Play all audios:
ABSTRACT Regular endurance exercise training is an effective intervention for the maintenance of metabolic health and the prevention of many age-associated chronic diseases. Several
metabolic and inflammatory factors are involved in the health-promoting effects of exercise training, but regulatory mechanisms remain poorly understood. Cellular senescence—a state of
irreversible growth arrest—is considered a basic mechanism of aging. Senescent cells accumulate over time and promote a variety of age-related pathologies from neurodegenerative disorders to
cancer. Whether long-term intensive exercise training affect the accumulation of age-associated cellular senescence is still unclear. Here, we show that the classical senescence markers p16
and IL-6 were markedly higher in the colon mucosa of middle-aged and older overweight adults than in young sedentary individuals, but this upregulation was significantly blunted in
age-matched endurance runners. Interestingly, we observe a linear correlation between the level of p16 and the triglycerides to HDL ratio, a marker of colon adenoma risk and cardiometabolic
dysfunction. Our data suggest that chronic high-volume high-intensity endurance exercise can play a role in preventing the accumulation of senescent cells in cancer-prone tissues like colon
mucosa with age. Future studies are warranted to elucidate if other tissues are also affected, and what are the molecular and cellular mechanisms that mediate the senopreventative effects of
different forms of exercise training. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECT OF MIDLIFE EXERCISE ON LIPID METABOLISM IN AGING MICE: COMPARABLE TO LIFELONG EXERCISE, BETTER THAN
CEASING MIDLIFE EXERCISE Article Open access 11 April 2025 AGE-DEPENDENT IMPACT OF TWO EXERCISE TRAINING REGIMENS ON GENOMIC AND METABOLIC REMODELING IN SKELETAL MUSCLE AND LIVER OF MALE
MICE Article Open access 27 June 2022 EFFECTS OF SHORT-TERM MODERATE INTENSITY EXERCISE ON THE SERUM METABOLOME IN OLDER ADULTS: A PILOT RANDOMIZED CONTROLLED TRIAL Article Open access 04
May 2024 Regular physical exercise is one of the key pillars for health promotion since ancient times, although our ancestors did not know the biological processes responsible for its
beneficial effects. Data from animal and human randomized trials indicate that aerobic exercise training improves glucose tolerance, insulin sensitivity and lipid metabolism through multiple
mechanisms, including mitochondrial biogenesis, increased expression of the insulin responsive glucose transporter type 4 (GLUT4) and lipoprotein lipase in the skeletal muscle1,2. Regular
exercise training also promotes visceral fat loss, reduces inflammation and oxidative stress, and improves left ventricular diastolic function in overweight men and women1,3,4,5,6.
Accumulating data show that physical activity evokes profound metabolic and molecular responses not only in key metabolic organs (skeletal muscle, adipose tissues, and liver), but also in
tissues at high risk of neoplastic transformation. Epidemiological studies suggest an inverse association between physical activity and risk for 13 different types of cancer, in particular
for colon and breast cancer7,8,9. Regular exercise training can also improve prognosis among breast and colorectal cancer survivors10,11 by long-term regulation of various metabolic,
inflammatory and aging pathways that promote DNA and cellular repair, proteostasis, replicative stress resistance, and apoptosis of permanently damaged cells12. One of the fundamental
cellular mechanisms regulating aging and tumor development is cellular senescence13. Cellular senescence is a state of irreversible proliferative arrest triggered by diverse DNA or
mitochondrial damages to prevent propagation of damaged cells14. Senescent cells are characterized by the engagement of the Cyclin-dependent kinase inhibitors p16_Ink4a_ (p16) and p21CIP1
(p21)15, enhanced lysosomal activity, and a hypersecretory phenotype known as Senescence-Associated Secretory Phenotype (SASP). The SASP remain highly heterogeneous and dependent on various
intrinsic and extrinsic factors16,17. However, persistent senescent cells can cause chronic low-level inflammation and aberrant tissue growth and remodeling via SASP
factors18,19,20,21,22,23. Lifestyle factors can have consequences on induction and accumulation of cellular senescence. For example, caloric restriction (CR), a well-known and highly
conserved anti-aging and anti-cancer intervention, is associated to reduced accumulation of senescent cells in both mice and humans24,25. Interestingly, we have also recently shown that, at
least in mice, high-protein and high-fat diets lead to premature hepatic accumulation of hyper-inflammatory senescent cells26. Besides dietary approaches, it has been shown that a 12-week
exercise program reduces circulating senescence biomarkers in older adults27 and a recent human study suggests that the number of senescent cells of the adipose tissue is inversely
correlated to physical function in older women28. However, whether regular vigorous aerobic exercise can prevent accumulation of age-associated senescence, especially in highly proliferating
cancer prone tissues, remains controversial29. Here, we studied the effects of chronic intensive endurance exercise training on cardiometabolic health and candidate biomarkers of cell
senescence in colon mucosa biopsies of master athletes who ran an average 48 miles/week (range 30 to 90 miles/week) for an average of 21 years (range 5–35 years). Participants in this study
were endurance runners (mean age 57 ± 10 years) consuming usual American diets (EX); age- and sex-matched sedentary (regular exercise < 1 h per week) controls eating Western diets (WD-o);
and very young (mean age 24.3 ± 2 years) sedentary controls (WD-y) who should have negligible numbers of senescent cells. Average calorie intake in the EX group was 2806 ± 618 kcal/day, 13
and 7% higher than in the WD-o (2443 ± 407 kcal/day) and WD-y (2618 ± 712 kcal/day) groups, respectively (_p_ < 0.05 for EX vs. WD-o). The percentages of total energy intake derived from
protein, carbohydrate, and fat were similar among the groups: 15.7%, 51.8%, and 32.5%, respectively, in EX; 15.1%, 50.4%, and 32.8%, in WD-o; 17.2%, 48.2%, and 33.4% in WD-y. In Table 1, we
reported the study sample’s summary statistics, including the distribution of age, sex, body mass index, DXA body fat percentage and lean mass, and a range of fitness and cardiometabolic
parameters. BMI, body fat, resting heart rate, LDLc, total cholesterol HDL ratio, triglycerides, triglycerides to HDL ratio, fasting glucose, fasting insulin, HOMA-IR, and total white blood
cell count were significantly lower in the EX group than in the WD-o group (_p_ < 0.05). As expected, EX volunteers had significantly higher VO2max and HDLc than WD-o participants (_p_
< 0.05). To investigate the effects of long-term EX on biomarkers of cell senescence, we collected colon mucosa biopsies in a subset of 11 middle-aged (58.6 ± 8.3 years), weight-stable
and lean (BMI, 24.5 ± 2.8 kg/m2) master athletes, 10 age- and sex-matched nonobese (BMI, 27.1 ± 2.3 kg/m2) and 6 sedentary young (24.3 ± 2 years) and lean (BMI, 25.7 ± 1 kg/m2) control
subjects (Supplementary table 1). Because p16 is still considered one of the most relevant cell senescence markers in human specimens, and because p16 measurements were included in most of
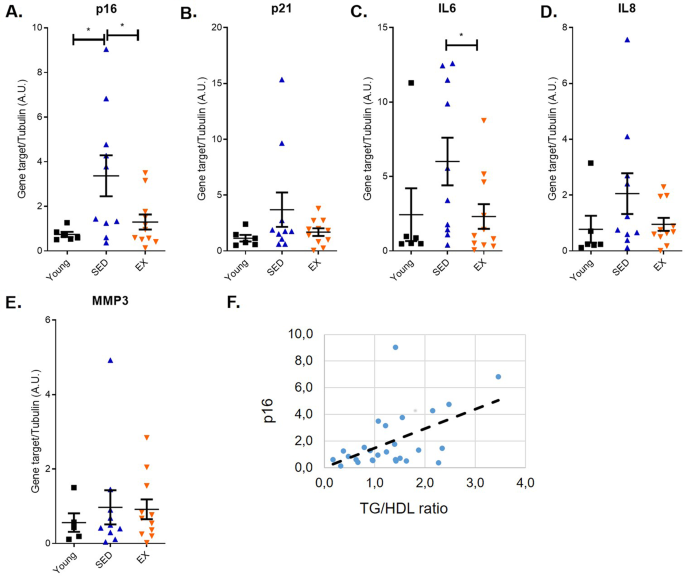
the previous studies on the effect of physical exercise on senescence markers, we measured its mRNA abundance. As expected, p16 levels were markedly higher in older than younger sedentary
individuals consuming Western diets (Fig. 1A). Strikingly, this upregulation was significantly blunted in endurance runners (Fig. 1A). p21 is another important regulator of cell cycle arrest
often dysregulated during senescence. Similar to what observed for p16, p21 levels were upregulated in older sedentary individuals compared to young sedentary or endurance runners (Fig.
1B). However, the difference in expression between groups did not reach statistical significance. The detrimental functions of cellular senescence are, at least partly, mediated by
pro-inflammatory secreted factors. The composition of pro-inflammatory SASP is variable, but IL-6 remains one of the most consistent SASP factors13. In accordance, IL-6 mRNA levels of colon
mucosa were significantly higher in old sedentary individuals consuming Western diet, whereas IL-6 levels in master athletes where low and similar to those of very young sedentary people
consuming Western diets (Fig. 1C). We then measured levels of two additional SASP factors, IL8 and MMP3. We observed a trend for the upregulation of MMP3 and IL8 in older sedentary
individuals compared to young sedentary and endurance runners, but there was no statistical significance (Fig. 1D, E). p16 mRNA levels correlated linearly with p21 (_r_ = 0.758; _p_ <
0.001) and IL-6 (_r_ = 0.798; _p_ < 0.001) mRNA levels (not shown). To evaluate potential links between the senescence burden and metabolic alterations, we then studied the association of
p16 levels with various metabolic parameters. Strikingly, p16 mRNA levels were linearly correlated with the triglycerides to HDL ratio, a well-accepted marker of metabolic syndrome,
coronary heart disease and colon adenoma risk (Fig. 1F)30,31,32. Interestingly, in patients with early-stage colorectal cancer, the combination of obesity and low HDL-cholesterol and high
triglycerides levels predicts worst cancer survival33. Our results are preliminary and limited, in particular in light of characterizing senescence-associated phenotypes and the type of
cells more affected by these changes. Nevertheless, the findings shown here suggest that chronic high-volume high-intensity, unlike low-volume34, endurance exercise can play a major role in
preventing the accumulation of senescent cells in cancer prone tissues like colon mucosa with age. This is important because data from transgenic mouse models, including our p16-3MR mouse19,
have shown that ablation of senescent cells is sufficient to systemically reduce inflammation, rejuvenate tissue functions, alleviate various age-related conditions, improve health and
extend longevity13. As senescent cells are likely contributor to dysregulated inflammatory responses, our data are in line with a previous report showing that the level of stress-induced
(acute exercise) inflammatory markers is reduced in muscle and blood of lifelong aerobic exercising older men compared to old healthy nonexercisers35. In addition, prevention of cell
senescence could partly explain the anti-cancer effect of lifelong aerobic exercise36. Future studies should be focusing on understanding which tissues are most affected, and what are the
molecular and cellular mechanisms that mediate the senopreventative effect of endurance exercise training. Moreover, it will be key to analyze individuals following different physical
exercise regimens, including resistance and high-intensity interval training. METHODS PATIENTS AND TISSUE COLLECTION This study sample includes three groups of volunteers, named from
hereafter EX, WD-o, and WD-y. The EX group consisted of 44 master athletes who ran at least 30 miles/week (range 30–90 miles/week) or expended similar amount of energy by cycling or
swimming, for at least the previous three years (range 3–35 years). The control (WD-o) group comprised 44 age- and sex-matched individuals reporting less than 1 hour of physical activity per
week, recruited from the St. Louis metropolitan area. A third control group with negligible numbers of senescent cells consisted of 6 very young (mean age 24.3 ± 2 years) sedentary controls
(WD-y) consuming Western diets. All the participants reported weight stability, defined as less than a 2-kg change in body weight in the preceding 6 months. Participants recorded all food
and beverage intake for 7 consecutive days. Food records were analyzed by our dietitian by using the NDS-R pro-gram (v.4.03_31) and used to define the western diet consumers. None of the
participants had evidence of chronic disease, smoked cigarettes, or took medications that could affect the outcome variables. The present study (HRPO #: 01-0804) was approved by the Human
Studies Committee of Washington University School of Medicine, and all subjects gave written informed consent before their participation. Height and body weight were obtained in the morning
after an overnight fast, with the participants wearing only underwear and a hospital gown. Total body fat mass and fat-free mass were determined by dual-energy X-ray absorptiometry (DXA; QDR
1000/w; Hologic). VO2max was determined by indirect calorimetry during an incremental exercise test to exhaustion37. Participants walked on a level treadmill at a pace that elicited 60–70%
of age-predicted maximal heart rate for a 5-minute warm-up. The speed was then set at the fastest comfortable pace, and the grade was increased 1–2% every 1–2 minutes until volitional
exhaustion, electrocardiographic changes, or other abnormalities that rendered it unsafe to continue. Blood pressure was measured with an oscillometric blood pressure monitor (Dinamap
Procare 200; GE Healthcare, Waukesha, WI) in the morning after a 12-h fast. In the EX group, blood pressure was measured at least 48 h after the last exercise session. A venous blood sample
was taken to determine lipid and hormone concentrations after subjects had fasted overnight. In the EX group, blood samples were obtained ≥48 h after the last exercise session. Measurement
of serum lipid and lipoprotein-cholesterol concentrations, glucose, insulin, C-reactive protein was performed in the Barnes-Jewish Hospital Laboratory by automated enzymatic,
radioimmunoassay and ELISA commercial kits. Insulin resistance was calculated using homeostasis model assessment of insulin resistance 9HOMA-IR = [fasting glucose (mmol/l) × fasting insulin
59]/22.5). RNA ISOLATION AND CDNA SYNTHESIS Biopsy specimens of normal-appearing sigmoidal colon mucosa were collected from a subset of 11 EX, 11 WD-o, and 5 WD-y volunteers in the morning
after an overnight fast and a preparation with an enema containing water. Colonic mucosal specimens were immediately washed in PBS and then flash-frozen in liquid nitrogen and stored at −80
°C until processed. Tissues were homogenized in liquid nitrogen. For each sample, 20 mg of tissue powder was used to isolate total RNA using the Isolate II RNA Mini Kit (Bioline). In all,
250–500 ng of RNA was reverse transcribed into cDNA using a kit (Applied Biosystems). REAL TIME-QPCR qRT-PCR reactions were performed with the LightCycler 480 Instrument II (Roche) using UPL
system (Roche) with a SensiFast Probe kit (Bioline). The reactions were carried out in a total volume of 10 _μ_l using a TaqMan assay. Tubulin was used for normalization of the CT values.
List of primers/probe combination: Tubulin: FW- cttcgtctccgccatcag; RV-cgtgttccaggcagtagagc; Probe #40 Cdkn2a (p16): FW-gagcagcatggagcctc; RV-cgtaactattcggtgcgttg; Probe #67 Cdkn1a (p21):
FW-tcactgtcttgtacccttgtgc; RV-ggcgtttggagtggtagaaa; Probe #32 IL6: FW-caggagcccagctatgaact; RV-gaaggcagcaggcaacac; Probe #45 IL8: FW-gagcactccataaggcacaaa; RV-atggttccttccggtggt; Probe #72
MMP3: FW-caaaacatatttctttgtagaggacaa; RV-ttcagctatttgcttgggaaa; Probe #36 STATISTICAL ANALYSIS One-way analysis of variance (ANOVA) was used to compare group variables, followed by Tukey
post-hoc testing when indicated. One-way ANOVA with Games-Howell was performed for distributions where equal variances could not be assumed. Pearson correlation was used to assess
associations between continuous variables. Statistical significance was set at _P_ < 0.05 for all tests. All data were analyzed by using SPSS software, version 28.0 (SPSS Inc, Chicago).
Data are expressed as mean ± SEM or SD (indicated). A difference with _P_-values < 0.05 were considered statistically significant. DATA AVAILABILITY Data are available from the
corresponding authors upon reasonable request. REFERENCES * Weiss, E. P. et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing
energy intake: a randomized controlled trial. _Am. J. Clin. Nutr._ 84, 1033–1042 (2006). Article CAS PubMed Google Scholar * Fontana, L. et al. Calorie restriction or exercise: effects
on coronary heart disease risk factors. A randomized, controlled trial. _Am. J. Physiol.-Endocrinol. Metab._ 293, E197–E202 (2007). Article CAS PubMed Google Scholar * Racette, S. B. et
al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. _J. Gerontol. Seri. A: Biol. Sci. Med. Sci._ 61, 943–950 (2006).
Article Google Scholar * Hofer, T. et al. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. _Rejuvenation Res._
11, 793–799 (2008). Article CAS PubMed PubMed Central Google Scholar * Fontana, L., Klein, S. & Holloszy, J. O. Effects of long-term calorie restriction and endurance exercise on
glucose tolerance, insulin action, and adipokine production. _Age_ 32, 97–108 (2010). Article CAS PubMed Google Scholar * Riordan, M. M. et al. The effects of caloric restriction- and
exercise-induced weight loss on left ventricular diastolic function. _Am. J. Physiol. Heart Circ. Physiol._ 294, H1174–H1182 (2008). Article CAS PubMed Google Scholar * Matthews, C. E.
et al. Amount and intensity of leisure-time physical activity and lower cancer risk. _J. Clin. Oncol._ 38, 686–697 (2020). Article PubMed Google Scholar * Moore, S. C. et al. Association
of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. _JAMA Int. Med._ 176, 816–825 (2016). Article Google Scholar * Giovannucci, E. et al. Physical
activity, obesity, and risk for colon cancer and adenoma in men. _Ann. Int. Med._ 122, 327–334 (1995). Article CAS PubMed Google Scholar * Meyerhardt, J. A. et al. Physical activity and
survival after colorectal cancer diagnosis. _J. Clin. Oncol._ 24, 3527–3534 (2006). Article PubMed Google Scholar * Zhong, S. et al. Association between physical activity and mortality in
breast cancer: a meta-analysis of cohort studies. _Eur. J. Epidemiol._ 29, 391–404 (2014). Article PubMed Google Scholar * McTiernan, A. Mechanisms linking physical activity with cancer.
_Nat. Rev. Cancer_ 8, 205–211 (2008). Article CAS PubMed Google Scholar * Borghesan, M., Hoogaars, W. M. H., Varela-Eirin, M., Talma, N. & Demaria, M. A senescence-centric view of
aging: implications for longevity and disease. _Trends Cell Biol._ 30, 777–791 (2020). Article CAS PubMed Google Scholar * Sharpless, N. E. & Sherr, C. J. Forging a signature of in
vivo senescence. _Nat. Rev. Cancer_ 15, 397–408 (2015). Article CAS PubMed Google Scholar * Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence.
_Genes Dev._ 24, 2463–2479 (2010). Article CAS PubMed PubMed Central Google Scholar * Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. _Curr.
Biol._ 27, 2652–2660. e2654 (2017). Article CAS PubMed PubMed Central Google Scholar * Casella, G. et al. Transcriptome signature of cellular senescence. _Nucleic Acids Res._ 47,
7294–7305 (2019). Article CAS PubMed PubMed Central Google Scholar * van Deursen, J. M. The role of senescent cells in ageing. _Nature_ 509, 439–446 (2014). Article PubMed PubMed
Central Google Scholar * Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. _Dev. Cell_ 31, 722–733 (2014). Article CAS
PubMed PubMed Central Google Scholar * Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. _Nature_ 530, 184–189 (2016). Article CAS PubMed
PubMed Central Google Scholar * Burd, C. E. et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. _Cell_ 152, 340–351 (2013). Article CAS PubMed
PubMed Central Google Scholar * Yamakoshi, K. et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. _J. Cell Biol._ 186, 393–407 (2009). Article CAS PubMed PubMed
Central Google Scholar * Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. _Nat. Med._ 24, 1246–1256 (2018). Article CAS PubMed PubMed Central Google
Scholar * Fontana, L. et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. _Aging Cell_ 17, e12746 (2018).
Article PubMed PubMed Central Google Scholar * Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. _Nat. Rev. Mol.
Cell Biol._ 23, 56–73 (2022). Article CAS PubMed Google Scholar * Nehme, J. et al. High dietary protein and fat contents exacerbate hepatic senescence and SASP in mice. _FEBS J_.
https://doi.org/10.1111/febs.16292 (2021). * Englund, D. A. et al. Exercise reduces circulating biomarkers of cellular senescence in humans. _Aging Cell_ 20, e13415 (2021). Article CAS
PubMed PubMed Central Google Scholar * Justice, J. N. et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women.
_J. Gerontol. A Biol. Sci. Med. Sci._ 73, 939–945 (2018). Article CAS PubMed Google Scholar * Chen, X. K. et al. Is exercise a senolytic medicine? A systematic review. _Aging Cell_ 20,
e13294 (2021). Article CAS PubMed Google Scholar * Manninen, V. et al. Joint effects of serum triglyceride and ldl cholesterol and Hdl cholesterol concentrations on coronary
heart-disease risk in the helsinki heart-study - implications for treatment. _Circulation_ 85, 37–45 (1992). Article CAS PubMed Google Scholar * McLaughlin, T. et al. Use of metabolic
markers to identify overweight individuals who are insulin resistant. _Ann. Int. Med._ 139, 802–809 (2003). Article PubMed Google Scholar * Bayerdorffer, E. et al. Decreased
high-density-lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. _Ann. Int. Med._ 118, 481–487 (1993). Article CAS PubMed Google
Scholar * Cespedes Feliciano, E. M. et al. Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. _J. Clin. Oncol._ 34, 3664 (2016). Article PubMed
PubMed Central Google Scholar * Kammire, M. S. et al. Does walking during chemotherapy impact p16(INK4a) levels in women with early breast cancer. _J. Clin. Lab. Anal._ 36, e24753
(2022). Article CAS PubMed PubMed Central Google Scholar * Lavin, K. M. et al. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. _J.
Appl. Physiol. (1985)_ 129, 1493–1504 (2020). Article CAS PubMed PubMed Central Google Scholar * Nilsson, M. I. et al. Lifelong aerobic exercise protects against inflammaging and
cancer. _PLoS ONE_ 14, e0210863 (2019). Article CAS PubMed PubMed Central Google Scholar * Kohrt, W. M. et al. Effects of gender, age, and fitness level on response of VO2max to
training in 60–71 yr olds. _J. Appl. Physiol. (1985)_ 71, 2004–2011 (1991). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS All the other authors have no financial
conflicts of interest to disclose. M.D. is supported from by grants from the Dutch Cancer Foundation (KWF) and from the Dutch Research Council (Vidi-NWO). L.F. is supported by grants from
the Bakewell Foundation, the Australian NHMRC Investigator Grant (APP1177797), Australian Youth and Health Foundation, and Philip Bushell Foundation. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * European Research Institute for the Biology of Ageing (ERIBA), University Medical Center Groningen (UMCG), University of Groningen (RUG), Groningen, Netherlands Marco Demaria
* Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA Beatrice Bertozzi, Valeria Tosti & Dayna S. Early * Geriatric Unit, Department of Internal Medicine
and Geriatrics, University of Palermo, Palermo, Italy Nicola Veronese * Geriatric Unit, AULSS 9 Scaligera, “Mater Salutis” Hospital, Legnago, Verona, Italy Francesco Spelta * Unit of
Dietetic and Clinical Nutrition, San Camillo, Forlanini Hospital, Rome, Italy Edda Cava * Department of Neurology, Washington University, St.Louis, MO, USA Laura Piccio * Brain and Mind
Centre, University of Sydney, Sydney, NSW, Australia Laura Piccio * Charles Perkins Centre, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, 2006, Australia Laura Piccio
& Luigi Fontana * Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, NSW, 2006, Australia Luigi Fontana * Department of Clinical and Experimental Sciences, Brescia
University School of Medicine, Brescia, Italy Luigi Fontana Authors * Marco Demaria View author publications You can also search for this author inPubMed Google Scholar * Beatrice Bertozzi
View author publications You can also search for this author inPubMed Google Scholar * Nicola Veronese View author publications You can also search for this author inPubMed Google Scholar *
Francesco Spelta View author publications You can also search for this author inPubMed Google Scholar * Edda Cava View author publications You can also search for this author inPubMed Google
Scholar * Valeria Tosti View author publications You can also search for this author inPubMed Google Scholar * Laura Piccio View author publications You can also search for this author
inPubMed Google Scholar * Dayna S. Early View author publications You can also search for this author inPubMed Google Scholar * Luigi Fontana View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: M.D. and L.F.; Data curation: M.D., B.B, N.V., F.S., E.C., V.T., L.P., D.S.E., and L.F.; Formal analysis: M.D. and L.F.;
Funding acquisition: M.D. and L.F.; Investigation: M.D., B.B, N.V., F.S., E.C., V.T., L.P., D.S.E., and L.F.; Sample collection: B.B, N.V., F.S., E.C., V.T., L.P., D.S.E., and L.F.;
Supervision: M.D. and L.F.; Writing – original draft: M.D. and L.F.; Writing – review & editing: M.D. and L.F. CORRESPONDING AUTHORS Correspondence to Marco Demaria or Luigi Fontana.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests but the following competing non-financial interests: M.D. is the scientific co-founder and served
as scientific advisor of Cleara Biotech and is member of the scientific advisory board of Oisin Biotechnologies. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Demaria, M., Bertozzi, B., Veronese, N. _et al._
Long-term intensive endurance exercise training is associated to reduced markers of cellular senescence in the colon mucosa of older adults. _npj Aging_ 9, 3 (2023).
https://doi.org/10.1038/s41514-023-00100-w Download citation * Received: 17 November 2022 * Accepted: 17 February 2023 * Published: 27 February 2023 * DOI:
https://doi.org/10.1038/s41514-023-00100-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative