h) but none of the receptor signals were found to localize in the TGN/EE, the identified ligand-release compartment (Fig. 3g, i). This raised the question of whether
and how a _cis-_Golgi-localizing VSR can mediate the transportation of ligands in the vacuolar route. Based on our ligand-binding analysis, we hypothesized that the _cis_-Golgi-localizing
VSR7 could travel downstream to the TGN/EE for ligand release. We have previously implemented nanobody/epitope-tagged fusion proteins to demonstrate the retrograde upstream recycling of the
TGN/EE-localizing VSR4 to the _cis_-Golgi27. We wanted to take further advantage of the specificity and sensitivity of nanobody-epitope interaction-triggered intermolecular assembly
reactions to test our hypothesis regarding putative downstream trafficking of the _cis_-Golgi-localizing VSR7 to the TGN/EE. For this, we envisaged using NbG-tagged receptors together with
GFP epitope-labeled TGN/EE-localizing membrane anchor proteins, which would catch and trap transiting receptors immediately upon their arrival in the TGN/EE via nanobody-epitope interaction
(Fig. 4a). However, the realization of such an approach in vivo is complicated by the fact that translational nanobody- and epitope-tagged fusion proteins cannot simply be coexpressed for
this purpose because they will bind to each other already in the ER, immediately after synthesis and folding is completed. In this case, the proteins would travel alongside, and it would be
impossible to set up a trap for incoming receptors at a downstream location. To overcome these constraints, we outsourced the synthesis of the required GFP-epitope and opted for an
early-endosomal-location-specific posttranslational GFP-labelling of the TGN/EE-localizing membrane anchor, instead of using a translational GFP-fusion protein. To achieve this, we employed
an additional nanobody-epitope pair, the α-synuclein binding nanobody (NbS), and its 23 amino acid-long epitope, SYN, to drive the posttranslational attachment of the GFP-epitope as an
SYN-tagged GFP protein, GFP-SYN27, to an NbS-tagged TGN/EE membrane anchor. This dual-epitope protein GFP-SYN is produced as a secretory protein (secGFP-SYN) by a separate population of
protoplasts, is recovered from their culture medium, and is used in a second step for the posttranslational labeling in endocytic uptake experiments with protoplasts, expressing the
respective NbG/NbS-tagged receptor/anchor proteins24,27. In this scenario, the SYN epitope of the endocytosed dual-epitope protein secGFP-SYN would posttranslationally label the NbS-tagged
cyan fluorescent TGN/EE marker SYP61-CFP-NbS, while the GFP epitope would serve as the bait for trapping the arriving NbG-tagged red fluorescent VSRs, NbG-RFP-VSR7 (Fig. 4aII, see
Supplementary Fig. 6 for quantification). For the successful application of this strategy, it was important to ensure that the NbG-tagging of the red fluorescent VSR7 does not alter the
_cis_-Golgi localization of the receptor (Fig. 4b). Most important however was to demonstrate that the endocytosed dual-epitope linker secGFP-SYN follows the default route to the vacuole,
but does not reach the _cis_-Golgi. For this we performed endocytic uptake assays showing that the endocytosed secGFP-SYN does not colocalize with the _cis-_Golgi marker Man1-CFP (Fig. 4c),
or even with the NbG-tagged _cis-_Golgi marker Man1-RFP-NbG (Fig. 4d). Of similar importance was to ensure that the coexpression of the two nanobody-tagged proteins NbG-RFP-VSR7 and
SYP61-CFP-NbS, does not result in any colocalization of the fusion proteins in the absence of the dual-epitope linker protein due to unforeseeable unspecific interactions (Fig. 4e). In sharp
contrast to the above controls, incubation of NbG-RFP-VSR7 and SYP61-CFP-NbS-coexpressing cells with the protoplast-secreted secGFP-SYN caused an almost complete overlap of the signals from
the VSR7, the TGN/EE anchor and the dual epitope linker secGFP-SYN (Fig. 4f). Such colocalizations between the VSR7 and the TGN/EE anchor were never observed in the absence of either the
linking secGFP-SYN (e), or if the conventional TGN/EE marker SYP61-CFP, which lacks the NbS, is used, instead (Fig. 4g). This demonstrates that the _cis_-Golgi-localizing NbG-RFP-VSR7 does
indeed travel downstream of its steady-state location, and reaches the TGN/EE, where it is linked to the posttranslationally secGFP-SYN-labelled TGN/EE anchor via the NbG-GFP
nanobody-epitope interaction. Interestingly, incubation of cells coexpressing the NbG-RFP-VSR7 and the TGN/EE marker SYP61-CFP with the secGFP-SYN results in a clear overlap of signals of
only the NbG-RFP-VSR7 and the endocytosed secGFP-SYN linker, while no colocalization of these signals was detected with the SYP61-CFP (Fig. 4g). This colocalization, however, shows that the
NbG-tagged VSR7 came in contact with the endocytosed secGFP-SYN in the TGN/EE and consequently, became posttranslationally labeled. This furthermore demonstrates that the secGFP-SYN-labeled
NbG-RFP-VSR7 did indeed transit the TGN/EE, and we speculated whether the non-TGN/EE-localizing secGFP-SYN-labeled VSR7 are receptors that have already recycled from the TGN/EE. To test for
this, we subjected NbG-RFP-VSR7 and _cis_-Golgi marker Man1-CFP-expressing cells to endocytic uptake assays with secGFP-SYN (Fig. 4aIII, see Supplementary Fig. 6 for quantification) and
found that signals from the posttranslationally secGFP-SYN-labeled NbG-RFP-VSR7 colocalize of with the _cis_-Golgi marker (Fig. 4h). Together, this demonstrates that the
_cis_-Golgi-localizing VSR7 already experienced downstream traveling, TGN/EE transit and upstream recycling to the _cis_-Golgi. The demonstrated TGN/EE transit of the VSR7 is also supported
by its detection in fractions of immunoisolated SYP61-CFP or vacuolar-type H+-ATPase subunit a1 (VHA-a1)-GFP compartments in proteomic analyses28,29 even though they are non-discriminative
for proteins that have multiple locations and proteins that reside exclusively in these compartments29. THE RECYCLING OF THE _CIS_-GOLGI-LOCALIZING VSR7 DEPENDS ON CLATHRIN Our data show
that the VSR7 localizes at steady-state conditions in the _cis_-Golgi, which differs from the TGN/EE localization of the best-characterized member of the VSR protein family, the VSR4 (Fig.
3a and Supplementary Fig. 4). We speculated that the differences in the localization could be due to differences in their Golgi trafficking. Golgi trafficking might involve coat protein I
(COPI)-coated vesicles that are formed after the activation and membrane recruitment of the COPI-specific GTPase ADP-ribosylation factor 1, ARF1, which in turn, recruits the heptameric
cargo-recognizing coatomer complex. To test for differences in the transport of the two receptors, we coexpressed them with the GTP-locked dominant-negative ARF1 mutant, ARF1M30, and the
_cis_-Golgi marker or the TGN/EE marker, respectively (Fig. 5a–c, Supplementary Fig. 8, see Supplementary Fig. 7 for quantification). The coexpression of ARF1M altered the colocalization of
the RFP-VSR7 with Man1-CFP and caused its colocalization with GFP-VSR4, instead (Fig. 5a), which was never seen in the absence of the ARF1M (Fig. 5b, Supplementary Fig. 8), suggesting, that
the VSR7 has lost its _cis_-Golgi localization. However, the expression of the ARF1M did not affect the TGN/EE localization of the VSR4 (Fig. 5c, compared to Supplementary Fig. 4),
demonstrating that the mutant did not affect the anterograde transport across the Golgi stack and beyond to the TGN/EE. Therefore, we speculated that the ARF1M-caused TGN/EE colocalization
of the VSR7 with the VSR4 hints at an ARF1-dependent VSR7 recycling mechanism from the TGN/EE, ultimately leading to the _cis_-Golgi localization of the VSR7 at steady-state conditions. ARF1
has been shown to localize at the TGN/EE in addition to the Golgi stack, while the localization of the coatomer complex seems to be restricted to the Golgi stack. Interestingly, ARF1 also
plays a crucial role in the recruitment and activation of tetrameric clathrin adaptor complexes that mediate the cargo selection and formation of clathrin-coated vesicles (CCVs)31, the most
prominent type of transport vesicles, found to bud at the TGN/EE. Based on this, we speculated that the VSR7 recycles after the release of the ligand in the TGN/EE via a clathrin-dependent
transport back to the _cis_-Golgi. To test this hypothesis, we assessed the localization of the VSR7 regarding its clathrin dependency by coexpressing the dominant-negative mutant of the
clathrin heavy chain, the clathrin hub32, to inhibit clathrin-mediated trafficking (Fig. 5d–h, see Supplementary Fig. 7 for quantification). Coexpression of the fluorescent hub fragment,
GFP-Hub, with the RFP-VSR7 and SYP61-CFP trapped the VSR7 in the TGN/EE, as judged by the colocalization of RFP-VSR7 and SYP61-CFP signals (Fig. 5d), similar to the signals obtained by the
coexpression of the ARF1M (compare to overlapping red/blue peaks in the line intensity plots in Fig. 5c). Remarkably, some of the RFP-VSR7 signals did not colocalize with the TGN/EE marker.
Therefore, we tested whether the Hub-caused recycling inhibition could have resulted in the downstream progression of RFP-VSR7 to the MVB/LE, but this was not the case and no colocalization
between the RFP-VSR7 and the MVB/LE marker CFP-BP80 was evident (Fig. 5e). However, we speculated, that the inhibition of the receptor recycling might not have been completed under these
conditions. To test this hypothesis, we increased the expression time. After 48 h incubation, we found largely overlapping signals between the RFP-VSR7 and the CFP-BP80, demonstrating the
downstream progression of the VSR due to the inhibited recycling (Fig. 5f). Strikingly, the GFP-Hub did not alter the TGN/EE localization of the RFP-VSR4, and even after 48 h expression, the
RFP-VSR4 did not reach the MVB/LE at detectable amounts (Fig. 5g) and remained in the TGN/EE (Fig. 5h). Together, this suggests, that the VSR7 recycles via a clathrin-dependent transport
mechanism from the TGN/EE to the _cis_-Golgi, while the VSR4 does not join this trip. DISCUSSION Despite the apparent differences in morphology and organization of the intracellular
compartments of eukaryotic cells, receptor-mediated transport of soluble proteins follows a common principle: the sorting receptors capture the soluble proteins via their ligand binding
domain (LBD) in the compartmental lumen, thereby linking the soluble cargo protein via their cytosolic domain to the membrane trafficking machinery, which in turn fulfills the sorting and
targeting of the receptor-ligand complex. As far as the ligand is concerned, receptor-mediated transport ends with the dissociation of the receptor-ligand complex in the chemically different
environment of the target compartment. The receptor, however, undergoes retrograde recycling back to the donor compartment and performs further transport rounds. This principle is conserved
among eukaryotes, but the implementation seems to vary to take into account the morphological peculiarities of the respective system. Despite the different nature of vacuolar/lysosomal
sorting signals, MPRs and VSRs exhibit pH-dependent receptor-ligand interaction14,33,34, with ligand binding occurring at neutral to slightly acidic pH while ligand release occurs at acidic
or alkaline pH. In plants, neutral to slightly alkaline conditions are found in the ER and the Golgi stack, while the _trans_-Golgi network, which is also the early endosome (TGN/EE)26,35,
is the most acidic compartment of the vacuolar transport route25,36. This supports the findings from the compartment-specific receptor-ligand interaction analysis, showing that plant
receptors bind ligands in the early secretory pathway3,24,37 and release them already in the TGN/EE24, rather than in the multivesicular body, which is the late endosome (MVB/LE)38, as was
initially proposed14,39, before they recycle upstream to the _cis_-Golgi for further rounds of transport27. The post-TGN/EE trafficking of the released ligands to the vacuole occurs then,
together with all of the endocytosed soluble material independent of VSRs24,40 by the TGN/EE maturation-based formation of MVBs/LEs41, which ultimately fuse with the tonoplast24,41. Our
direct comparison between VSR7 and VSR4 shows, that both receptors bind the ssVSS ligand in the ER and the Golgi, but not in the TGN/EE, suggesting that the VSR7 transports ligands from the
early secretory pathway to the TGN/EE, as was previously demonstrated for the VSR424. However, both receptors exhibit differential locations at steady-state conditions, with the VSR4
localizing to the TGN/EE and the VSR7 localizing at the _cis_-Golgi. Protein transport across the Golgi stack is not fully understood. It has been suggested that anterograde transport occurs
via a cisternal maturation process that is driven by arriving material at the _cis_-face, while the Golgi-residing enzymes are constantly retrieved via COPI vesicles to maintain cisternal
functionality, thereby resulting in a constant forward movement of the cisternae, which ultimately develop into a TGN that detaches from the stack thereby becoming a Golgi-independent
TGN/EE42,43. In such a scenario, the anterograde movement of membrane proteins like the VSRs would occur at the same speed. Therefore, it is plausible to assume that the differential
location of the VSRs might be due to differences in their recycling from the TGN/EE after ligand release. In mammals, MPRs recycle from the EE to the TGN via the retromer complex44, which
was suggested to form tubular carriers45. In plants, recycling from the TGN/EE is controversial. However, subunits of the retromer complex have also been detected at the TGN/EE29,46,
biochemical evidence for a VSR1-retromer interaction was presented47, and retromer subunit VPS29 knockdown mutants showed perturbed VSR1 recycling48, suggesting that such a tubular carrier
could indeed facilitate recycling of the genetically redundant VSRs. On the other, MPRs and VSRs alike possess characteristic tyrosine-based sorting motifs, which are recognized by the
µ-adaptin subunit of tetrameric clathrin adapter protein complexes49, to facilitate the sorting of membrane proteins into clathrin-coated vesicles. Together with our observation that the
inhibition of clathrin-mediated trafficking perturbed only the recycling of the VSR7, but not the VSR4, it seems that these receptors use different recycling mechanisms that might differ in
the time it takes to form and move a carrier for efficient cargo export from the TGN/EE. In this regard it is tempting to speculate that the loading and formation of a large tubular
retromer-coated carrier could take longer than the formation of a smaller, spherical, clathrin-coated vesicle. However, this could account for a longer TGN/EE transit time and, thus,
extended visibility of the departing VSR4 compared to the VSR7 (Fig. 5i). The TGN/EE is characterized by budding clathrin-coated vesicles (CCVs) and it was recently demonstrated that it
possesses noticeably different subdomains to facilitate differential cargo export42. In this regard, it was suggested that the clathrin adaptor complex 1 (AP1) facilitates transport to the
PM. Interestingly, AP1 seems to mediate multiple transport routes in mammals and yeats50, including the recycling from a late-stage TGN to an early-stage51. Our data regarding the
compartment-specific receptor-ligand interactions and the trafficking routes of the VSRs VSR4 and VSR7 point to a sorting and transport mechanism for the VSRs, that differs from the basic
concept of receptor-mediated transport. Ligand transport is assumed to occur via a vesicle shuttle that connects spatially separated but persistent compartments, which provide ligand-binding
or ligand-release conditions. However, such a model seems not apply to the morphological situation in plants. It is, therefore, tempting to speculate that plant VSRs mediate transport not
between spatially separate persistent compartments but temporally distinct units. The TGN/EE as the target compartment ultimately emerges from the initial starting compartment in a
maturation-based process. It appears as if the sorting function of a VSR is the ligand binding at a neutral pH in the _cis-_Golgi cisternae and thus its immobilization to prevent its
secretory loss throughout the cisternae’s maturation into a VHA-a1-acidified Golgi-independent TGN with EE functionality. This is in agreement with our demonstration of the pH-dependent
ligand release in the TGN/EE, and also supports a recently suggested transport model in which the VSRs segregate vacuolar and secretory cargo in domains within the maturing TGN
compartment52. However, there are still many open questions regarding retrograde transport mechanisms that allow for the efficient recycling of sorting receptors in the endomembrane system
of plants, which will be exciting research projects for the future. METHODS PLANT MATERIALS _Nicotiana tabacum_ L. SR1 was grown on Murashige and Skoog’s medium supplemented with 2% (w/v)
sucrose, 0.5 g L–1 MES, and 0.8% (w/v) Agar at pH 5.7 in 16/8 h light-dark cycles at 22 °C. PROTOPLAST ISOLATION AND TRANSFECTION Electrotransformation-competent tobacco protoplasts were
isolated and transfected as previously described53. In short, about 2.5 million protoplasts in a total volume of 600 µL electro-transfection-buffer were electro-transfected with 1-10 µg
plasmid DNA per construct, using a 160 V single square wave pulse for 10 ms (Gene Pulser XcellTM, Bio-Rad Laboratories ltd., Shanghai). After transfection, each sample was supplemented with
2 ml incubation buffer and incubated for 16–24 h, if not indicated otherwise, at 25 °C in the dark. GENETIC CONSTRUCTS DNA manipulations were performed according to established procedures,
using pGreenII-based vectors and _Escherichia coli_ MC1061. All VSR constructs are based on _At_VSR7 (GenBank accession No. NM_001203848). The coding sequences of the GFP/α-synuclein-binding
nanobodies were synthesized according to the _Arabidopsis_ codon usage and were described previously24,27. All constructs used in this study are given in Supplementary Table 1. CONFOCAL
MICROSCOPY AND IMAGE ANALYSIS Image acquisition was performed using a confocal laser scanning microscope (Nikon A1 plus, Nikon, Japan) with a 40 × 1.15 NA water immersion objective. The
BFP2, CFP, GFP, and RFP-containing fusion proteins were excited at λ 405 nm, 455 nm, 488 nm, and 561 nm, and emission was recorded in the range of 425–475 nm, 425–475 nm, 500–550 nm, and
570–620 nm, respectively. Pinholes were adjusted to 1 Airy unit for each wavelength. Post-acquisition image processing and analysis were performed using the software ImageJ (v.1.51,
https://imagej.nih.gov/ij/index.html) and GraphPad Prism 8.0 (https://www.graphpad.com/scientific-software/prism). STATISTICS & REPRODUCIBILITY The sample size for FLIM analysis was
estimated based on previously achieved effect sizes in our lab: Effect size f (ANOVA) for the data shown in Fig. 1e was 1.24; with our desired error values (=0.001, (1-β) = 0.95) and 5
different groups of samples, this computes to a minimum of 35 total samples or 7 samples per group. Effect size f (ANOVA) for the data shown in Fig. 2e was 0.96; with our desired error
values (=0.001, (1-β) = 0.95) and first 4 different groups of samples in the absence of the drug, the other 4 different groups of samples in the presence of the drug, this computes to a
minimum of 17 total samples or 5 samples per group. The calculation was performed using G*Power Version 3.1.9.2. We increased this number to 10 samples per group to accommodate for possible
slightly weaker effect sizes or data point distributions, which would necessitate alternative non-parametric tests. For quantification of signal colocalization, the linear Pearson’s
correlation coefficient (rP) and nonlinear Spearman’s rank correlation coefficient (rS) of fluorescent signals were calculated. The calculations were performed using ImageJ software with the
PSC colocalization plug-in, and threshold levels were set to 10. For statistics, correlation coefficients of 10 individually analyzed cells per experiment were considered and are given as
average values with SD. Statistical significance was calculated using ANOVA, followed by Student’s t-test. All experiments were repeated with similar results.The number of repetitions of
each experiment is given in the respective figure legend. DATA EXCLUSION STATEMENT Protoplast that were not completely turgescent or exhibited any signs of damage and protoplasts that did
not express the respective fluorescent proteins were excluded from image acquisition. For FLIM analysis regions of interest (ROIs) were chosen in a way, that all fluorescent signals, which
did not obviously originate from Chlorophyll autofluorescence, were used to calculate average lifetimes. RANDOMIZATION STATEMENT All protoplasts for an experiments were derived from a single
pool, allocation into different transformations samples was performed by pipetting ~3*10^6 protoplasts at once. Thus individual cells were completely randomly distributed to the
transformation samples. BLINDING STATEMENT Blinding was not performed during experiments and outcome assessment. Due to characteristic morphology, distribution, and movement of fluorescently
tagged compartmental marker proteins in the cells, the investigators are able to deduct which sample they are observing. FLUORESCENCE LIFETIME IMAGING MICROSCOPY (FLIM) Experimental setup,
data acquisition, and analysis were performed as previously described54. FLIM recordings were performed using a Nikon A1R confocal laser scanning microscope (Nikon, Japan) equipped with a
PicoHarp 300 time-correlated single-photon counting (TCSPC) module and a PDL800-D picosecond diode laser driver (PicoQuant GmbH, Berlin, Germany). The energy donor GFP (GFP-CNX) was excited
at λ 485-nm with 40-MHz pulse frequency. Emission was recorded at λ 482/35 nm until a count of at least 400 photons was reached in the brightest pixel. Per sample, 10-15 cells were recorded.
FLIM data were analyzed using SymphoTime64 v2.0 (PicoQuant GmbH, Berlin, Germany). Values result from the analysis of selected regions of the cells using the software’s “region of interest”
(ROI) tools, allowing for the elimination of background noise. To calculate the fluorescence lifetimes of the donor, TCSPC histograms were reconvoluted with an instrumental response
function (IRF) and fitted against a multiexponential (_n_ = 2) decay model. Only fittings with Chi-squared values between 0.9 and 1.5 were considered. The calculated lifetimes of the
respective cells are presented as box plots with whiskers showing all data points from min to max, with the box showing the interquartile range of the middle 50%, and the median is indicated
by a line. Statistical significance was calculated as given above. CELL EXTRACTION AND IMMUNODETECTION Extraction of cells and analysis by SDS–PAGE/WB was performed with minor modifications
as previously reported55. All processed samples were mixed with an equal amount of 2× Xtreme loading dye (900 μL of sample buffer (0.1% (w/v) bromophenol blue, 5 mM EDTA, 200 mM Tris-HCl,
pH 8.8, 1 M sucrose), supplemented with 300 μL 10% (w/v) SDS and 20 μl of 1 M DTT) and denatured for 5 min at 95 °C. The antibody used was a rat monoclonal anti-HA–Peroxidase antibody (Roche
12013819001, 1:5,000). PHYLOGENETIC ANALYSIS The amino acid sequences of the members of the VSR protein families from the given species were retrieved from the National Center for
Biotechnology Information (https://www.ncbi.nlm.nih.gov) and aligned using ClustalW (https://www.ebi.ac.uk/clustalw). The aligned VSR protein sequences were further processed using Molecular
Evolutionary Genetics Analysis (MEGA) version 11 (https://www.megasoftware.net/) to generate Neighbor-Joining trees under 1000 replicates of bootstraps iterations. REPORTING SUMMARY Further
information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data supporting the findings of this study are available
within the paper and its Supplementary Information. Source data are provided with this paper. REFERENCES * Rohrer, J. & Kornfeld, R. Lysosomal hydrolase mannose 6-phosphate uncovering
enzyme resides in the trans-Golgi network. _Mol. Biol. Cell_ 12, 1623–1631 (2001). Article CAS PubMed Central PubMed Google Scholar * Gonzalez-Noriega, A., Grubb, J. H., Talkad, V.
& Sly, W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. _J. Cell Biol._ 85, 839–852 (1980). Article CAS
PubMed Google Scholar * Niemes, S. et al. Sorting of plant vacuolar proteins is initiated in the ER. _Plant J._ 62, 601–614 (2010). Article CAS PubMed Google Scholar * Holwerda, B. C.,
Padgett, H. S. & Rogers, J. C. Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. _Plant Cell_ 4, 307–318 (1992). CAS PubMed Central PubMed Google
Scholar * Matsuoka, K. & Nakamura, K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. _Proc. Natl Acad. Sci. USA_ 88, 834–838 (1991). Article ADS
CAS PubMed Central PubMed Google Scholar * Koide, Y., Hirano, H., Matsuoka, K. & Nakamura, K. The N-terminal propeptide of the precursor to sporamin acts as a vacuole-targeting
signal even at the C terminus of the mature part in tobacco cells. _Plant Physiol._ 114, 863–870 (1997). Article CAS PubMed Central PubMed Google Scholar * Frigerio, L., de Virgilio,
M., Prada, A., Faoro, F. & Vitale, A. Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. _Plant Cell_ 10, 1031–1042 (1998). Article CAS PubMed
Central PubMed Google Scholar * Dombrowski, J. E., Schroeder, M. R., Bednarek, S. Y. & Raikhel, N. V. Determination of the functional elements within the vacuolar targeting signal of
barley lectin. _Plant Cell_ 5, 587–596 (1993). CAS PubMed Central PubMed Google Scholar * Viotti, C. et al. The Endoplasmic Reticulum Is the Main Membrane Source for Biogenesis of the
Lytic Vacuole in Arabidopsis. _Plant Cell_ 25, 3434–3449 (2013). Article CAS PubMed Central PubMed Google Scholar * Hinz, G., Colanesi, S., Hillmer, S., Rogers, J. C. & Robinson, D.
G. Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. _Traffic_ 8, 1452–1464 (2007). Article CAS PubMed Google
Scholar * Paris, N., Stanley, C. M., Jones, R. L. & Rogers, J. C. Plant cells contain two functionally distinct vacuolar compartments. _Cell_ 85, 563–572 (1996). Article CAS PubMed
Google Scholar * Zheng, H. & Staehelin, L. A. Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell
type-specific mechanisms. _Plant Physiol._ 155, 2023–2035 (2011). Article CAS PubMed Central PubMed Google Scholar * Shimada, T., Takagi, J., Ichino, T., Shirakawa, M. &
Hara-Nishimura, I. Plant Vacuoles. _Annu. Rev. plant Biol._ 69, 123–145 (2018). Article CAS PubMed Google Scholar * Kirsch, T., Paris, N., Butler, J. M., Beevers, L. & Rogers, J. C.
Purification and initial characterization of a potential plant vacuolar targeting receptor. _Proc. Natl Acad. Sci. USA_ 91, 3403–3407 (1994). Article ADS CAS PubMed Central PubMed
Google Scholar * Ahmed, S. U., Bar-Peled, M. & Raikhel, N. V. Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting
receptors of eukaryotic cells. _Plant Physiol._ 114, 325–336 (1997). Article CAS PubMed Central PubMed Google Scholar * Shimada, T. et al. Vacuolar sorting receptor for seed storage
proteins in Arabidopsis thaliana. _Proc. Natl Acad. Sci. USA_ 100, 16095–16100 (2003). Article ADS CAS PubMed Central PubMed Google Scholar * Miao, Y., Yan, P. K., Kim, H., Hwang, I.
& Jiang, L. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. _Plant Physiol._
142, 945–962 (2006). Article CAS PubMed Central PubMed Google Scholar * Park, J. H., Oufattole, M. & Rogers, J. C. Golgi-mediated vacuolar sorting in plant cells: RMR proteins are
sorting receptors for the protein aggregation/membrane internalization pathway. _Plant Sci._ 172, 728–745 (2007). Article CAS Google Scholar * Park, M., Lee, D., Lee, G. J. & Hwang,
I. AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. _J. Cell Biol._ 170, 757–767 (2005). Article CAS PubMed Central PubMed Google Scholar *
Wang, H., Rogers, J. C. & Jiang, L. Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. _FEBS J._ 278, 59–68 (2011). Article
CAS PubMed Google Scholar * Shimada, T. et al. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. _Plant Cell Physiol._
47, 1187–1194 (2006). Article CAS PubMed Google Scholar * Zouhar, J., Munoz, A. & Rojo, E. Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4
sort vacuolar storage cargo in seeds and vegetative tissues. _Plant J._ 64, 577–588 (2010). Article CAS PubMed Google Scholar * De Marcos Lousa, C., Gershlick, D. C. & Denecke, J.
Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. _Plant Cell_ 24, 1714–1732 (2012). Article PubMed Central PubMed Google
Scholar * Künzl, F., Früholz, S., Fäßler, F., Li, B. & Pimpl, P. Receptor-mediated sorting of soluble vacuolar proteins ends at the trans-Golgi network/early endosome. _Nat. Plants_ 2,
16017 (2016). Article PubMed Google Scholar * Shen, J. et al. Organelle pH in the _Arabidopsis_ Endomembrane System. _Mol. Plant_ 6, 1419–1437 (2013). Article CAS PubMed Google Scholar
* Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D. & Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. _Plant Cell_ 18,
715–730 (2006). Article CAS PubMed Central PubMed Google Scholar * Früholz, S., Fässler, F., Kolukisaoglu, U. & Pimpl, P. Nanobody-triggered lockdown of VSRs reveals ligand
reloading in the Golgi. _Nat. Commun._ 9, 643 (2018). Article ADS PubMed Central PubMed Google Scholar * Drakakaki, G. et al. Isolation and proteomic analysis of the SYP61 compartment
reveal its role in exocytic trafficking in Arabidopsis. _Cell Res_ 22, 413–424 (2012). Article CAS PubMed Google Scholar * Groen, A. J. et al. Identification of trans-Golgi network
proteins in _Arabidopsis thaliana_ root tissue. _J. Proteome Res_ 13, 763–776 (2014). Article CAS PubMed Google Scholar * Pimpl, P., Hanton, S. L., Taylor, J. P., Pinto-DaSilva, L. L.
& Denecke, J. The GTPase ARF1p Controls the Sequence-Specific Vacuolar Sorting Route to the Lytic Vacuole. _Plant Cell_ 15, 1242–1256 (2003). Article CAS PubMed Central PubMed Google
Scholar * Ren, X., Farias, G. G., Canagarajah, B. J., Bonifacino, J. S. & Hurley, J. H. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1.
_Cell_ 152, 755–767 (2013). Article CAS PubMed Central PubMed Google Scholar * Liu, S. H., Marks, M. S. & Brodsky, F. M. A dominant-negative clathrin mutant differentially affects
trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. _J. Cell Biol._ 140, 1023–1037 (1998). Article CAS PubMed Central
PubMed Google Scholar * Tong, P. Y. & Kornfeld, S. Ligand interactions of the cation-dependent mannose 6-phosphate receptor. Comparison with the cation-independent mannose 6-phosphate
receptor. _J. Biol. Chem._ 264, 7970–7975 (1989). Article CAS PubMed Google Scholar * Tong, P. Y., Gregory, W. & Kornfeld, S. Ligand interactions of the cation-independent mannose
6-phosphate receptor. The stoichiometry of mannose 6-phosphate binding. _J. Biol. Chem._ 264, 7962–7969 (1989). Article CAS PubMed Google Scholar * Lam, S. K. et al. Rice SCAMP1 defines
clathrin-coated, _trans_-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. _Plant Cell_ 19, 296–319 (2007). Article CAS PubMed Central PubMed Google
Scholar * Martiniere, A., Desbrosses, G., Sentenac, H. & Paris, N. Development and properties of genetically encoded pH sensors in plants. _Front. plant Sci._ 4, 523 (2013). Article
PubMed Central PubMed Google Scholar * Watanabe, E. et al. An ER-Localized Form of PV72, a Seed-Specific Vacuolar Sorting Receptor, Interferes the Transport of an NPIR-Containing
Proteinase in Arabidopsis Leaves. _Plant Cell Physiol._ 45, 9–17 (2004). Article CAS PubMed Google Scholar * Tse, Y. C. et al. Identification of multivesicular bodies as prevacuolar
compartments in Nicotiana tabacum BY-2 cells. _Plant Cell_ 16, 672–693 (2004). Article CAS PubMed Central PubMed Google Scholar * Paris, N. et al. Molecular cloning and further
characterization of a probable plant vacuolar sorting receptor. _Plant Physiol._ 115, 29–39 (1997). Article CAS PubMed Central PubMed Google Scholar * Etxeberria, E., Gonzalez, P.,
Baroja-Fernandez, E. & Romero, J. P. Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells: evidence for the distribution of
solutes to different intracellular compartments. _Plant Signal Behav._ 1, 196–200 (2006). Article PubMed Central PubMed Google Scholar * Scheuring, D. et al. Multivesicular bodies mature
from the trans-Golgi network/early endosome in Arabidopsis. _Plant Cell_ 23, 3463–3481 (2011). Article CAS PubMed Central PubMed Google Scholar * Shimizu, Y. et al. Cargo sorting zones
in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. _Nat. Commun._ 12, 1901 (2021). Article ADS CAS PubMed Central PubMed Google
Scholar * Kang, B. H., Nielsen, E., Preuss, M. L., Mastronarde, D. & Staehelin, L. A. Electron tomography of RabA4b- and PI-4Kbeta1-labeled trans Golgi network compartments in
Arabidopsis. _Traffic_ 12, 313–329 (2011). Article CAS PubMed Google Scholar * Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R. & Bonifacino, J. S. Role of the mammalian
retromer in sorting of the cation-independent mannose 6-phosphate receptor. _J. Cell Biol._ 165, 123–133 (2004). Article CAS PubMed Central PubMed Google Scholar * Hierro, A. et al.
Functional architecture of the retromer cargo-recognition complex. _Nature_ 449, 1063–1067 (2007). Article ADS CAS PubMed Central PubMed Google Scholar * Niemes, S. et al. Retromer
recycles vacuolar sorting receptors from the trans-Golgi network. _Plant J._ 61, 107–121 (2010). Article CAS PubMed Google Scholar * Oliviusson, P. et al. Plant retromer, localized to
the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. _Plant Cell_ 18, 1239–1252 (2006). Article CAS PubMed Central PubMed Google
Scholar * Kang, H. et al. Trafficking of vacuolar proteins: the crucial role of Arabidopsis vacuolar protein sorting 29 in recycling vacuolar sorting receptor. _Plant Cell_ 24, 5058–5073
(2012). Article CAS PubMed Central PubMed Google Scholar * Happel, N. et al. _Arabidopsis_ micro A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1.
_Plant J._ 37, 678–693 (2004). Article CAS PubMed Google Scholar * Duncan, M. C. New directions for the clathrin adaptor AP-1 in cell biology and human disease. _Curr. Opin. Cell Biol._
76, 102079 (2022). Article CAS PubMed Central PubMed Google Scholar * Casler, J. C., et al. Clathrin adaptors mediate two sequential pathways of intra-Golgi recycling. _J. Cell Biol_.
221, https://doi.org/10.1083/jcb.202103199 (2022). * Robinson, D. G. & Neuhaus, J. M. Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model. _J. Exp.
Bot._ 67, 4435–4449 (2016). Article CAS PubMed Google Scholar * Früholz, S. & Pimpl, P. Analysis of Nanobody-Epitope Interactions in Living Cells via Quantitative Protein Transport
Assays. _Methods Mol. Biol._ 1662, 171–182 (2017). Article PubMed Google Scholar * Fässler, F. & Pimpl, P. In Vivo Interaction Studies by Measuring Forster Resonance Energy Transfer
Through Fluorescence Lifetime Imaging Microscopy (FRET/FLIM). _Methods Mol. Biol._ 1662, 159–170 (2017). Article PubMed Google Scholar * Pimpl, P. et al. Golgi-mediated vacuolar sorting
of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. _Plant Cell_ 18, 198–211 (2006). Article CAS PubMed Central PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China No. 31970185 P.P, and No. 32270742.P.P, and the Key
Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes (2019KSYS006), and the Shenzhen Science and Technology Program (No. KQTD20190929173906742). We
thank the staff at SUSTech Cryo-EM Center for their assistance. We thank Dr. Yelin Zhou and Dr. Xibin Lu for their technical support on FRET–FLIM. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Harbin Institute of Technology, Harbin, China Xiaoyu Shao * Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Institute of Plant and Food
Science, Department of Biology, School of Life Sciences, Southern University of Science and Technology (SUSTech), Shenzhen, Guangdong, 518055, China Xiaoyu Shao, Hao Xu & Peter Pimpl
Authors * Xiaoyu Shao View author publications You can also search for this author inPubMed Google Scholar * Hao Xu View author publications You can also search for this author inPubMed
Google Scholar * Peter Pimpl View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X. S. and H. X. performed experiments and data acquisition.
X.S. and P.P. evaluated the data and wrote the manuscript, and P. P. conceived the study. CORRESPONDING AUTHOR Correspondence to Peter Pimpl. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this
work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shao, X., Xu, H. & Pimpl, P. Nanobody-based VSR7 tracing shows
clathrin-dependent TGN to Golgi recycling. _Nat Commun_ 14, 6926 (2023). https://doi.org/10.1038/s41467-023-42331-1 Download citation * Received: 07 March 2023 * Accepted: 06 October 2023 *
Published: 30 October 2023 * DOI: https://doi.org/10.1038/s41467-023-42331-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative