Play all audios:
ABSTRACT To shed light on the enigmatic origin of the vertebrate head, our study employs an integrated approach that combines single-cell transcriptomics, perturbations in signaling
pathways, and cis-regulatory analysis in amphioxus. As a representative of a basal lineage within the chordate phylum, amphioxus retains many characteristics thought to have been present in
the common chordate ancestor. Through cell type characterization, we identify the presence of prechordal plate-like, pre-migratory, and migratory neural crest-like cell populations in the
developing amphioxus embryo. Functional analysis establishes conserved roles of the Nodal and Hedgehog signaling pathways in prechordal plate-like populations, and of the Wnt signaling
pathway in neural crest-like populations’ development. Furthermore, our trans-species transgenic experiments highlight similarities in the regulatory environments that drive neural
crest-like and prechordal plate-like developmental programs in both vertebrates and amphioxus. Our findings provide evidence that the key features of vertebrate head development can be
traced back to the common ancestor of all chordates. SIMILAR CONTENT BEING VIEWED BY OTHERS AN AMPHIOXUS NEURULA STAGE CELL ATLAS SUPPORTS A COMPLEX SCENARIO FOR THE EMERGENCE OF VERTEBRATE
HEAD MESODERM Article Open access 29 May 2024 STEP-WISE EVOLUTION OF NEURAL PATTERNING BY HEDGEHOG SIGNALLING IN CHORDATES Article 13 July 2020 EVOLUTIONARY ORIGIN OF THE CHORDATE NERVOUS
SYSTEM REVEALED BY AMPHIOXUS DEVELOPMENTAL TRAJECTORIES Article 18 July 2024 INTRODUCTION Deciphering the enigmatic evolution and origin of the vertebrate head stands as a pivotal challenge
in the realm of chordate evolutionary developmental biology. Central to this enigma are the neural crest (NC), placodes, and prechordal plate (PrCP), innovations fundamental to vertebrate
head evolution, yet intriguingly absent in amphioxus, a representative of basal chordate lineage1,2,3,4,5. The PrCP is a transient embryonic structure that forms as a band of thickened
mesendoderm, rostrally to the notochord6,7,8,9. It significantly influences the induction and patterning of the forebrain10,11 and contributes to the cranial mesoderm and endoderm12,13.
Based on single-cell RNA sequencing (scRNA-seq) analysis in zebrafish, a transcriptional divergence between the PrCP and the notochord emerges during gastrulation, leading to the formation
of two distinct cell populations with specific transcriptomic profiles14. Several studies demonstrated the existence of a gastrula organizer in amphioxus, with a compelling degree of
homology to the dorsal organizer of vertebrates15,16,17,18. Despite this, a prevailing view suggests the absence of a PrCP in amphioxus4,19. This assertion stems primarily from two
distinctive features observed in amphioxus - the extension of the notochord beyond the brain vesicle in adults, and the inconsistent gene expression patterns that defy the conventional model
of PrCP formation in vertebrates4,19,20. A recent study revealed that the cerebral vesicle of amphioxus at the early mid-neurula stage displays vertebrate-like partitioning, including an
area termed the hypothalamo-prethalamic primordium (HyPth), which exhibits molecular partitions similar to those of the vertebrate floor, basal, and alar/roof plates21. In vertebrates, these
partitions are influenced by signals from the PrCP10. The work on HyPth21 has revived the debate on the existence of a PrCP-like region in amphioxus. However, despite generating new
hypotheses, the question remained unanswered due to a lack of conclusive experimental data20,22. Here, to address the question of head evolution in the chordate lineage, we utilized a
single-cell transcriptomic approach to analyze embryonic cells across four developmental stages in amphioxus. Initially, we identified a PrCP-like population, as well as pre-migratory and
migratory neural crest-like populations in the amphioxus head region. These populations are characterized by a unique combination of differentially expressed genes (DEGs), the orthologs of
which are found in the vertebrate PrCP14, as well as in neural crest (NC) or its derivatives23,24,25,26,27,28,29,30. Nodal and Hh signaling are fundamental to PrCP and head development in
vertebrates7,9,31, and Wnt signaling is required for NC specification32. Therefore, we examined the roles of the Nodal and Hh (Hedgehog) signaling pathways in the development of amphioxus
PrCP-like, along with Wnt signaling in neural crest-like populations. Trans-species transgenic experiments further provided evidence of the conservation of PrCP and NCC developmental
programs within the chordate lineage at the cis-regulatory level. RESULTS PRCP-LIKE CELL POPULATION IS PRESENT IN AMPHIOXUS EMBRYO To determine the existence of a PrCP in amphioxus, we
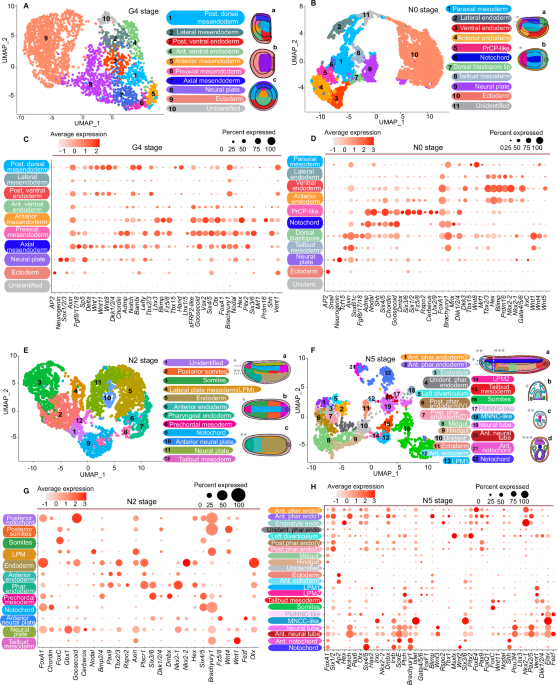
clustered scRNA-seq datasets from mid-gastrula to neurula stages (G4, N0, N2 and N5) and annotated the resulting clusters based on the spatial expression patterns of marker genes (Fig. 1;
Supplementary Figs. 1, 1a–d, 2, 2a–e, 3, 3e–f, 4, 4a–e). Our analysis primarily targeted clusters in the axial and anterior mesendoderm territory. At G4, we identified axial, preaxial and
anterior mesendoderm cell populations (Fig. 1, A, C). The preaxial mesendoderm is characterized by the abundant expression of _Chordin_, _Shh_, _Six3/6_, _Six4/5_, _Nodal_, _Goosecoid_,
_Pitx2_ and _Lefty_ genes. While axial and anterior mesendoderm share small number of common genes between each other, preaxial mesendoderm exhibits a higher degree of overlap in expressed
genes with both axial and anterior mesendoderm populations. At N0, we identified a cluster expressing _Lhx2/9_ and _Dmbx_ (Fig. 1B, D and Supplementary Fig. 2, panels D1–E3), along with
predominant expression of _Six3/6_, _Nodal_, _Fz5/8_, _Cerberus_, and _Dkk1/2/4_, all recognized as PrCP markers in vertebrates14,33. These cells, representing the anterior dorsal
mesendoderm domain (Fig. 1Ba, b), contrast the notochord by exhibiting a markedly lower _Brachyury1_ level and lacking _Fgf8/17/18_ and _Mnx_ expression. Despite slightly reduced levels,
they maintain _Chordin_ and _Goosecoid_ expression. We designated this population as the PrCP-like and propose its homology with the vertebrate PrCP. Additionally, we integrated our datasets
from the G4 and N0 stages with previously published datasets from the late gastrula and early neurula stages of _Branchiostoma japonicum_, obtained using similar technology34. We
successfully identified PrCP-like populations within the individual datasets (Supplementary Fig. 5) and in the newly generated datasets resulting from the integration with those from Satoh
et al. (Supplementary Fig. 6, 7). Furthermore, the integration of our N0 stage and N0 stage dataset from Ma et al.35 revealed a cluster of cells expressing PrCP-like genes, consistent with
the PrCP-like population found in our dataset (Supplementary Fig. 8). Recently, top-ranking DEG specifying the PrCP and notochord were identified from scRNA-seq data in zebrafish14. We found
orthologs of the corresponding genes in amphioxus and examined their expression in our dataset (Fig. 2A–H; Supplementary Fig. 2, panels A, B5 and F1, F2). At G4, PrCP-specific genes were
predominantly expressed in the preaxial and axial mesendoderm (Fig. 2A). By N0, most of the genes showed preferential expression in either the PrCP-like or notochord, reflecting patterns in
zebrafish (Fig. 2A, B). These observations suggest that the separation of the PrCP-like and notochord in amphioxus occurs during gastrulation, similar to vertebrates6,9,14. Calculated cell
state transition probabilities further support this notion (Supplementary Fig. 9A). To understand the molecular differences between the notochord and PrCP-like populations at the N0 stage,
we analyzed top DEG (Supplementary Fig. 10). The PrCP-like primarily exhibited genes linked to migration and cell motility, similar to the migrating PrCP cells in vertebrates8,36. The
notochord displayed a distinct molecular signature, with a preponderance of genes involved in autophagy and energy metabolism. At N2, we identified a cluster corresponding to the anterior
dorsal mesendoderm, termed ‘prechordal mesoderm’ (Fig. 1E, G). This population is marked by high _Six3/6_, _Dmbx_, and _Bmp2/4_ expression, but shows notably lower levels of _Brachyury1_ and
_Mnx_ compared to the notochord. In our comparative analysis of vertebrate PrCP and notochord markers within amphioxus embryos, we observed a more distinct separation between the notochord
and PrCP-like at the N0 stage compared to the notochord and prechordal mesoderm at N2 (Fig. 2A, B). Certain notochord-specific markers, absent at N0, were present in the prechordal mesoderm
at N2, though at lower levels (Fig. 2B). The preaxial mesendoderm at G4 displays a transcriptomic profile more similar to vertebrate PrCP than the prechordal mesoderm at N2 (Fig. 2A).
_Goosecoid_ persists in the PrCP-like until N1 but disappears by N2 (Fig. 2H). These findings suggest that the amphioxus PrCP-like most closely mirrors its vertebrate counterpart during late
gastrula to early neurula stages. NEURAL CREST-LIKE CELLS ARE PRESENT IN AMPHIOXUS At the N5 stage, a cluster termed ‘MNCC-like’ (Migratory Neural Crest Cells-like) was identified (Fig. 1F,
H), marked by high _Elav_, _Islet_, and _Six3/6_ expression. The abundant _Six3/6_ suggests that these cells are located in the embryo’s anterior domain (Supplementary Fig. 4, panels J-J3).
UMAP clustering revealed proximity between this cluster and the ‘PMNCC-like’ (Pre-migratory Neural Crest Cells-like) anterior neural tube population, characterized by increased _Otx_
expression (Fig. 3A, I; Supplementary Fig. 11A, panels B1–B3). Computations of cell fate transition probabilities, which critically rely on transcriptomic profile similarity, indicate a
strong transcriptional resemblance and a high probability that MNCC-like cells at N5 originate from the anterior neural plate at N2. Additionally, a high transition probability of MNCC-like
origin from PMNCC-like populations was revealed. These computations suggest that the most probable origin of MNCC-like lies within these domains (Supplementary Figs. 9B; 14). ScRNA-seq
analysis and double immunostaining revealed _Elav_ co-expression with _Otx_ in PMNCC, and a distinct strong expression of Elav alone in cells between the ectoderm and endoderm (Fig. 3A and
panels B-B2). We observed dynamic changes in Elav expression at N5, particularly a shift in the concentration of Elav+ cells from the dorsorostral to the rostral region (Fig. 3, panels
C3-C4). MNCC-like also exhibited heightened expression of _Hand_ (Fig. 3A and panels D1, D2). In vertebrates, _Hand_ is associated with the cranial NC and lateral plate mesoderm (LPM)29,37,
a pattern mirrored in amphioxus where _Hand_ is notably expressed in the LPM38 (Fig. 3A). Additionally, _Hand_ expression was especially strong in the MNCC-like cluster, the only population
in our scRNA-seq dataset exhibiting extensive co-expression of _Hand_ and _Elav_. Analysis of DEG in MNCC-like identified genes including _Trk, Shox2, Lingo2, Otof, Prdm12, Tlx, Scrt2,
Skor2, Pou4f3, Neto2_, and _Runx1_ (Fig. 3I; Supplementary Figs. 11F; 15, panels D1-D5 E1-E5, H1-H5; 16, panels A1-A4, C1-C3, F1-F2, I1-i3). These markers in vertebrates typically signify
developing sensory neurons within sensory ganglia27,39,40,41,42. Notably, vertebrate sensory ganglia originate from NC and cranial placodes. Further examination of DEG in MNCC-like revealed
numerous orthologs to genes associated with developing or migrating NC, including _Mitf, Slit1, Robo1, Ebf, Meis, FoxN3, Ets, Id, Idh1, Zeb2, Elk, Rxrg, Arnt2, Itgb1, Ptn, Tle, Tmem132C,
Bmpr1B, Rho_ and _Adam_ genes24,28,30,32,43,44 (Fig. 3I; Supplementary Figs. 11A_,_ panels D1-E9; 11_F;_ 15, panels B1-C4, 9G1-G4; 16, panels D1-D4). While _SoxE_ is absent, _SoxD_ and
_SoxC_ are present, with _SoxD_ being highly elevated (Fig. 3I, Supplementary Figs. 15, panels A1–A4; 16, panels B1-b3). In vertebrates, the representatives of _SoxC_ and _SoxD_ groups are
expressed in NC progenitors or derivatives45,46. The PMNCC-like population, likely a precursor to MNCC-like, exhibits a similar gene profile and additional vertebrate NC markers like _FoxD,
Lhx1, Ets, Snail, Zic, Btg, Dlx, Ap-2, Atf2_, and _Zfhx3_ (Fig. 3I; Supplementary Fig. 11A, panels A1–A3, C1–C3, F1–F3, G1–G3; 11F). Furthermore, we explored the dataset representing the
late neurula stage from Satoh et al.47 According to the schematic diagram provided in their study, this dataset corresponds to an earlier phase of the late neurula stage. As indicated by
Elav staining, we did not observe a significant presence of MNCC-like cells at this stage (Supplementary Fig. 11B). Consistent with our observations, we identified only a PMNCC-like
population, forming a distinct single cluster in this dataset (Supplementary Fig. 12). However, some genes characteristic of the MNCC-like population were present in the PMNCC-like
population, albeit in low quantities. In the integrated dataset, we successfully identified both MNCC-like and PMNCC-like populations (Supplementary Fig. 13). Previous studies have shown
that from the early neurula stage, amphioxus has Elav+ cells in the ventral ectoderm, which migrate between the ectoderm and endoderm48,49. In our scRNA-seq data, Elav+ cells were identified
in the ectoderm at the G4, N0, and N5 stages. UMAP clustering and the calculation of cell fate transition probabilities revealed that these cells are distinct from the MNCC-like population
(Fig. 1; Supplementary Figs. 9B, 14). Wnt signaling promotes NC induction and affects pre-placodal ectoderm induction in vertebrates32. In our study, we noted high expression of _Wnt7b_ in
PMNCC-like and MNCC-like populations, with _Tcf, Lrp6_ and _Dkk1/2/4_ also elevated in PMNCC-like (Fig. 3I; Supplementary Fig. 11E-F). _Dkk1/2/4_, orthologous to vertebrate _Dkk1_ and
_Dkk2_, have contrasting roles in NC development: _Dkk2_ activates Wnt/β-catenin signaling for NC specification50, while _Dkk1_, when secreted from the PrCP, inhibits it in the rostral
neural plate border51. To explore the effect of Wnt signaling on PMNCC-like and MNCC-like, we applied the pathway inhibitor C59 from the N1 stage. Low concentrations of C59 did not
noticeably alter embryo morphology but led to reduced _FoxD_ expression in PMNCC-like and decreased Elav in both MNCC-like and PMNCC-like, while other Elav+ cells remained unaffected (Fig.
3F-F’). Higher doses disrupted the overall development, but ectodermal cells persisted (Supplementary Fig. 11D). These findings suggest that PMNCC-like and MNCC-like populations are
differently regulated by Wnt signaling compared to ventral ectoderm sensory cells. To further examine our hypothesis that the amphioxus MNCC-like population resembles the vertebrate NC, we
utilized a trans-species transgenic reporter assay involving _crestin_, a zebrafish-specific gene expressed exclusively in NC52. Previous studies have demonstrated that a regulatory element
located 4.5 kb upstream of the predicted _crestin_ open reading frame when linked to a reporter gene, accurately marks emerging and migrating NC53. In transgenic amphioxus embryos,
_Crestin::mCherry_ reporter gene activity was present in the MNCC-like cells at N5, but absent in the most anterior neural tube (Fig. 3H-H3). At the N3 stage, the reporter signal appeared
only sporadically in individual cells of the anterior neural plate (Supplementary Fig. 11C). In zebrafish, the transcription factors Sox10, Ap-2 and Mitf/Myc are crucial for _crestin_
activity in NC53. In the MNCC-like, while _Mitf_ was present, _Ap-2_ was only found in PMNCC-like. Despite the absence of SoxE, other family members like SoxD and SoxC might bind to the SoxE
motif, particularly given SoxD’s flexible DNA binding sequence54. Together, our data suggest that the migratory population, which exhibits features of the migrating and differentiating NC,
originates from the specific domain in the anterior neuroectoderm. PRCP-LIKE DEVELOPMENT IN AMPHIOXUS IS REGULATED BY NODAL AND HH SIGNALING Motivated by evidence of Nodal7,9 and Hh
signaling11,31 critical roles in PrCP development in vertebrates, we performed a pharmacological inhibition of these pathways in amphioxus to explore conservation of the respective PrCP
programs at the gene regulatory level. We conducted pharmacological treatments at the gastrula stage to target the early separation phase of the PrCP-like from the axial mesendoderm,
focusing on PrCP-like formation rather than organizer formation. After applying the Nodal inhibitor at G4, we observed no significant reduction in _Six3/6_ expression in the PrCP-like at N1,
nor any morphological deficiencies in larvae (Fig. 4). In contrast, G2 stage treatment eliminated _Six3/6_, _Goosecoid_, and _FoxA1_ expression in the PrCP-like domain (Fig. 4A-g’),
significantly shortened the notochord’s anterior tip in larvae, and resulted in a deficient cerebral vesicle (Fig. 4I-T’). Expressions of _Six3/6_ and _Fz5/8_ (Fig. 4L-P2) diminished in the
notochord’s anterior tip and anterior cerebral vesicle, while _Lhx3_ was absent from the ventral domain of the cerebral vesicle (Fig. 4R-T’). The absence of _Lhx3_ expression in the left
diverticulum in G4-treated embryos aligns with previously described disruptions in left-right asymmetry during neurulation55. Administering the Shh signaling inhibitor cyclopamine at the G2
stage inhibited _Ptch_, a target of Hh signaling, and led to severe phenotypic malformations in larvae (Fig. 5). These included the absence of the notochord’s anterior tip, defects in
anterior neural tube closure, pharyngeal endoderm, and the first pair of somites. _FoxA1_ and _Six3/6_ expression vanished in the anterior notochord (Fig. 5C-F2), _Myl1_ was reduced in
anterior somites (Fig. 5G-H2), and _Otx_ was absent from the anterior cerebral vesicle (Fig. 5I-J2). The defects must be related to Shh activity in the preaxial mesendoderm at mid gastrula,
as late gastrula cyclopamine application didn’t cause a similar effect56 (Fig. 5K). No severe rostral malformations were observed in amphioxus upon the Hh signaling components knockout,
including _Shh_57, _Smo_ and _Ptch_58, possibly due to the presence of maternal mRNAs or other Patched/Shh-like genes (Supplementary Fig. 17). GENE REGULATORY CONSERVATION OF PRCP
DEVELOPMENTAL PROGRAMS IN CHORDATES To further explore the conservation of PrCP development in chordates, we employed cross-species transgenic reporter assays37,59,60. Specifically, we
examined the activity of reporters driven by amphioxus _Goosecoid_ (_BfGoosecoid::EGFP_), _Chordin_ (_BfChordin::EGFP_), and _Six3/6_ (_BfSix3/6::EGFP_) cis-regulatory elements in developing
zebrafish embryos. _BfGoosecoid::EGFP_ reporter gene activity was observed in the anterior mesendoderm and PrCP-like of amphioxus (Supplementary Fig. 18A) and zebrafish embryos (Fig.
6A–D’). This reporter also exhibited strong activity in the axial mesoderm, similar to its endogenous pattern. Likewise, _BfChordin::EGFP_18 drove expression in the axial domain and PrCP at
the 80% epiboly stage in zebrafish (Fig. 6I), more closely aligning with the endogenous expression of _Chordin_ in amphioxus than with that in zebrafish61. A recent functional annotation of
the amphioxus genome using ATAC-seq identified potential cis-regulatory DNA elements59. Testing three open chromatin regions in the amphioxus _Six3/6_ locus at gastrula to mid neurula stages
(Supplementary Fig. 18C), revealed an enhancer matching the endogenous anterior ectoderm and PrCP-specific expression of _Six3/6_ (Fig. 6J). In zebrafish embryos, this enhancer was active
in the PrCP and anterior ectoderm domain (Fig. 6K), resembling the expression pattern of endogenous zebrafish _six3b_62. Treating zebrafish embryos with a Nodal signaling inhibitor at the
early gastrula stage abolished _BfGoosecoid::EGFP_ and _BfChordin::EGFP_ expression in the PrCP, but left axial mesoderm expression unaffected (Fig. 6L–O”, Supplementary Fig. 18B). This
response mirrors amphioxus results under similar conditions (Fig. 4) and aligns with zebrafish patterns, where endogenous _goosecoid_ is lost from the PrCP-like after the treatment the at
the early gastrula-stage7 (Fig. 6P). These results provide additional support for the existence of an evolutionarily conserved regulatory program underlying PrCP development in chordates
(Fig. 7). DISCUSSION PRECHORDAL PLATE AS AN ANCESTOR FEATURE OF CHORDATES Our study contributes to the debate on vertebrate head evolution by substantiating the ancestral origin of the PrCP
in chordates. The obtained data suggest that the PrCP-like in amphioxus is specified and functions during the late gastrula to early neurula stages. By the N2 stage, the partitioning of the
anterior neural plate in amphioxus resembles that in vertebrates21, indicating that vital brain regionalization signaling events likely occur during earlier stages, aligning with the period
when the PrCP’s transcriptomic profile closely mirrors that of vertebrates. In vertebrates, Nodal signaling from the PrCP is essential for its development and for establishing the
hypothalamus63. In amphioxus, cerebral vesicle deficiencies, including the absence of Lhx3 in the ventral domain, were noted only with treatment at the G2 stage, not G4. This underscores the
late gastrula/early neurula period as critical for Nodal signaling role in anterior neuroectoderm patterning, given that transcription inhibition by Nodal inhibitors can start within 30 to
45 minutes7. At N0, Nodal signaling is active in the PrCP-like but not in the anterior neuroectoderm (Supplementary Fig. 19A, panels A-C), indicating its role in patterning from the
PrCP-like during this phase. By N2, nuclear phospho-Smad2 is not detected in the prechordal mesoderm (Supplementary Fig. 19A, panels D-E). In early amphioxus neurula stages, Nodal signaling
shifts to regulating left-right asymmetry55,57 (Supplementary Fig. 19B). Conversely, in vertebrates, the PrCP-specific genes expression endures longer64, with asymmetrical _Pitx_ expression
starting later compared to the progression of neurulation and forebrain development. Similarly, Hh signaling during the late gastrula/early neurula phase is required for forming the head
mesoderm, endoderm and cerebral vesicle in amphioxus, likely influenced by signals from the PrCP-like and preaxial mesendoderm, where Shh is abundant but absent in the neuroectoderm (Fig.
1C). Post-N0 stage, Shh expression in the anterior mesendoderm significantly decreases56,57 (Supplementary Fig. 19B). In vertebrates, Hh signaling from the prechordal mesoderm is crucial for
head development, a function extending over a significant duration of neurulation10,11. Meister _et al_. observed differences between the anterior tip and the central domain of the
notochord during amphioxus neurulation, raising a question about the transient presence of vertebrate-like PrCP22. Our findings corroborate the observation of notochord regionalization at
the early mid-neurula and late neurula stages, revealing separate clusters for the notochord anterior domain at N2 and N5. However, the structure we described here as homologous to the
vertebrate PrCP is distinct, and by the N2 stage, it no longer exists in a bona fide vertebrate-like manner. In addition, at the late gastrula/early neurula stages, PrCP-like includes
additional lateral and anterior dorsal mesendoderm domains. Further, our observations are supported by SAMap (Self-Assemling Manifold mapping) analysis. SAMap algorithm enables mapping of
single-cell transcriptomes between phylogenatically remote species by relaxing the constraints imposed by sequence orthology65. However, combining amphioxus with vertebrates introduces
certain limitations due to two rounds of genome duplication in vertebrates, internal gene duplication in amphioxus, and imperfections in the sequencing and assembly of the amphioxus genome,
which can result in significant noise. Even with these limitations, the amphioxus preaxial mesendoderm at G4 and prechordal plate-like population at N0 strongly correlate with the prechordal
plate in zebrafish (Supplementary Figs. 20–24). The PrCP-like in amphioxus, a transient cell population, likely transitions into various cell types. Specifically, our computation of cell
fate transition probabilities suggests that the N0 PrCP-like may transition to the pharyngeal endoderm and prechordal mesoderm at N2, which then contributes to the anterior notochord and the
Hatschek’s left diverticulum (HLD) (Supplementary Fig. 9A). This aligns with the concept of the PrCP being part of the anterior mesendoderm, encompassing both dorsal and lateral regions.
Accordingly, revisiting Goodrich’s hypothesis that HLD is of mesodermal origin and serves as a homolog of the vertebrates’ cranial premandibular coelom66 is pertinent, especially considering
that the premandibular coelom is also derived from the PrCP12. Previous studies have shown the complex gene expression patterns within HLD66,67. Our cell fate transition probability
analysis supports this complexity, indicating a probable contribution from the anterior neural plate, likely due to high transcriptomic similarity and implying a sharing of gene regulatory
network features. Post-metamorphosis, HLD fuses with the surface ectoderm to form Hatschek’s pit, potentially analogous to the vertebrate adenohypophysis1,68, originating from cranial
placode and endoderm1,69. This high transcriptomic similarity aligns with the hypothesis that certain transcription factors in the chordate lineage may have expanded from the endomesodermal
regions of HLD to adjacent ectoderm1. IDENTIFICATION OF NEURAL CREST-LIKE CELLS IN AMPHIOXUS Studies suggest the presence of sensory cells migrating from the ventral ectoderm48,49, with a
significant fraction of their genes found in vertebrates’ dorsally forming sensory cells70. The MNCC-like population closely resembles vertebrates’ placode/NC-derived sensory neurons and
migrating NC. However, it is distinct from the ventral ectoderm-derived peripheral sensory cells and most likely originates from the anterior neural tube. Alternatively, MNCC-like may
originate from the rostral or ventral anterior ectoderm. Our findings reveal that, unlike MNCC-like, Wnt signaling is not necessary for _Elav_ expression in late neurula stage ventral and
posterior sensory cells. A recent study indicated that similar treatment marginally increases Elav+ cells in the mid-neurula ventral ectoderm70. Conversely, activating canonical Wnt
signaling inhibits _Elav_ and _Tlx_ in the anterior ventral ectoderm70. This indicates that the sensory cells’ development in the ventral anterior ectoderm follows a regulatory mechanism
distinct from that in MNCC-like. Moreover, the reduced _FoxD_ and _Elav_ expression in PMNCC-like due to Wnt inhibition indicates the necessity of Wnt signaling for the specification of
these cells, similar to MNCC-like. Notably, _Elav_, absent in the rostral domain during neurula stages, is detected in the rostral ectoderm in larvae17,47, alongside _Islet_38. These Elav+
cells may correspond to the rostral sensory cells described by Lacalli _et al_.71, potentially originating from the MNCC-like. We also considered the possibility that MNCC-like originate
from the dorsorostral ectoderm, as they share some genes with this region, though less than with the PMNCC-like (Fig. 3I). Immunohistochemistry revealed dorsal/rostral Elav+ MNCC-like in
embryos starting at early N5 stage, absent at N3 or N4 stages (Supplementary Fig. 11B), suggesting their emergence occurs during late N4 or early N5. Yet, the appearance of a population
resembling PMNCC-like more than the anterior ectoderm (Supplementary Fig. 14) during this period is improbable. We propose that PMNCC-like specification occurs during the second phase of
neurulation, with delamination at the end of neurulation. The PMNCC-like population in our dataset likely includes both pre-migratory and delaminating cells, starting to express sensory
neuron markers. In this context, the MNCC-like population more closely resembles cranial placodes morphogenesis, where delaminating cells predominantly adopt a neuronal fate72. In contrast
to the PrCP-like populations, SAMap analysis did not reveal a correlation between MNCC-like/PMNCC-like populations and the midbrain/neural crest lineage in zebrafish (Supplementary Figs.
25-26). This may be due to the higher multipotency of vertebrate neural crest cells compared to the more specialized neural/sensory cell “blueprint” of the amphioxus PMNCC/MNCC-like cells.
However, MNCC-like cells correlate with the spinal cord and olfactory placode lineages, indicating that they embody gene expression programs of both neural and sensory neurons. PMNCC-like
cells show the highest alignment score with the telencephalon. Intriguingly, the vertebrate olfactory system, which originates from both neural crest and placode73,74, uniquely arises from
the anterior neural fold75. This fold includes a mix of precursors for the epidermis, olfactory, and lens placodes, as well as parts of the telencephalon. Altogether, this allows us to
speculate that PMNCC/MNCC-like populations may exhibit characteristics of both neural crest and placodal developmental programs. A key argument against placodes’ existence in amphioxus is
the lack of key markers like _Six1/2_, _Eya_, and _Six4/5_ in the dorsal non-neural ectoderm during late gastrula/early neurula stages67. Supporting this, our scRNA-seq data found no
_Six1/2/Eya/Six4/5_-positive clusters in the ectoderm at G4, N0 and N5, nor any expression in the anterior ectoderm at N2. In _Ciona intestinalis_, sensory cells arise from proto-placodal or
proto-NC regions, with potential interconversion following genetic disruptions76,77,78. In amphioxus, PMNCC-like are situated in the most anterior neural tube, in close contact with the
anterior ectoderm. The signals from the dorsal anterior ectoderm may contribute to the specification of PMNCC-like. In vertebrates, cranial NC is not specified at the rostral neural plate
border, likely due to Wnt signaling inhibitors from the PrCP32,51. However, amphioxus PrCP resembles vertebrate PrCP only during the late gastrula/early neurula stages, and at N5, Wnt
inhibitors _Dkk1/2/4_ and _Dkk3_ are absent in the anterior notochord of amphioxus (Supplementary Fig. 11F). Some genes identified in amphioxus MNCC-like are not present in vertebrate
sensory cells originating from NC or placodes. Notably, vertebrate _Mitf_ is expressed in NC-derived melanocytes79 and in melanocytes derived from Schwann cell progenitors80. Likewise,
_Hand2_ is expressed in various NC-derived cells, including sympathetic ganglion and enteric neurons29. This suggests two possibilities: either amphioxus and vertebrates have different
sensory cell specification programs, or amphioxus MNCC-like may give rise to more than just sensory cells. In summary, we propose that some cornerstone innovations in vertebrate head
development, such as the PrCP and NC, can be traced back to ancestral features within the chordate lineage (Fig. 7). METHODS ETHICAL COMPLIANCE STATEMENT This research was conducted in
accordance with all applicable ethical regulations. The study protocol was reviewed and approved by the Animal Welfare and Protection Committee at Institute of Molecular Genetics of the
Czech Academy of Sciences. MAINTENANCE AND AMPHIOXUS HUSBANDRY Amphioxus _Branchiostoma floridae_ adults were housed in 5 litres of natural seawater at a temperature of 28 °C and were fed
with algae on a daily basis. To induce spawning, the animals were transferred to a temperature of 18 °C for at least 6 weeks before being exposed to a heat shock induced by elevating the
temperature to 28 °C for 24 hours. One hour before artificial sunset, the animals were separated into 0.5-liter plastic cups containing 30-50 ml of seawater. Most of the animals spawned
within an hour after the lights were switched off. The resulting embryos were raised at a temperature of 25 °C. OLIGONUCLEOTIDES Oligonucleotides used for the generation of constructs used
in WMISH, protein expression, and transgenesis are shown in Supplementary Table S1. ANTIBODY PRODUCTION Amphioxus-specific antibodies were prepared as previously described81. Selected
protein-coding sequences were cloned into pET42a(+) vector (Novagen) using primers shown in Supplementary Table S1. Proteins were expressed in BL21 (DE3) RIPL bacteria (Stratagene) and
purified using Ni-NTA agarose beads (Qiagen). Three mice of the B10A-H2xBALB/CJ strain were immunized four times in 4 weeks’ intervals with 30 mg of purified protein in PBS mixed with
Freund’s adjuvant (Sigma-Aldrich). The serum was collected 10 days after the 4th immunization. IMMUNOHISTOCHEMISTRY STAINING OF AMPHIOXUS EMBRYOS After fixation with 4% PFA/MOPS (0.1 M
3-(N-morpholino)propanesulfonic acid, 1 mM EGTA, 2 mM MgSO4, 0.5 M NaCl, pH = 7.5) for 15 minutes on ice, the embryos were transferred through a series of 30% and 70% methanol mixed with
1xTBS and 0.1% Triton-X100 (TBST) to 100% methanol and stored at −20 °C. The immunostaining procedure followed the general protocol81 with minor modifications, such as the use of 1xTBS
buffer with 0.05% Triton in all washing solutions, a 10% BSA and 10% donkey serum blocking solution and donkey anti-mouse secondary antibodies (ThermoFisherScientific) at the dilution 1:500.
The embryos were imaged with Leica SP8 or Dragonfly confocal microscope and processed with Fiji ImageJ analysis software. IN-SITU HYBRIDIZATION OF AMPHIOXUS AND ZEBRAFISH EMBRYOS The
methodology used in this study involved conducting in-situ hybridization of amphioxus embryos, as previously described82. The embryos were imaged with Leica SP8 confocal microscope withLeica
application Suite X3.5.7.23225 and processed with Fiji ImageJ (version 1.54d) analysis software. Adjustments to individual color channels were made for the merged images. 3D reconstruction
was performed using the 3D Viewer plugin of the Fiji ImageJ (version 1.54d) analysis software. All experiments with zebrafish were performed in compliance with the European Communities
Council Directive of 24 November 1986 (86/609/EEC) and guidelines of the Institute of Molecular Genetics of the Czech Academy of Sciences approved by the Animal Care Committee (approval
numbers 11/2018 and 16-2023-P). Zebrafish embryos, collected from adult zebrafish (_Danio rerio_, strain AB), were fixed overnight in 4% PFA/PBT solution at 4 °C with shaking. Subsequently,
they were stored in methanol at −20 °C. In-situ was performed as previously described83. Colored signal was developed by incubation in BM-Purple (Roche) or Vector® Blue Substrate Kit at room
temperature (RT) and then stored in glycerol at 4 °C. Embryos were imaged with Olympus SZX10 with Quick PhotoMicro2.3 and data were processed with Fiji ImageJ (version 1.54d) analysis
software. CLONING OF THE REPORTER GENE CONSTRUCTS, GENERATION, AND ANALYSIS OF STABLE LINES IN ZEBRAFISH The regulatory sequences of amphioxus _Goosecoid_ and _Six3/6_ genes were amplified
from B.floridae genomic DNA with oligonucleotides shown in (Supplementary Table S1). Enhancer sequences were inserted into pZED vector upstream of the zebrafish gata2a minimal promoter84 and
promoter sequences into promoter-less pZED derivative. Transgenesis was performed with Tol2 transposon/transposase method85. A mixture of 30 ng/µl of transposase mRNA, 30 ng/µl of
Tol2-based transgenic construct, and 0.05% phenol red was injected into the one-cell stage embryos. Two independent transgenic lines were generated for _BfGoosecoid::EGFP_ and
_BfSix3/6::EGFP_ constructs. The embryos of F1 and later generations were analyzed with in-situ hybridization. Previously generated transgenic line containing _BfCordin::EGFP_18 was analyzed
with light sheet fluorescent microscopy. The embryos were imaged with a Zeiss Light Sheet Z.1 microscope imaged with Zen3.1 (black version) software and processed with ZEN 3.4 (blue
version), Imarisx64 9.6.0, and Fiji ImageJ software. TRANSIENT TRANSGENESIS IN AMPHIOXUS Intronic enhancer of amphioxus _Six3/6_ and zebrafish −4.5 kb _crestin_ regulatory element were
cloned upstream of the minimal actin promoter and tdTomato or mCherry reporter in the vector carrying _PiggyBac_ transposon terminal repeats59. The −4.4 kb promoter of amphioxus _Goosecoid_
was cloned into the promoter-less version of the same vector. For microinjection of amphioxus eggs, mixture of _BfGoosecoi::tdTomato_ or _Six3/6::tdTomato_ (200 ng/µl) or _Crestin::mCherry_
with PiggyBac transposase mRNA (100 ng/µl) in 15% glycerol was used. Transgenic embryos were allowed to develop until G4, N0 or N1 stage, fixed overnight in 4% MOPS/PFA (pH = 8.0) at 4 °C,
stained with DAPI, mounted with Vectashield (Vector Laboratories), and analyzed using Leica SP8 confocal microscope. The confocal images were processed with Fiji ImageJ. PHARMACOLOGICAL
TREATMENT OF ZEBRAFISH AND AMPHIOXUS EMBRYOS The Nodal signaling pathway was inhibited with SB505124. Zebrafish embryos were placed in E3 medium and allowed to develop until the 40% epiboly
stage when SB505124 was added at a concentration of 25 µM. Subsequently, the embryos were fixed at either the 80% or 90% epiboly stage and analyzed with in situ hybridization. Amphioxus
embryos were treated with 3 µM SB505124 in seawater at either the G2 or G4 stage and fixed at the N1 or T1 stage for analysis. The expression of Six3/6, Goosecoid, and Fz5/8 genes was
analyzed with in situ hybridization, and FoxA1 expression was analyzed with immunohistochemistry staining. Hedgehog signaling pathway was inhibited with 4 µM cyclopamine. The embryos were
treated with DMSO, EtOH or cyclopamine at the G1 stage and allowed to develop until T0 stage for the analysis. The expression of Ptch, Six3/6, Myl1 was analyzed with in situ hybridization,
FoxA1 and Otx expression was analyzed with immunohistochemistry staining. Wnt signaling was inhibited with 3 and 10 µM C59. The embryos were treated at N1 and allowed to develop until N5
stage for the analysis. The expression of FoxD was analyzed with in situ hybridization, Elav and Otx expression was analyzed with immunohistochemistry staining. PREPARATION OF SINGLE-CELL
SAMPLES AND 10X GENOMICS SINGLE-CELL LIBRARY FROM AMPHIOXUS For each sample, 15 to 20 morphologically normal embryos were randomly picked and transferred into 4 well plates pre-coated with
BSA and filled with filtered sea water (FSW). The embryos were washed with Ca2+, Mg2+-free artificial seawater (Ca2+, Mg2+-free ASW, 0.5 M KCl, 2 M NaCl, 0.5 mM NaHCO3, 0.2 M Tris–HCl, pH=8,
0.33 M Na2SO4). Then, the embryos were transferred into tubes pre-coated with 5% BSA in Ca2+, Mg2+-free artificial sea water and immediately dissociated with preheated 1 to 2.5% trypsin in
Ca2+, Mg2+-free ASW for 3–10 minutes (from the gastrula to late neurula stages) at 37 °C and 150 rcf, while pipetting to complete the dissociation. The digestion was then inhibited with 20%
FBS. Cells were collected by centrifugation at room temperature for 5 minutes at 100 rcf and then resuspended in ASW and filtered. The cell concentration and viability of each sample was
checked using Trypan Blue solution (Sigma Aldrich) with the Automated Cell Counter TC20TM (Biorad). Samples with a viability above 90% were used for single-cell sequencing. Samples
containing the cells from the embryos at the G4, N0, N2, and N5 stages were collected and washed with 1.2 M glycine and then dissociated. Single-cell encapsulation, cDNA synthesis, and
library preparation were performed using Chromium nextGEM single cells 3’reagent kit v3.1, samples were targeted to 5000 cells (Supplementary Table S2). Sequencing was performed on Illumina
NextSeq500 with mRNA fragment read length of 130 bases. UMI (Unique Molecular Identifiers) were quality controlled and counted by Cell Ranger Single-Cell Software Suite 6.1.1 from 10 ×
Genomics using _B. floridae_ genome86 with added manually curated genome models from _B. lanceolatum_ genome (https://genome.jgi.doe.gov/) and (GitHubrRpository > 10X_matrices >
gene_id_conversion_table.csv). SINGLE-CELL RNA-SEQ DATA ANALYSIS Analysis of gene expression matrices, clustering, and projections construction were conducted using the Seurat package
v4.3.0.1 in R. The datasets from the G4, N0, N2, and N5 stages were analyzed individually, adhering to the parameters specified in (Supplementary information: Githubrepository >
Individual_timepoints (local copy provided with submission)). To identify cell populations, we utilized a semi-supervised approach for cluster identification and annotation. Initially,
unbiased clustering was applied to delineate distinct clusters. Following this, the top differentially expressed genes within each cluster were assessed based on their spatial embryonic
expression patterns, which were sourced either from previously published resources or our own investigations (Supplementary Figs. 1, 1a–d, 2, 2a–e, 3, 3a–e, 4, 4a–e). The cluster identities
were ascertained through an analysis of the combinatorial spatial gene expression codes depicted in (Fig. 1). The head area was the main focus of our research. Therefore, extensive analysis
of spatial gene expression was performed for the clusters corresponding to the anterior mesendoderm and preaxial mesendoderm at the G4 stage, anterior endoderm and prechordal plate at the N0
stage, prechordal mesoderm, pharyngeal endoderm, and anterior neural plate at the N2 stage, and anterior tip of notochord, left diverticulum, anterior neural tube, PMNCC and MNCC
populations at the N5 stage. Extensive analysis of combinatorial spatial gene expression allowed us to directly annotate these clusters. Due to the complexity of the datasets, some clusters
were challenging to annotate accurately without further extensive spatial gene expression analysis. These clusters were not the primary focus of our research, and we did not conduct in-depth
analysis on them. For instance, there were several clusters that corresponded to the subpopulation of somites or ectoderm, but their precise spatial localization was not determined. To
streamline the analysis, we grouped some of these clusters together into a single ‘somites’ or ‘ectoderm’ cluster for simplicity (see GitHubRepository > Individual_timepoints)). At the N2
stage, ectodermal cells did not settle during centrifugation and were removed with the supernatant. As a result, we obtained a sample enriched with cells from the sub-ectodermal populations
and were able to correctly identify and verify all non-ectodermal relevant populations within the N2 stage embryo. Additionally, we were not able to accurately distinguish the location of
peripheral sensory neurons and neural tube neurons. Therefore, some Tlx+, Elav+, or Trk+ cells within the anterior neural tube and neural tube clusters may represent peripheral sensory
neurons. To infer probable fates of annotated cell types across timepoints, we utilized a modified URD approach used in ref. 14. URD calculates cell fate transition probabilities by first
utilizing defined timepoints, the initial and final stages in a dataset. It then critically employs the transcriptomic profiles of individual cells, analyzing changes in gene expression
across these timepoints to determine the likelihood of cell fate transitions. First, we integrated expression data from individual stages using mutual nearest neighbors (MNN) algorithm87.
Then, diffusion map was generated based on the corrected data. The transition probabilities were then determined by the Euclidean distance between cells in gene expression space, as
represented on a diffusion map, and converted to transition matrix. For subsequent pseudotime estimation within URD framework, we aimed to identify root cells (pseudotime = 0) with more
fidelity than simply selecting all cells from G4 stage. To achieve that, cell differentiation potential was computed using CytoTRACE algorithm88 implemented in CellRank89. As a root cells,
we then identified G4 cells exhibiting CytoTRACE score <0.15. As a next step, the cell transition probabilities matrix was biased by the differences in each cell’s assigned pseudotime,
favoring transitions that align with the direction of real time according to logistic function. Parameters of the logistic function were estimated based on overall pseudotime distribution in
the dataset. Next, transition probabilities were averaged across groups of cells defined by cell types annotated during individual stages analyses and the stages of origin. The resulting
transition matrix, representing probability of differentiation of individual cell types to cell types in consecutive stages, were z-scaled for more feasible interpretation and visualization
(GitHubRepository > Transitions). To visualize the transition probabilities as shown in Supplementary Fig. 9, we employed a series of data processing steps. First, transition matrix
containing cell types of interest was converted to a directed graph with edge weights represented by scaled transition probabilities. To de-noise the graphs, we restricted edges to only
connect to their respective consecutive stages (nodes). We further applied an arbitrary weight threshold—0.9 for the prechordal plate lineage and 1.5 for the crest lineage - while retaining
the highest-weight edge to prevent nodes from becoming disconnected (GitHubRepository > Transitions). Raw FASTQ files from Satoh et al.47, spanning the late gastrula, early neurula, and
late neurula stages, were processed using the Cell Ranger Single-Cell Software Suite 8.0.1 from 10x Genomics. Expression matrices generated for the GCA_000003815.2 assembly were converted
into Seurat objects, and the relevant stages were analyzed and integrated with our data using the default Seurat integration method (Canonical Correlation Analysis). Integrated expression
values were computed for all expressed genes. The combined single-nuclei RNA gene expression matrix presented in Ma et al.35 along with the transcript sequences and the map of initial
SPLiT-seq barcodes corresponding to embryonic stages. The matrix was segmented into individual stages based on the provided initial barcode sequences, and the resulting per-stage matrices
were filtered for expressed gene counts using the parameters described in Ma et al.‘s methods. Only nuclei processed with polyT RNA-capturing primers from relevant stages were used in the
SAMap analysis. The obtained transcript sequences were compared to amphioxus GCA_000003815.2 transcripts using BLAST as part of the SAMap workflow preprocessing step. Finally, we integrated
the N0 stage data from Ma et al. with our data using SAMap under default settings. SAMap analysis was performed according to algorithm described previously65. STATISTICS AND REPRODUCIBILITY
For the single-cell analysis, the maximum possible number of embryos was chosen to ensure with high viability of single cells during trypsinization. To examine the gene expression patterns
in wild-type embryos, in-situ hybridization or immunohistochemistry was performed on at least 20 embryos. At least six embryos were analyzed using a confocal microscope. Each experiment
involving pharmacological treatment was conducted at least three times, consistently yielding similar results. For the gene expression analysis of pharmacologically treated amphioxus
embryos, 10–20 treated embryos were used for each condition. The first number indicated in the panels represents the count of embryos displaying the observed pattern, while the second number
indicates the total number of analyzed embryos in the representative experiment. A minimum of ten treated embryos were analyzed using confocal microscopy, with at least three being imaged
as a complete z-stack. For gene expression analysis of pharmacologically treated zebrafish embryos, 20–40 embryos were used per condition in each experiment. Two independent germline
founders were identified for each zebrafish transgenic line, and in each case, they were verified to show similarly specific activity in at least five independent animals. For transient
transgenesis in amphioxus, at least 100 eggs were injected, and 30 embryos were analyzed using confocal microscopy. The first number indicated in the panels represents the count of embryos
displaying the observed pattern, while the second number indicates the total number of positive embryos. REPORTING SUMMARY Further information on research design is available in the Nature
Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The scRNAseq datasets generated in this study have been deposited in the European Nucleotide Archive (ENA) database
under accession code PRJEB67341. The combined single-nuclei RNA gene expression matrix presented in Ma et al.35 was obtained from https://lifeomics.shinyapps.io/shinyappmulti/. Zebrafish
single-cell RNA-Seq data were obtained from https://singlecell.broadinstitute.org/single_cell/study/SCP162/. CODE AVAILABILITY The detailed code used for the analysis in this study is
available at https://github.com/jakubovciak/Vertebrate_Head. REFERENCES * Schlosser, G. From so simple a beginning - what amphioxus can teach us about placode evolution. _Int J. Dev. Biol._
61, 633–648 (2017). Article CAS PubMed Google Scholar * Patthey, C., Schlosser, G. & Shimeld, S. M. The evolutionary history of vertebrate cranial placodes–I: cell type evolution.
_Dev. Biol._ 389, 82–97 (2014). Article CAS PubMed Google Scholar * York, J. R. & McCauley, D. W. The origin and evolution of vertebrate neural crest cells. _Open Biol._ 10, 190285
(2020). Article CAS PubMed PubMed Central Google Scholar * Yasuoka, Y., Tando, Y., Kubokawa, K. & Taira, M. Evolution of cis-regulatory modules for the head organizer gene goosecoid
in chordates: comparisons between Branchiostoma and Xenopus. _Zool. Lett._ 5, 27 (2019). Article Google Scholar * Holland, L. Z. & Holland, N. D. Evolution of neural crest and
placodes: amphioxus as a model for the ancestral vertebrate? _J. Anat._ 199, 85–98 (2001). Article CAS PubMed PubMed Central Google Scholar * Vesque, C. et al. Development of chick
axial mesoderm: specification of prechordal mesoderm by anterior endoderm-derived TGFbeta family signalling. _Development_ 127, 2795–2809 (2000). Article CAS PubMed Google Scholar *
Hagos, E. G. & Dougan, S. T. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. _BMC Dev. Biol._ 7, 22 (2007). Article PubMed PubMed Central Google
Scholar * Dumortier, J. G., Martin, S., Meyer, D., Rosa, F. M. & David, N. B. Collective mesendoderm migration relies on an intrinsic directionality signal transmitted through cell
contacts. _Proc. Natl Acad. Sci. USA_ 109, 16945–16950 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Gritsman, K., Talbot, W. S. & Schier, A. F. Nodal signaling
patterns the organizer. _Development_ 127, 921–932 (2000). Article CAS PubMed Google Scholar * Pera, E. M. & Kessel, M. Patterning of the chick forebrain anlage by the prechordal
plate. _Development_ 124, 4153–4162 (1997). Article CAS PubMed Google Scholar * Sagai, T., Amano, T., Maeno, A., Ajima, R. & Shiroishi, T. SHH signaling mediated by a prechordal and
brain enhancer controls forebrain organization. _Proc. Natl Acad. Sci. USA_ 116, 23636–23642 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Kuratani, S. & Adachi,
N. What are head cavities? - A history of studies on vertebrate head segmentation. _Zool. Sci._ 33, 213–228, (2016). Article Google Scholar * Kirby, M. L. et al. Hensen’s node gives rise
to the ventral midline of the foregut: implications for organizing head and heart development. _Dev. Biol._ 253, 175–188 (2003). Article CAS PubMed Google Scholar * Farrell, J. A. et al.
Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. _Science_ 360, eaar3131 (2018). Article PubMed PubMed Central Google Scholar * Yu, J. K. et al.
Axial patterning in cephalochordates and the evolution of the organizer. _Nature_ 445, 613–617 (2007). Article CAS PubMed Google Scholar * Onai, T., Yu, J. K., Blitz, I. L., Cho, K. W.
& Holland, L. Z. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. _Dev. Biol._ 344, 377–389 (2010). Article CAS PubMed PubMed
Central Google Scholar * Kozmikova, I. & Kozmik, Z. Wnt/beta-catenin signaling is an evolutionarily conserved determinant of chordate dorsal organizer. _Elife_ 9, e56817 (2020).
Article CAS PubMed PubMed Central Google Scholar * Machacova, S., Kozmik, Z. & Kozmikova, I. Identification of Nodal-dependent enhancer of amphioxus Chordin sufficient to drive gene
expression into the chordate dorsal organizer. _Dev. Genes Evol._ 232, 137–145 (2022). Article CAS PubMed Google Scholar * Onai, T., Irie, N. & Kuratani, S. The evolutionary origin
of the vertebrate body plan: the problem of head segmentation. _Annu Rev. Genom. Hum. Genet_ 15, 443–459 (2014). Article CAS Google Scholar * Ferran, J. L., Irimia, M. & Puelles, L.
Is there a prechordal region and an acroterminal domain in amphioxus? _Brain Behav. Evol._ 96, 334–352 (2022). Article PubMed Google Scholar * Albuixech-Crespo, B. et al. Molecular
regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. _PLoS Biol._ 15, e2001573 (2017). Article PubMed PubMed Central Google Scholar
* Meister, L., Escriva, H. & Bertrand, S. Functions of the FGF signalling pathway in cephalochordates provide insight into the evolution of the prechordal plate. _Development_ 149,
dev200252 (2022). Article CAS PubMed PubMed Central Google Scholar * Samaan, G. et al. Foxn3 is essential for craniofacial development in mice and a putative candidate involved in human
congenital craniofacial defects. _Biochem Biophys. Res Commun._ 400, 60–65 (2010). Article CAS PubMed Google Scholar * Williams, R. M. et al. Reconstruction of the global neural crest
gene regulatory network in vivo. _Dev. Cell_ 51, 255–276.e257 (2019). Article CAS PubMed PubMed Central Google Scholar * Nassif, A. et al. Transcriptional regulation of jaw osteoblasts:
development to pathology. _J. Dent. Res_ 101, 859–869 (2022). Article CAS PubMed PubMed Central Google Scholar * Machon, O., Masek, J., Machonova, O., Krauss, S. & Kozmik, Z. Meis2
is essential for cranial and cardiac neural crest development. _BMC Dev. Biol._ 15, 40 (2015). Article PubMed PubMed Central Google Scholar * Ray, P. et al. Comparative transcriptome
profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. _Pain_ 159, 1325–1345 (2018). Article CAS PubMed PubMed Central
Google Scholar * Rogers, C., Phillips, J. & Bronner, M. Elk3 is essential for the progression from progenitor to definitive neural crest cell. _Dev. Biol._ 374, 255–263 (2012).
Article PubMed PubMed Central Google Scholar * Hendershot, T. J. et al. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression
for development of neural crest-derived noradrenergic sympathetic ganglion neurons. _Dev. Biol._ 319, 179–191 (2008). Article CAS PubMed PubMed Central Google Scholar * Simoes-Costa, M.
& Bronner, M. E. Establishing neural crest identity: a gene regulatory recipe. _Development_ 142, 242–257 (2015). Article CAS PubMed PubMed Central Google Scholar * Aoto, K. et al.
Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. _Dev. Biol._ 327, 106–120 (2009). Article CAS PubMed Google Scholar * Schlosser, G.
Do vertebrate neural crest and cranial placodes have a common evolutionary origin? _Bioessays_ 30, 659–672 (2008). Article CAS PubMed Google Scholar * Patten, I., Kulesa, P., Shen, M.
M., Fraser, S. & Placzek, M. Distinct modes of floor plate induction in the chick embryo. _Development_ 130, 4809–4821 (2003). Article CAS PubMed Google Scholar * Satoh, N. et al. A
preliminary single-cell RNA-Seq analysis of embryonic cells that express brachyury in the amphioxus, Branchiostoma Japonicum. _Front Cell Dev. Biol._ 9, 696875 (2021). Article PubMed
PubMed Central Google Scholar * Ma, P. et al. Joint profiling of gene expression and chromatin accessibility during amphioxus development at single-cell resolution. _Cell Rep._ 39, 110979
(2022). Article CAS PubMed Google Scholar * Heisenberg, C. P. & Tada, M. Zebrafish gastrulation movements: bridging cell and developmental biology. _Semin Cell Dev. Biol._ 13,
471–479 (2002). Article PubMed Google Scholar * Prummel, K. D. et al. A conserved regulatory program initiates lateral plate mesoderm emergence across chordates. _Nat. Commun._ 10, 3857
(2019). Article ADS PubMed PubMed Central Google Scholar * Pascual-Anaya, J. et al. The evolutionary origins of chordate hematopoiesis and vertebrate endothelia. _Dev. Biol._ 375,
182–192 (2013). Article CAS PubMed Google Scholar * Vermeiren, S., Bellefroid, E. J. & Desiderio, S. Vertebrate sensory ganglia: common and divergent features of the transcriptional
programs generating their functional specialization. _Front Cell Dev. Biol._ 8, 587699 (2020). Article PubMed PubMed Central Google Scholar * Vernon, C. G. & Swanson, G. T. Neto2
assembles with Kainate receptors in DRG neurons during development and modulates neurite outgrowth in adult sensory neurons. _J. Neurosci._ 37, 3352–3363 (2017). Article CAS PubMed PubMed
Central Google Scholar * Haines, B. & Rigby, P. Expression of the Lingo/LERN gene family during mouse embryogenesis. _Gene Expr. patterns: GEP_ 8, 79–86 (2008). Article CAS PubMed
Google Scholar * Patthey, C. et al. Identification of molecular signatures specific for distinct cranial sensory ganglia in the developing chick. _Neural Dev._ 11, 3 (2016). Article PubMed
PubMed Central Google Scholar * Shiau, C. E., Lwigale, P. Y., Das, R. M., Wilson, S. A. & Bronner-Fraser, M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the
trigeminal ganglion. _Nat. Neurosci._ 11, 269–276 (2008). Article CAS PubMed Google Scholar * Christian, L., Bahudhanapati, H. & Wei, S. Extracellular metalloproteinases in neural
crest development and craniofacial morphogenesis. _Crit. Rev. Biochem. Mol. Biol._ 48, 544–560 (2013). Article CAS PubMed Google Scholar * Hong, C. S. & Saint-Jeannet, J. P. Sox
proteins and neural crest development. _Semin Cell Dev. Biol._ 16, 694–703 (2005). Article CAS PubMed Google Scholar * Uy, B. R., Simoes-Costa, M., Koo, D. E., Sauka-Spengler, T. &
Bronner, M. E. Evolutionarily conserved role for SoxC genes in neural crest specification and neuronal differentiation. _Dev. Biol._ 397, 282–292 (2015). Article CAS PubMed Google Scholar
* Satoh, G., Wang, Y., Zhang, P. & Satoh, N. Early development of amphioxus nervous system with special reference to segmental cell organization and putative sensory cell precursors: a
study based on the expression of pan-neuronal marker gene Hu/elav. _J. Exp. Zool._ 291, 354–364 (2001). Article CAS PubMed Google Scholar * Kaltenbach, S. L., Yu, J. K. & Holland,
N. D. The origin and migration of the earliest-developing sensory neurons in the peripheral nervous system of amphioxus. _Evol. Dev._ 11, 142–151 (2009). Article PubMed Google Scholar *
Benito-Gutierrez, E., Nake, C., Llovera, M., Comella, J. X. & Garcia-Fernandez, J. The single AmphiTrk receptor highlights increased complexity of neurotrophin signalling in vertebrates
and suggests an early role in developing sensory neuroepidermal cells. _Development_ 132, 2191–2202, (2005). Article CAS PubMed Google Scholar * Devotta, A., Hong, C.-S. &
Saint-Jeannet, J.-P. Dkk2 promotes neural crest specification by activating Wnt/β-catenin signaling in a GSK3β independent manner. _eLife_ 7, e34404 (2018). Article PubMed PubMed Central
Google Scholar * Carmona-Fontaine, C., Acuña, G., Ellwanger, K., Niehrs, C. & Mayor, R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory
mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. _Dev. Biol._ 309, 208–221 (2007). Article CAS PubMed Google Scholar * Luo, R., An, M., Arduini, B. L. & Henion,
P. D. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. _Dev. Dyn._ 220, 169–174 (2001). Article CAS PubMed Google Scholar * Kaufman, C. K. et
al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. _Science_ 351, aad2197 (2016). Article PubMed PubMed Central Google Scholar *
Lefebvre, V. The SoxD transcription factors–Sox5, Sox6, and Sox13–are key cell fate modulators. _Int J. Biochem Cell Biol._ 42, 429–432 (2010). Article CAS PubMed Google Scholar *
Soukup, V. et al. The Nodal signaling pathway controls left-right asymmetric development in amphioxus. _Evodevo_ 6, 5 (2015). Article PubMed PubMed Central Google Scholar * Ono, H.,
Koop, D. & Holland, L. Z. Nodal and Hedgehog synergize in gill slit formation during development of the cephalochordate Branchiostoma floridae. _Development_ 145, dev162586 (2018).
Article PubMed Google Scholar * Hu, G., Li, G., Wang, H. & Wang, Y. Hedgehog participates in the establishment of left-right asymmetry during amphioxus development by controlling
Cerberus expression. _Development_ 144, 4694–4703 (2017). Article CAS PubMed Google Scholar * Ren, Q. et al. Step-wise evolution of neural patterning by Hedgehog signalling in chordates.
_Nat. Ecol. Evol._ 4, 1247–1255 (2020). Article PubMed Google Scholar * Marlétaz, F. et al. Amphioxus functional genomics and the origins of vertebrate gene regulation. _Nature_ 564,
64–70 (2018). Article ADS PubMed PubMed Central Google Scholar * Kozmikova, I. & Kozmik, Z. Gene regulation in amphioxus: An insight from transgenic studies in amphioxus and
vertebrates. _Mar. Genomics_ 24, 159–166 (2015). Article PubMed Google Scholar * Miller-Bertoglio, V., Fisher, S., Sánchez, A., Mullins, M. & Halpern, M. Differential regulation of
chordin expression domains in mutant Zebrafish. _Dev. Biol._ 192, 537–550 (1998). Article Google Scholar * Xu, X., He, Y., Sun, L., Ma, S. & Luo, C. Maternal Vsx1 plays an essential
role in regulating prechordal mesendoderm and forebrain formation in zebrafish. _Dev. Biol._ 394, 264–276 (2014). Article CAS PubMed Google Scholar * Mathieu, J., Barth, A., Rosa, F. M.,
Wilson, S. W. & Peyriéras, N. Distinct and cooperative roles for Nodal and Hedgehog signals during hypothalamic development. _Development_ 129, 3055–3065 (2002). Article CAS PubMed
Google Scholar * Wang, H., Holland, P. & Takahashi, T. Gene profiling of head mesoderm in early zebrafish development: insights into the evolution of cranial mesoderm. _EvoDevo_ 10, 14
(2019). Article PubMed PubMed Central Google Scholar * Tarashansky, A. J. et al. Mapping single-cell atlases throughout Metazoa unravels cell type evolution. _eLife_ 10, e66747 (2021).
Article CAS PubMed PubMed Central Google Scholar * Goodrich, E. S. Memoirs: Proboscis pores in craniate vertebrates, a suggestion concerning the premandibular somites and hypophysis.
_Q. J. Microsc. Sci._ 62, 539–553 (1917). Google Scholar * Kozmik, Z. et al. Pax-Six-Eya-Dach network during amphioxus development: conservation in vitro but context specificity in vivo.
_Dev. Biol._ 306, 143–159 (2007). Article CAS PubMed Google Scholar * Glardon, S., Holland, L. Z., Gehring, W. J. & Holland, N. D. Isolation and developmental expression of the
amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. _Development_ 125, 2701–2710 (1998). Article CAS PubMed Google Scholar * Fabian, P. et al. Lineage
analysis reveals an endodermal contribution to the vertebrate pituitary. _Science_ 370, 463–467 (2020). Article CAS PubMed PubMed Central Google Scholar * Chowdhury, R. et al. Highly
distinct genetic programs for peripheral nervous system formation in chordates. _BMC Biol._ 20, 152 (2022). Article CAS PubMed PubMed Central Google Scholar * Lacalli, T. C., Gilmour,
T. H. J. & Kelly, S. J. The Oral nerve plexus in amphioxus larvae: function, cell types and phylogenetic significance. _Proc.: Biol. Sci._ 266, 1461–1470 (1999). Google Scholar *
Schlosser, G. Making senses development of vertebrate cranial placodes. _Int Rev. Cell Mol. Biol._ 283, 129–234 (2010). Article CAS PubMed Google Scholar * Saxena, A., Peng, B. N. &
Bronner, M. E. Sox10-dependent neural crest origin of olfactory microvillous neurons in zebrafish. _eLife_ 2, e00336 (2013). Article PubMed PubMed Central Google Scholar * Katoh, H. et
al. The dual origin of the peripheral olfactory system: placode and neural crest. _Mol. Brain_ 4, 34 (2011). Article CAS PubMed PubMed Central Google Scholar * Whitlock, K. A new model
for olfactory placode development. _Brain. Behav. Evol._ 64, 126–140 (2004). Article PubMed Google Scholar * Wagner, E., Stolfi, A., Gi Choi, Y. & Levine, M. Islet is a key
determinant of ascidian palp morphogenesis. _Development_ 141, 3084–3092 (2014). Article CAS PubMed PubMed Central Google Scholar * Horie, R. et al. Shared evolutionary origin of
vertebrate neural crest and cranial placodes. _Nature_ 560, 228–232 (2018). Article ADS CAS PubMed PubMed Central Google Scholar * Stolfi, A., Ryan, K., Meinertzhagen, I. A. &
Christiaen, L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. _Nature_ 527, 371–374 (2015). Article ADS CAS PubMed PubMed Central Google Scholar *
Steingrímsson, E., Copeland, N. G. & Jenkins, N. A. Melanocytes and the microphthalmia transcription factor network. _Annu. Rev. Genet_ 38, 365–411 (2004). Article PubMed Google
Scholar * Adameyko, I. et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. _Cell_ 139, 366–379 (2009). Article CAS PubMed Google Scholar
* Bozzo, M., Pergner, J., Kozmik, Z. & Kozmikova, I. Novel polyclonal antibodies as a useful tool for expression studies in amphioxus embryos. _Int J. Dev. Biol._ 61, 793–800 (2017).
Article CAS PubMed Google Scholar * Bozzo, M., Candiani, S. & Schubert, M. Whole mount in situ hybridization and immunohistochemistry for studying retinoic acid signaling in
developing amphioxus. _Methods Enzymol._ 637, 419–452 (2020). Article CAS PubMed Google Scholar * Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount
zebrafish embryos. _Nat. Protoc._ 3, 59–69 (2008). Article CAS PubMed Google Scholar * Bessa, J. et al. Zebrafish enhancer detection (ZED) vector: a new tool to facilitate transgenesis
and the functional analysis of cis-regulatory regions in zebrafish. _Dev. Dyn._ 238, 2409–2417 (2009). Article CAS PubMed Google Scholar * Kawakami, K. et al. A transposon-mediated gene
trap approach identifies developmentally regulated genes in zebrafish. _Dev. Cell_ 7, 133–144 (2004). Article CAS PubMed Google Scholar * Simakov, O. et al. Deeply conserved synteny
resolves early events in vertebrate evolution. _Nat. Ecol. Evol._ 4, 820–830 (2020). Article PubMed PubMed Central Google Scholar * Haghverdi, L., Lun, A. T. L., Morgan, M. D. &
Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. _Nat. Biotechnol._ 36, 421–427 (2018). Article CAS PubMed PubMed
Central Google Scholar * Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. _Science_ 367, 405–411 (2020). Article ADS CAS PubMed
PubMed Central Google Scholar * Lange, M. et al. CellRank for directed single-cell fate mapping. _Nat. Methods_ 19, 159–170 (2022). Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to Halyna Klymets for technical support during the manuscript improvement, Lucie Kozmikova for her drawings of animal representatives,
and Veronika Noskova for amphioxus facility maintenance. We acknowledge the Light Microscopy Core Facility, IMG CAS, Prague, Czech Republic, supported by MEYS (LM2023050,
CZ.02.1.01/0.0/0.0/18_046/0016045), for their support with the microscopy presented herein. This work was supported by Czech Science Foundation grant GA20-25377S to ZK. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Laboratory of Transcriptional Regulation, Institute of Molecular Genetics of the Czech Academy of Sciences, Videnska 1083, Prague, Czech Republic Anna Markos,
Simona Mikula Mrstakova, Anna Zitova, Simona Machacova, Zbynek Kozmik & Iryna Kozmikova * Laboratory of Genomics and Bioinformatics, Institute of Molecular Genetics of the Czech Academy
of Sciences, Videnska 1083, Prague, Czech Republic Jan Kubovciak, Jan Paces & Zbynek Kozmik-Jr Authors * Anna Markos View author publications You can also search for this author inPubMed
Google Scholar * Jan Kubovciak View author publications You can also search for this author inPubMed Google Scholar * Simona Mikula Mrstakova View author publications You can also search
for this author inPubMed Google Scholar * Anna Zitova View author publications You can also search for this author inPubMed Google Scholar * Jan Paces View author publications You can also
search for this author inPubMed Google Scholar * Simona Machacova View author publications You can also search for this author inPubMed Google Scholar * Zbynek Kozmik-Jr View author
publications You can also search for this author inPubMed Google Scholar * Zbynek Kozmik View author publications You can also search for this author inPubMed Google Scholar * Iryna
Kozmikova View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: I.K.; Methodology: I.K., A.M., Z.K., Z.K.-Jr, S.M.M., J.P.,
J.K.; Investigation: I.K., A.M., A.Z., Z.K., Z.K.-Jr., S.M.M., J.P., J.K., S.M.; Visualization: I.K., S.M.M., J.K.; Funding acquisition: Z.K.; Project administration: Z.K., I.K.;
Supervision: IK; Writing – original draft: I.K.; Writing – review & editing: I.K., Z.K., J.K. CORRESPONDING AUTHOR Correspondence to Iryna Kozmikova. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of
this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Markos, A., Kubovciak, J., Mikula Mrstakova, S. _et al._ Cell type and
regulatory analysis in amphioxus illuminates evolutionary origin of the vertebrate head. _Nat Commun_ 15, 8859 (2024). https://doi.org/10.1038/s41467-024-52938-7 Download citation *
Received: 26 January 2024 * Accepted: 25 September 2024 * Published: 14 October 2024 * DOI: https://doi.org/10.1038/s41467-024-52938-7 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative