Play all audios:
ABSTRACT We introduce a classification of breast tumors into seven classes which are more clearly defined by interpretable mRNA signatures along the PAM50 gene set than the five traditional
PAM50 intrinsic subtypes. Each intrinsic subtype is partially concordant with one of our classes, and the two additional classes correspond to division of the classes concordant with the
Luminal B and the Normal intrinsic subtypes along expression of the Her2 gene group. Our Normal class shows similarity with the myoepithelial mammary cell phenotype, including TP63
expression (specificity: 80.8% and sensitivity: 82.8%), and exhibits the best overall survival (89.6% at 5 years). Though Luminal A tumors are traditionally considered the least aggressive,
our analysis shows that only the Luminal A tumors which are now classified as myoepithelial have this phenotype, while tumors in our luminal class (concordant with Luminal A) may be more
aggressive than previously thought. We also find that patients with basal tumors surviving to 48 months exhibit favorable continued survival rates when certain markers for B lymphocytes are
present and poor survival rates when they are absent, which is consistent with recent findings. SIMILAR CONTENT BEING VIEWED BY OTHERS THE BREAST CANCER CLASSIFIER REFINES MOLECULAR BREAST
CANCER CLASSIFICATION TO DELINEATE THE HER2-LOW SUBTYPE Article Open access 20 February 2025 BREAST CANCER CONSENSUS SUBTYPES: A SYSTEM FOR SUBTYPING BREAST CANCER TUMORS BASED ON GENE
EXPRESSION Article Open access 12 October 2021 PROGNOSTIC AND PREDICTIVE PARAMETERS IN BREAST PATHOLOGY: A PATHOLOGIST’S PRIMER Article 05 November 2020 INTRODUCTION Multiparametric genetic
tests such as the PAM50/Prosigna Risk of Recurrence (ROR) for breast cancer prognostication are becoming commonplace.1,2 However, due to limited accuracy and poor concordance with biological
phenotypes, their clinical utility is still under investigation.3 In this paper, we address these issues in the context of one of the most prevalent assays, the PAM50 ROR, which is mainly
driven by an intrinsic subtype classification along a 50-gene mRNA expression profile. We reclassify these profiles using topological data analysis, incorporating prior knowledge of
biological phenotype (basal/luminal stratification). Unlike the five traditional PAM50 intrinsic subtypes, our seven classes are accurately defined by clear patterns of activation and
inactivation of gene groups directly interpretable in terms of specific normal mammary cell types: basal, luminal/ER, myoepithelial, and Her2-related gene groups. The basal/luminal
terminology refers to mammary cell differentiation from basal–epithelial cells near the basement membrane to the more differentiated luminal–epithelial cells near the lumen or ducts. It was
the basis for the systematic molecular classification of breast cancer initiated by Perou et al.4 Myoepithelial refers to a mammary cell type playing a key role in breast duct secretion.5,6
Overexpression of Her2 (ERBB2) and a group of related genes marks the Her2 + cohort well-known since the 1990s for highly favorable response to the drug trastuzumab (herceptin). Figure 1
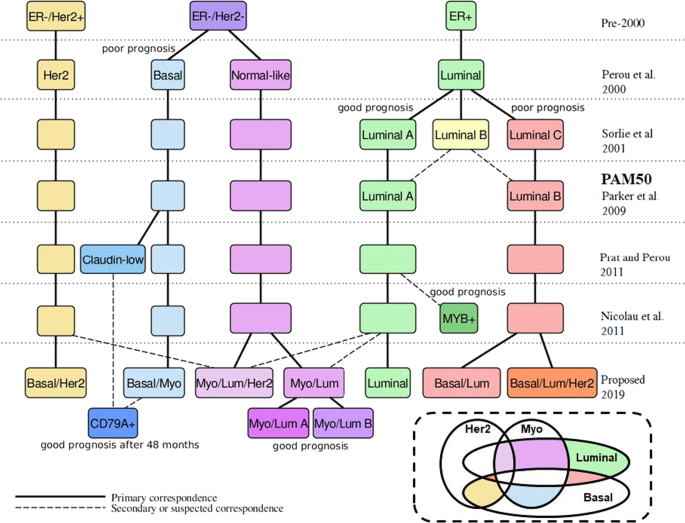
summarizes the history of the molecular classification and our contribution. Table 1 lists the new classes. RESULTS CLEARLY DEFINED 50-GENE SIGNATURES The signature classes we defined show
partial concordance with the PAM50 subtypes, with a Normalized Mutual Information (NMI) of 0.19 (29.1 times the maximum NMI found in 10,000 random permutation bootstrapping trials) (Fig. 2).
However, our classes show tighter clustering along the 50-gene profile: the k-mean for the PAM50 subtypes is 87.9% of the total variance, and for our classification is only 82.7% (both
using the L1 norm). To assess the quality of the signatures themselves, we consider the average silhouette width7 of each class. The silhouette width is the average distance _a_(_i_) between
a sample _i_ and the cluster to which it belongs subtracted from the smallest average distance _b_(_i_) between _i_ and the other clusters, normalized by max (_a_(_i_), _b_(_i_)). The
average silhouette width over a given cluster (abbreviated SW) measures the tightness of the cluster with respect to the clustering scheme, with larger SW (closer to 1) indicating a good
cluster and smaller SW (closer to −1) indicating a poor cluster. Our Luminal class SW = 0.151 is greater than the PAM50 Luminal A SW by 0.107; Luminal/Basal SW = 0.131 is greater than the
PAM50 Luminal B SW by 0.112; Myo/Luminal SW = 0.0422 is greater than the PAM50 Normal SW by 0.0432 (silhouette widths range from −1 to 1). The SWs of our Her2 and Basal/Myo SWs are very
close to the SW of the PAM50 Her2 and Basal subtypes. As shown in Fig. 2, the main example of a clear new signature is the heterogeneous expression of the myoepithelial gene group in the
PAM50 Luminal A subtype, resolved by division into Luminal and Myo/Luminal classes. One exception is that the Basal/Her2 class binds together the PAM50 Her2 with several PAM50 Luminal B
samples. However, the Luminal B here clearly differ from the Her2 by the presence of Luminal markers, so to address this we divide this class into Basal/Her2 and Basal/Her2/Luminal. Also,
the two myoepithelial gene groups are small and closely related, so we merge them together into a single myoepithelial group and accordingly merge the classes denoted Myo/Luminal A and
Myo/Luminal B. The seven resulting signatures are shown in Table 1. MYO/LUMINAL CLASS WITH GOOD SURVIVAL The Kaplan–Meier survival analysis of the new classes is shown in Fig. 3 for both
1904 METABRIC and 1082 TCGA samples (Figs 3–5). The plots show that the Myo/Luminal class exhibits the greatest survival rate, even greater than PAM50 Luminal A (the log-rank test for
statistically significant difference between Normal and Myo/Luminal survival curves yields _p_ = 0.003). Many of the Myo/Luminal tumors are designated PAM50 Luminal A, and since the Luminal
A subtype is already the one with the best prognosis in the PAM50 scheme, we conclude that the Myo/Luminal class preferentially selects from Luminal A subtype the patients with especially
good prognosis even among Luminal A. The Myo/Luminal and Myo/Luminal/Her2 subtypes have signatures with the most new features. Kaplan–Meier analysis shows that the Myo/Luminal A
(FOXC1-/MIA-/PHGDH-) phenotype has the best prognosis of all, with 93% survival at 5 years (Fig. 4). To investigate the Myo/Luminal class further, we drew upon the classification of normal
mammary cell types of Santagata et al.5 in terms of marker genes/proteins ESR1, AR, VDR, KRT5, MKI67, KRT18, MME, SMN1, and TP63. Figure 5 shows the Mapper analysis of the 290 normal breast
tissue samples of the GTEx RNA expression database.8 We found normal tissue expression patterns were similar to one of our class’ signatures along the PAM50, and also similar to one of the
cell type patterns of Santagata et al.5 along their marker genes. One of the clearest patterns was activation of only the basal gene group along the normal cell type denoted L1,
characterized by expression of the proliferation marker MKI67. In addition, a clear subset of samples, displaying a superposition of the pattern of normal myoepithelial cell-type M2 and
normal cell-type L7 (KRT5+/VDR+), also displayed the signature Myo/Luminal/Her2. The main characteristic of M2 is expression of TP63. We found that TP63 expression can be used as a single
marker for the Myo/Luminal class (specificity: 80.8%, sensitivity: 82.8%). BASAL/MYOEPITHELIAL (TRIPLE-NEGATIVE) SUBCLASS WITH IMMUNE-RELATED SURVIVAL ADVANTAGE Since the Myo/Luminal class
is heterogeneous with respect to FOXC1, MIA, and PHGDH expression, we expected that FOXC1+/MIA+/PHGDH+ would be associated with a more aggressive phenotype (Fig. 6). After all, these genes
are highly expressed in the PAM50 Basal subtype (Basal/Myo). We found that while this is true for the first 48 months after diagnosis, the FOXC1+, MIA+, and PHGDH+ phenotypes all showed very
favorable survival rates contingent on survival to 48 months (Fig. 6). We hypothesized that this phenomenon might generalize to the PAM50 Basal subtype. To test this, we sought genes from
the set of 18,543 genes available for the METABRIC cohort which would separate the long-term and short-term survivors in the FOXC1+/MIA+/PHGDH+ group. The 100 most significant genes with
respect to the _t_ test for difference of mean expression (−log10(_p_) value > 6.7) included the genes coding for the B-cell antigen receptor complex-associated protein alpha and beta
chains, the B-cell-specific coactivator OBF-1, the pre-B-lymphocyte-specific protein-2, and B-cell maturation factor (CD79A, CD79B, POU2AF1, IGLL1, and TNFRSF17), as well as CD38, expressed
by many immune cells. (In fact, CD79A is one of the major positive expression markers for the Claudin-low subtype introduced by Prat and Perou.9 The Claudin-low subtype and our
CD79A+/CD38+/IGLL1+ type are both subgroups of the Basal group). Figure 6 shows that expression of each of CD79A, CD38, and IGLL1 strongly stratifies the Basal tumors into a poor prognosis
group and another group with much better prognosis after 48 months. DISCUSSION Only certain combinations of the elementary phenotypes we identified, Basal, Luminal, Myoepithelial, and Her2,
are observed in breast tumors. For example, the Luminal/Basal, Basal/Myo, and Myo/Luminal are all observed, but the combination Luminal/Basal/Myo is not. We conclude that in the tumor
development process, the activation of any two of the Luminal, Basal, and Myoepithelial gene groups precludes the further activation of the third. Some of the genes in the new Myoepithelial
gene group (denoted b in Fig. 2) concurrently stratify the Myo/Lum class. These include FOXC1, MIA, and PHGDH. The protein product of PHGDH, phosphoglycerate dehydrogenase, is a key enzyme
participating in biosynthesis of serine. Maddocks et al.10 find that functioning p53 is required for complete activation of the serine synthesis pathway in human cancer cells. Since the
Myo/Luminal tumors have a very low TP53 mutant rate of only 15.6% in comparison to 78% for Basal/Myo, the Myo/Luminal tumors, with functioning p53, are probably capable of synthesis of
serine in response to serine starvation. Only the Myo/Luminal B subclass of Myo/Luminal actually expresses PHGDH, suggesting serine synthesis and metabolism. Since Myo/Luminal A exhibits
better survival rates than Myo/Luminal B, our findings are consistent with the results of Labuschagne et al.11 and Amelio et al.12 implicating serine metabolism in promoting tumor growth.
TP63 is one of the myoepithelial markers in the work of Santagata et al.5 and also a key marker for our Myo/Luminal class. From the Kaplan–Meier analysis in Fig. 4, we conclude that TP63
expression confers a survival advantage even greater than the well-known survival advantage conferred by PGR expression across the whole METABRIC cohort. The PAM50 subtype most resembling
the Myo/Luminal class is Normal-like. The status of the Normal-like subtype has been uncertain since its introduction by Perou et al.4 It is often thought to represent non-cancer tissue
which is incidentally present in bulk tissue samples. For example, the PAM50 classifier uses actual normal tissue samples to train the centroid of the Normal class. However, in our analysis
all of the classes of breast cancer show similarity to some combination of normal mammary cell types. We found a significantly lower death rate after 4 years for patients with basal tumors
expressing key B-lymphocyte-related markers CD79A, CD79B, POU2AF1, IGLL1, and TNFRSF17. This group is 80.3% of all patients with basal tumors surviving to 4 years. We conclude that the
remaining 19.7% of these patients, with basal tumors lacking these markers, are still at high risk of mortality. This observation is consistent with the finding of Rueda et al.13 that a
certain subgroup of triple-negative breast cancers can be defined which rarely recurs after 5 years. Regarding future work, responses to specific drugs or therapies should be investigated to
decide whether some patients with Luminal but not Myo/Luminal tumors are under treated. Moreover, future work should address the question of why the four main gene groups appear. One
possible explanation is that the four prototypical expression patterns Luminal, Basal, Myoepithelial, and Her2-related represent types of clones derived from an original transformation, and
the combinations of these prototypes correspond to a certain clonal mixture. Another possibility is that the observed expression patterns are superpositions of actual tumor expression,
expression of tumor microenvironmental normal cells with types related to the four prototypes, or expression patterns similar to original normal ancestor cells. New techniques of single-cell
sequencing, potentially in conjunction with tumor-level spatial mapping, may provide answers to these questions. Finally, the differential prognosis among triple-negative tumors observed
with respect to the B-lymphocyte-related stratification suggests that the immune systems of ~51% of patients with triple-negative tumors can naturally and reliably mount a successful
response to the tumor. If this hypothesis is correct, a longitudinal study monitoring the immune system of triple-negative patients should be able to discover exactly what response is
mounted, which could lead to potential new therapies that induce this natural response. METHODS TOPOLOGICAL DATA ANALYSIS Topological data analysis (TDA) methods, employing ideas from the
mathematical field of topology, have gained popularity in recent years. More precisely, discrete algorithmic counterparts of topological concepts have emerged in response to the availability
of large data sets harboring hidden structures. Mapper,14 a discrete analogue of a Morse-theoretic analysis of a manifold with respect to a height function, or “filter” function, has
received particular attention with regards to both its theoretical foundations15,16 and, following Nicolau et al.,17 its application to cancer genomics.18,19,20 Mapper builds a graphical
summary of a given sample set with respect to a chosen stratification (filter) function. We use three sample sets: TCGA, METABRIC,21,22 and GTEx.8 The 1082 TCGA and 1904 METABRIC mRNA
expression _z_-score data sets along the PAM50 gene set were retrieved from cBioPortal.23,24 The 290 GTEx normal breast data set was downloaded from the GTEx portal; metadata supporting data
files may be found in.25 Due to the retrospective nature of this study using only publicly available data, ethics approval for the study was not required. FILTER FUNCTION The “filter
function” or initial stratification is taken to be a basal–luminal epithelial differentiation score, calculated as the average expression _z_-score of luminal–epithelial markers (XBP1,
FOXA1, GATA3, ESR1, and ANXA9) minus the average expression _z_-score of basal–epithelial markers (KRT17, KRT5, DST, ITGB4, LAMC2, CDH3, LAD1, and ITGA7). Selected largely on the basis of
Perou et al.,4 the basal markers are all associated with anchorage of epithelial cell layers to the basement membrane, while the luminal markers are all expressed in well-differentiated or
mature luminal epithelial cells. The Mapper graph and 50-gene signatures determined from the METABRIC breast tumor samples are shown in Fig. 7. Correlation-based clustering along small
contiguous subsets with respect to the graph yielded the five main gene groups. A simple classifier was constructed from the table of observed signatures (see Fig. 7) as follows: For a given
sample and a given signature or profile, the average values for each gene group are calculated, then added together with the signature signs as weights. The resulting number is a similarity
score between the sample and the signature. The sample is assigned to the highest-scoring signature. Finally, the classes and gene groups shown in Fig. 2 were adjusted: The two
myoepithelial gene groups were merged, the Myo/Luminal A and Myo/Luminal B classes were merged as a result, and Luminal expression was used to delineate classes Basal/Her2 and
Basal/Luminal/Her2. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data
generated and analysed during this study are publicly available in GitHub as described in the following data record: https://doi.org/10.6084/m9.figshare.9199289.25 The study used three
publicly available datasets and the raw data can be accessed from cBioPortal at https://identifiers.org/cbioportal:brca_metabric (METABRIC data) and at
https://identifiers.org/cbioportal:brca_tcga_pan_can_atlas_2018 (TCGA data). The raw normal breast data can be accessed from the GTEx portal at https://gtexportal.org/home/datasets.8,21,22
CODE AVAILABILITY The code written in Python and R is available upon request. The analysis methodology is described in detail in the Supplementary Information file. REFERENCES * Duffy, M. et
al. Clinical use of biomarkers in breast cancer: updated guidelines from the european group on tumor markers (egtm). _Eur. J. Cancer_ 75, 284–298 (2017). Article CAS Google Scholar *
Coates, A. S. et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. _Ann.
Oncol._ 26, 1533–1546 (2015). Article CAS Google Scholar * Untch, M. et al. Primary therapy of patients with early breast cancer: evidence, controversies, consensus. _Geburtshilfe und
Frauenheilkd._ 75, 556–565 (2015). Article CAS Google Scholar * Perou, C. M. et al. Molecular portraits of human breast tumours. _Nature_ 406, 747–752 (2000). Article CAS Google Scholar
* Santagata, S. et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. _J. Clin. Invest._ 124, 859–870 (2014). Article CAS Google Scholar * Gudjonsson, T.,
Adriance, M. C., Sternlicht, M. D., Petersen, O. W. & Bissell, M. J. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. _J. Mammary Gland Biol.
Neoplasia_ 10, 261–272 (2005). Article Google Scholar * Rousseeuw, P. J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. _J. Comput. Appl. Math._ 20,
53–65 (1987). Article Google Scholar * Lonsdale, J. et al. The genotype-tissue expression (GTEx) project. _Nat. Genet._ 45, 580–585 (2013). Article CAS Google Scholar * Prat, A. &
Perou, C. M. Deconstructing the molecular portraits of breast cancer. _Mol. Oncol._ 5, 5–23 (2011). Article CAS Google Scholar * Maddocks, O. D. et al. Serine starvation induces stress
and p53-dependent metabolic remodelling in cancer cells. _Nature_ 493, 542–546 (2013). Article CAS Google Scholar * Labuschagne, C. F., van den Broek, N. J., Mackay, G. M., Vousden, K. H.
& Maddocks, O. D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. _Cell Rep._ 7, 1248–1258 (2014). Article CAS Google Scholar * Amelio, I.,
Cutruzzola, F., Antonov, A., Agostini, M. & Melino, G. Serine and glycine metabolism in cancer. _Trends Biochem. Sci._ 39, 191–198 (2014). Article CAS Google Scholar * Rueda, O. M.
et al. Dynamics of breast-cancer relapse reveal late-recurring er-positive genomic subgroups. _Nature_. https://doi.org/10.1038/s41586-019-1007-8 (2019). Article PubMed Google Scholar *
Singh, G., Memoli, F. & Carlsson, G. Topological methods for the analysis of high dimensional data sets and 3D object recognition. In _Eurographics Symposium on Point-Based Graphics_
(eds. Botsch, M., Pajarola, R., Chen, B. & Zwicker, M.) (The Eurographics Association, 1–11 2007). * Carrière, M. & Oudot, S. Structure and stability of the one-dimensional mapper.
_Found. Comput. Math._ https://doi.org/10.1007/s10208-017-9370-z (2017). Article Google Scholar * Dey, T. K., Mémoli, F. & Wang, Y. Multiscale Mapper: Topological Summarization via
Codomain Covers. In: Krautgamer, R. (ed.) _Proc. Twenty-Seventh Annual ACM-SIAM Symposium on Discrete Algorithms_, 997–1013 (SIAM Publications, Philadelphia, PA, 2016). * Nicolau, M.,
Levine, A. J. & Carlsson, G. Topology based data analysis identifies a subgroup of breast cancers with a unique mutational profile and excellent survival. _Proc. Natl Acad. Sci. USA_
108, 7265–7270 (2011). Article CAS Google Scholar * Lum, P. Y. et al. Extracting insights from the shape of complex data using topology. _Sci. Rep._ 3, 1236 (2013). Article CAS Google
Scholar * Lockwood, S. & Krishnamoorthy, B. Topological features in cancer gene expression data. Preprint at: http://arxiv.org/abs/1410.3198v1 (2014). * Jeitziner, R. et al. Two-tier
mapper: a user-independent clustering method for global gene expression analysis based on topology. Preprint at: https://arxiv.org/pdf/1801.01841.pdf (2017). * Curtis, C. et al. The genomic
and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. _Nature_ 486, 346–352 (2012). Article CAS Google Scholar * Pereira, B. et al. The somatic mutation
profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. _Nat. Commun._ 7, 11479 (2016). Article CAS Google Scholar * Gao, J. et al. Integrative analysis of
complex cancer genomics and clinical profiles using the cBioPortal. _Sci. Signal_ 6, pl1 (2013). Article Google Scholar * Cerami, E. et al. The cBio cancer genomics portal: an open
platform for exploring multidimensional cancer genomics data. _Cancer Disco._ 2, 401–404 (2012). Article Google Scholar * Mathews, J. C. et al. Metadata supporting data files of the
related article: robust and interpretable PAM50 reclassification exhibits survival advantage for myoepithelial and immune phenotypes. _figshare_. https://doi.org/10.6084/m9.figshare.9199289
(2019). * Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. _Proc. Natl Acad. Sci. USA_ 98, 10869–10874 (2001). Article
CAS Google Scholar * Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. _J. Clin. Oncol._ 27, 1160–1167 (2009). Article Google Scholar
Download references ACKNOWLEDGEMENTS This study was supported by AFOSR grant (FA9550-17-1-0435), NIA grant (R01-AG048769), MSK Cancer Center Support Grant/Core Grant (P30 CA008748), and a
grant from Breast Cancer Research Foundation (grant BCRF-17-193). The article was previously published as a preprint: Ref. Mathews, J. C. et al. Robust and interpretable PAM50
reclassification exhibits survival advantage for myoepithelial andimmune phenotypes. Preprint at: https://doi.org/10.1101/480723v3 (2019). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, 10065, USA James C. Mathews, Saad Nadeem, Maryam Pouryahya & Joseph O. Deasy * Institute for Advanced
Study, School of Natural Sciences, Princeton, NJ, 08540, USA Arnold J. Levine * Departments of Computer Science & Applied Mathematics, Stony Brook University, Stony Brook, NY, 11794,
USA Allen Tannenbaum Authors * James C. Mathews View author publications You can also search for this author inPubMed Google Scholar * Saad Nadeem View author publications You can also
search for this author inPubMed Google Scholar * Arnold J. Levine View author publications You can also search for this author inPubMed Google Scholar * Maryam Pouryahya View author
publications You can also search for this author inPubMed Google Scholar * Joseph O. Deasy View author publications You can also search for this author inPubMed Google Scholar * Allen
Tannenbaum View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.C.M. performed research and analyzed data. J.C.M. and S.N. drafted the paper.
A.L. contributed a number of key suggestions for the present version. S.N., A.L., M.P., J.O.D. and A.T. edited the paper. A.T. and J.O.D. directed the research. CORRESPONDING AUTHOR
Correspondence to Allen Tannenbaum. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mathews, J.C., Nadeem, S.,
Levine, A.J. _et al._ Robust and interpretable PAM50 reclassification exhibits survival advantage for myoepithelial and immune phenotypes. _npj Breast Cancer_ 5, 30 (2019).
https://doi.org/10.1038/s41523-019-0124-8 Download citation * Received: 17 April 2019 * Accepted: 05 August 2019 * Published: 09 September 2019 * DOI:
https://doi.org/10.1038/s41523-019-0124-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative