Play all audios:
ABSTRACT Anemia is highly prevalent globally, especially in young children in low-income countries, where it often overlaps with a high burden of diarrheal disease. Distribution of iron
interventions (as supplements or iron-containing multiple micronutrient powders, MNPs) is a key anemia reduction strategy. Small studies in Africa indicate iron may reprofile the gut
microbiome towards pathogenic species. We seek to evaluate the safety of iron and MNPs based on their effects on diversity, composition, and function of the gut microbiome in children in
rural Bangladesh as part of a large placebo-controlled randomized controlled trial of iron or MNPs given for 3 months (ACTRN12617000660381). In 923 infants, we evaluate the microbiome
before, immediately following, and nine months after interventions, using 16S rRNA gene sequencing and shotgun metagenomics in a subset. We identify no increase in diarrhea with either
treatment. In our primary analysis, neither iron nor MNPs alter gut microbiome diversity or composition. However, when not adjusting for multiple comparisons, compared to placebo, children
receiving iron and MNPs exhibit reductions in commensal species (e.g., _Bifidobacterium, Lactobacillus_) and increases in potential pathogens, including _Clostridium_. These increases are
most evident in children with baseline iron repletion and are further supported by trend-based statistical analyses. SIMILAR CONTENT BEING VIEWED BY OTHERS MICRONUTRIENT SUPPLEMENTS CAN
PROMOTE DISRUPTIVE PROTOZOAN AND FUNGAL COMMUNITIES IN THE DEVELOPING INFANT GUT Article Open access 18 November 2021 INFANT GUT MICROBIOTA CHARACTERISTICS GENERALLY DO NOT MODIFY EFFECTS OF
LIPID-BASED NUTRIENT SUPPLEMENTATION ON GROWTH OR INFLAMMATION: SECONDARY ANALYSIS OF A RANDOMIZED CONTROLLED TRIAL IN MALAWI Article Open access 09 September 2020 BIOACTIVE GLYCANS IN A
MICROBIOME-DIRECTED FOOD FOR CHILDREN WITH MALNUTRITION Article Open access 13 December 2023 INTRODUCTION Anemia affects almost 300 million pre-school-aged children worldwide, and is most
prevalent in children in low- and middle-income countries (LMICs).1 In 2019, the prevalence of anemia in children aged 6–59 months was 49.0% in South-East Asia, and 43.1% in Bangladesh.2
Anemia in young children is often accompanied by a concomitant burden of diarrhea in low-income settings. Diarrhea causes 10% of all child deaths in Asia and Africa,3 precipitated by unsafe
water, sanitation and hygiene (WASH) conditions. The World Health Organization (WHO) recommends universal distribution of iron (either as iron-containing multiple micronutrient powders—MNPs,
or iron supplements, e.g., drops) to all young children where anemia is prevalent.4,5 However, the safety of this approach is uncertain, with large field trials of iron-containing MNPs
suggesting an increased risk of diarrhea.6 Trials in sub-Saharan Africa have reported pathogenic reprofiling of the gut microbiome (an increase in pathogenic enterobacteria and a decrease in
commensal species) in young children receiving iron-containing MNPs, associated with evidence of increased intestinal inflammation. This may relate to an increase in colonic iron that
facilitates the growth of potentially pathogenic bacteria at the expense of commensal species, leading to dysbiosis.7 An effect of iron interventions on the microbiome has been observed in
studies conducted in sub-Saharan Africa. A study of Kenyan children receiving iron-containing MNPs reported that iron promoted growth of enteropathogens e.g., _Escherichia_, _Salmonella_ and
_Clostridium_, and increased intestinal inflammation.8 A second Kenyan study reported that even low-dose iron pathogenically reprofiled the microbiota and exacerbated intestinal
inflammation.9 In both cases, these conclusions were drawn from statistical analyses of the microbiome that were not adjusted for multiple comparisons, and could represent false discovery
(i.e., false positive) findings. Crucially, there remains limited data evaluating microbiome effects in South Asia, where effects from iron on the clinical endpoint of diarrhea have been
observed.6 Iron status (deficiency or repletion) influences intestinal iron uptake10 and could, therefore, potentially modify the effects of interventions on the microbiome. Defining the
safety of iron interventions is critical, given the ongoing public health recommendation for their distribution.11 There is an urgent need for well-powered, placebo-controlled clinical
trials that evaluate the safety of iron interventions on the gut microbiome using a rigorous study design to examine causal relationships. To address this, we leverage the BRISC (Benefits
and Risks of Iron InterventionS in Children) trial, a large, placebo-controlled double-blind, double-dummy randomized controlled trial conducted in rural Bangladesh in which infants were
randomized to receive three months of daily iron syrup, MNPs or placebo.12 In this large sub-study we present here, we evaluate stool samples from a subset of BRISC participants at baseline,
after three months of intervention, and after a further 9-month post-intervention follow-up. Samples are analyzed using 16S rRNA amplicon sequencing, with a subset also analyzed with
shotgun metagenomic sequencing. This combined methodology enables us to define causal iron-induced gut microbiome reprofiling by exploring the effects of iron and MNPs compared to placebo on
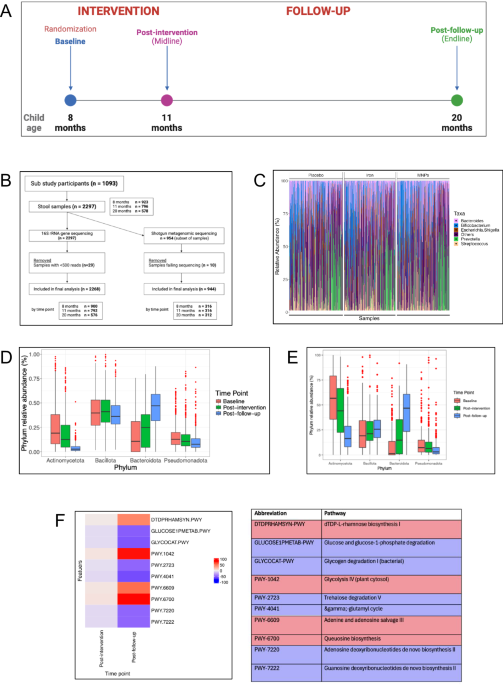
diversity and composition across an unprecedented sample size while also undertaking a high-resolution evaluation of the effect of iron on species and function. RESULTS Between September
2018 and February 2019, we collected 923 baseline stool samples, 796 samples at the subsequent post-intervention time point 13 weeks later, and 578 samples at the post-follow-up time point
(Fig. 1A, Supplementary Fig. 1). Overall, a sample was provided at baseline from 84% of participants approached for this sub-study, and of these, 86% provided a sample at midline and 63% at
endline. Baseline characteristics of the cohort are summarized in Table 1. 16S rRNA amplicon sequencing was performed on 923 samples at baseline, 796 at midline, and 578 at endline; a subset
of 319, 320 and 315 samples also underwent shotgun metagenomic sequencing at these time points, respectively (Fig. 1B). Baseline characteristics of the shotgun metagenomic sub-cohort are
summarized in Table S1_(Supplementary Material)_. The BRISC trial found that across all participants, neither iron nor MNPs significantly increased parent-reported days with diarrhea
(incidence rate ratio (IRR) 1.13 [95% CI 0.89–1.42] for iron versus placebo, IRR 1.17 [0.93–1.48] for MNPs versus placebo).12 Among children in the microbiome sub-study, again, neither iron
nor MNPs statistically significantly increased parent-reported days with diarrhea (IRR 1.43 [0.89–2.27] for iron versus placebo, IRR 1.24 [0.77–1.98] for MNPs versus placebo). For the gut
microbiome analysis we first examined baseline taxonomic profiles and changes with age. The top five genera detected at baseline are presented in Fig. 1C (source data in _Supplementary
Material_). No significant baseline differences were seen between arms at the genus or species levels. We then examined changes to the microbiome as children grew from 8 to 20 months of age.
Using 16S and shotgun metagenomics, we observed reductions in Actinomycetota and Pseudomonadota phyla and increased Bacteroidota over this 12-month timeframe (Fig. 1D, E). Compared to
baseline, many key functional pathways altered as children reached the 3-month post-intervention period (11 months of age); 155 pathways were differentially abundant compared to baseline (91
increased and 64 reduced). At 9 months follow-up (age 20 months), 349 pathways were differentially abundant (113 up and 236 down) compared to baseline. For both comparisons the pathway with
the greatest increase was dTDP-L-rhamnose biosynthesis I (log2-fold change 0.001, SD 0.0004, adj. _p_-value 0.003 at 11 months; log2-fold change 0.006, SD 0.0004, adj. _p_ < 0.001 at 20
months) and greatest reduction was glucose and glucose-1-phosphate degradation (log2-fold change −0.002, SD 0.0003, adj. _p_ < 0.001 at 11 months; log2-fold change −0.005, SD 0.0003, adj.
_p_ < 0.001 at 20 months) (Fig. 1F, with additional pathways detailed in Supplementary Fig. 2 and in source data in _Supplementary Material_). Next, using 16S rRNA gene sequencing data,
we evaluated the effects of iron interventions on microbial diversity. Neither iron supplements nor MNPs altered alpha diversity (Shannon and inverse Simpson indices) at the immediate
post-intervention time point. Likewise, we did not find an effect from the interventions on beta diversity (Fig. 2A, B). Furthermore, there were no differences between trial arms in
abundance at the genus level, after FDR adjustment. Shotgun metagenomic analysis by the trial arm revealed no significant changes in differential abundance at the species level, nor by
functional pathways measured by _HUMAnN 3.0_ (Fig. 3A, B), after FDR adjustment (source data in _Supplementary Material_). We reasoned that iron interventions may differentially impact the
gut microbiome in iron-deficient compared with iron-replete children, as intestinal iron absorption may be higher in children with iron depletion (mediated by suppressed hepcidin
levels),13,14 leaving less luminal iron to influence the microbiome.15 To evaluate this, we undertook a subgroup analysis by baseline iron status on the effect of oral iron interventions on
the microbiome. We defined iron deficiency in two ways: (1) evidence of low baseline iron stores (iron deficiency, defined as a ferritin concentration <12 µg/L or <30 µg/L if
C-reactive protein >5 mg/L, accounting for effect of inflammation on ferritin)12,16 compared to iron repletion, and (2) evidence of increased iron uptake by suppressed baseline hepcidin
(<10 ng/mL) compared to non-low hepcidin (10 ng/mL or higher).17 Using 16S rRNA gene sequencing data, no differences in alpha diversity were observed between iron, MNP and placebo groups
comparing baseline iron-deficient and iron-replete subgroups or baseline low hepcidin and non-low hepcidin (Fig. 4A–D). Likewise, we did not detect any significant differences at genus or
species levels between trial arms in any subgroup, nor did we detect a difference in effect from iron between these subgroups in functional analysis using the shotgun metagenomic data, all
using FDR-adjusted analyses. Previous studies reporting effects of iron on gut microbiome profile have evaluated differential abundance of particular taxa without adjusting statistical
significance thresholds for false discovery.8,9 Importantly, our study sought to evaluate the safety of a public health intervention that is distributed to children worldwide. We therefore
also report findings using unadjusted _p_ values to evaluate any differential abundance in gut genera or species between trial arms. Through this analysis, by 16S rRNA gene sequencing, no
genera were found to be differentially abundant between the intervention arms compared to placebo at the midline time point (Fig. 5A). However, shotgun metagenomic unadjusted analysis at the
species level indicated that iron supplementation was associated with higher abundance of _Enterococcus faecalis_ (log2-fold change 0.12, SD 0.04, _p_ = 0.002, FDR-adjusted _p_ = 0.452) at
the midline time point, while children who received MNPs had small increases in _Clostridium_ species (_C. saccharolyticum_ log2-fold change 0.08, SD 0.04, _p_ = 0.026, FDR-adjusted _p_ =
0.994 and _C. neonatale_ log2-fold change 0.04, SD 0.02, _p_ = 0.048, FDR-adjusted _p_ = 0.994) and reductions in _Lactobacillus gasseri_ (log2-fold change −0.07, SD 0.04, _p_ = 0.049,
FDR-adjusted _p_ = 0.994) and _Escherichia coli_ (log2-fold change −0.27, SD 0.13, _p_ = 0.040, FDR-adjusted _p_ = 0.994) (Fig. 5B). We next report unadjusted analysis to the previously
defined baseline iron status subgroups utilizing the shotgun metagenomic data. Children with baseline iron repletion or non-low baseline hepcidin receiving MNPs exhibited an increase in
_Clostridium_ species after intervention. Iron-replete children receiving iron had a reduction in _Bifidobacterium bifidum_ at the same time point (Fig. 5D, F). Likewise, the iron
supplementation-related increase in _E. faecalis_ was only statistically significant in the non-low hepcidin subgroup (Fig. 5F compared to Fig. 5C, E). To further interrogate the effects of
iron interventions on differential abundance, we finally explored two key phyla previously reported to change after oral iron interventions.8 Using shotgun metagenomic data from samples
obtained immediately post-intervention, we conducted an enrichment analysis in which species abundance patterns within each of phylum Bacteroidota and phylum Bacillota were compared between
iron/MNPs and placebo. We found that Bacteroidota species were significantly enriched with placebo (10 species, net decrease with iron compared to placebo, _p_ = 0.01) and Bacillota species
were significantly enriched with iron supplements (57 species, net increase to placebo, _p_ = 0.04) (Fig. 6A, B). However, no statistically significant effects were observed in the
enrichment analysis of MNPs versus placebo for either phylum (Fig. 6C, D). There was high overall parent-reported adherence to the intervention during the 13-week intervention period, with
those with 70% or higher adherence to syrup and sachet 74.7%, 72.3% and 76.9% in the iron, MNPs and placebo arms respectively. A further subset analysis was undertaken to evaluate the effect
of adherence on the microbiome. Restricting the analysis to samples from participants with at least 70% adherence to their interventions did not influence results compared to analysis of
the overall study population (Supplementary Fig 3A–D). Finally, we evaluated the effects of interventions 9 months following completion, at the endline time point, using FDR-adjusted
analyses and the overall group only. These analyses indicate higher alpha diversity among the MNP group compared to the placebo or the iron group, reduced abundance of _Prevotella copri_ in
the MNP group compared to placebo, and differential abundance of several genes relating to metabolic pathways (Supplementary Fig 4A–D). DISCUSSION There is concern regarding the safety and
hence net benefit of universal iron interventions for young children living in settings where anemia is highly prevalent but exposure to infectious diseases is intense.18 To define the
effect of iron interventions on the gut microbiome we leveraged a randomized placebo-controlled trial of iron supplements and MNPs in young children in rural Bangladesh, using 16S rRNA gene
sequencing across over almost 1000 children, and shotgun metagenomics in a subsample for higher resolution assessment. We found no evidence that iron interventions adversely reprofile the
gut microbiome when an unbiased bioinformatic approach with statistical significance adjusted for false discoveries was used. However, unadjusted findings indicate that iron interventions
given to children with baseline iron repletion reduce abundance of commensals and may increase abundance of some potentially pathogenic species. These findings are supported by evidence from
shotgun metagenomic data of differential enrichment of two phyla previously linked to iron-microbiome interactions, suggesting these changes may not be due to false discovery errors.
Previous studies in sub-Saharan Africa have shown that iron may increase the prevalence of intestinal pathogens. Children in Côte d’Ivoire randomized to 6 months of iron-fortified biscuits
(_n_ = 60) exhibited increased pathogenic enterobacteria (measured using PCR) and intestinal inflammation.19 In 6-month-old Kenyan children (_n_ = 115) where there was high baseline carriage
of pathogenic species (e.g., _C. difficile_, _C. perfringens_, _Salmonella_, pathogenic _E. coli_), MNPs with iron promoted a greater increase (or less of a decrease) in taxa including
_Bacillota_ (phylum), _Escherichia/Shigella_ and _Clostridium_ (genera) measured using PCR and 16S rRNA gene sequencing.8 In a third study, also in Kenya (_n_ = 155), even low-dose MNPs (5
mg iron) pathogenically reprofiled the microbiota (measured by 16S rRNA gene sequencing and PCR) and exacerbated intestinal inflammation.9 Notably, differential abundance testing in these
studies was not adjusted for false discovery. In contrast to these three trials, several others have not identified an effect of iron on gut microbiome composition.20,21,22 For example,
iron-fortified lipid nutrient supplements did not reprofile the gut microbiome in a trial of 213 Malawian infants measured with 16S rRNA gene sequencing.20 In another small Kenyan study (_n_
= 33), _Escherichia_ and _Bifidobacterium_ abundances in infants who received iron-containing MNPs versus placebo or a non-iron MNP showed no significant differences from control measured
using 16S rRNA gene sequencing.21 Finally, a small South African study (_n_ = 49), where baseline prevalence of pathogenic bacteria was low, no adverse effects from iron were observed on
microbiota or inflammation by PCR.22 Our 16S rRNA gene sequencing study exceeds the cumulative sample size across these studies; in addition, by applying shotgun metagenomics to this
problem, our study provides higher sequencing resolution to address this problem. We recognize the risk of false discoveries when analysing the effects of interventions across hundreds of
potential genera or species and aimed to present conservative findings through the rigorous control of the false discovery rate (FDR) and transparent reporting. Importantly, our analyses
represent a safety (rather than efficacy) study, where conservative application of multiple testing corrections may be prudent to avoid the risk of false negative findings.23 To further
challenge our findings, we undertook enrichment analysis that revealed iron-induced changes within members of Bacillota and Bacteroidota phyla. These tests evaluate overall changes in the
distribution of all members of each phylum and do not require correction for multiple testing. Although they do not reveal which species within a phylum are changing with iron treatment,
they do show that there is a statistically significant increase in Bacillota and reduction in Bacteroidota with iron relative to placebo. Our findings exhibit biological plausibility and
consistency with previous findings. In the colon, commensal bacteria (e.g., _Lactobacillus_ and _Bifidobacterium_) are crucial in preventing colonization by pathogenic species.24
Lactobacilli do not require iron, and most _Bifidobacterium_ species require only little iron compared to other taxa.8 In contrast, iron is essential for virulence and colonization by
enteric pathogens (i.e., gram-negative organisms such as _Salmonella_, pathogenic _E. coli_ and _Shigella_). Oral iron supplements (especially iron in MNPs) are only partially absorbed, with
most iron reaching the colon, where it can influence the gut microbiome.7 In our study, we observed possible iron-induced reductions in _Lactobacillus_ and _Bifidobacterium_ species and
increases in _Clostridium_ species, broadly consistent with previous positive findings.8,9 However, in our study, iron did not increase _E. coli_ (rather, caused reductions) or other
Enterobacteriaceae. The effect size of iron on differential abundance was relatively small, thus, the clinical significance of these changes remains uncertain. The master iron-regulatory
hormone, hepcidin, regulates iron absorption from the intestine.10 We reasoned that iron-deplete children (with suppressed hepcidin) absorb more iron from the supplement, whereas
iron-replete children may absorb less iron, with more iron reaching the colon and influencing the gut microbiome. Overall, iron interventions did not have a differential impact on the gut
microbiome between iron-deficient or iron-replete children. However, unadjusted analyses indicated that reprofiling of the microbiome was most profound among children with baseline iron
repletion, where higher doses of iron may reach the colon, providing biological plausibility to this finding. These findings were supported by our enrichment analyses. These results may
indicate that iron may be best deployed for treatment of iron deficiency or iron deficiency anemia, rather than for universal use. However, a screen-and-treat program would require
near-patient testing for iron deficiency prior to provision of iron, which would raise the cost and complexity of this public health program and may leave still some anemia untreated. Such
cost-effective testing technology remains an unmet need. This approach was explored in two Gambian randomized trials of pre-iron supplementation hepcidin screening in pregnancy and in
infants that did not demonstrate non-inferiority in terms of anemia control.14,25 Our study has several strengths. The parent trial was a placebo-controlled (blinded) randomized trial in a
rural, low-income South Asian setting, with high adherence. Wet, bioinformatic and statistical analyses were undertaken prior to unblinding of the researchers to the study arms. Crucially,
ours is the largest and one the first studies to incorporate measurement of iron biomarkers, including the first to assess the iron-regulatory hormone hepcidin in a subgroup analysis, to
enable assessment of the interaction between host iron status (and iron uptake), iron interventions, and effects on the microbiome. One limitation of our study is that we did not evaluate
non-bacterial organisms in the gut microbiome, such as fungi and viruses. Viruses are among the leading causes of diarrheal infections requiring hospitalization in children in Bangladesh,
and this is likely the case in many other LMICs.26 For example, adenovirus is among the most common causes of diarrhea in Bangladesh in both children and adults.26 Future analysis will
evaluate these. Our study was not designed to measure any effects from iron that may cause changes to intestinal function unrelated to microbiota reprofiling (for example, impaired barrier
function), which may also cause diarrhea. Furthermore, antibiotic use was common this population and an analysis of the association between antibiotic use and the microbiome, including the
interaction with iron interventions, has been previously presented27. Finally, although efforts were taken to minimize environmental contamination of samples, this remains a potential
limitation of this field-based study in rural Bangladesh. Ultimately, although our results do not definitively confirm that iron interventions adversely reprofile the infant gut microbiome,
an adverse effect of iron on the gut microbiome remains biologically plausible. Thus, the risk-benefit of iron should be carefully considered before implementing these interventions. METHODS
INCLUSION AND ETHICS STATEMENT The BRISC trial was conceived within a long standing partnership between the International Center for Diarrheal Disease Research, Bangladesh (icddr,b), The
Walter and Eliza Hall Institute of Medical Research (WEHI, Australia), and The Peter Doherty Institute, University of Melbourne (Australia). icddr,b, WEHI and Doherty Institute researchers
co-developed the study design and methods and were named co-investigators on research grants. Local health workers were involved in design of study procedures. Through the BRISC trial
program, one Bangladeshi student has undertaken a PhD program at WEHI in Australia. The trial protocol, including stool collection and microbiome analyses, was approved by ethics committees
at the icddr,b and Melbourne Health, Melbourne, Australia, and overseen by an independent Data Safety and Monitoring Board. The full protocol is available with the main BRISC outcome
publication, and sub-study methods have been published previously.12,27 Parents or guardians of participants gave written informed consent prior to enrollment and were compensated for travel
costs for visits. STUDY DESIGN This microbiome sub-study recruited the final 1093 children from the BRISC trial (ACTRN12617000660381), set in rural parts of Rupganj Upazila, Bangladesh.
Briefly, BRISC was a three-arm, individually randomized, placebo-controlled trial that recruited 3300 children aged eight months and randomized them 1:1:1 to receive one of iron syrup (and
placebo MNPs); iron, zinc, ascorbic acid, vitamin A and folate-containing MNPs (and placebo syrup) or placebo (placebo syrup and placebo MNPs) daily for three months, reflecting WHO
guidelines.4,5 Iron interventions were 12.5 mg ferrous sulphate in syrup and 12.5 mg ferrous fumarate in MNPs. Individual block randomization method was used, with stratification by sex and
region (Union) to maintain balance between intervention arms. Blinding was achieved through the use of identical packaging that carried the randomization code for each participant. All
participants were required to take two interventions each day: a syrup and a powder. Participants in the trial attended major visits at three time points: baseline – 8 months of age; midline
– 11 months of age, immediately following completion of the intervention; and endline – 20 months of age. Data regarding daily adherence to the intervention and parent-reported diarrhea
incidence (using the WHO definition of ≥3 loose or liquid stools per day28) was obtained by field staff during weekly visits over the 3-month intervention period. The primary outcome was
child development after intervention, with a range of other developmental, anthropometric and laboratory outcomes also measured.29 For this sub-study, we aimed to include the final 1000
participants randomized during the final 6 months of recruitment. Samples were collected at baseline, midline and endline visits. We sought to perform 16S rRNA gene amplicon sequencing from
all stool samples. This allows analysis of microbiome alpha and beta diversity, and taxonomic profiling at the genus level. Additionally, in approximately one-third of samples, we performed
shotgun metagenomic sequencing (with priority given where canonical baseline, midline and endline samples were available), enabling us to further interrogate composition to species level and
to perform functional analysis. All sample collection, processing, and bioinformatic and statistical data analysis were planned while blinded to the intervention arm. STOOL COLLECTION AND
TRANSPORT At recruitment, guardians of sub-study participants were given instructions for collection of stool samples, and nappies and specimen containers were provided. Guardians were asked
to collect a sample of stool passed within 3 h of the visit. Study staff were sent to retrieve the samples if stool was passed after a visit. Once provided to the field team, samples were
stored on ice and transported to the central laboratory in the field within 3 h. There, aliquots were made, DNA/RNA Shield (Zymo Research) was added and aliquots were stored at −20 °C.
Specimens were then transported on dry ice to a −80 °C freezer at the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) facility in Dhaka, Bangladesh, then shipped on
dry ice to The Walter and Eliza Hall Institute of Medical Research (WEHI), Australia. LABORATORY MEASURES Venous blood samples were obtained at baseline, midline and endline visits.
Hemoglobin was measured in the study field office (HemoCue 301+), and ferritin and C-reactive protein (CRP) were measured at icddr,b. Remaining sample aliquots were transported frozen to
WEHI, Melbourne, where hepcidin ELISA assays (Intrinsic Life Sciences) were performed. DNA EXTRACTION FROM STOOL FOR MICROBIOME ANALYSIS Aliquots of samples were added to PowerBead Pro bead
tubes along with lysis buffer (Solution CD1 from DNeasy PowerSoil Pro Kit, comprising sodium thiocyanate >= 1 - <10% w/w) and were homogenized on a TissueLyser LT (Qiagen, Venlo,
Netherlands) for 10 min at maximum speed. DNA was then extracted with the DNeasy PowerSoil Pro Kit (Qiagen, Venlo, Netherlands) according to manufacturer protocol. 16S RRNA GENE AMPLICON
SEQUENCING The V4 hypervariable region of the 16S rRNA gene was targeted using universal primers (Integrated DNA Technologies) in an initial PCR reaction (_Supplementary Material_). Next,
dual-index barcodes (forward and reverse) were introduced in a second PCR reaction to join the common overhang sequences in the first reaction to enable sample identification during
analysis. This 16S rRNA amplicon sequencing method was developed by the WEHI genomics core facility and has been used successfully in previous applications.30 PCR plates each contained at
least one positive control (a healthy donor stool that underwent DNA extraction in parallel with sub-study samples), one negative DNA extraction control and several PCR negative controls
(blanks). Libraries, including negative and positive controls, were sequenced at the WEHI genomics core facility on a MiSeq instrument (Illumina, San Diego, USA) using a 300 bp paired-end
protocol with 600 cycles. SHOTGUN METAGENOMIC SEQUENCING A subset of samples and controls underwent shotgun metagenomic sequencing using the QIAseq FX DNA Library Kit (Qiagen, Venlo,
Netherlands), with input DNA and reagents used at 0·5x the specified volumes. DNA from samples was first enzymatically fragmented for eight minutes to achieve a fragment size of 450 base
pairs. Adapter ligation was then performed, followed by 8 cycles of PCR amplification. Qubit™ dsDNA Assay kit (Thermo Fisher Cat#Q32851) was used to quantify library concentrations, and
library size was calculated using D1000 ScreenTape (Agilent Cat# 5067-5582) in the TapeStation 4200 (Agilent Cat# G2991BA). Equimolar amounts of libraries were pooled and sequenced using
NovaSeq (Illumina, San Diego, USA). BIOINFORMATIC AND STATISTICAL ANALYSIS 16S rRNA amplicon sequence files were processed with the _DADA2_ package in RStudio.31 Data generated from this
pipeline were used to create an object in _phyloseq_.32 Samples with <500 reads and amplicon sequence variants (ASVs) with zero reads were removed, and ASVs were agglomerated at the genus
level before further analysis. Alpha diversity analysis used the _microbiomeSeq_ package in RStudio using Shannon and inverse Simpson indices.33 Group differences between trial arms were
calculated using pair-wise analysis of variance (ANOVA) for each diversity measure. Beta diversity calculation used _phyloseq_ to calculate and plot Bray-Curtis dissimilarity. _Vegan_ was
used to calculate numerical values and significance testing. _ANCOM-BC_ was used with default settings to calculate differential abundance at the genus level. Where an adjusted _p_-value is
shown, this was calculated using the Benjamini-Hochberg method to control for the FDR at a level of 5%.34 Taxa were also required not to be sensitive to pseudo-count addition in order to be
considered differentially abundant.35 Our primary analyses applied correction for FDR. However, because this study evaluates a safety outcome for which false positive and false negative
findings could have important public health impacts, we also explored and transparently reported findings based on the unadjusted _p_-value. This aligns with the conservative approach of the
main trial in which efficacy outcomes were adjusted for multiple testing, but safety outcomes were reported without adjustment.12,23 Shotgun metagenomic samples were processed using the
_bioBakery workflows_ tool, comprising the default quality control steps in _KneadData_ and taxonomic classification in _MetaPhlAn_.36 Taxonomic differential abundance by trial metadata was
performed at the species level using _MaAsLin2_ with a minimum species prevalence cut-off of 10% of samples.37 Centered log ratio normalization/transformation was performed on relative
abundance data in _MaAsLin2_, with outputs for differentially abundant species including coefficient (approximating log2-fold change), standard deviation, and adjusted _p_-values (using
Benjamini-Hochberg method with an FDR cut-off of 0·05).34 As above, our primary analyses applied adjustment for false discovery; in a further analysis, we explored and transparently report
unadjusted effects. Competitive phylum set enrichment analysis was conducted for two phyla previously reported to be impacted by iron interventions,8,9 using _cameraPR_ from the R package
_limma_ (version 3.56.2), using t-statistics for the ranking of species.38,39 This approach evaluates the rank order of expression intensity for species within a specific subset in contrast
to all other species. It examines whether there are statistically significant variations in the aggregate mean rank order for the entire set between different treatment conditions, thus
providing insight into the differential expression patterns induced by the treatments. Microbial functional pathways were profiled using _HUMAnN_ in the _bioBakery workflow_s using
_ChocoPhlAn UniRef90_, _MetaCyc_ and _MinPath_ databases.36,40,41,42 The resulting pathway abundance files were merged, unstratified from corresponding organisms, and renormalized from reads
per kilobase (RPKs) to relative abundances. _HUMAnN_ outputs were then processed using _MaAsLin2_ as above to calculate differential abundance at the unstratified pathway level according to
trial arm, adjusted for multiple comparisons using Benjamini-Hochberg method at a level of 5%. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio
Reporting Summary linked to this article. DATA AVAILABILITY The sequencing data used in this study have been deposited in the NCBI Sequence Read Archive (SRA) database under BioProject
accession PRJNA1081952 [https://www.ncbi.nlm.nih.gov/sra]. Source data are provided with this paper. CODE AVAILABILITY Code used to process sequencing files (in R for 16S rRNA files and
using Python for shotgun metagenomic files), perform statistical analysis and generate figures (in R) is based on the respective packages cited in the manuscript. Custom code was not used.
However, code is available from the authors by request. REFERENCES * World Health Organization. _The global prevalence of anaemia in 2011_ (World Health Organization, Geneva, 2015). * World
Health Organization. Global Health Observatory data repository: Anaemia in children estimates by country and WHO region (World Health Organization, Geneva, 2021). * United Nations Children’s
Fund (UNICEF). One is too many: Ending child deaths from pneumonia and diarrhea. (UNICEF, New York, 2016). * World Health Organization. Daily iron supplementation in children 6–23 months of
age. _e-Library of Evidence for Nutrition Actions (eLENA)_, Vol. 2016 (World Health Organization, Geneva, 2016). * WHO Guidelines Approved by the Guidelines Review Committee. Use of
multiple micronutrient powders for point-of-use fortification of foods consumed by infants and young children aged 6–23 months and children aged 2–12 years (World Health Organization,
Geneva, 2016). * Soofi, S. et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial.
_Lancet_ 6736, 60437 (2013). Google Scholar * Kortman, G. A., Raffatellu, M., Swinkels, D. W. & Tjalsma, H. Nutritional iron turned inside out: intestinal stress from a gut microbial
perspective. _FEMS Microbiol. Rev._ 38, 1202–1234 (2014). Article CAS PubMed Google Scholar * Jaeggi, T. et al. Iron fortification adversely affects the gut microbiome, increases
pathogen abundance and induces intestinal inflammation in Kenyan infants. _Gut_ 64, 731–742 (2015). Article CAS PubMed Google Scholar * Paganini, D. et al. Prebiotic
galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: a randomised controlled study in Kenyan infants. _Gut_ 66, 1956–1967 (2017). Article CAS
PubMed Google Scholar * Nemeth, E. & Ganz, T. Hepcidin and iron in health and disease. _Annu. Rev. Med._ 74, 261–277 (2022). * United Nations Children’s Fund (UNICEF). Home
Fortification with Multiple Micronutrient Powders for the Prevention of Iron Deficiency Anaemia in Early Childhood. Brief Guidance Note (UNICEF, New York, 2023). * Pasricha, S.-R. et al.
Benefits and risks of iron interventions in infants in rural Bangladesh. _N. Engl. J. Med._ 385, 982–995 (2021). Article CAS PubMed Google Scholar * Young, M. F. et al. Serum hepcidin is
significantly associated with iron absorption from food and supplemental sources in healthy young women. _Am. J. Clin. Nutr._ 89, 533–538 (2009). Article CAS PubMed Google Scholar *
Bah, A. et al. Hepcidin-guided screen-and-treat interventions against iron-deficiency anaemia in pregnancy: a randomised controlled trial in The Gambia. _Lancet Glob. Health_ 7, e1564–e1574
(2019). Article PubMed PubMed Central Google Scholar * Seyoum, Y., Baye, K. & Humblot, C. Iron homeostasis in host and gut bacteria - a complex interrelationship. _Gut Microbes_ 13,
1–19 (2021). Article CAS PubMed Google Scholar * WHO Guidelines Approved by the Guidelines Review Committee. _WHO guideline on use of ferritin concentrations to assess iron status in
individuals and populations_ (World Health Organization, Geneva, 2020). * Gutschow, P. et al. Clinical immunoassay for human Hepcidin predicts iron deficiency in first-time blood donors. _J.
Appl. Lab Med._ 5, 943–953 (2020). Article PubMed PubMed Central Google Scholar * Pasricha, S. R. et al. Net benefit and cost-effectiveness of universal iron-containing multiple
micronutrient powders for young children in 78 countries: a microsimulation study. _Lancet Glob. Health_ 8, e1071–e1080 (2020). Article PubMed PubMed Central Google Scholar * Zimmermann,
M. B. et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. _Am. J. Clin. Nutr._ 92, 1406–1415 (2010). Article
CAS PubMed Google Scholar * Cheung, Y. B. et al. Gut microbiota in Malawian infants in a nutritional supplementation trial. _Trop. Med. Int. Health_ 21, 283–290 (2016). Article PubMed
Google Scholar * Tang, M. et al. Iron in micronutrient powder promotes an unfavorable gut microbiota in Kenyan Infants. _Nutrients_ 9, 776 (2017). Article PubMed PubMed Central Google
Scholar * Dostal, A. et al. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in
South African children. _Br. J. Nutr._ 112, 547–556 (2014). Article CAS PubMed Google Scholar * Braat, S. et al. The Benefits and Risks of Iron interventions in Children (BRISC) trial:
statistical analysis plan. _F1000Research_ 9, 427 (2020). * Abt, M. C. & Pamer, E. G. Commensal bacteria mediated defenses against pathogens. _Curr. Opin. Immunol._ 29, 16–22 (2014).
Article CAS PubMed Google Scholar * Wegmuller, R. et al. Hepcidin-guided screen-and-treat interventions for young children with iron-deficiency anaemia in The Gambia: an individually
randomised, three-arm, double-blind, controlled, proof-of-concept, non-inferiority trial. _Lancet Glob. Health_ 11, e105–e116 (2023). Article CAS PubMed Google Scholar * Taniuchi, M. et
al. Etiology of diarrhea requiring hospitalization in Bangladesh by quantitative polymerase chain reaction, 2014–2018. _Clin. Infect. Dis._ 73, e2493–e2499 (2020). Article PubMed Central
Google Scholar * Baldi, A. et al. Community use of oral antibiotics transiently reprofiles the intestinal microbiome in young Bangladeshi children. _Nat. Commun._ 15, 6980 (2024). Article
CAS PubMed PubMed Central Google Scholar * World Health Organization. Diarrhoeal disease. (World Health Organization, Geneva, 2010). * Hasan, M. I. et al. Benefits and risks of Iron
interventions in children (BRISC): protocol for a three-arm parallel-group randomised controlled field trial in Bangladesh. _BMJ Open_ 7, e018325 (2017). Article PubMed PubMed Central
Google Scholar * Penington, J. S. et al. Influence of fecal collection conditions and 16S rRNA gene sequencing at two centers on human gut microbiota analysis. _Sci. Rep._ 8, 4386 (2018).
Article ADS PubMed PubMed Central Google Scholar * Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. _Nat. Methods_ 13, 581–583 (2016). Article
CAS PubMed PubMed Central Google Scholar * McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. _PLoS
ONE_ 8, e61217 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Ssekagiri, A. T., Sloan, W. & Ijaz, U. Z. microbiomeSeq: an R package for analysis of microbial
communities in an environmental context. in _ISCB Africa ASBCB Conference_ (Kumasi, Ghana, 2017). * Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and
powerful approach to multiple testing. _J. R. Stat. Soc. Ser. B_ 57, 289–300 (1995). Article MathSciNet Google Scholar * Lin, H. & Peddada, S. D. Analysis of compositions of
microbiomes with bias correction. _Nat. Commun._ 11, 3514 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Beghini, F. et al. Integrating taxonomic, functional, and
strain-level profiling of diverse microbial communities with bioBakery 3. _eLife_ 10, e65088 (2021). Article CAS PubMed PubMed Central Google Scholar * Mallick, H. et al. Multivariable
association discovery in population-scale meta-omics studies. _PLoS Comput. Biol._ 17, e1009442 (2021). Article CAS PubMed PubMed Central Google Scholar * Ritchie, M. E. et al. limma
powers differential expression analyses for RNA-sequencing and microarray studies. _Nucleic Acids Res._ 43, e47 (2015). Article PubMed PubMed Central Google Scholar * Wu, D. & Smyth,
G. K. Camera: a competitive gene set test accounting for inter-gene correlation. _Nucleic Acids Res._ 40, e133 (2012). Article CAS PubMed PubMed Central Google Scholar * UniProt
Consortium. UniProt: the Universal Protein Knowledgebase in 2023. _Nucleic Acids Res._ 51, D523–d531 (2023). Article Google Scholar * Caspi, R. et al. The MetaCyc database of metabolic
pathways and enzymes - a 2019 update. _Nucleic Acids Res._ 48, D445–d453 (2020). Article CAS PubMed Google Scholar * Ye, Y. & Doak, T. G. A parsimony approach to biological pathway
reconstruction/inference for genomes and metagenomes. _PLoS Comput. Biol._ 5, e1000465 (2009). Article ADS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We
thank the local field workers for their support, and all participants and their families for their involvement in the study. The authors gratefully acknowledge the WEHI Advanced Genomics
Facility for their support and assistance in this work. This work was supported by the Australian National Health and Medical Research Council GNT1103262 (B.A.B.), GNT1159151 (S.R.P.),
GNT1158696 (S.R.P.) and GNT2009047 (S.R.P.), and by The Geok Hua Wong Charitable Trust. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing
core/unrestricted support. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. AB was supported by a
Research Training Program Scholarship from the University of Melbourne and a stipend from WEHI. The study funder had no role in study design, data collection and analysis or manuscript
writing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia Andrew Baldi,
Sabine Braat, Mohammed Imrul Hasan, Cavan Bennett, Naomi Jones, Imadh Abdul Azeez, Pradip Kumar Roy, Ricardo Ataide, Danielle Clucas, Rory Bowden, Aaron Jex & Sant-Rayn Pasricha *
Department of Medical Biology, The University of Melbourne, Parkville, VIC, Australia Andrew Baldi, Mohammed Imrul Hasan, Cavan Bennett, Imadh Abdul Azeez, Danielle Clucas, Rory Bowden,
Aaron Jex & Sant-Rayn Pasricha * Centre for Epidemiology and Biostatistics, University of Melbourne School of Population and Global Health, Carlton, Carlton, VIC, Australia Sabine Braat
* Department of Infectious Diseases at the Peter Doherty Institute of Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia Sabine Braat, Ricardo Ataide &
Beverley-Ann Biggs * International Center for Diarrheal Diseases Research, Bangladesh (icddr,b), Dhaka, Bangladesh Mohammed Imrul Hasan, Mohammad Saiful Alam Bhuiyan & Jena Hamadani *
Advanced Technology and Biology Division, Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia Marilou Barrios, Stephen Wilcox & Rory Bowden * Faculty of
Science, University of Melbourne, Melbourne, VIC, Australia Pradip Kumar Roy & Aaron Jex * Diagnostic Haematology, The Royal Melbourne Hospital, Parkville, VIC, Australia Danielle Clucas
& Sant-Rayn Pasricha * Department of Health Promotion, Education, and Behavior, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA Leila M. Larson * Medical
Research Council Translational Immune Discovery Unit, MRC Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, England, UK Michael Zimmermann *
Victorian Infectious Diseases Service, Royal Melbourne Hospital, Parkville, VIC, Australia Beverley-Ann Biggs * Clinical Haematology at The Royal Melbourne Hospital and the Peter MacCallum
Cancer Centre, Parkville, VIC, Australia Sant-Rayn Pasricha Authors * Andrew Baldi View author publications You can also search for this author inPubMed Google Scholar * Sabine Braat View
author publications You can also search for this author inPubMed Google Scholar * Mohammed Imrul Hasan View author publications You can also search for this author inPubMed Google Scholar *
Cavan Bennett View author publications You can also search for this author inPubMed Google Scholar * Marilou Barrios View author publications You can also search for this author inPubMed
Google Scholar * Naomi Jones View author publications You can also search for this author inPubMed Google Scholar * Imadh Abdul Azeez View author publications You can also search for this
author inPubMed Google Scholar * Stephen Wilcox View author publications You can also search for this author inPubMed Google Scholar * Pradip Kumar Roy View author publications You can also
search for this author inPubMed Google Scholar * Mohammad Saiful Alam Bhuiyan View author publications You can also search for this author inPubMed Google Scholar * Ricardo Ataide View
author publications You can also search for this author inPubMed Google Scholar * Danielle Clucas View author publications You can also search for this author inPubMed Google Scholar * Leila
M. Larson View author publications You can also search for this author inPubMed Google Scholar * Jena Hamadani View author publications You can also search for this author inPubMed Google
Scholar * Michael Zimmermann View author publications You can also search for this author inPubMed Google Scholar * Rory Bowden View author publications You can also search for this author
inPubMed Google Scholar * Aaron Jex View author publications You can also search for this author inPubMed Google Scholar * Beverley-Ann Biggs View author publications You can also search for
this author inPubMed Google Scholar * Sant-Rayn Pasricha View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.B. designed the study, led the
fieldwork, performed the microbiome experiments, undertook bioinformatic data analysis, interpreted the findings, and wrote the manuscript. S.B. designed the study, undertook statistical
analysis, and wrote the manuscript. M.I.H. and J.H. designed the study, led the fieldwork, and interpreted the findings. M.B., N.J., S.W., and R.B. assisted in designing and performing the
microbiome experiments. A.J., I.A.A., and P.K.R. assisted with bioinformatic data analysis. L.M.L., C.B., R.A., D.C., M.Z., and A.J. interpreted the findings. M.S.A.B. assisted with
fieldwork. B.A.B. designed the study and the parent trial. S.R.P. designed the study and parent trial, interpreted the findings, and wrote the manuscript. All authors reviewed and approved
the final version of the manuscript. CORRESPONDING AUTHORS Correspondence to Andrew Baldi or Sant-Rayn Pasricha. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Thao Ho, Judd Walson and the other, anonymous, reviewer(s) for their contribution to the peer review of this
work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Baldi, A., Braat, S., Hasan, M.I. _et al._ Effects of iron
supplements and iron-containing micronutrient powders on the gut microbiome in Bangladeshi infants: a randomized controlled trial. _Nat Commun_ 15, 8640 (2024).
https://doi.org/10.1038/s41467-024-53013-x Download citation * Received: 09 April 2024 * Accepted: 27 September 2024 * Published: 05 October 2024 * DOI:
https://doi.org/10.1038/s41467-024-53013-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative