Play all audios:
ABSTRACT Contactless microscale tweezers are highly effective tools for manipulating, patterning, and assembling bioparticles. However, current tweezers are limited in their ability to
comprehensively manipulate bioparticles, providing only partial control over the six fundamental motions (three translational and three rotational motions). This study presents a joint
subarray acoustic tweezers platform that leverages acoustic radiation force and viscous torque to control the six fundamental motions of single bioparticles. This breakthrough is significant
as our manipulation mechanism allows for controlling the three translational and three rotational motions of single cells, as well as enabling complex manipulation that combines controlled
translational and rotational motions. Moreover, our tweezers can gradually increase the load on an acoustically trapped cell to achieve controllable cell deformation critical for
characterizing cell mechanical properties. Furthermore, our platform allows for three-dimensional (3D) imaging of bioparticles without using complex confocal microscopy by rotating
bioparticles with acoustic tweezers and taking images of each orientation using a standard microscope. With these capabilities, we anticipate the JSAT platform to play a pivotal role in
various applications, including 3D imaging, tissue engineering, disease diagnostics, and drug testing. SIMILAR CONTENT BEING VIEWED BY OTHERS MOVABLE SURFACE ACOUSTIC WAVE TWEEZERS: A
VERSATILE TOOLBOX FOR MICROMANIPULATION Article Open access 28 October 2024 ACOUSTIC BLACK HOLE EFFECT ENHANCED MICRO-MANIPULATOR Article Open access 12 October 2024 SPATIALLY SELECTIVE
MANIPULATION OF CELLS WITH SINGLE-BEAM ACOUSTICAL TWEEZERS Article Open access 25 August 2020 INTRODUCTION Precise single-cell manipulation, encompassing translation1,2, rotation3, and
deformation4,5, is essential for cellular biology, biophysics, and biomedical engineering. The ability to translate living cells in a contact-free manner significantly enhances the
capabilities of tissue engineering technologies to produce biomimetic tissues for numerous applications in regenerative medicine, and disease modeling2. Moreover, controllable rotational
manipulation enables high-resolution 3D reconstruction of cells3,6, and small organisms7, thereby unveiling hidden details pertaining to cellular structure and organization. This ability has
proven valuable in various areas, including cell profiling, disease diagnostics, and drug screening3. Furthermore, the ability to gradually deform a cell allows for the characterization of
cell mechanical properties, particularly those sensitive to changes in cytoskeletal and nuclear components, offering a label-free method for evaluating these alterations5. This ability is
also critical for mechanical phenotyping, cell classification, and the tracking of cellular metabolic dynamics8,9,10 with wide-ranging applications in biophysics and
biology11,12,13,14,15,16. Although various techniques have been developed to precisely manipulate cells, including optical tweezers17,18,19,20, optoelectronic tweezers21, magnetic
tweezers22,23, acoustic tweezers24,25,26,27,28,29, acousto-dielectric tweezers30, dielectrophoresis31,32,33, electrorotation3, and high-speed hydrodynamic flow14, the ability to manipulate
cells with complete control over all six fundamental motions remained a formidable challenge. Optical tweezers have gained popularity as a technique for manipulating micro/nano-particles18;
however, they require complex equipment and precise alignment of optical components while also introducing the risk of laser-induced heating that may cause physiological damage to
bioparticles. Optoelectronic tweezers offer the advantage of dynamically trapping cells with less power than traditional optical methods21. However, they fall short when attempting to
achieve in-plane cell rotation and deformation. Moreover, their usages are limited by the requirement of high ionic concentration environments due to ionic shielding and Joule heating21.
Magnetic tweezers are constrained by the complexity involved in embedding or attaching magnetic nanoparticles to cells, which may lead to cell damage34. While dielectrophoresis33,
electrorotation3, and high-speed hydrodynamic manipulation14,35 can accomplish cell translation, rotation, or deformation, respectively, none of these techniques possess all of the
aforementioned functions. In addition, they lack the ability to achieve and control all six fundamental motions. In recent years, acoustofluidics has emerged as a promising contact-free
strategy for cell manipulation, offering the advantages of label-free operation and excellent biocompatibility36,37,38,39,40,41,42,43. With these features, acoustofluidic technologies have
found numerous applications in bioparticle separation44,45,46,47,48,49, exosome enrichment50,51,52, and cell patterning53,54,55, among others56,57,58,59,60,61,62. These applications
typically leverage the acoustic radiation force that results from bioparticle-induced acoustic field changes63,64,65,66,67, as well as the acoustic streaming that stems from acoustic energy
dissipation in fluids68,69,70. Acoustic tweezers based on orthogonally arranged two pairs of transducers with phase and amplitude modulation capabilities enable 3D translation of single
cells2. For rotational object manipulation, an early study by Busse and Wang presented a theoretical framework to predict the torque induced by orthogonal acoustic waves71. To achieve both
translational and rotational manipulation of acoustically trapped objects, Marzo and Drinkwater developed holographic acoustic tweezers, leveraging an array of transducers to generate
airborne acoustic waves and reshape the acoustic energy field to versatile patterns72. However, as this method uses airborne acoustic waves, it is limited to manipulating objects in the
air72. In addition to translational and rotational object manipulation, bulk acoustic wave- and streaming-based approaches have been developed to deform cells6,15,73,74,75,76. Given the
successes of previous studies, in the context of single-cell manipulation using surface acoustic waves (SAWs), no SAW device achieves the three critical cell manipulation functions:
controllable translation, rotation, and deformation of cells. Moreover, few studies investigate the mechanisms to achieve complex manipulation that combines controlled translation and
rotation of single cells. This study presents an acoustic tweezer platform, termed the joint subarray acoustic tweezers (JSAT) system, which allows for controlling the six fundamental (three
translational and three rotational) motions of single cells, achieving complex motions with controlled translation and rotation, and deforming an acoustically trapped cell, by leveraging
SAW-induced radiation force and acoustic streaming vortex-induced shear force. By tuning the phases and amplitudes of orthogonal standing SAWs at low frequencies, the JSAT system traps cells
and controls the trapped cell’s three translational motions (i.e., _u__x_, _u__y_, and _u__z_). To control the cell’s rotational motions (i.e., _θ__x_ and _θ__y_), high-frequency standing
SAW-induced acoustic streaming vortices are utilized to apply viscous torques on the trapped cell. Similarly, acoustic streaming vortices77,78 induced by high-frequency traveling SAWs
control the trapped cell’s rotational degree-of-freedom (DoF) _θ__z_. Unlike existing acoustic tweezers, our JSAT system is able to control all six fundamental motions and achieve complex
manipulation combined with controlled translational and rotational motions, thus facilitating comprehensive cell manipulation in a 3D space. In addition to these features, our system can
control the force applied to an acoustically trapped cell to gradually deform the cell. This ability allows for the introduction of controllable mechanical perturbations to single cells for
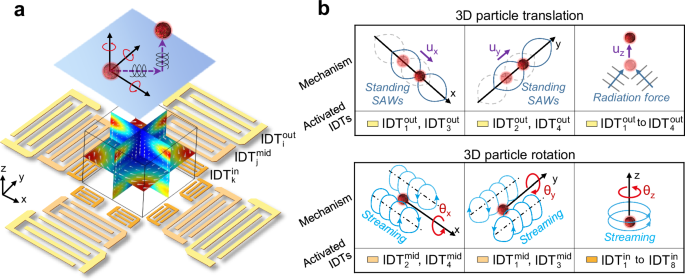
detailed characterization of cellular responses at different levels of mechanical perturbations. RESULTS MECHANISMS OF JSAT The JSAT system can exert and control both the acoustic radiation
force and viscous torque applied on a cell to enable precise and controllable six fundamental motions (three translational and three rotational motions) manipulation of the cell. To enable
this ability, our JSAT system leverages a unique array of interdigital transducers (IDTs) deposited on a LiNbO3 substrate to generate and control both the SAW and acoustic streaming fields
inside a polydimethylsiloxane (PDMS)-based microfluidic chamber. As illustrated by a device schematic in Fig. 1a (also see Supplementary Fig. S1), the entire array is composed of three
(i.e., inner, middle, and outer) subarrays that have collections of 4, 4, and 8 IDTs (denoted as {\({{\mbox{IDT}}}_{{{\mathrm{i}}}}^{{{\mathrm{out}}}}\)}4,
{\({{\mbox{IDT}}}_{{{\mathrm{j}}}}^{{{\mathrm{mid}}}}\)}4, and {\({{\mbox{IDT}}}_{{{\mathrm{k}}}}^{{{\mathrm{in}}}}\)}4, respectively) for generating SAWs at different frequencies. Such
design is difficult to achieve using thickness-mode piezoelectric transducers (such as PZTs), as the bulk acoustic waves generated by a PZT are impeded by other PZTs placed in the wave
propagation path. This limits the options of integrating multiple PZTs for achieving complex cell/particle manipulation functions. Conversely, SAWs generated by an IDT exhibit good
transmissibility through regions with IDTs working at different frequencies, thereby allowing more options for designing complex IDT arrays. Supplementary Fig. S2 shows a photo of a
fabricated JSAT device with an IDT array and a microfluidic chamber. The mechanisms for achieving multi-DoF manipulation of single cells using the three subarrays are presented below. The
outer subarray enables cell trapping by leveraging acoustic radiation force and controlling the trapped cell’s three translational motions (i.e., _u__x_, _u__y_, and _u__z_) through phase
and amplitude modulations of SAWs. This subarray has two orthogonal pairs of IDTs positioned along the _x_- and _y_-axes, respectively. When applying excitation signals to these IDTs at
their resonance frequencies, a grid-like standing SAW field is formed within the microfluidic chamber. Consequently, a cell can be trapped by one of multiple Gor’kov potential wells due to
the acoustic radiation force79,80 applied to the cell. Moreover, the trapped cell can be precisely translated with a displacement of _u__x_ (or _u__y_), by moving the Gor’kov potential
well’s position through phase modulation, i.e., changing the phase difference between the two IDTs along the _x_- (or _y_-) axis. In addition, the SAW energy leaked into the fluid domain in
the PDMS chamber induces an out-of-plane acoustic radiation force, as illustrated in Fig. 1b (top right). The out-of-plane translation _u__z_ of the acoustically trapped cell can be
precisely controlled by adjusting the IDT excitation voltage, which regulates the force component responsible for the translation. The middle subarray controls the trapped cell’s rotational
DoFs _θ__x_ and _θ__y_ by utilizing standing SAW-induced acoustic streaming vortices. The middle subarray’s two IDTs positioned along the _x_-axis generate acoustic streaming vortices with
non-zero angular momentums, enabling precise control of cell rotation _θ__x_, as shown in Fig. 1b (bottom left). Similarly, the two IDTs along the _y_-axis can generate vortices, enabling
precise control of cell rotation _θ__y_, as illustrated in Fig. 1b (bottom middle). The operation frequencies of these middle subarray IDTs are higher than the frequencies of the outer
subarray IDTs, so the diameter (~ 25 µm) of each streaming vortex is comparable to the cell size. The generated streaming vortices can apply a viscous torque to the cell, introducing angular
momentum. Moreover, the cell angular velocities can be altered by adjusting the input voltages applied to the middle subarray. The inner subarray controls the trapped cell’s rotation _θ__z_
by manipulating the traveling SAW-induced acoustic streaming vortices. Here, the inner subarray’s four IDTs with even subscripts (see Supplementary Fig. S1) are utilized to generate
traveling SAWs whose energy beam directions are tangential to a circle. These traveling SAWs can induce an in-plane acoustic streaming vortex with a clockwise angular momentum, resulting in
the ability to rotate a cell clockwise. On the other hand, the four IDTs with odd subscripts can be used to generate a counterclockwise acoustic streaming vortex to enable the
counterclockwise rotation of a cell, as illustrated in Fig. 1b (bottom right). Moreover, by tuning the input voltages for these IDTs, the angular movement of the generated streaming vortex
can be adjusted to control the angular velocity of the cell. As presented above, by leveraging three subarrays of IDTs, our JSAT system can trap a cell and control the cell’s six fundamental
motions. Moreover, through the superposition of forces/torques generated by IDTs to control different DoFs, our JSAT system enables complex manipulation of a trapped cell, allowing for
simultaneous translation and rotation. Furthermore, by slowly increasing the force applied on a cell, our device can gradually deform an acoustically trapped cell. We have performed
simulations and experiments with key results reported below to achieve and validate these functions. 3D TRANSLATION VIA JSAT To trap a cell and control its 3D translational motion, the outer
subarray leverages two orthogonal pairs of IDTs (see Fig. 2a) to generate SAWs with the same wavelength (λout = 200 μm) in both the _x-_ and _y-_ directions. The interaction of SAWs
generated from these four IDTs leads to a square grid-like distribution of pressure nodes (or antinodes) with the same period of λout/2 along the _x-_ and _y-_ directions. At each pressure
node, the resulting Gor’kov potential well can trap a cell with a positive acoustic contrast factor81. Since cells typically have dimensions in tens of microns, the low frequencies used
ensure the in-plane Stokes drag force resulting from acoustic streaming is negligible compared to the in-plane acoustic radiation force for trapping the cell. To achieve controllable
in-plane translations _u__x_ and _u__y_ of a trapped cell, our system leverages the phased modulation approach, which tunes the phase differences _φ__x_ and _φ__y_ between excitation signals
for the two IDTs along the _x-_ and _y-_directions, respectively. As shown by the simulated acoustic energy fields, when phase differences change from _Φ_ (Fig. 2b, left) to _Φ_ + Δ_Φ_
(Fig. 2b, right), where _Φ_ = [_φ__x_, _φ__y_] and Δ_Φ_ = [Δ_φ__x_, Δ_φ__y_], the potential well with the trapped cell can be shifted by a short translation vector _U_ = [_u__x_, _u__y_, 0]
with _u__x_ = Δ_φ__x_λout/(4π) and _u__y_ = Δ_φ__y_λout/(4π). Based on this mechanism, a long and complex translation path can be achieved by discretizing the path into a series of short
translation vectors, calculating the required phase adjustments for these vectors, and sequentially applying the phase adjustments to IDT excitation signals. To execute this translation
process automatically, the phase adjustments can be sequentially applied through two dual-channel function generators controlled by MATLAB code. To demonstrate this ability, we successfully
guided an MCF7 cell’s movement to depict the letters ‘D’, ‘U’, ‘K’, and ‘E’ (see Supplementary Movie 1). The actual cell trajectories (see Fig. 2c) were revealed by stacking the microscopic
images captured during the dynamic cell translation process. As shown in Supplementary Fig. S3, the actual cell positions closely agree with the predicted positions using relations _u__x_ =
Δ_φ__x_λout/(4π) and _u__y_ = Δ_φ__y_λout/(4π). As the phase modulation-based translational manipulation mechanism is known for its good predictability2, we didn’t perform any calibration
before translating an MCF7 cell following complex trajectories. To achieve controllable out-of-plane translation _u__z_ of a trapped cell, our system leverages the amplitude modulation
approach, which adjusts the excitation amplitudes of the outer subarray’s four IDTs. The experimental results demonstrate that the position of an MCF7 cell can be shifted in the +
_z_-direction through acoustic waves generated by the IDTs (see Fig. 2e and Supplementary Movie 2). To better elucidate the manipulation mechanism, finite element simulations were performed.
As SAWs propagate in the LiNbO3 substrate, their energy leaks into the fluid domain above the substrate, leading to an energy flux along the Rayleigh angle direction, as illustrated in Fig.
2d. Therefore, a cell within the vicinity is subjected to an out-of-plane acoustic radiation force component \({{{\mathrm{F}}}}_{{{{\mathrm{rad}}}}\_{{{\mathrm{z}}}}}^{{{\mathrm{out}}}}\),
which points to the + _z_-direction acting as the levitation driving force, as predicted by simulation results in Supplementary Fig. S4. Additional theoretical investigation of the
out-of-plane acoustic radiation force can be found in our previous article82. An acoustofluidic simulation was also performed to analyze the acoustic streaming-induced out-of-plane Stokes
drag force. As shown in the simulation results (Supplementary Fig. S5 and Fig. 2d, middle), there is a downward acoustic streaming at the pressure node, thus inducing a – _z_-direction drag
force that gradually diminishes from the microfluidic chamber’s center to the bottom and top (see Supplementary Fig. S5). The out-of-plane translation _u__z_ depends on the interplay of all
the out-of-plane forces, including the position-dependent acoustic radiation and drag forces, the buoyancy force FBuo, and the gravitational force Fg, as shown in Fig. 2d (right). Most
cells, including MCF7 cells, have a density slightly higher than the culture medium (water with additives), so the _z_-directional manipulation can be controlled theoretically by applying a
precise input power to match to account for the aforementioned out-of-plane forces. However, the control of out-of-plane translation cannot be as precise and stable as the control of
in-plane translation due to the absence of a Gor’kov potential well-like trap. In addition, when the cell experiences other motions, especially rotational motions, they affect the
out-of-plane translation. The out-of-plane translation precision is also affected by the two-dimensional imaging nature of our current microscope, as the translation is difficult to be
quantitively monitored. 3D ROTATION VIA JSAT To control an acoustically trapped cell’s 3D rotational motion, our JSAT device leverages the middle and inner subarrays of IDTs to generate and
control acoustic streaming vortices for applying and controlling viscous torques on the cell. The \({{{\mathrm{IDT}}}}_{2}^{{{\mathrm{mid}}}}\) and
\({{{\mathrm{IDT}}}}_{4}^{{{\mathrm{mid}}}}\) of the middle subarray are for controlling the cell rotation _θ__x_ using acoustic streaming, as illustrated in Fig. 3a. They generate standing
SAWs with a wavelength _λ_mid of 100 μm, further inducing tunnel-like streaming vortices with counter chiralities at different sides of each pressure node, as revealed by the results in
Supplementary Fig. S6 and Supplementary Movie 3. Therefore, for a cell trapped at a pressure node, the two counter-chirality vortices both apply torques to the cell, and the competition
between the two vortices determines the cell’s angular motion. To better elucidate the cell manipulation mechanism, we performed numerical simulations with the excitation voltage
\({{{{\rm{V}}}}}_{2}^{{{{\mathrm{mid}}}}}\) for \({{{\mathrm{IDT}}}}_{2}^{{{\mathrm{mid}}}}\) slightly higher than the voltage \({{{{\rm{V}}}}}_{4}^{{{{\rm{mid}}}}}\) for
\({{{\mathrm{IDT}}}}_{4}^{{{\mathrm{mid}}}}\). As shown in Supplementary Fig. S6, the streaming fields of vortices on different sides of a pressure node become slightly asymmetric. These
asymmetric vortices can lead to a non-zero viscous torque applied on the cell, consequently inducing cell rotation. Moreover, our simulation results (Supplementary Fig. S6d, S6e) reveal that
the tangential streaming velocities can lead to cell rotation in the + _θ__x_-direction being dominant. Furthermore, during the streaming-induced cell rotation, the acoustic potential well
generated by the standing SAWs can still effectively trap the cell, ensuring no translational motion or eccentricity. The experimental validation results (top of Fig. 3b and Supplementary
Movie 3) show that an MCF7 cell could be successfully trapped and rotated in the + _θ__x_-direction when \({{{{\rm{V}}}}}_{4}^{{{{\rm{mid}}}}}\)<\({{{{\rm{V}}}}}_{2}^{{{{\rm{mid}}}}}\),
agreeing with the numerically predicted rotation direction. Therefore, we can reliably execute the rotational manipulation as planned. Similarly, under the condition of
\({{{{\rm{V}}}}}_{4}^{{{{\rm{mid}}}}}\)>\({{{{\rm{V}}}}}_{2}^{{{{\rm{mid}}}}}\), an acoustically trapped MCF7 cell exhibited rotation in the – _θ__x_-direction (Fig. 3b, bottom and
Supplementary Movie 3). Note that the aforementioned rotation control approach is for a cell located near the center of the microfluidic chamber, as the SAWs emitted from a pair of IDTs
travel to the center region with similar attenuation lengths. When the cell position is close to either \({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\) or
\({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\), asymmetric streaming vortices can still be generated at different sides of a pressure node even
\({{{{\rm{V}}}}}_{2}^{{{{\rm{mid}}}}}\)=\({{{{\rm{V}}}}}_{4}^{{{{\rm{mid}}}}}\), enabling cell rotation in the ± _θ__x_-directions. To investigate the relationship between input voltage and
cell rotation speed, we loaded an MCF7 cell at a distance of _λ_mid/2 away from the center, followed by applying the same voltage to \({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\) and
\({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\). As depicted in Fig. 3c, the rotation speed exhibited a positive correlation with the input voltage and could reach values as high as ~ 450 RPM in
the cell culture medium. Furthermore, the cell rotation revolution shows a nearly linear relationship with time (see Supplementary Fig. S7). These findings indicate that the cell’s spinning
rate remains relatively stable during the streaming generation period. In addition, by activating \({{{{\rm{IDT}}}}}_{1}^{{{{\rm{mid}}}}}\) and \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{mid}}}}}\) to
generating streaming vortices, we successfully induced the rotation of an MCF7 cell in the ±_θ__y_-directions (Fig. 3e and Supplementary Movie 4). The corresponding relationship between
input voltage and rotation speed is summarized in Fig. 3f. For enabling cell rotation in the _θ__z_-direction, the inner subarray (Fig. 3g) is used. When signals at resonant frequencies are
applied to the four IDTs with odd subscripts, the traveling SAW from each IDT produces a volume force that propels the fluid away from the IDT. Consequently, the combined effect of the four
driving flows forms a counterclockwise streaming vortex. Note that when only activating \({{{{\rm{IDT}}}}}_{1}^{{{{\rm{in}}}}}\) and \({{{{\rm{IDT}}}}}_{5}^{{{{\rm{in}}}}}\), a
counterclockwise streaming vortex can still be generated, as proven by numerical and experimental results (Supplementary Fig. S8 and Supplementary Movie 5). Therefore, an MCF7 cell, driven
by the viscous torque induced by the counterclockwise streaming vortex, can be rotated in the + _θ__z_-direction (see Fig. 3h, top and Supplementary Movie 5). Similarly, the IDTs with even
subscripts in the inner subarray can induce cell rotation in the – _θ__z_-direction. Through experiments with \({{{{\rm{IDT}}}}}_{2}^{{{{\rm{in}}}}}\) and
\({{{{\rm{IDT}}}}}_{6}^{{{{\rm{in}}}}}\) turned on, the results (Fig. 3h, bottom and Supplementary Movie 5) successfully confirm the induced cell rotation in the – _θ__z_-direction.
Furthermore, we experimentally investigated the relationship between input voltage and rotation speed. The findings reveal that the rotation speed exhibited an increasing trend as the input
voltage increased (Fig. 3i). The slight spin rate drops at 10 Vpp could be induced by measurement error. TUNABLE ROTATION VIA JSAT Our device can steer the cell rotation axis by
simultaneously activating the two orthogonal pairs of IDTs in the middle subarray for rotating a cell in the _θ__x_- and _θ__y_- directions (Fig. 3j). Supplementary Fig. S9a presents the
numerical results of acoustic streaming when _x_- and _y_-axes standing SAWs have the same amplitude. In the vicinity of a pressure node, the results show four vortices with angular momentum
vectors in − 45°, 45°, 135°, and − 135° directions, respectively. Similar to controlling the cell rotation in the _θ__x_-direction, the middle subarray can rotate a trapped cell in the same
direction as any one of those four vortices by adjusting the voltages applied to the four IDTs or placing the cell off-center. For instance, an MCF7 cell trapped at _x_ = _λ_mid/2 and _y_ =
_λ_mid/2 was successfully spun to carry an angular momentum in the direction of − 45° (see Fig. 3k and Supplementary Movie 6), _x_- and _y_-axes SAWs had similar amplitudes. Moreover, by
carefully changing the amplitude ratio between _x_- and _y_-axes SAWs, the axis of cell rotation can be gradually steered. For example, when the ratio is 0.57:1, the simulation
(Supplementary Fig. S9b) predicts four vortices surrounding a pressure node carrying angular momentums in − 30°, 30°, 150°, and − 150°. The experimental results (bottom row of Fig. 4k and
Supplementary Movie 6) show a rotating MCF7 cell with respect to an axis at − 29°, close to the predicted direction of − 30°. When the SAW amplitude ratio further changes to 0.3:1, numerical
simulations in Supplementary Fig. S9c reveal the four vortices with angular momentums at − 15°, 15°, 165°, and − 165°. The experimental results (Supplementary Fig. S9d and Supplementary
Movie 6) confirm that an MCF7 cell trapped at _x_ = _λ_mid/2 and _y_ = _λ_mid/2 can be rotated to carry an angular momentum in the direction of − 15°, agreeing with the numerical prediction.
Note that when adjusting input voltages for IDTs, the different SAW generation efficiencies in the _x_- and _y_- directions of the used Y128-cut LiNbO3 wafer must be considered.
SIMULTANEOUS TRANSLATION AND ROTATION VIA JSAT A unique feature of our JSAT platform is its ability to perform simultaneous cell translation and rotation. To understand such compound motion,
we conducted simulations and experiments involving different combinations of translation and rotation directions. Figure 4a (left) illustrates the mechanism to simultaneously translate a
trapped cell in the _x_-direction and rotate the cell in the _θ__x_-direction. The translation is controlled by tuning the input phase difference between
\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\) and \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\), and the rotation is controlled by the streaming vortex generated by
\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\) and \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\). Supplementary Fig. S10 compares acoustic energy distributions of standing SAWs generated by one
transducer pair {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and two transducer pairs {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\),
\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\)}. The _x_ position with minimum energy is unchanged upon
introducing the _y_-axis SAWs from {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\)}, meaning that the positional stability of a trapped cell is
unaffected by the _y_-axis SAWs. On the other hand, we found that the _x_-axis standing SAW exhibits negligible impact on the generation of streaming vortices with angular momentum vectors
in ± _x_-directions. These simulation results support the simultaneous execution of cell translation and rotation and control these motions using transducer pairs
{\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\)},
respectively. For the proof-of-concept, we successfully utilized these two transducer pairs to achieve controlled translation in the _x_-direction and rotation in the _θ__x_-direction
(Supplementary Movie 7 and Fig. 4a, right). As the _x_-axis standing SAW generated from {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} exhibits
negligible impact on the streaming vortices generated by {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\)} and the two streaming vortex tunnels are
parallel to the cell translation direction, our approach achieves continuous cell rotation during the translation process. Similarly, we manipulated an MCF7 cell to undergo both translation
in the _y_-direction and rotation in the _θ__y_-direction (Supplementary Movie 7 and Fig. 4b, right) by using transducer pairs {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{out}}}}}\)
\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{mid}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{mid}}}}}\)}. The
mechanism to enable simultaneous cell translation in the _x_-direction and rotation in the _θ__y_-direction is illustrated in Fig. 4c (left). Here, the translation control is achieved by
tuning the phase difference between inputs for \({{{{\rm{IDT}}}}}_{1}^{{{{\rm{mid}}}}}\) and \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{mid}}}}}\), and the rotation is enabled by streaming vortices
generated by the _x_-axis standing SAWs. To demonstrate this approach, we performed an experiment with \({{{{\rm{IDT}}}}}_{1}^{{{{\rm{mid}}}}}\) and \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{mid}}}}}\)
turned it on. By gradually adjusting the phase difference, our results (Supplementary Movie 8 and Fig. 4c, right) show that the generated standing SAWs can translate an MCF7 cell in the
_x-_direction while inducing acoustic streaming to spin the cell in the _θ__y_-directions. Although both acoustic radiation force and streaming are generated by the same IDTs, the
translation _u__x_ is controlled by the input phase difference, and the rotation _θ__y_ is controlled by the input amplitude difference between the two IDTs. Hence, the involved
translational and rotational motions could be independently controlled. Moreover, when translating a cell through phase modulation, the positions of two streaming vortex tunnels (illustrated
in Fig. 4c) shift synchronously, thus ensuring continuous cell rotation during the translation process. By using the above mechanisms with \({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\) and
\({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\), we achieved simultaneous translation of an MCF7 in the _y-_direction and rotation in the _θ__x_-direction (Fig. 4d and Supplementary Movie 8). The
mechanism to achieve simultaneous cell translation in the _x_-direction and rotation in the _θ__z_-direction is illustrated in Fig. 4e (left). By tuning the phase difference between inputs
for \({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\) and \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\), the pressure node position of an _x_-axis standing SAW can be adjusted to translate a trapped
cell in the _x_-direction. On the other hand, the _θ__z_-direction cell rotation can be achieved using the streaming vortex induced by the traveling SAWs from
\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{in}}}}}\) and \({{{{\rm{IDT}}}}}_{7}^{{{{\rm{in}}}}}\). By comparing the simulated acoustic energy fields (Supplementary Fig. S11) generated by one transducer
pair {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and two transducer pairs {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\),
\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{in}}}}}\), \({{{{\rm{IDT}}}}}_{7}^{{{{\rm{in}}}}}\)}, the acoustic energy field experiences small changes when
considering the traveling SAWs from {\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{in}}}}}\), \({{{{\rm{IDT}}}}}_{7}^{{{{\rm{in}}}}}\)}. On the other hand, the simulated streaming field (Supplementary Fig.
S11) shows a counterclockwise streaming vortex. These simulation results support the simultaneous control of the cell translation in the _x_-direction and the rotation in the
_θ__z_-direction by leveraging transducer pairs {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{3}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{3}^{{{{\rm{in}}}}}\),
\({{{{\rm{IDT}}}}}_{7}^{{{{\rm{in}}}}}\)}, respectively. Our experiment successfully achieved simultaneous translation of an MCF7 cell in the _x_-direction and rotation in the
_θ__z_-direction (Supplementary Movie 9 and Fig. 4e, right). Similarly, we achieved concurrent translation and rotation of an MCF7 cell in the _y_-and _θ__z_-directions (Supplementary Movie
9 and Fig. 4f, right) by using transducer pairs {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{out}}}}}\), \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{out}}}}}\)} and {\({{{{\rm{IDT}}}}}_{1}^{{{{\rm{in}}}}}\),
\({{{{\rm{IDT}}}}}_{5}^{{{{\rm{in}}}}}\)}. DEFORMING SINGLE CELLS VIA JSAT Our JSAT platform can locally deform single cells and control cell motion. As illustrated in Fig. 5a, when an
acoustically trapped cell is positioned within a high angular momentum region of streaming vortices induced by standing SAWs generated by {\({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\),
\({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\)}, the streaming velocity in the cell’s viscous boundary layer drops rapidly to the same value as the cell’s surface velocity, thereby subjecting the
cell to a large shear stress. Notably, the cell’s surface velocity and shear stress exhibit a decreasing trend from the equator to the poles, prompting cell elongation along the axis of
rotation (i.e., _x_-axis). The acoustic radiation force induced by _y_-axis standing SAWs also contributes to cell elongation. To experimentally deform an MCF7 cell, we applied 10 Vpp
excitation signals to \({{{{\rm{IDT}}}}}_{2}^{{{{\rm{mid}}}}}\) and \({{{{\rm{IDT}}}}}_{4}^{{{{\rm{mid}}}}}\). The obtained time-sequential images (Fig. 5b) and Supplementary Movie 10
clearly show the dynamic process of gradually deforming a trapped cell over time. We also conducted a quantitative analysis by evaluating the gradual cell deformation over time and
investigating the effect of the IDT excitation voltage on cell deformation. At 6 Vpp, the shape of an MCF7 cell remains almost unchanged throughout the 100-second observation period (see
Fig. 5c). At a higher voltage of 8 Vpp, the cell deformation data exhibits a nearly linear trend with time. When the input voltage was further increased to 10 Vpp, the cell deformation
initially experienced a quick increase, followed by a slight decrease, and then converged to a relatively stable value. These findings indicate that the time-dependent cell deformation
processes demonstrate distinct trends under different IDT excitation voltages, highlighting our JSAT device’s ability to controllably deform acoustically trapped cells by tuning the
generated SAWs and streaming. DISCUSSION In this study, we successfully developed and demonstrated a JSAT platform that is capable of controlling the six fundamental motions of single cells,
including three translational (i.e., _u__x_, _u__y_, and _u__z_) and three rotational (i.e., _θ__x_, _θ__y_, and _θ__z_) components. Our unique IDT array, composed of an outer, middle, and
inner subarray, enables this ability. The in-plane translations (_u__x_ and _u__y_) of a cell trapped in a Gor’kov potential well are achieved by shifting the potential well’s position and
tuning input signal phases for the outer subarray. By adjusting the input signal voltages for the outer subarray, our JSAT platform allows for controlling the out-of-plane translation
(_u__z_), i.e., controlling a cell’s _z_ position with acoustic radiation, buoyancy, drag, and gravitational forces. The controllable rotations (_θ__x_ and _θ__y_) of an acoustically trapped
cell are accomplished based on acoustic streaming vortices induced by high-frequency standing SAWs from the middle subarray, and the rotation _θ__z_ is achieved by using acoustic streaming
vortices generated by multiple traveling SAWs, whose energy beam directions are all tangential to a circle. With the aforementioned features, such as achieving the six fundamental motions of
single cells and locally deforming a cell, our JSAT platform represents a significant advancement compared to previous technologies, such as optical, magnetic, and acoustic tweezers. Our
experiments successfully validated the ability to independently control the multi-DoF motions of an acoustically trapped cell, while none of the previously developed tweezers could provide
this ability. Moreover, our experiments demonstrated the ability to realize complex combined motions, such as _u__x_ and _θ__x_, _u__x_ and _θ__z_, _u__y_ and _θ__z_, etc., while previous
tweezers could not perform these complex manipulations. Furthermore, our JSAT platform can gradually deform an acoustically trapped cell without directly touching the cell by controlling the
applied shear force induced by streaming and acoustic radiation force from standing SAWs. Recording the dynamic cell deformation process makes it possible to characterize cell mechanical
properties83. Therefore, contactless tweezers that can gradually deform a cell hold significant potential for cell phenotyping, disease diagnosis, and drug testing applications. Beyond the
experiments conducted in this study, we will further test the ability to simultaneously deform multiple cells trapped in an array of potential wells. We also plan to introduce a feedback
control loop that integrates live imaging, real-time decision-making, and programmable input signal modulation to enable platform automation. In addition, beyond manipulating cells, we
expect that our tweezing mechanisms can be used for manipulating other bioparticles, such as embryos, bacteria, and extracellular vesicles, and these manipulations will be tested in our
future studies. In the long run, we anticipate that the JSAT platform can become a widely used tool facilitating various applications such as 3D cell imaging, single-cell analysis, tissue
engineering, disease diagnostics, and mechanobiology. METHODS DEVICE FABRICATION A schematic and a photo of the fabricated JSAT device are shown in Supplementary Figs. S1 and S2,
respectively. The JSAT device is composed of a PDMS microfluidic chamber and an array of IDTs on a Y128-cut LiNbO3 wafer (500 mm thick). To fabricate IDTs, we transferred the designed
electrode patterns to a wafer by standard photolithography84. Then, we conducted e-beam evaporation of 10 nm Cr and then 100 nm Au. After a lift-off process, we obtained a wafer with an IDT
array composed of outer, middle, and inner subarrays, whose electrode widths were 50 μm, 25 μm, and 10 μm, respectively. On the other hand, the PDMS micro-chamber was fabricated by using
standard soft-lithography and mold-replica steps. First, a 60 μm layer of photoresist (SU-8 50, KAYAKU) was coated on a 4-inch silicon wafer, followed by soft lithography and application of
an SU-8 Developer (KAYAKU). Then, PDMS was poured over the SU-8 mold, degassed, and cured at 65 °C for 1 h. The cured PDMS was peeled off the silicon wafer and cut into the desired
small-size blocks containing microfluidic chambers. The inlet and outlet of a microfluidic chamber were created with a puncher. The PDMS chamber and the SAW substrate with IDTs were surface
treated by oxygen plasma for 5 mins (Plasma Cleaner Atto, Diener Electronic) and then bonded, followed by a heat treatment at 65 °C for 24 h. PARTICLE AND CELL SAMPLE PREPARATION Polystyrene
particles (PSF-200NM, Magsphere) were suspended in deionized water to a concentration of ~ 1 × 106 cells/mL. MCF7 cells (ATCC) were cultured in DMEM (Gibco, Life Technologies), which
contains 10% fetal bovine serum (Gibco, Life Technologies) and 1% penicillin-streptomycin (Mediatech) in an incubator (Heracell Vios 160i CO2 incubator, Thermo Scientific) with a temperature
of 37 °C and a CO2 level of 5%. Before each experiment, cells were detached from the culture dish with trypsin-EDTA (Gibco, Life Technology) and resuspended in DMEM to a concentration of ~
1 × 106 cells/mL. DEVICE OPERATION To perform cell manipulation experiments using our fabricated JSAT device, the device was mounted on the sample stage of an inverted optical microscope
(TE2000U, Nikon). MCF7 cells suspended in DMEM were injected into the microfluidic chamber using a 1-mL syringe (309659, Becton Dickinson). To generate SAWs for applying acoustic radiation
and viscous shear forces on cells, the input signals were generated by two dual-channel arbitrary function generators (AFG3102C, Tektronix) and then directly sent to IDTs. Note that the
function generators were controlled by a MATLAB program to achieve automatic manipulation of acoustic and flow fields, along with the desired translation and rotation of an acoustically
trapped MCF7 cell in the microfluidic chamber. To translate a cell along the desired complex paths, the frequencies and voltages for the IDTs remain constant, while shifts are made to the
input phases. The phase shifts are automatically performed using customized MATLAB codes, having key features including adjusting the phases, frequencies, and amplitudes of multiple signal
channels, as well as gradually changing the phases following predetermined sequences of phases for achieving step-by-step translation of a cell. Images and videos were taken by a
charge-coupled device camera (CoolSNAP HQ2, Photometrics). The videos for cell rotation speed measurement were recorded by a high-speed camera (Fastcam SA4, Photron). NUMERICAL SIMULATIONS
The details of numerical simulation methods were provided in Supplementary Notes 1 and 2. CELL DEFORMATION MEASUREMENT To evaluate cell deformation from an acquired microscopic image, we
used feature and edge detection codes in Matlab to get the cell shape from the image. Then, the cell’s projected area _A_ and perimeter _l_ were extracted from the identified cell shape. By
further using a relation \(1-2\sqrt{\pi A}/l\), the cell deformation induced by our JSAT platform was elevated85. In addition, we measured the SAW device’s temperature, as well as the
post-treatment cell viability. Their detailed procedures and results are in Supplementary Note 3 and Supplementary Figs. 13–15. REPORTING SUMMARY Further information on research design is
available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The authors declare that all data and code supporting the findings of this study are available
within the article and the supplementary materials. Further information is available from the corresponding author upon request. REFERENCES * Berthelot, J. et al. Three-dimensional
manipulation with scanning near-field optical nanotweezers. _Nat. Nanotechnol._ 9, 295–299 (2014). Article ADS PubMed CAS Google Scholar * Guo, F. et al. Three-dimensional manipulation
of single cells using surface acoustic waves. _Proc. Natl. Acad. Sci. USA_ 113, 1522–1527 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Huang, L., Zhao, P. & Wang,
W. 3D cell electrorotation and imaging for measuring multiple cellular biophysical properties. _Lab Chip_ 18, 2359–2368 (2018). Article PubMed CAS Google Scholar * Urbanska, M. et al. A
comparison of microfluidic methods for high-throughput cell deformability measurements. _Nat. Methods_ 17, 587–593 (2020). Article PubMed PubMed Central CAS Google Scholar * Wu, P.-H.
et al. A comparison of methods to assess cell mechanical properties. _Nat. Methods_ 15, 491–498 (2018). Article PubMed PubMed Central CAS Google Scholar * Ahmed, D. et al. Rotational
manipulation of single cells and organisms using acoustic waves. _Nat. Commun._ 7, 11085 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Chen, C. et al. Acoustofluidic
rotational tweezing enables high-speed contactless morphological phenotyping of zebrafish larvae. _Nat. Commun._ 12, 1118 (2021). Article ADS PubMed PubMed Central CAS Google Scholar *
Maloney, J. M. et al. Mesenchymal stem cell mechanics from the attached to the suspended state. _Biophys. J._ 99, 2479–2487 (2010). Article ADS PubMed PubMed Central CAS Google Scholar
* Lautenschläger, F. et al. The regulatory role of cell mechanics for migration of differentiating myeloid cells. _Proc. Natl. Acad. Sci. USA_ 106, 15696 (2009). Article ADS PubMed
PubMed Central Google Scholar * Nyberg, K. D. et al. Quantitative deformability cytometry: Rapid, calibrated measurements of cell mechanical properties. _Biophys. J._ 113, 1574–1584
(2017). Article ADS PubMed PubMed Central CAS Google Scholar * Di Carlo, D. A mechanical biomarker of cell state in medicine. _J. Lab. Autom._ 17, 32–42 (2012). Article PubMed Google
Scholar * Fregin, B. et al. High-throughput single-cell rheology in complex samples by dynamic real-time deformability cytometry. _Nat. Commun._ 10, 415 (2019). Article ADS PubMed
PubMed Central CAS Google Scholar * Guck, J. et al. The optical stretcher: A novel laser tool to micromanipulate cells. _Biophys. J._ 81, 767–784 (2001). Article ADS PubMed PubMed
Central CAS Google Scholar * Rosendahl, P. et al. Real-time fluorescence and deformability cytometry. _Nat. Methods_ 15, 355–358 (2018). Article PubMed CAS Google Scholar * Xie, Y. et
al. Probing cell deformability via acoustically actuated bubbles. _Small_ 12, 902–910 (2016). Article PubMed CAS Google Scholar * Nematbakhsh, Y. & Lim, C. T. Cell biomechanics and
its applications in human disease diagnosis. _Acta Mech. Sin._ 31, 268–273 (2015). Article ADS CAS Google Scholar * Grier, D. G. A revolution in optical manipulation. _Nature_ 424,
810–816 (2003). Article ADS PubMed CAS Google Scholar * Yang, A. H. J. et al. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. _Nature_ 457,
71–75 (2009). Article ADS PubMed CAS Google Scholar * Juan, M. L., Righini, M. & Quidant, R. Plasmon nano-optical tweezers. _Nat. Photon._ 5, 349–356 (2011). Article ADS CAS
Google Scholar * Juan, M. L., Gordon, R., Pang, Y., Eftekhari, F. & Quidant, R. Self-induced back-action optical trapping of dielectric nanoparticles. _Nat. Phys._ 5, 915–919 (2009).
Article CAS Google Scholar * Wu, M. C. Optoelectronic tweezers. _Nat. Photon._ 5, 322–324 (2011). Article ADS CAS Google Scholar * De Vlaminck, I. & Dekker, C. Recent advances in
magnetic tweezers. _Annu. Rev. Biophys._ 41, 453–472 (2012). Article PubMed Google Scholar * Trick, A. Y. et al. Filtration-assisted magnetofluidic cartridge platform for HIV RNA
detection from blood. _Lab Chip_ 22, 945–953 (2022). Article PubMed PubMed Central CAS Google Scholar * Alghane, M. et al. Streaming phenomena in microdroplets induced by Rayleigh
surface acoustic wave. _J. Appl. Phys._ 109, 114901 (2011). Article ADS Google Scholar * Baudoin, M. et al. Folding a focalized acoustical vortex on a flat holographic transducer:
Miniaturized selective acoustical tweezers. _Sci. Adv._ 5, eaav1967 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Bernassau, A. L., MacPherson, P. G. A., Beeley, J.,
Drinkwater, B. W. & Cumming, D. R. S. Patterning of microspheres and microbubbles in an acoustic tweezers. _Biomed. Microdevices_ 15, 289–297 (2013). Article PubMed CAS Google Scholar
* Collins, D. J. et al. Acoustic tweezers via sub–time-of-flight regime surface acoustic waves. _Sci. Adv._ 2, e1600089 (2016). Article ADS PubMed PubMed Central Google Scholar *
Delsing, P. et al. The 2019 surface acoustic waves roadmap. _J. Phys. D_ 52, 353001 (2019). Article CAS Google Scholar * Dholakia, K., Drinkwater, B. W. & Ritsch-Marte, M. Comparing
acoustic and optical forces for biomedical research. _Nat. Rev. Phys._ 2, 480–491 (2020). Article Google Scholar * Shen, L. et al. Acousto-dielectric tweezers for size-insensitive
manipulation and biophysical characterization of single cells. _Biosens. Bioelectron._ 224, 115061 (2023). Article PubMed CAS Google Scholar * Yafouz, B., Kadri, N. A. & Ibrahim, F.
Dielectrophoretic manipulation and separation of microparticles using microarray dot electrodes. _Sensors_ 14, 6356–6369 (2014). Article ADS PubMed PubMed Central CAS Google Scholar *
Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. _Biomicrofluidics_ 4, 022811 (2010). Article PubMed PubMed Central Google Scholar * Kim, D., Sonker, M.
& Ros, A. Dielectrophoresis: From molecular to micrometer-scale analytes. _Anal. Chem._ 91, 277–295 (2019). Article PubMed CAS Google Scholar * Tanase, M., Biais, N., & Sheetz,
M. Magnetic tweezers in cell biology. _Methods Cell Biol._ 83, 473–493 (2007). * Gossett, D. R. et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping.
_Proc. Natl. Acad. Sci. USA_ 109, 7630–7635 (2012). Article ADS PubMed PubMed Central CAS Google Scholar * Wiklund, M. Acoustofluidics 12: Biocompatibility and cell viability in
microfluidic acoustic resonators. _Lab Chip_ 12, 2018–2028 (2012). Article PubMed CAS Google Scholar * Tao, R. et al. Bimorph material/structure designs for high sensitivity flexible
surface acoustic wave temperature sensors. _Sci. Rep._ 8, 9052 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Shpak, O. et al. Acoustic droplet vaporization is
initiated by superharmonic focusing. _Proc. Natl. Acad. Sci. USA_ 111, 1697 (2014). Article ADS PubMed PubMed Central CAS Google Scholar * Melde, K., Mark, A. G., Qiu, T. &
Fischer, P. Holograms for acoustics. _Nature_ 537, 518–522 (2016). Article ADS PubMed CAS Google Scholar * Laurell, T., Petersson, F. & Nilsson, A. Chip integrated strategies for
acoustic separation and manipulation of cells and particles. _Chem. Soc. Rev._ 36, 492–506 (2007). Article PubMed CAS Google Scholar * Kooiman, K., Vos, H. J., Versluis, M. & de
Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. _Adv. Drug Del. Rev._ 72, 28–48 (2014). Article CAS Google Scholar * Friend, J. & Yeo, L. Y. Microscale
acoustofluidics: Microfluidics driven via acoustics and ultrasonics. _Rev. Mod. Phys._ 83, 647–704 (2011). Article ADS Google Scholar * Gu, J. & Jing, Y. Modeling of wave propagation
for medical ultrasound: a review. _IEEE Trans. Ultrason Ferroelectr. Freq. Control_ 62, 1979–1992 (2015). Article PubMed Google Scholar * Ding, X. et al. Cell separation using
tilted-angle standing surface acoustic waves. _Proc. Natl. Acad. Sci. USA_ 111, 12992–12997 (2014). Article ADS PubMed PubMed Central CAS Google Scholar * Li, P. et al. Acoustic
separation of circulating tumor cells. _Proc. Natl. Acad. Sci. USA_ 112, 4970 (2015). Article ADS PubMed PubMed Central CAS Google Scholar * Nam, J., Lim, H., Kim, C., Yoon Kang, J.
& Shin, S. Density-dependent separation of encapsulated cells in a microfluidic channel by using a standing surface acoustic wave. _Biomicrofluidics_ 6, 024120 (2012). Article PubMed
Central Google Scholar * Bruus, H. et al. Forthcoming Lab on a Chip tutorial series on acoustofluidics: Acoustofluidics—exploiting ultrasonic standing wave forces and acoustic streaming in
microfluidic systems for cell and particle manipulation. _Lab Chip_ 11, 3579–3580 (2011). Article PubMed CAS Google Scholar * Rufo, J., Cai, F., Friend, J., Wiklund, M. & Huang, T.
J. Acoustofluidics for biomedical applications. _Nat. Rev. Methods Prim._ 2, 30 (2022). Article CAS Google Scholar * Zhang, P., Bachman, H., Ozcelik, A. & Huang, T. J. Acoustic
Microfluidics. _Annu. Rev. Anal. Chem._ 13, 17–43 (2020). Article CAS Google Scholar * Habibi, R. et al. Exosome trapping and enrichment using a sound wave activated nano-sieve (SWANS).
_Lab Chip_ 20, 3633–3643 (2020). Article PubMed CAS Google Scholar * Rufo, J., Zhang, P., Zhong, R., Lee, L. P. & Huang, T. J. A sound approach to advancing healthcare systems: the
future of biomedical acoustics. _Nat. Commun._ 13, 3459 (2022). Article ADS PubMed PubMed Central CAS Google Scholar * Gu, Y. et al. Acoustofluidic centrifuge for nanoparticle
enrichment and separation. _Sci. Adv._ 7, eabc0467 (2021). Article ADS PubMed PubMed Central CAS Google Scholar * Ding, X. et al. Tunable patterning of microparticles and cells using
standing surface acoustic waves. _Lab Chip_ 12, 2491–2497 (2012). Article PubMed PubMed Central CAS Google Scholar * Collins, D. J. et al. Two-dimensional single-cell patterning with
one cell per well driven by surface acoustic waves. _Nat. Commun._ 6, 8686 (2015). Article ADS PubMed CAS Google Scholar * Yang, S. et al. Acoustic tweezers for high-throughput
single-cell analysis. _Nat. Protoc._ 18, 2441–2458 (2023). Article PubMed PubMed Central CAS Google Scholar * Hao, N. et al. Acoustofluidics-assisted fluorescence-SERS bimodal
biosensors. _Small_ 16, 2005179 (2020). Article CAS Google Scholar * Hao, N. et al. Acoustofluidic multimodal diagnostic system for Alzheimer’s disease. _Biosens. Bioelectron._ 196,
113730 (2022). Article PubMed CAS Google Scholar * Lu, H. et al. Rapid additive-free bacteria lysis using traveling surface acoustic waves in microfluidic channels. _Lab Chip_ 19,
4064–4070 (2019). Article PubMed CAS Google Scholar * Chen, Z. et al. Acoustofluidic micromixers: From rational design to lab-on-a-chip applications. _Appl. Mater. Today_ 26, 101356
(2022). Article Google Scholar * Reboud, J. et al. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. _Proc. Natl. Acad. Sci. USA_ 109,
15162–15167 (2012). Article ADS PubMed PubMed Central CAS Google Scholar * Ren, T., Steiger, W., Chen, P., Ovsianikov, A. & Demirci, U. Enhancing cell packing in buckyballs by
acoustofluidic activation. _Biofabrication_ 12, 025033 (2020). Article ADS PubMed CAS Google Scholar * Wang, W. et al. Acoustic propulsion of nanorod motors inside living cells. _Angew.
Chem. Int. Ed._ 53, 3201–3204 (2014). Article CAS Google Scholar * Liang, S. & Chaohui, W. Acoustic radiation force on a compressible cylinder in the standing surface acoustic wave
(SSAW). _J. Appl. Phys._ 123, 044504 (2018). Article ADS Google Scholar * Liang, S. & Chaohui, W. Revised model for the radiation force exerted by standing surface acoustic waves on a
rigid cylinder. _Phys. Rev. E_ 97, 033103 (2018). Article ADS PubMed CAS Google Scholar * Liang, S., Chaohui, W. & Qiao, H. The radiation force on a rigid sphere in standing
surface acoustic waves. _J. Appl. Phys._ 124, 104503 (2018). Article ADS Google Scholar * Yang, S. et al. Harmonic acoustics for dynamic and selective particle manipulation. _Nat. Mater._
21, 540–546 (2022). Article ADS PubMed PubMed Central CAS Google Scholar * He, Y. et al. Acoustofluidic interfaces for the mechanobiological secretome of MSCs. _Nat. Commun._ 14, 7639
(2023). Article ADS PubMed PubMed Central CAS Google Scholar * Bruus, H., Winckelmann, B. G., Bach, J. S., & Skov, N. R. 3D modeling of acoustofluidics in a liquid-filled cavity
including streaming, viscous boundary layers, surrounding solids, and a piezoelectric transducer. AIMS _Math._ 4, 99–111 (2019). * Barnkob, R. et al. Acoustically driven fluid and particle
motion in confined and leaky systems. _Phys. Rev. Appl._ 9, 014027 (2018). Article ADS CAS Google Scholar * Garg, N. et al. Rapid immunodiagnostics of multiple viral infections in an
acoustic microstreaming device with serum and saliva samples. _Lab Chip_ 19, 1524–1533 (2019). Article PubMed PubMed Central CAS Google Scholar * Busse, F. H. & Wang, T. G. Torque
generated by orthogonal acoustic waves–Theory. _Acoust. Soc. Am. J._ 69, 1634–1638 (1981). Article ADS MathSciNet Google Scholar * Marzo, A. & Drinkwater, B. W. Holographic acoustic
tweezers. _Proc. Natl. Acad. Sci. USA_ 116, 84 (2019). Article ADS PubMed CAS Google Scholar * Mishra, P., Hill, M. & Glynne-Jones, P. Deformation of red blood cells using acoustic
radiation forces. _Biomicrofluidics_ 8, 034109 (2014). Article PubMed PubMed Central Google Scholar * Link, A. & Franke, T. Acoustic erythrocytometer for mechanically probing cell
viscoelasticity. _Lab Chip_ 20, 1991–1998 (2020). Article PubMed CAS Google Scholar * Silva, G. T. et al. Acoustic deformation for the extraction of mechanical properties of lipid
vesicle populations. _Phys. Rev. E_ 99, 063002 (2019). Article ADS PubMed CAS Google Scholar * Liu, Y. & Xin, F. Deformation dynamics of spherical red blood cells in viscous fluid
driven by ultrasound. _Phys. Fluids_ 35, 012011 (2023). * Ni, Z. et al. Modelling of SAW-PDMS acoustofluidics: physical fields and particle motions influenced by different descriptions of
the PDMS domain. _Lab Chip_ 19, 2728–2740 (2019). Article PubMed CAS Google Scholar * Destgeer, G. & Sung, H. J. Recent advances in microfluidic actuation and micro-object
manipulation via surface acoustic waves. _Lab Chip_ 15, 2722–2738 (2015). Article PubMed CAS Google Scholar * Marston, P. L. & Zhang, L. Radiation torques and forces in scattering
from spheres and acoustical analogues. In _Advances in Imaging_ (Optica Publishing Group, 2009). * Settnes, M. & Bruus, H. Forces acting on a small particle in an acoustical field in a
viscous fluid. _Phys. Rev. E_ 85, 016327 (2012). Article ADS Google Scholar * Augustsson, P., Karlsen, J. T., Su, H.-W., Bruus, H. & Voldman, J. Iso-acoustic focusing of cells for
size-insensitive acousto-mechanical phenotyping. _Nat. Commun._ 7, 11556 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Liang, S., Chaohui, W. & Qiao, H. Force on a
compressible sphere and the resonance of a bubble in standing surface acoustic waves. _Phys. Rev. E_ 98, 043108 (2018). Article ADS CAS Google Scholar * Suresh, S. Biomechanics and
biophysics of cancer cells. _Acta Mater._ 55, 3989–4014 (2007). Article ADS CAS Google Scholar * Mei, J., Zhang, N., & Friend, J. Fabrication of surface acoustic wave devices on
lithium niobate. _J. Vis. Exp._ https://doi.org/10.3791/61013 (2020). * Otto, O. et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. _Nat. Methods_ 12, 199–202
(2015). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge the support from the National Science Foundation (CMMI-2104526 (Z.T.) and CMMI-2243771
(Z.T.)), the National Science Foundation Graduate Research Fellowship Program (2139754 (J.R.)), and the National Institutes of Health (R01GM144417 (Z.T.)). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Mechanical Engineering and Materials Science, Duke University, Durham, NC, USA Liang Shen, Kaichun Yang, Jianping Xia, Neil Upreti, Jinxin Zhang, Chuyi Chen,
Nanjing Hao, Zhichao Pei & Tony Jun Huang * Department of Mechanical Engineering, Virginia Polytechnical Institute and State University, Blacksburg, VA, USA Liang Shen & Zhenhua Tian
* Department of Biomedical Engineering, Duke University, Durham, NC, USA Joseph Rich Authors * Liang Shen View author publications You can also search for this author inPubMed Google
Scholar * Zhenhua Tian View author publications You can also search for this author inPubMed Google Scholar * Kaichun Yang View author publications You can also search for this author
inPubMed Google Scholar * Joseph Rich View author publications You can also search for this author inPubMed Google Scholar * Jianping Xia View author publications You can also search for
this author inPubMed Google Scholar * Neil Upreti View author publications You can also search for this author inPubMed Google Scholar * Jinxin Zhang View author publications You can also
search for this author inPubMed Google Scholar * Chuyi Chen View author publications You can also search for this author inPubMed Google Scholar * Nanjing Hao View author publications You
can also search for this author inPubMed Google Scholar * Zhichao Pei View author publications You can also search for this author inPubMed Google Scholar * Tony Jun Huang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.S. conceived the idea. L.S. developed the models for simulations. L.S. designed, fabricated, and
characterized JSAT devices. K.Y. and J.Z. fabricated the JSAT devices. L.S., N.H., Z.P., J.X., and C.C. conducted the experiments. L.S. analyzed the data. L.S., Z.T., J.R., N.U., and T.J.H.
wrote the paper. T.J.H. and Z.T. supervised the study. CORRESPONDING AUTHORS Correspondence to Zhenhua Tian or Tony Jun Huang. ETHICS DECLARATIONS COMPETING INTERESTS T.J.H. has co-founded a
start-up company, Ascent Bio-Nano Technologies Inc., to commercialize technologies involving acoustofluidics and acoustic tweezers. All other authors declare no competing interests. PEER
REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Per Augustsson, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file
is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY MOVIE 1 SUPPLEMENTARY MOVIE 2 SUPPLEMENTARY MOVIE 3 SUPPLEMENTARY MOVIE 4
SUPPLEMENTARY MOVIE 5 SUPPLEMENTARY MOVIE 6 SUPPLEMENTARY MOVIE 7 SUPPLEMENTARY MOVIE 8 SUPPLEMENTARY MOVIE 9 SUPPLEMENTARY MOVIE 10 SUPPLEMENTARY MOVIE 11 REPORTING SUMMARY RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third
party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shen, L., Tian, Z., Yang, K.
_et al._ Joint subarray acoustic tweezers enable controllable cell translation, rotation, and deformation. _Nat Commun_ 15, 9059 (2024). https://doi.org/10.1038/s41467-024-52686-8 Download
citation * Received: 26 February 2024 * Accepted: 18 September 2024 * Published: 20 October 2024 * DOI: https://doi.org/10.1038/s41467-024-52686-8 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative